Abstract
Objective:
To determine the frequency and type of histopathologic lesions in placentas delivered by women with a normal pregnancy outcome.
Methods:
This retrospective cohort study included placental samples from 944 women with a singleton gestation who delivered at term without obstetrical complications. Placental lesions were classified into the following four categories as defined by the Society for Pediatric Pathology and by our Unit: 1) acute placental inflammation, 2) chronic placental inflammation, 3) maternal vascular malperfusion, and 4) fetal vascular malperfusion.
Results:
1) 78% of placentas had lesions consistent with inflammatory or vascular lesions; 2) acute placental inflammatory lesions were the most prevalent, observed in 42.3% of the placentas, but only 1.0% of the lesions were severe; 3) acute inflammatory lesions were more common in the placentas of women with labor than in those without labor; 4) chronic inflammatory lesions of the placenta were present in 29.9%; and 5) maternal and fetal vascular lesions of malperfusion were detected in 35.7% and 19.7%, respectively. Two or more lesions of maternal or fetal vascular features consistent with malperfusion (high-burden lesions) were present in 7.4% and 0.7%, respectively.
Conclusion:
Most placentas had lesions consistent with inflammatory or vascular lesions, but severe and/or high-burden lesions were infrequent. Mild placental lesions may be interpreted as either acute changes associated with parturition or representative of a subclinical pathological process that did not affect the course of pregnancy.
Keywords: acute histologic chorioamnionitis, amniotic fluid infection, chronic chorioamnionitis, chronic deciduitis, chronic placental inflammation, fetal vascular malperfusion, funisitis, maternal vascular malperfusion, normal or uncomplicated pregnancy, villitis of unknown etiology
INTRODUCTION
In the evaluation of pregnancy complications, neglect of the placenta in developed countries has become a thing of the past. In 1997, the American College of Pathologists published guidelines for examining placentas in high-risk pregnancies (1); subsequently, the Perinatal Section of the Society for Pediatric Pathology in the United States published a series of reports led by Redline et al. about the classification and reproducibility of the diagnosis of placental lesions (2–9). Such lesions were broadly categorized as those consistent with 1) amniotic fluid infection (2, 9), 2) maternal vascular underperfusion (2, 8), and 3) fetal vascular underperfusion (2, 7), to which was added a fourth category—chronic placental inflammation (5, 10–20). During a workshop organized in Amsterdam in 2014, the recommendations for sampling and the definitions of placental lesions were updated (21).
However, to date, there is very little information on how frequently histologic inflammatory or vascular lesions are found in placentas of patients with normal pregnancy outcomes (22–24), and this information is required to interpret the significance of placental histopathologic findings in patients who have obstetrical complications. Moreover, this information may also be useful in counseling patients about the outcome of the index pregnancy (25–40), the prognosis for subsequent pregnancies (41–47), and in a medicolegal context (48–56). Often, the placenta has been considered a “witness” in litigation (57).
Therefore, the objective of this study was to determine the frequency with which different pathological lesions can be detected histologically in the placenta in a large cohort of women who had a singleton pregnancy, no obstetrical complications, and who delivered appropriate-for-gestational-age neonates at term without perinatal complications.
METHODS
Study Design
This report is based on a retrospective analysis of the placental histological lesions diagnosed by pediatric pathologists experienced in the assessment of placental lesions (CJK, JSK, YMK, SMJ, and FQ). Subjects recruited into a cohort study between January 11, 2006, and June 11, 2014, had no medical or obstetrical complications and delivered a singleton, term (37–42 weeks) neonate with a 5-minute Apgar score ≥7 and a birthweight between the 10th and 90th percentiles of a United States’ reference population (58). Patients provided written informed consent before the collection of placentas. The collection and use of clinical data and specimens were approved by the Human Investigation Committee of Wayne State University (WSU) and the Institutional Review Board of the National Institute of Child Health and Human Development (NICHD). Pathologic examination of the placentas was undertaken for research purposes.
Placental Pathology Examination
Placentas were collected in the Labor and Delivery Unit or Operating Room at Hutzel Women’s Hospital of the Detroit Medical Center (DMC) and transferred to the Perinatology Research Branch (PRB) laboratory. For all placentas, the maternal surface (basal plate), fetal surface (chorionic plate), and the umbilical cord were photographed prior to dissection. The gross morphologic description and placental measurements were documented, and the placental weight was recorded before and after the trimming of the chorioamniotic membranes and umbilical cord. Sampling of the placentas was conducted according to protocols of the PRB and the Department of Pathology of the WSU School of Medicine and the DMC.
Samples from the placenta were obtained using two approaches:
1) Systematic random sampling at the PRB and 2) targeted sampling in the laboratory of the WSU/DMC Department of Pathology.
A). Systematic random sampling at the PRB
1). Sampling of chorioamniotic membranes and the umbilical cord:
Samples were taken from the extraplacental membranes in the form of membrane rolls (n=3), and the umbilical cord (n=3). The chorioamniotic membrane roll was dissected into a 30-mm piece and placed into a labeled specimen cup containing ~20 mL of 10% formalin. The umbilical cord was bisected at 3 cm from the point of insertion into the chorionic plate, followed by the collection of three samples from the umbilical cord at these locations: 1) the distal end attached to the placenta, 2) the middle portion, and 3) the proximal end closest to the fetus.
2). Sampling of the placental disc:
Systematic random sampling of the placental disc was performed using a random sequence generator designed for this purpose, known as the PRB DICE software (Supplementary Material and Figures). A minimum of six blocks of the placental disc were obtained using random sampling; then, three of the six blocks were embedded in paraffin and stained with hematoxylin and eosin (H&E), while the remaining three blocks were stored for further research. Additional samples were obtained at the discretion of the pathologist. The placenta was then transferred to the WSU/DMC Department of Pathology where targeted sampling was performed, according to standard clinical protocols (1).
B). Targeted sampling in the laboratory of the WSU/DMC Department of Pathology
This processing technique entailed taking representative sections of the selected non-randomized predefined areas from the placenta. A minimum of one section from the membrane roll and two sections of umbilical cord were placed together in one cassette. The sections taken from the villous parenchyma included the entire thickness of the placenta, extending from the chorionic plate (fetal surface) to the basal plate (maternal surface), and both the amnion and decidua. A thick placenta necessitated bisecting the section to fit the cassette. The margins of the placenta were not sampled as they are often non-representative. Therefore, only appropriate sections from the central region were targeted. For those placentas that appeared grossly normal, a minimum of three full-thickness sections from the central portion of the placenta and three slivers from the basal plate were processed for histologic examination and stained with H&E, whereas for placentas with gross lesions, additional sections were taken at the discretion of the pathologist.
After sampling (both systematic random and targeted), tissues were fixed with 10% neutral buffered formalin (NBF) at a minimum 1:20 ratio (tissue: NBF) for at least 12 hours, but not more than 48 hours, before further processing. After an NBF fixation, the samples were trimmed, processed, and paraffin-embedded. A 5-μm-thick section was taken from each paraffin block and stained with H&E for microscopic evaluation.
All H&E-stained slides of the chorioamniotic membrane roll, umbilical cord, and placental disc (minimum of seven: at least three by systematic random sampling and four by targeted sampling) were examined by pediatric pathologists who are specialists in the assessment of placental lesions (CJK, JSK, YMK, SMJ, FQ). Tables 1A and1B show the terminology used in this study, which was based mainly on the criteria established by the Perinatal Section of the Society for Pediatric Pathology (2). Subsequently, the terminology was revised by the Amsterdam Placental Workshop Group Consensus (2, 21) and we used this for the purpose of this study. The grading and staging of chronic chorioamnionitis were scored following the publication of diagnostic criteria by our group (14).
Table 1A.
Histopathology of placental inflammatory lesions.
| Histologic Lesions |
|---|
| I. Acute inflammatory lesions |
| A. Maternal inflammatory response |
| Staging (location of neutrophil infiltration) |
| Stage 1: early acute subchorionitis or chorionitis |
| Stage 2: acute chorioamnionitis |
| Stage 3: necrotizing chorioamnionitis |
| Grading (intensity) |
| Grade 1 (not-severe) |
| Grade 2 (severe): subchorionic microabscess |
| B. Fetal inflammatory response |
| Staging (location of neutrophil infiltration) |
| Stage 1: chorionic vasculitis or umbilical phlebitis |
| Stage 2: umbilical arteritis |
| Stage 3: necrotizing funisitis |
| Grading (intensity) |
| Grade 1 (not-severe) |
| Grade 2 (severe): intense chorionic and/or umbilical vasculitis |
| II. Chronic inflammatory lesions |
| A. Maternal inflammatory response |
| Chronic deciduitis |
| Lymphocytic (without plasma cells) |
| Lymphoplasmacytic |
| Chronic villitis of unknown etiology |
| Low-grade lesions: < 10 contiguous villi in any one focus |
| High-grade lesions: ≥ 10 contiguous villi in at least one focus |
| Chronic chorioamnionitis |
| Staging (location of lymphocyte infiltration) |
| Stage 1: lymphocytic infiltration limited to the chorionic trophoblast layer |
| Stage 2: lymphocytic infiltration into the chorioamniotic connective tissue |
| Grading (Intensity) |
| Grade 1: <2 foci of inflammation or patchy inflammation |
| Grade 2: diffuse inflammation |
| Chronic histiocytic intervillositis |
| Villitis of infectious origin |
| B. Fetal inflammatory response |
| Eosinophilic T-cell vasculitis |
Table 1B.
Placental histopathology of maternal and fetal vascular malperfusion.
| Histologic Lesions |
|---|
| I. Findings consistent with maternal vascular malperfusion |
| A. Villous changes |
| Villous infarct(s) |
| Increased syncytial knots |
| Villous agglutination |
| Increased intervillous fibrin deposition |
| Distal villous hypoplasia |
| B. Vascular lesions |
| Persistent muscularization of basal plate arteries |
| Mural hypertrophy of decidual arterioles |
| Acute atherosis of basal plate arteries and/or decidual arterioles |
| Spiral artery fibrinoid necrosis |
| Decidual vascular thrombosis |
| Persistence endovascular trophoblast |
| Retroplacental hemorrhage |
| II. Findings consistent with fetal vascular malperfusion |
| A. Villous changes |
| Early |
| Villous stromal-vascular karyorrhexis |
| Late |
| Hyalinized avascular villi, small foci |
| Hyalinized avascular villi, variable sized foci |
| Severe |
| Fetal thrombotic vasculopathy |
| B. Vascular lesions |
| Thrombi in large fetal vessels |
| Intimal fibrin deposition, large fetal vessels |
| Fibromuscular sclerosis, intermediate-sized fetal vessels |
Statistical Analysis
All data analyses were performed in R (59). For continuous data, comparisons between the groups were done using the Kruskal-Wallis test without making any distributional assumptions. For categorical data, proportions were compared between groups using exact tests for contingency tables. Generalized linear models were used to examine the associations between covariates, such as ethnicity (African-American or others), nulliparity (nulliparous or multiparous), labor (yes or no), gestational age at delivery (continuous variable), duration of membrane rupture (continuous variable), and frequency of histopathologic lesions. Associations among the four major categories of histologic lesions (acute inflammatory, chronic inflammatory, maternal vascular malperfusion, and fetal vascular malperfusion) were studied by fitting hierarchically nested log linear models for multiway contingency tables. A p-value of 0.05 was considered significant.
RESULTS
Demographic and clinical characteristics of the study population
The placentas from 946 women were examined. The demographic and clinical characteristics of the study population are shown in Table 2. The majority of subjects were multiparous (66.2%; 626/946), of self-identified African-American origin (81.1%; 766/945), and delivered vaginally (73.9%; 699/946) (Table 2).
Table 2.
Demographic and clinical characteristics of 946 singleton normal pregnant women at term.
| Characteristics | Vaginal Delivery with Labor (n=699) | Cesarean Delivery (n=247) | p-valuea | |
|---|---|---|---|---|
| Cesarean Delivery with Labor (n=90) |
Elective Cesarean Delivery (n=157) |
|||
| Maternal age (years) | 23 (20–28) | 24 (20–28.75) | 28 (23–31) | <0.0001 |
| Nulliparous | 37.2% (260/699) |
54.4% (49/90) |
7% (11/157) |
<0.0001 |
| African American ethnicityb | 82.4% (575/698) |
80% (72/90) |
75.8% (119/157) |
0.1588 |
| Tobacco use during pregnancyc | 10.6% (74/696) |
10% (9/90) |
17.9% (28/156) |
0.0399 |
| Alcohol use during pregnancyd | 0.7% (5/695) |
0% (0/90) |
1.3% (2/156) |
0.6640 |
| History of preterm births | 6.7% (47/699) |
7.8% (7/90) |
8.9% (14/157) |
0.5730 |
| Obesitye,f | 24.7% (164/663) |
33.7% (28/83) |
45.8% (65/142) |
<0.0001 |
| Gestational age at delivery (weeks) | 39.6 (38.7-40.4) |
40 (38.9-40.9) |
39.1 (39-39.4) |
<0.0001 |
| Birth weight (grams) | 3300 (3100-3565) |
3330 (3145-3630) |
3430 (3160-3620) |
<0.01 |
| Male sex neonatec | 50.3% (350/696) |
59.6% (53/89) |
53.5% (84/157) |
0.2309 |
Data are presented as median (interquartile range) or % (n/N).
The p-values come from the Kruskal-Wallis test in the case of continuous variables and exact test for 2 by 3 contingency tables in the case of categorical variables.
Due to missing demographic data, total number of each case was
n=945,
n=942,
n=941,
n=888.
Obesity was defined as pre-pregnancy body mass index (BMI) >30 kg/m2.
Of the 247 women who underwent a cesarean delivery, 63.6% (157/247) had the procedure performed before the onset of labor. Women who underwent cesarean delivery without labor had a statistically significant higher rate of advanced maternal age, multiparity, tobacco use during pregnancy, obesity (body mass index [BMI] > 30 kg/m2), lower median gestational age at delivery, and higher median birthweight than those in the other study groups (Table 2).
The indications for cesarean delivery were as follows: previous cesarean section, 64.8% (160/247); non-reassuring fetal heart tracing, 15.4% (38/247); malpresentation, 8.9% (22/247); failure to progress, 8.5% (21/247); failed induction, 0.8% (2/247); suspected macrosomia, 0.8% (2/247); a previous uterine surgical scar, 0.4% (1/247); and maternal request, 0.4% (1/247).
Placental histopathologic findings
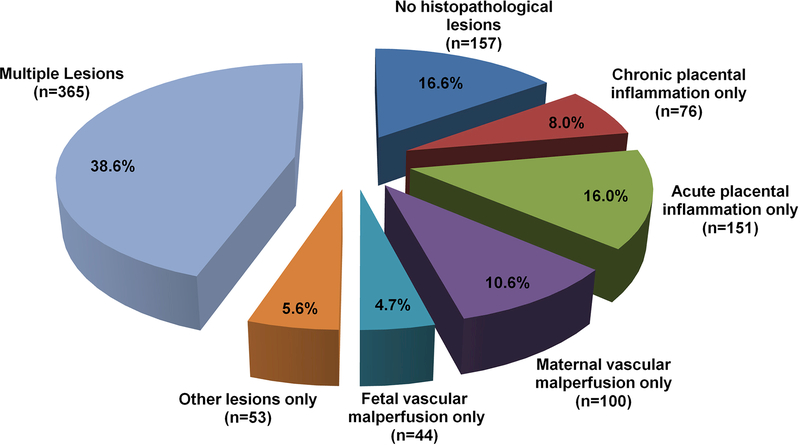
The frequency of single and combined histopathological placental lesions is displayed in Supplementary Table A. Placental histologic inflammatory or vascular lesions were present in 77.8% (736/946) of the cases. Twenty-two percent (210/946) of the cases had neither inflammatory nor vascular lesions and 16.6% (157/946) of the cases had no placental histopathologic lesion. Figure 1 shows the frequency of the histological placental lesions.
Figure 1 – Distribution of placental lesions in term pregnancies with normal outcome.
The distribution of histopathologic placental lesions in women with normal pregnancies indicates that the majority of cases had multiple lesions (consistent with more than one type of histopathologic lesion). Among women with isolated placental lesions the most frequent were acute placental inflammation, followed by maternal vascular malperfusion, chronic placental inflammation and fetal vascular malperfusion.
Other lesions include: Laminar Necrosis Decidua Capsularis; Meconium laden macrophages in fetal membranes; Hemosiderin laden macrophages in fetal membranes; Nucleated Red Blood cells; Massive Perivillous Fibrin Deposition; Basal plate myometrial fibers;and Hypervascularity of chorionic villi
The frequency of lesions classified as other than inflammatory or vascular was as follows: meconium-laden macrophages in fetal membranes, 12.9% (122/943); laminar necrosis of the decidua capsularis, 5.5% (52/944); basal plate myometrial fibers, 3.0% (28/944); hemosiderin-laden macrophages in the fetal membranes, 0.3% (3/944); massive perivillous fibrin deposition, 0.2% (2/944); intervillous thrombus, 10.3% (97/944); hypervascularity of the chorionic villi, 3.5% (33/940); and villous edema, 0.1% (1/944). These findings did not alter the classification of inflammatory or vascular lesions.
A. Acute placental inflammation
The frequency of all acute placental inflammatory lesions was 42.3% (400/946) (Table 3), but severe lesions (defined as stage 3 and/or grade 2 maternal and/or fetal inflammatory responses) were present in only 1.0% (9/946) of the cases. The distribution of acute placental inflammatory lesions according to the mode of delivery is shown in Figure 2.
Table 3.
Frequency of acute inflammatory placental lesions of normal singleton pregnant women at term.
| Histologic Lesionsa | % (n/N) |
|---|---|
| Acute inflammatory lesions | 42.3% (400/946) |
| A. Maternal inflammatory response | 36.2% (342/946) |
| Stage 1: early acute subchorionitis or chorionitis | 23.3% (220/946) |
| Grade 1 | 23.3% (220/946) |
| Grade 2 | 0% (0/946) |
| Stage 2: acute chorioamnionitis | 12.2% (115/946) |
| Grade 1 | 12.2% (115/946) |
| Grade 2 | 0% (0/946) |
| Stage 3: necrotizing chorioamnionitis | 0.7% (7/946) |
| Grade 1 | 0.6% (6/946) |
| Grade 2 | 0.1% (1/946) |
| B. Fetal inflammatory response | 23.3% (220/946) |
| Stage 1: chorionic vasculitis or umbilical phlebitis | 18.7% (177/946) |
| Grade 1 | 18.6% (176/946) |
| Grade 2 | 0.1% (1/946) |
| Stage 2: umbilical arteritis | 4.4% (42/946) |
| Grade 1 | 4.4% (42/946) |
| Grade 2 | 0% (0/946) |
| Stage 3: necrotizing funisitis | 0.1% (1/946) |
| Grade 1 | 0.1% (1/946) |
| Grade 2 | 0% (0/946) |
Placentas may have more than one type of acute placental inflammatory lesions.
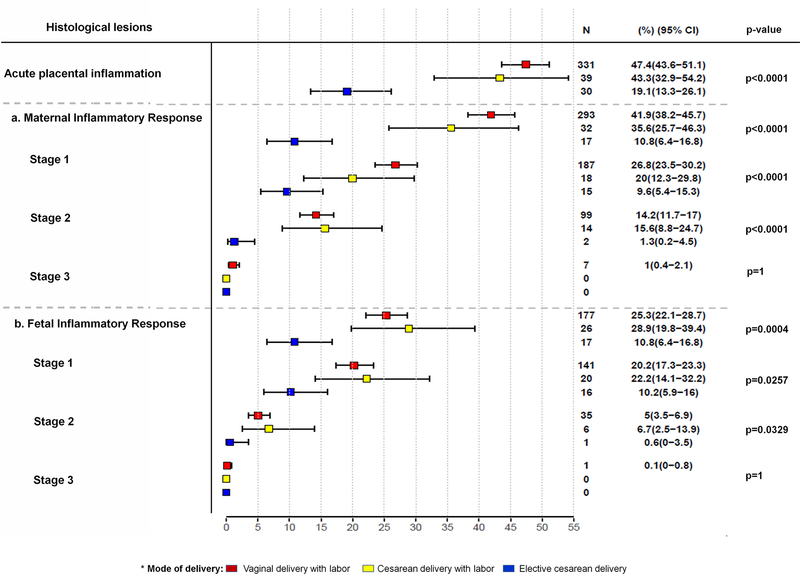
Figure 2 -. The distribution of acute placental inflammatory lesions according to the mode of delivery.
2a - The distribution of lesions consistent with a maternal inflammatory response according to mode of delivery
2b - The distribution of lesions consistent with a fetal inflammatory response, according to mode of delivery
Red box: vaginal delivery with labor
Yellow box: cesarean delivery with labor
Blue box: elective cesarean delivery
The rate of acute placental inflammation was significantly higher in women who underwent labor than for those without labor: 46.9% (370/789) versus 19.1% (30/157), p<0.0001. Among women with labor, African-American ethnicity, nulliparity, and duration of membrane rupture were independently associated with an increased frequency of acute placental histologic lesions [African American versus Other: prevalence ratio = 1.3, p = 0.02; nulliparity versus multiparity: prevalence ratio = 1.3, p < 0.01; and a two-hour duration of labor: prevalence ratio = 1.02, p = 0.01].
Maternal inflammatory response
The frequency of acute maternal inflammatory lesions (n=342) according to grading and staging were as follows: 1) stage 1 (early acute subchorionitis/chorionitis) in 64.3% (220/342) of the cases; 2) stage 2 (acute chorioamnionitis) in 33.6% (115/342); 3) the frequency of stage 3 (necrotizing chorioamnionitis) and/or grade 2 maternal acute placental inflammatory lesions was 2.0% (7/342); of note, these lesions were only observed in patients who delivered vaginally (Table 3). The frequency of lesions consistent with a maternal inflammatory response was associated with the presence of labor (p<0.0001), African-American ethnicity (p<0.01), gestational age (p<0.0001), and nulliparity (p<0.001) (Supplementary Table B).
Fetal inflammatory response
The frequency of acute fetal inflammatory lesions (n=220) according to grading and staging were as follows: 1) stage 1 (chorionic vasculitis/umbilical phlebitis) in 80.5% (177/220) of the cases; 2) stage 2 (umbilical arteritis) in 19.1% (42/220); 3) the frequency of stage 3 (necrotizing funisitis) and/or grade 2 fetal acute placental inflammatory lesions was 0.9% (2/220) (Table 3). A fetal inflammatory response was associated with labor (p<0.001), and gestational age (p<0.0001), adjusting for ethnicity and nulliparity (Supplementary Table B).
B. Chronic placental inflammation
The overall frequency of chronic inflammatory lesions was 29.9% (283/946), however, severe lesions (defined as high-grade lesions of chronic villitis of unknown etiology or grade 2 chronic chorioamnionitis) were present in only 2.5% (23/923) of chronic inflammatory lesions. Table 4 shows the frequency of the specific lesions. The most common lesion was chronic deciduitis in 19.2% (182/946) of the cases, followed by chronic villitis of unknown etiology present in 18.6% (176/946), and chronic chorioamnionitis present in 12.7% (120/946) of the cases. Nulliparity was negatively associated with chronic inflammatory lesions (p <0.001), adjusting for the presence of labor, ethnicity, and gestational age (Supplementary Table B).
Table 4.
Frequency of chronic inflammatory placental lesions of normal singleton pregnant women at term.
| Histologic Lesionsa | % (n/N) |
|---|---|
| Chronic inflammatory lesions | 29.9% (283/946) |
| A. Maternal inflammatory response | 29.8% (282/946) |
| Chronic deciduitis | 19.2% (182/946) |
| Lymphocytic (without plasma cells) | 10.3% (97/946) |
| Lymphoplasmacytic | 9.0% (85/946) |
| Chronic villitis of unknown etiology | 18.6% (176/946 ) |
| Low-grade lesions | 13.2% (122/927) |
| High-grade lesions | 1.2% (11/927) |
| Chronic chorioamnionitis | 12.7% (120/946) |
| Stage 1 | 3.8% (36/942) |
| Grade 1 | 3.6% (34/942) |
| Grade 2 | 0.2% (2/942) |
| Stage 2 | 7.6% (72/942) |
| Grade 1 | 6.6% (62/942) |
| Grade 2 | 1.1% (10/942) |
| Chronic histiocytic intervillositis | 0.4% (4/944) |
| Villitis of infectious origin | 0% (0/944) |
| B. Fetal inflammatory response | |
| Eosinophilic T-cell vasculitis | 0.4% (4/944) |
Placentas may have more than one type of chronic placental inflammatory lesions.
C. Maternal vascular malperfusion
The overall rate of lesions consistent with maternal vascular malperfusion was 35.6% (337/946). The frequency of specific lesion groups were as follows: 1) villous changes, 18.6% (176/946); 2) vascular lesions, 20.5% (194/946); and 3) a high burden of lesions (defined as two or more lesions of maternal vascular malperfusion) were present in only 7.4% (70/946) of the cases (Table 5).
Table 5.
Frequency of placental maternal and fetal vascular malperfusion lesions of normal singleton pregnant women at term.
| Histologic Lesionsa | % (n/N) |
|---|---|
| Maternal vascular malperfusion | 35.6% (337/946) |
| A. Villous Changes | 18.6% (176/946) |
| Villous infarct(s) | 5.6% (53/945) |
| Increased syncytial knots | 8.5% (80/946) |
| Villous agglutination | 2.0% (19/946) |
| Increased intervillous fibrin deposition | 6.3% (60/946) |
| Distal villous hypoplasia | 0.1% (1/946) |
| B. Vascular Lesions | 20.5% (194/946) |
| Persistent muscularization of basal plate arteries | 16.3% (154/946) |
| Mural hypertrophy of decidual arterioles | 1.8% (17/946) |
| Acute atherosis of basal plate arteries and/or decidual arterioles | 0.8% (8/946) |
| Spiral artery fibrinoid necrosis | 0.3% (3/944) |
| Decidual vascular thrombosis | 1.7% (16/944) |
| Persistence of endovascular trophoblast | 0.3% (3/944) |
| Retroplacental hemorrhage | 0.8% (8/944) |
| ≥ 2 lesions of maternal vascular malperfusion | 7.4% (70/946) |
| ≥ 3 lesions of maternal vascular malperfusion | 1.2% (11/946) |
| Fetal vascular malperfusion | 19.7% (186/946) |
| A. Villous Changes | 11.3% (107/946) |
| Early | |
| Villous stromal-vascular karyorrhexis | 2.5% (24/946) |
| Late | |
| Hyalinized avascular villi, small foci | 6.6% (62/946) |
| Hyalinized avascular villi, variable sized foci | 2.0% (19/946) |
| Severe | |
| Fetal thrombotic vasculopathy | 0.2% (2/946) |
| B. Vascular Lesions | 9% (85/946) |
| Thrombi in large fetal vessels | 2.6% (25/946) |
| Intimal fibrin deposition, large fetal vessels | 6.4% (61/946) |
| Fibromuscular sclerosis, intermediate-sized fetal vessels | 0% (0/946) |
| ≥ 2 lesions of fetal vascular malperfusion | 0.7% (7/946) |
Placentas may have more than one type of maternal and fetal vascular malperfusion lesions.
D. Fetal vascular malperfusion
The prevalence of lesions consistent with fetal vascular malperfusion was 19.7% (186/946). The frequency of specific lesion groups were as follows: 1) villous changes, 11.3% (107/946); 2) vascular lesions, 9.0% (85/946); and 3) a high burden of lesions (defined as two or more lesions of fetal vascular malperfusion) were present in only 0.7% (7/946) of the cases (Table 5).
Neither maternal nor fetal vascular malperfusion lesions were significantly associated with the presence of labor, gestational age at delivery, African-American ethnicity, and nulliparity (Supplementary Table B).
Associations among placental histologic lesions
While almost 40 percent of placentas in normal pregnancies showed multiple histological lesions, we investigated whether they co-occur in the same placenta purely by chance. A four-level contingency table was constructed by cross-classifying acute and chronic inflammatory lesions, and fetal and maternal vascular malperfusion lesions, and examined with log-linear models to determine whether the associations between different types of lesions could have occurred by chance. Only the association between chronic inflammatory and fetal malperfusion lesions of the villous type was significant (p < 0.01), and only chronic villitis of unknown etiology was associated with fetal malperfusion lesions of the villous type (p < 0.01).
DISCUSSION
Principal findings of the study:
1) 78% of placentas had lesions consistent with acute or chronic inflammation or maternal or fetal vascular malperfusion; 2) acute placental inflammatory lesions were the most prevalent, observed in 42.3% of the placentas – however, only 1.0% had severe acute inflammatory lesions; 3) acute inflammatory lesions were more common in the placentas of women with labor than in those without labor; 4) chronic inflammatory lesions of the placenta were present in 29.9%; and 5) multiple histologic lesions consistent with acute or chronic inflammation or maternal or fetal vascular malperfusion were present in 38.6% of the cases.
Why was this study performed?
Original research, review articles (60–79), monographs (80–82), and textbooks of placental pathology (83–96) have described the morphologic features of this organ. Placenta, a Journal dedicated specifically to the biology of the organ, reports regularly on histologic findings of human placentas (97).
Recent studies suggest that placental examination provides information that may be helpful in understanding the mechanisms of disease responsible for adverse pregnancy outcome (98–104) as well as in assessing the risk for adult disease (105–124). The importance of placentology is increasingly recognized as evidenced, in part, by the launching of the Human Placenta Project by the Eunice Kennedy Shriver NICHD, National Institutes of Health, U. S. Department of Health and Human Services (125).
However, to date, there is very little information available about the frequency and type of histologic lesions in the placentas from uncomplicated pregnancies with normal outcome (22–24). Therefore, we sought to address this question to assist in the interpretation of the results of placental pathology reports.
Maternal and fetal acute inflammatory lesions of the placenta are more frequent in placentas delivered after labor
The reported prevalence of acute placental inflammation in uncomplicated term pregnancies ranges from 3.8% to 54.0% (18, 23, 24, 126–131). This broad range may be due to 1) the lack of uniformity of placental pathologic diagnostic and reporting criteria, e.g., different definitions of inflammation (23, 132) and inclusion of decidual inflammation (22); 2) variations in the methods of placental sampling (23, 127, 129, 132); 3) socioeconomic status and ethnicity (133); 4) the presence of spontaneous labor (130, 134–136); and 5) the duration of labor (130, 136, 137).
In our study, the frequency of acute placental inflammatory lesions increased with the presence of labor. Among women in labor, African-American ethnicity, nulliparity, and duration of membrane rupture were independently associated with the increased frequency of these lesions. Our findings are consistent with previous reports that the frequency of acute histologic chorioamnionitis in women with spontaneous labor was significantly higher than in those without labor (24, 130, 134, 136, 138). Specifically, Lee et al (136), in a seminal study, reported that the risk of intra-amniotic infection or intra-amniotic inflammation (defined by analysis of amniotic fluid) increased with cervical dilatation in a group of patients who underwent cesarean delivery during labor at term (136). In addition, the current study found that the frequency of the early stages (stages 1 and 2) of the maternal inflammatory response was associated with the presence of labor (Figure 2).
Acute placental inflammation at term most often represents the inflammatory process related to parturition rather than acute infection
The presence of early stage acute inflammatory lesions in the placenta may represent the inflammatory process associated with labor per se, and not necessarily infection (135, 139–141). Whether some forms of acute chorioamnionitis reflect mild forms of microbial invasion of the amniotic cavity during spontaneous labor or sterile intra-amniotic inflammation generated by the presence of danger signals is not known. While microbial invasion of the amniotic cavity has been reported in 13% to 19% of women in spontaneous labor at term with intact membranes (140, 142, 143), intra-amniotic inflammation (defined as an elevated interleukin (IL)-6, IL-8, or other inflammatory mediators) is also more common in patients with spontaneous labor at term than in those without labor (129, 144–156). Spontaneous labor at term is considered prototypical of a sterile inflammatory phenomenon accompanied by an activation of the inflammasome (157–162). Therefore, the presence of acute inflammatory lesions of the placenta should not be construed as indicators of the presence of intra-amniotic infection.
Acute histologic chorioamnionitis represents a maternal host response, as neutrophils infiltrating the chorion and amnion are of maternal origin (163) that, in some cases, can reach the amniotic cavity (139, 164).
By contrast, funisitis and chorionic vasculitis represent a fetal inflammatory response, and these lesions are considered the histologic counterpart of the fetal inflammatory response syndrome (139, 165–169). Although the Society of Pediatric Pathologists and the Amsterdam group categorized these lesions under the label “amniotic fluid infection,” it is now clear that such lesions can also be present in the context of sterile intra-amniotic inflammation (139, 170–176). Therefore, we recommend that, when referring to acute placental inflammatory lesions, the term “amniotic fluid infection” be abandoned (139). Our interpretation is consistent with the results of Roberts et al. (177) who reported, based on placental cultures and molecular microbiologic studies for the identification of microbial footprints, that among nulliparous women who delivered at term following an uneventful pregnancy, the overall rate of histological chorioamnionitis at term was 34% (67/195). However, in 96% (64/67) of the cases, microorganisms could not be identified by either cultivation or molecular techniques (177). Indeed, using cultures and molecular techniques for the identification of viruses and bacteria, our group has shown that a subset of women with clinical or mild histologic chorioamnionitis at term do not have an intra-amniotic infection (169, 174). Collectively, these findings suggest that mild histologic acute placental inflammation represents the inflammatory process related to parturition or the aging of a placenta (178–180) rather than to acute infection.
Chronic placental inflammatory lesions in normal term gestation
Chronic inflammatory lesions are of maternal origin and are either caused by viruses (181–185), bacteria (185, 186), and/or parasites (183) or are a reaction to the foreign antigens of the fetus (19). Such lesions can also be of fetal origin, e.g., eosinophilic T-cell vasculitis (20, 187).
The rate of chronic placental inflammation in our study population was 29.9%, and the most frequent lesion was chronic deciduitis (19.2%), followed by chronic villitis of unknown etiology (18.6%) and chronic chorioamnionitis (12.7%).
There are few reports on the frequency of chronic placental inflammatory lesions in normal term pregnancy, and of these, most have been case-control studies that reported solely on individual lesions and in which normal pregnancies served as the control (18, 24, 131). The frequency of chronic villitis of unknown etiology ranged between 5% and 33.8% (5, 12, 14, 19, 129, 188–192) and that of chronic chorioamnionitis between 10% and 19% (18, 19), which agree with our findings, but the current study is the first report on the entire spectrum of chronic inflammatory lesions in normal pregnancy.
We doubt that nearly 30% of women with a normal pregnancy would have an infectious agent as an explanation of chronic placental inflammatory lesions, and therefore, favor an immunological mechanism of disease to support these findings. It is very likely that an immunological stand-off between the mother and fetus occurs at the maternal-fetal interface throughout normal pregnancy, but if maternal lymphocyte infiltration of the fetal tissues is not associated with substantial damage of the chorioamniotic membranes, villous tree, or decidua basalis, a pregnancy without clinical complications is possible. Then, the presence of maternal lymphocytes infiltrating the chorioamniotic membranes would represent evidence of maternal-fetal cellular interaction without necessarily indicating a pathologic process.
Maternal and fetal placental vascular lesions in normal pregnancy at term
The frequencies of placental lesions consistent with maternal vascular malperfusion and fetal vascular malperfusion in the current study were 35.6% and 19.7%, respectively. Bar et al. (24) reported that the frequency of placental lesions consistent with maternal and fetal vascular malperfusion in an Israeli population was 24.5% and 7.9%, respectively. In contrast, Lee et al. reported that placental lesions with maternal and fetal vascular malperfusion were 1.3% and 2.7 %, respectively, in an Asian population (18). The high frequency of maternal and/or fetal vascular malperfusion lesions found in the current study may be attributed to a larger number of placentas studied, or the use of systematic random sampling and targeted sampling, leading to a higher number of sections evaluated and a meticulous adherence to the placental sampling protocol.
It has been proposed that some lesions consistent with fetal vascular malperfusion may be related to vascular stasis, and tissue damage that may occur in the placenta at the end of gestation (193). Alternatively, these lesions may reflect placental senescence as gestational age advances toward term (178–180).
Co-occurrence of placental lesions
We did not find significant correlation among the placental histological lesions with the exception of the co-occurrence patterns between chronic inflammatory lesions (i.e., villitis of unknown etiology) and fetal vascular malperfusion lesions of the villous type. This finding is consistent with previous reports indicating that avascular villi may be part of the spectrum of villitis of unknown etiology (5, 7, 181). The association of villitis of unknown etiology with villous lesions of fetal vascular malperfusion is most likely a reflection of villous injury caused by inflammation that can lead to luminal obliteration and resulting in downstream regions of avascular villi (5, 7). In general, if villitis of unknown etiology is present in the proximal villi, the distal changes of avascular villi should not be considered as fetal vascular malperfusion (5). Current terminology for occasional avascular villi seen in a placenta with villitis should be reported as “chronic villitis with associated avascular villi” (21). Therefore, we do not interpret this association as a true co-occurrence.
The placenta has a large functional reserve
Severe compromise of placental anatomy and function must occur before clinical disease becomes evident. There is a substantial body of clinical and experimental evidence in support of this view (89, 194–199).
Embolization of the umbilical artery in pregnant sheep can result in a decrease in blood flow, an increase in umbilical artery resistance and the systolic/diastolic ratio, and a progression of the diastolic waveform from normal to absent and, subsequently, the reversal of end-diastolic flow (200, 201). Substantial embolization of the placental cotyledons must occur before Doppler abnormalities are observed. Giles et al. (202) reported that placental microvascular anatomy was dramatically changed in patients with abnormal Doppler velocimetry findings. Specifically, the modal small-artery count in tertiary stem villi was significantly lower in patients with abnormal umbilical artery Doppler velocimetry than in those with normal Doppler indices (202). Such findings have been confirmed by the observation that absent end-diastolic flow in the umbilical artery of growth-restricted fetuses is demonstrated after there is an approximate 50% reduction of the vessel area in the placental terminal villi (203). Moreover, mathematical modeling of the placental circulation has shown that obliteration of 80% of the vascular tree is required before dramatic changes in the pulsatility index occur (204). Similarly, a substantial proportion of the placenta affected by perivillous fibrinoid deposition is required before an adverse pregnancy event will occur, such as severe fetal growth restriction or fetal death (205).
Strengths and Limitations
The strengths of this study include the following: 1) numerous term placentas from women without any obstetrical complications; 2) extensive examination of the placental sections; 3) comprehensive description of histologic placental pathology and its correlation to demographic and clinical parameters; and 4) the use of standardized protocols for histopathologic examination of the placenta. The limitations of the study included 1) the non-consecutive enrollment of patients over an extended time period and 2) the lack of follow-up beyond the neonatal period.
Conclusion
We report the frequency and type of histologic lesions from a large series of placentas of women with a normal pregnancy outcome. Most placentas had some type of lesion (either inflammatory or vascular), but most were mild. The results of this study can be used as a reference when interpreting the results of placental pathology in patients with perinatal complications. Placental histopathological lesions and the extent to which they can cause obstetrical complications needs to be rigorously evaluated and quantified.
Current pathologic examination of the placenta relies on limited sampling of a large organ and taxonomy based on morphologic criteria that are largely qualitative. The application of advances in molecular pathology that address sampling (206–213), the identification of cellular markers of pathological processes, and the computational methods utilized to summarize and analyze data can be utilized in placental pathology to improve the characterization of this important organ.
Supplementary Material
Acknowledgement:
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services Contract No. HHSN275201300006C.
Footnotes
Disclosure: The authors report no conflicts of interest.
References:
- 1.Langston C, Kaplan C, Macpherson T, Manci E, Peevy K, Clark B, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Archives of pathology & laboratory medicine. 1997;121(5):449–76. [PubMed] [Google Scholar]
- 2.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation - A workshop report. Placenta. 2005;26:S114–7. [DOI] [PubMed] [Google Scholar]
- 3.Redline RW. Clinical and pathological umbilical cord abnormalities in fetal thrombotic vasculopathy. Human pathology. 2004;35(12):1494–8. [DOI] [PubMed] [Google Scholar]
- 4.Redline RW. Placental inflammation. Seminars in neonatology : SN. 2004;9(4):265–74. [DOI] [PubMed] [Google Scholar]
- 5.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Human pathology. 2007;38(10):1439–46. [DOI] [PubMed] [Google Scholar]
- 6.Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008;29 Suppl A:S86–91. [DOI] [PubMed] [Google Scholar]
- 7.Redline RW, Ariel I, Baergen RN, Desa DJ, Kraus FT, Roberts DJ, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2004;7(5):443–52. [DOI] [PubMed] [Google Scholar]
- 8.Redline RW, Boyd T, Campbell V, Hyde S, Kaplan C, Khong TY, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2004;7(3):237–49. [DOI] [PubMed] [Google Scholar]
- 9.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, et al. Amniotic infection syndrome: Nosology and reproducibility of placental reaction patterns. Pediatric and Developmental Pathology. 2003;6(5):435–48. [DOI] [PubMed] [Google Scholar]
- 10.Gersell DJ, Phillips NJ, Beckerman K. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists. 1991;10(3):217–29. [PubMed] [Google Scholar]
- 11.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Human pathology. 1998;29(12):1457–61. [DOI] [PubMed] [Google Scholar]
- 12.Becroft DM, Thompson JM, Mitchell EA. Placental villitis of unknown origin: epidemiologic associations. American journal of obstetrics and gynecology. 2005;192(1):264–71. [DOI] [PubMed] [Google Scholar]
- 13.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182(6):3919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Modern Pathology. 2010;23(7):1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogge G, Romero R, Lee DC, Gotsch F, Than NG, Lee J, et al. Chronic chorioamnionitis displays distinct alterations of the amniotic fluid proteome. The Journal of pathology. 2011;223(4):553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J, Romero R, Dong Z, Xu Y, Qureshi F, Jacques S, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59(5):928–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, Romero R, Xu Y, Kim JS, Topping V, Yoo W, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PloS one. 2011;6(2):e16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Kim JS, Park JW, Park CW, Park JS, Jun JK, et al. Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta. 2013;34(8):681–9. [DOI] [PubMed] [Google Scholar]
- 19.Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. American journal of obstetrics and gynecology. 2015;213(4 Supplement):S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katzman PJ. Chronic inflammatory lesions of the placenta. Seminars in perinatology. 2015;39(1):20–6. [DOI] [PubMed] [Google Scholar]
- 21.Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Archives of pathology & laboratory medicine. 2016;140(7):698–713. [DOI] [PubMed] [Google Scholar]
- 22.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstetrics and gynecology. 1989;73(3 Pt 1):383–9. [PubMed] [Google Scholar]
- 23.Pathak S, Lees CC, Hackett G, Jessop F, Sebire NJ. Frequency and clinical significance of placental histological lesions in an unselected population at or near term. Virchows Archiv. 2011;459(6):565–72. [DOI] [PubMed] [Google Scholar]
- 24.Bar J, Schreiber L, Golan A, Kovo M. Placental factor in spontaneous term labor in uncomplicated pregnancy. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(12):2704–7. [DOI] [PubMed] [Google Scholar]
- 25.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. American journal of obstetrics and gynecology. 2002;187(5):1137–42. [DOI] [PubMed] [Google Scholar]
- 26.Sebire NJ, Backos M, El Gaddal S, Goldin RD, Regan L. Placental pathology, antiphospholipid antibodies, and pregnancy outcome in recurrent miscarriage patients. Obstetrics and gynecology. 2003;101(2):258–63. [DOI] [PubMed] [Google Scholar]
- 27.Ananth CV, Smulian JC, Vintzileos AM. Ischemic placental disease: maternal versus fetal clinical presentations by gestational age. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23(8):887–93. [DOI] [PubMed] [Google Scholar]
- 28.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. American journal of obstetrics and gynecology. 2003;189(4):1063–9. [DOI] [PubMed] [Google Scholar]
- 29.Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, et al. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. Journal of perinatal medicine. 2011;39(6):641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrone S, Toti P, Toti MS, Badii S, Becucci E, Gatti MG, et al. Perinatal outcome and placental histological characteristics: a single-center study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25 Suppl 1:110–3. [DOI] [PubMed] [Google Scholar]
- 31.Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(5):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arayici S, Kadioglu Simsek G, Oncel MY, Eras Z, Canpolat FE, Oguz SS, et al. The effect of histological chorioamnionitis on the short-term outcome of preterm infants </=32 weeks: a single-center study. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27(11):1129–33. [DOI] [PubMed] [Google Scholar]
- 33.Veerbeek JH, Nikkels PG, Torrance HL, Gravesteijn J, Post Uiterweer ED, Derks JB, et al. Placental pathology in early intrauterine growth restriction associated with maternal hypertension. Placenta. 2014;35(9):696–701. [DOI] [PubMed] [Google Scholar]
- 34.Labarrere CA, DiCarlo HL, Bammerlin E, Hardin JW, Kim YM, Chaemsaithong P, et al. Failure of physiologic transformation of spiral arteries, endothelial and trophoblast cell activation, and acute atherosis in the basal plate of the placenta. American journal of obstetrics and gynecology. 2017;216(3):287 e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanek J Placental examination in nonmacerated stillbirth versus neonatal mortality. Journal of perinatal medicine. 2017. [DOI] [PubMed] [Google Scholar]
- 36.Kovo M, Schreiber L, Bar J. Placental vascular pathology as a mechanism of disease in pregnancy complications. Thrombosis research. 2013;131 Suppl 1:S18–21. [DOI] [PubMed] [Google Scholar]
- 37.Vahanian SA, Lavery JA, Ananth CV, Vintzileos A. Placental implantation abnormalities and risk of preterm delivery: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S78–90. [DOI] [PubMed] [Google Scholar]
- 38.Lekovich J, Stewart J, Anderson S, Niemasik E, Pereira N, Chasen S. Placental malperfusion as a possible mechanism of preterm birth in patients with Mullerian anomalies. Journal of perinatal medicine. 2017;45(1):45–9. [DOI] [PubMed] [Google Scholar]
- 39.Choi J, Park JW, Kim BJ, Choi YJ, Hwang JH, Lee SM. Funisitis is more common in cervical insufficiency than in preterm labor and preterm premature rupture of membranes. Journal of perinatal medicine. 2016;44(5):523–9. [DOI] [PubMed] [Google Scholar]
- 40.Miller ES, Minturn L, Linn R, Weese-Mayer DE, Ernst LM. Stillbirth evaluation: a stepwise assessment of placental pathology and autopsy. American journal of obstetrics and gynecology. 2016;214(1):115.e1–6. [DOI] [PubMed] [Google Scholar]
- 41.Hackney DN, Tirumala R, Salamone LJ, Miller RK, Katzman PJ. Do placental histologic findings of chorion-decidual hemorrhage or inflammation in spontaneous preterm birth influence outcomes in the subsequent pregnancy? Placenta. 2014;35(1):58–63. [DOI] [PubMed] [Google Scholar]
- 42.Redline RW, Patterson P. Villitis of unknown etiology is associated with major infiltration of fetal tissue by maternal inflammatory cells. Am J Pathol. 1993;143(2):473–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Weiner E, Mizrachi Y, Grinstein E, Feldstein O, Rymer-Haskel N, Juravel E, et al. The role of placental histopathological lesions in predicting recurrence of preeclampsia. Prenatal diagnosis. 2016;36(10):953–60. [DOI] [PubMed] [Google Scholar]
- 44.Odibo AO, Patel KR, Spitalnik A, Odibo L, Huettner P. Placental pathology, first-trimester biomarkers and adverse pregnancy outcomes. Journal of perinatology : official journal of the California Perinatal Association. 2014;34(3):186–91. [DOI] [PubMed] [Google Scholar]
- 45.Srinivas SK, Ernst LM, Edlow AG, Elovitz MA. Can placental pathology explain second-trimester pregnancy loss and subsequent pregnancy outcomes? American journal of obstetrics and gynecology. 2008;199(4):402 e1–5. [DOI] [PubMed] [Google Scholar]
- 46.Redline RW, Abramowsky CR. Clinical and pathologic aspects of recurrent placental villitis. Human pathology. 1985;16(7):727–31. [DOI] [PubMed] [Google Scholar]
- 47.Russell P, Atkinson K, Krishnan L. Recurrent reproductive failure due to severe placental villitis of unknown etiology. The Journal of reproductive medicine. 1980;24(2):93–8. [PubMed] [Google Scholar]
- 48.Kraus FT. Perinatal pathology, the placenta, and litigation. Human pathology. 2003;34(6):517–21; discussion 22–7. [DOI] [PubMed] [Google Scholar]
- 49.Chang KT. Examination of the placenta: medico-legal implications. Semin Fetal Neonatal Med. 2014;19(5):279–84. [DOI] [PubMed] [Google Scholar]
- 50.Ito Y, Tsuda R, Kimura H. Diagnostic value of the placenta in medico-legal practice. Forensic science international. 1989;40(1):79–84. [DOI] [PubMed] [Google Scholar]
- 51.Benirschke K The placenta in the litigation process. American journal of obstetrics and gynecology. 1990;162(6):1445–8; discussion 8–50. [DOI] [PubMed] [Google Scholar]
- 52.Bateman C Discard the placenta at your peril, pathologist warns doctors. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2014;104(11):729–30. [DOI] [PubMed] [Google Scholar]
- 53.Schindler NR. Importance of the placenta and cord in the defense of neurologically impaired infant claims. Archives of pathology & laboratory medicine. 1991;115(7):685–7. [PubMed] [Google Scholar]
- 54.Altshuler G Placenta within the medicolegal imperative. Archives of pathology & laboratory medicine. 1991;115(7):688–95. [PubMed] [Google Scholar]
- 55.Naeye R, Travers H. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on the Role of the Pathologist in Malpractice Litigation Involving the Placenta. Archives of pathology & laboratory medicine. 1991;115(7):717–9. [PubMed] [Google Scholar]
- 56.Altshuler G Some placental considerations in alleged obstetrical and neonatology malpractice. Legal medicine. 1993:27–47. [PubMed] [Google Scholar]
- 57.Baergen RN. The placenta as witness. Clinics in perinatology. 2007;34(3):393–407. [DOI] [PubMed] [Google Scholar]
- 58.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3(4):225–31. [DOI] [PubMed] [Google Scholar]
- 59.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. 2015. [ [Google Scholar]
- 60.Roberts DJ, Oliva E. Clinical significance of placental examination in perinatal medicine. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(5):255–64. [DOI] [PubMed] [Google Scholar]
- 61.Lavery JP. The role of placental examination and its pathology in obstetric risk management. Journal of healthcare risk management : the journal of the American Society for Healthcare Risk Management. 1997;17(3):15–20. [DOI] [PubMed] [Google Scholar]
- 62.Rayne SC, Kraus FT. Placental thrombi and other vascular lesions. Classification, morphology, and clinical correlations. Pathology, research and practice. 1993;189(1):2–17. [DOI] [PubMed] [Google Scholar]
- 63.Redline RW. Classification of placental lesions. American journal of obstetrics and gynecology. 2015;213(4 Supplement):S21–8. [DOI] [PubMed] [Google Scholar]
- 64.Placental pathology. ACOG Committee Opinion: Committee on Obstetrics: Maternal and Fetal Medicine. Number 125-July 1993. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1993;42(3):318–9. [PubMed] [Google Scholar]
- 65.ACOG Practice Bulletin No. 102: management of stillbirth . Obstetrics and gynecology. 2009;113(3):748–61. [DOI] [PubMed] [Google Scholar]
- 66.Hargitai B, Marton T, Cox PM. Best practice no 178. Examination of the human placenta. Journal of clinical pathology. 2004;57(8):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox P, Evans C. The Royal College of Pathologists, London. Tissue pathway for histopathological examination of the placenta. Available at: https://www.rcpath.org/resourceLibrary/tissue-pathway-histopathological-placenta.html 2017.
- 68.Health N. Maternitiy - Indications for Placental Histologic Examination. NSW Health, Sydney, Available at: http://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2014_006.pdf. 2014. [Google Scholar]
- 69.Altshuler G, Deppisch LM. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on Indications for Placental Examination. Archives of pathology & laboratory medicine. 1991;115(7):701–3. [PubMed] [Google Scholar]
- 70.Burton GJ, Jauniaux E. What is the placenta? American journal of obstetrics and gynecology. 2015;213(4 Supplement ):S6 e1–4. [DOI] [PubMed] [Google Scholar]
- 71.Robinson J, Chidzanja S, Kind K, Lok F, Owens P, Owens J. Placental control of fetal growth. Reproduction, fertility, and development. 1995;7(3):333–44. [DOI] [PubMed] [Google Scholar]
- 72.Khong TY. Placental vascular development and neonatal outcome. Seminars in neonatology : SN. 2004;9(4):255–63. [DOI] [PubMed] [Google Scholar]
- 73.Cross JC. Placental function in development and disease. Reproduction, fertility, and development. 2006;18(1–2):71–6. [DOI] [PubMed] [Google Scholar]
- 74.Parolini O From fetal development and beyond: a continued role for placenta in sustaining life? Placenta. 2011;32 Suppl 4:S283–4. [DOI] [PubMed] [Google Scholar]
- 75.John R, Hemberger M. A placenta for life. Reproductive biomedicine online. 2012;25(1):5–11. [DOI] [PubMed] [Google Scholar]
- 76.Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. International journal of molecular sciences. 2014;15(9):16153–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longo LD, Reynolds LP. Some historical aspects of understanding placental development, structure and function. The International journal of developmental biology. 2010;54(2–3):237–55. [DOI] [PubMed] [Google Scholar]
- 78.Jansson T, Powell TL. Role of placental nutrient sensing in developmental programming. Clinical obstetrics and gynecology. 2013;56(3):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roos S, Powell TL, Jansson T. Placental mTOR links maternal nutrient availability to fetal growth. Biochemical Society transactions. 2009;37(Pt 1):295–8. [DOI] [PubMed] [Google Scholar]
- 80.Ramsey EM, Donner MW. Monograph-Placental vasculature and circulation. Stuttgart: Thieme; 1980. [Google Scholar]
- 81.Altshuler G The placenta, how to examine it, its normal growth and development In: Perinatal disease-Monograph of International Academy of Pathology. Baltimore: Williams & Wilkins; 1981. [PubMed] [Google Scholar]
- 82.Haust MD. Maternal diabetes mellitus - effects on the fetus and placenta In: Perinatal disease-Monograph of International Academy of Pathology. Baltimore: Williams & Wilkins; 1981. [PubMed] [Google Scholar]
- 83.Benirschke K, Burton GJ, Baergen RN. Pathology of the human placenta 6th ed. New York: Springer; 2012. [Google Scholar]
- 84.Boyd JD, Hamilton WJ. The human placenta Cambridge: Heffer; 1970. 365 p. [Google Scholar]
- 85.Naeye RL. Disorders of the placenta, fetus, and neonate: Diagnosis and clinical significance. St. Louis: Mosby-Year Book; 1992. 375 p. [Google Scholar]
- 86.Kraus FT, Redline R, Gersell DJ, Nelson MD, Dicke JM. Placental Pathology (Atlas of Nontumor Pathology) 1 ed. Washington DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2004. 331 p. [Google Scholar]
- 87.Faye-Petersen OM, Heller DS, Joshi VV. Handbook of placental pathology New York: Taylor and Francis group; 2005. 268 p. [Google Scholar]
- 88.Baergen RN. Manual of Pathology of the Human Placenta, 2nd edition. New York: Springer; 2011. 504 p. [Google Scholar]
- 89.Fox H, Sebire NJ. Pathology of the placenta. China: Saunders Elsevier; 2007. 574 p. [Google Scholar]
- 90.Pijnenborg R, Brosens I, Romero R. Placental bed disorders: Basic science and its translation to obstetrics 1st ed. New York: Cambridge University Press; 2010. 317 p. [Google Scholar]
- 91.Burton GJ, Barker DJ, Moffett A, Thornburg K. The human placenta and human developmental programming. New York: Cambridge University Press; 2011. [Google Scholar]
- 92.Kay HH, Nelson DM, Wang Y. The placenta: from development to disease. Singapore: Blackwell Publishing Ltd; 2011. 360 p. [Google Scholar]
- 93.Wooding P, Burton G. Comparative Placentation: Structures, Functions and Evolution. Springer Berlin Heidelberg; 2008. [Google Scholar]
- 94.Kaplan CG. Color Atlas of Gross Placental Pathology: Springer; New York; 2007. [Google Scholar]
- 95.Mills SE, Carter D, Greenson JK, Reuter VE, Stoler MH. Sternberg’s Diagnostic Surgical Pathology: Wolters Kluwer Health; 2012. [Google Scholar]
- 96.Beaconfield R, Birdwood GFB. Placenta: The Largest Human Biopsy New York: Pergamon Press; 1982. 174 p. [Google Scholar]
- 97.Fox H, Faulk WP. Editorial. Placenta. 1980;1(1):1. [Google Scholar]
- 98.Khong Y, Brosens I. Defective deep placentation. Best practice & research Clinical obstetrics & gynaecology. 2011;25(3):301–11. [DOI] [PubMed] [Google Scholar]
- 99.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Human reproduction update. 2006;12(6):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best practice & research Clinical obstetrics & gynaecology. 2011;25(3):313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salafia CM, Mill JF. The value of placental pathology in studies of spontaneous prematurity. Current opinion in obstetrics & gynecology. 1996;8(2):89–98. [PubMed] [Google Scholar]
- 102.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. American journal of obstetrics and gynecology. 2011;204(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. American journal of obstetrics and gynecology. 2016;215(1 Suppl):S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal diagnosis and therapy. 2014;36(2):117–28. [DOI] [PubMed] [Google Scholar]
- 105.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301(6746):259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barker DJ. Intrauterine programming of adult disease. Molecular medicine today. 1995;1(9):418–23. [DOI] [PubMed] [Google Scholar]
- 107.Godfrey K, Robinson S. Maternal nutrition, placental growth and fetal programming. The Proceedings of the Nutrition Society. 1998;57(1):105–11. [DOI] [PubMed] [Google Scholar]
- 108.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23 Suppl A:S20–7. [DOI] [PubMed] [Google Scholar]
- 109.Myatt L Placental adaptive responses and fetal programming. The Journal of physiology. 2006;572(Pt 1):25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond). 2007;113(1):1–13. [DOI] [PubMed] [Google Scholar]
- 111.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. Journal of neuroendocrinology. 2008;20(4):439–50. [DOI] [PubMed] [Google Scholar]
- 112.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. The International journal of developmental biology. 2010;54(2–3):525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thornburg KL, O’Tierney PF, Louey S. Review: The placenta is a programming agent for cardiovascular disease. Placenta. 2010;31 Suppl:S54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eriksson JG, Kajantie E, Thornburg KL, Osmond C, Barker DJ. Mother’s body size and placental size predict coronary heart disease in men. European heart journal. 2011;32(18):2297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Seminars in reproductive medicine. 2011;29(3):187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schug TT, Erlebacher A, Leibowitz S, Ma L, Muglia LJ, Rando OJ, et al. Fetal programming and environmental exposures: implications for prenatal care and preterm birth. Annals of the New York Academy of Sciences. 2012;1276:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burton GJ, Fowden AL. Review: The placenta and developmental programming: balancing fetal nutrient demands with maternal resource allocation. Placenta. 2012;33 Suppl:S23–7. [DOI] [PubMed] [Google Scholar]
- 118.Lewis RM, Cleal JK, Hanson MA. Review: Placenta, evolution and lifelong health. Placenta. 2012;33 Suppl:S28–32. [DOI] [PubMed] [Google Scholar]
- 119.Barker DJ, Thornburg KL. Placental programming of chronic diseases, cancer and lifespan: a review. Placenta. 2013;34(10):841–5. [DOI] [PubMed] [Google Scholar]
- 120.Thornburg KL, Marshall N. The placenta is the center of the chronic disease universe. American journal of obstetrics and gynecology. 2015;213(4 Supplement):S14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. The Journal of experimental biology. 2015;218(Pt 1):50–8. [DOI] [PubMed] [Google Scholar]
- 122.Nelson DM. How the placenta affects your life, from womb to tomb. American journal of obstetrics and gynecology. 2015;213(4 Supplement):S12–3. [DOI] [PubMed] [Google Scholar]
- 123.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. American journal of obstetrics and gynecology. 2016;215(4):484.e1–.e14. [DOI] [PubMed] [Google Scholar]
- 124.Brosens I, Benagiano M, Puttemans P, D’Elios MM, Benagiano G. The placental bed vascular pathology revisited: a risk indicator for cardiovascular disease. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2017:1–9. [DOI] [PubMed] [Google Scholar]
- 125.Guttmacher AE, Spong CY. The human placenta project: it’s time for real time. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S3–5. [DOI] [PubMed] [Google Scholar]
- 126.Russell P Inflammatory lesions of the human placenta. I. Clinical significance of acute chorioamnionitis. The American Journal of diagnostic Gynecology and Obstetrics. 1979;1(2):127–37. [Google Scholar]
- 127.Moller GH, Woods DL, Malan AF, Sinclair-Smith CC. Chorio-amnionitis in relation to mode of delivery at term. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1989;76(5):201–2. [PubMed] [Google Scholar]
- 128.Sebire NJ, Goldin RD, Regan L. Histological chorioamnionitis in relation to clinical presentation at 14–40 weeks of gestation. J Obstet Gynaecol. 2001;21(3):242–5. [DOI] [PubMed] [Google Scholar]
- 129.Houben ML, Nikkels PG, van Bleek GM, Visser GH, Rovers MM, Kessel H, et al. The association between intrauterine inflammation and spontaneous vaginal delivery at term: a cross-sectional study. PloS one. 2009;4(8):e6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Torricelli M, Voltolini C, Conti N, Vellucci FL, Orlandini C, Bocchi C, et al. Histologic chorioamnionitis at term: implications for the progress of labor and neonatal wellbeing. Journal of Maternal-Fetal & Neonatal Medicine. 2013;26(2):188–92. [DOI] [PubMed] [Google Scholar]
- 131.Romero R, Whitten A, Korzeniewski SJ, Than NG, Chaemsaithong P, Miranda J, et al. Maternal floor infarction/massive perivillous fibrin deposition: a manifestation of maternal antifetal rejection? Am J Reprod Immunol. 2013;70(4):285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Naeye RL. Causes of perinatal mortality excess in prolonged gestations. American journal of epidemiology. 1978;108(5):429–33. [DOI] [PubMed] [Google Scholar]
- 133.Chen Y, Huang L, Zhang H, Klebanoff M, Yang Z, Zhang J. Racial disparity in placental pathology in the collaborative perinatal project. International journal of clinical and experimental pathology. 2015;8(11):15042–54. [PMC free article] [PubMed] [Google Scholar]
- 134.Keski-Nisula L, Aalto ML, Katila ML, Kirkinen P. Intrauterine inflammation at term: A histopathologic study. Human pathology. 2000;31(7):841–6. [DOI] [PubMed] [Google Scholar]
- 135.Park HS, Romero R, Lee SM, Park CW, Jun JK, Yoon BH. Histologic Chorioamnionitis is More Common after Spontaneous Labor than after Induced Labor at Term. Placenta. 2010;31(9):792–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee SM, Lee KA, Kim SM, Park CW, Yoon BH. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta. 2011;32(7):516–21. [DOI] [PubMed] [Google Scholar]
- 137.Mi Lee S, Romero R, Lee KA, Jin Yang H, Joon Oh K, Park CW, et al. The frequency and risk factors of funisitis and histologic chorioamnionitis in pregnant women at term who delivered after the spontaneous onset of labor. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Herman HG, Schreiber L, Miremberg H, Bar J, Kovo M. 607: Histological chorioamnionitis at term according to labor onset: A prospective controlled study. American Journal of Obstetrics & Gynecology.218(1):S362. [DOI] [PubMed] [Google Scholar]
- 139.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. American journal of obstetrics and gynecology. 2015;213(4 Supplement):S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. American journal of obstetrics and gynecology. 2008;199(4):375 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. The Journal of reproductive medicine. 1993;38(7):543–8. [PubMed] [Google Scholar]
- 143.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32(3):200–10. [DOI] [PubMed] [Google Scholar]
- 144.Romero R, Parvizi ST, Oyarzun E, Mazor M, Wu YK, Avila C, et al. Amniotic fluid interleukin-1 in spontaneous labor at term. The Journal of reproductive medicine. 1990;35(3):235–8. [PubMed] [Google Scholar]
- 145.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. American journal of obstetrics and gynecology. 1991;165(4 Pt 1):813–20. [DOI] [PubMed] [Google Scholar]
- 146.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. American journal of obstetrics and gynecology. 1992;166(5):1576–87. [DOI] [PubMed] [Google Scholar]
- 147.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27(3–4):117–23. [DOI] [PubMed] [Google Scholar]
- 148.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. American journal of obstetrics and gynecology. 1999;181(6):1530–6. [DOI] [PubMed] [Google Scholar]
- 149.Cox SM, Casey ML, MacDonald PC. Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Human reproduction update. 1997;3(5):517–27. [DOI] [PubMed] [Google Scholar]
- 150.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein-1 increase during term parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2003;14(1):51–6. [DOI] [PubMed] [Google Scholar]
- 151.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutrition reviews. 2007;65(12 Pt 2):S194–202. [DOI] [PubMed] [Google Scholar]
- 152.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. American journal of obstetrics and gynecology. 2006;195(2):394 e1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chaiworapongsa T, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, et al. Amniotic fluid heat shock protein 70 concentration in histologic chorioamnionitis, term and preterm parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(7):449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(8):529–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. Journal of perinatal medicine. 2008;36(3):217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chaiyasit N, Romero R, Chaemsaithong P, Docheva N, Bhatti G, Kusanovic JP, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. Journal of perinatal medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pineles B, Romero R, Montenegro D, Tarca A, Than N, Hassan S, et al. “ The Inflammasome” in Human Parturition. REPRODUCTIVE SCIENCES. 2007;14(1). [Google Scholar]
- 158.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(9):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Romero R, Xu Y, Plazyo O, Chaemsaithong P, Chaiworapongsa T, Unkel R, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, et al. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci. 2017;24(6):934–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gomez-Lopez N, Romero R, Xu Y, Garcia-Flores V, Leng Y, Panaitescu B, et al. Inflammasome assembly in the chorioamniotic membranes during spontaneous labor at term. Am J Reprod Immunol. 2017;77(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, et al. In vivo evidence of inflammasome activation during spontaneous labor at term. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2018:1–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Steel JH, O’Donoghue K, Kennea NL, Sullivan MH, Edwards AD. Maternal origin of inflammatory leukocytes in preterm fetal membranes, shown by fluorescence in situ hybridisation. Placenta. 2005;26(8–9):672–7. [DOI] [PubMed] [Google Scholar]
- 164.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? American journal of obstetrics and gynecology. 2017;217(6):693.e1–.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. American journal of obstetrics and gynecology. 2001;185(2):496–500. [DOI] [PubMed] [Google Scholar]
- 166.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2002;11(1):18–25. [DOI] [PubMed] [Google Scholar]
- 167.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006;19(11):693–7. [DOI] [PubMed] [Google Scholar]
- 168.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17(1):20–5. [DOI] [PubMed] [Google Scholar]
- 169.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. Journal of perinatal medicine. 2016;44(1):33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72(5):458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71(4):330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28(12):1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. Journal of perinatal medicine. 2015;43(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. Journal of perinatal medicine. 2016;44(1):5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod. 2016;95(6):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute Histologic Chorioamnionitis at Term: Nearly Always Noninfectious. PloS one. 2012;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, et al. Chorioamniotic membrane senescence: a signal for parturition? American journal of obstetrics and gynecology. 2015;213(3):359.e1–16. [DOI] [PubMed] [Google Scholar]
- 179.Maiti K, Sultana Z, Aitken RJ, Morris J, Park F, Andrew B, et al. Evidence that fetal death is associated with placental aging. American journal of obstetrics and gynecology. 2017;217(4):441 e1–e14. [DOI] [PubMed] [Google Scholar]
- 180.Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? American journal of obstetrics and gynecology. 2017. [DOI] [PubMed] [Google Scholar]
- 181.Altshuler G, Russell P. The human placental villitides: a review of chronic intrauterine infection. Current topics in pathology Ergebnisse der Pathologie. 1975;60:64–112. [PubMed] [Google Scholar]
- 182.Garcia AG, Basso NG, Fonseca ME, Zuardi JA, Outanni HN. Enterovirus associated placental morphology: a light, virological, electron microscopic and immunohistologic study. Placenta. 1991;12(5):533–47. [DOI] [PubMed] [Google Scholar]
- 183.Benirschke K, Coen R, Patterson B, Key T. Villitis of known origin: varicella and toxoplasma. Placenta. 1999;20(5–6):395–9. [DOI] [PubMed] [Google Scholar]
- 184.Euscher E, Davis J, Holzman I, Nuovo GJ. Coxsackie virus infection of the placenta associated with neurodevelopmental delays in the newborn. Obstetrics and gynecology. 2001;98(6):1019–26. [DOI] [PubMed] [Google Scholar]
- 185.Satosar A, Ramirez NC, Bartholomew D, Davis J, Nuovo GJ. Histologic correlates of viral and bacterial infection of the placenta associated with severe morbidity and mortality in the newborn. Human pathology. 2004;35(5):536–45. [DOI] [PubMed] [Google Scholar]
- 186.Redline RW. Recurrent villitis of bacterial etiology. Pediatric pathology & laboratory medicine : journal of the Society for Pediatric Pathology, affiliated with the International Paediatric Pathology Association. 1996;16(6):995–1001. [DOI] [PubMed] [Google Scholar]
- 187.Jacques SM, Qureshi F, Kim CJ, Lee JH, Giorgadze T, Mittal P, et al. Eosinophilic/T-cell chorionic vasculitis: a clinicopathologic and immunohistochemical study of 51 cases. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2011;14(3):198–205. [DOI] [PubMed] [Google Scholar]
- 188.Russell P Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology. Placenta. 1980;1(3):227–44. [DOI] [PubMed] [Google Scholar]
- 189.Knox WF, Fox H. Villitis of unknown aetiology: its incidence and significance in placentae from a British population. Placenta. 1984;5(5):395–402. [DOI] [PubMed] [Google Scholar]
- 190.Labarrere CA, Faulk WP, McIntyre JA, Althabe OH. Materno-trophoblastic immunological balance. Am J Reprod Immunol. 1989;21(1):16–25. [DOI] [PubMed] [Google Scholar]
- 191.Labarrere CA, McIntyre JA, Faulk WP. Immunohistologic evidence that villitis in human normal term placentas is an immunologic lesion. American journal of obstetrics and gynecology. 1990;162(2):515–22. [DOI] [PubMed] [Google Scholar]
- 192.Hulthen Varli I, Petersson K, Kublickas M, Papadogiannakis N. Both acute and chronic placental inflammation are overrepresented in term stillbirths: a case-control study. Infect Dis Obstet Gynecol. 2012;2012:293867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kaplan CG. Fetal and maternal vascular lesions. Semin Diagn Pathol. 2007;24(1):14–22. [DOI] [PubMed] [Google Scholar]
- 194.Redman CW, Staff AC. Preeclampsia, biomarkers, syncytiotrophoblast stress, and placental capacity. American journal of obstetrics and gynecology. 2015;213(4 Suppl):S9 e1–S9–11. [DOI] [PubMed] [Google Scholar]
- 195.Bane AL, Gillan JE. Massive perivillous fibrinoid causing recurrent placental failure. BJOG : an international journal of obstetrics and gynaecology. 2003;110(3):292–5. [PubMed] [Google Scholar]
- 196.Roberts VH, Rasanen JP, Novy MJ, Frias A, Louey S, Morgan TK, et al. Restriction of placental vasculature in a non-human primate: a unique model to study placental plasticity. Placenta. 2012;33(1):73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Burton GJ, Fowden AL, Thornburg KL. Placental Origins of Chronic Disease. Physiol Rev. 2016;96(4):1509–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stanek J Utility of diagnosing various histological patterns of diffuse chronic hypoxic placental injury. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2012;15(1):13–23. [DOI] [PubMed] [Google Scholar]
- 199.Stanek J Hypoxic patterns of placental injury: a review. Archives of pathology & laboratory medicine. 2013;137(5):706–20. [DOI] [PubMed] [Google Scholar]
- 200.Trudinger BJ, Stevens D, Connelly A, Hales JR, Alexander G, Bradley L, et al. Umbilical artery flow velocity waveforms and placental resistance: the effects of embolization of the umbilical circulation. American journal of obstetrics and gynecology. 1987;157(6):1443–8. [DOI] [PubMed] [Google Scholar]
- 201.Morrow RJ, Adamson SL, Bull SB, Ritchie JW. Effect of placental embolization on the umbilical arterial velocity waveform in fetal sheep. American journal of obstetrics and gynecology. 1989;161(4):1055–60. [DOI] [PubMed] [Google Scholar]
- 202.Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. Br J Obstet Gynaecol. 1985;92(1):31–8. [DOI] [PubMed] [Google Scholar]
- 203.Hitschold T, Weiss E, Beck T, Hunterfering H, Berle P. Low target birth weight or growth retardation? Umbilical Doppler flow velocity waveforms and histometric analysis of fetoplacental vascular tree. American journal of obstetrics and gynecology. 1993;168(4):1260–4. [DOI] [PubMed] [Google Scholar]
- 204.Thompson RS, Trudinger BJ. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. Ultrasound in medicine & biology. 1990;16(5):449–58. [DOI] [PubMed] [Google Scholar]
- 205.Whitten AE, Romero R, Korzeniewski SJ, Tarca AL, Schwartz AG, Yeo L, et al. Evidence of an imbalance of angiogenic/antiangiogenic factors in massive perivillous fibrin deposition (maternal floor infarction): a placental lesion associated with recurrent miscarriage and fetal death. American journal of obstetrics and gynecology. 2013;208(4):310 e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Budhraja V, Sadovsky Y. Microarray analysis of trophoblast cells. Methods in molecular medicine. 2006;121:411–24. [DOI] [PubMed] [Google Scholar]
- 207.Montenegro D, Romero R, Kim SS, Tarca AL, Draghici S, Kusanovic JP, et al. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. The Journal of pathology. 2009;217(1):113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Szabo S, Xu Y, Romero R, Fule T, Karaszi K, Bhatti G, et al. Changes of placental syndecan-1 expression in preeclampsia and HELLP syndrome. Virchows Archiv : an international journal of pathology. 2013;463(3):445–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Varkonyi T, Nagy B, Fule T, Tarca AL, Karaszi K, Schonleber J, et al. Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar. Placenta. 2011;32 Suppl:S21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Acharya G, Albrecht C, Benton SJ, Cotechini T, Dechend R, Dilworth MR, et al. IFPA Meeting 2011 workshop report I: Placenta: Predicting future health; roles of lipids in the growth and development of feto-placental unit; placental nutrient sensing; placental research to solve clinical problems--a translational approach: Placenta. 2012. February;33 Suppl:S4–8. doi: 10.1016/j.placenta.2011.11.015. Epub 2011 Dec 10. [DOI] [PubMed] [Google Scholar]
- 211.Burton GJ, Sebire NJ, Myatt L, Tannetta D, Wang YL, Sadovsky Y, et al. Optimising sample collection for placental research. Placenta. 2014;35(1):9–22. [DOI] [PubMed] [Google Scholar]
- 212.Szabo S, Mody M, Romero R, Xu Y, Karaszi K, Mihalik N, et al. Activation of villous trophoblastic p38 and ERK1/2 signaling pathways in preterm preeclampsia and HELLP syndrome. Pathology oncology research : POR. 2015;21(3):659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Albrecht C, Baker JC, Blundell C, Chavez SL, Carbone L, Chamley L, et al. IFPA meeting 2016 workshop report I: Genomic communication, bioinformatics, trophoblast biology and transport systems. Placenta. 2017;60 Suppl 1:S5–S9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.