Abstract
Previous studies have demonstrated that sex differences in pain responsivity can be detected using various models of experimentally induced pain. The present study employed the mechanical pressure test in order to examine potential differences in pain report among men, normally menstruating women (NMW), and women taking monophasic oral contraceptives (OCW). Testing occurred during 5 phases of the menstrual cycle (menstrual, follicular, ovulatory, luteal, and late luteal) and all participants completed 10 sessions (2 sessions per phase). Menstrual-cycle phase was estimated for OCW based on their first day of menses. Men were tested at time points that roughly corresponded to the intervals during which the different phases occurred in NMW. During the mechanical pressure test, 4 different weights were placed on the fingers, one at a time, and ratings of pain were recorded for 30 seconds. The statistical decision-making model and a forced-choice procedure were used to analyze the response data. Two variables, based on signal detection theory, were thus generated: P(A), a measure of sensory pain, and B, a measure of response bias. P(A) is believed to be a measure of pain sensitivity while B measures stoicism. NMW tended to report lower P(A) values, indicating reduced ability to discriminate among different stimulus intensities, during the menstrual and late luteal phases compared to the luteal phase. OCW reported lower B values, indicating less stoicism, during the menstrual compared to the follicular and ovulatory phases. Men tended to have significantly lower B values than OCW, but not NMW. These results demonstrate subtle menstrual-cycle effects in NMW and OCW. Sex differences were few, with more group differences and trends emerging between OCW and men, as opposed to men and NMW.
Perspective:
The lack of consistent differences between men and NMW underscores the subtle impact of sex and hormonal changes in pain report. In addition, the data obtained in NMW support the notion that changes in hormone levels during the menstrual cycle can lead to changes in pain responsivity as NMW had trends for better discrimination in menstrual phases when estradiol levels were highest.
Keywords: Sex differences, pain, humans
Many studies show that women are more sensitive to pain than men.6,17,42 While few would argue that there are no differences, many researchers question the relevance of these differences, especially as it relates to treatment in the clinic.4,20,27 Of the different types of experimental pain, pressure pain in particular seems to be sensitive to sex differences.In a meta-analysis of gender differences in pain report,42 pressure pain had one of the highest effect sizes. However, this analysis may be skewed because the analysis included only 6 studies, all of which supported sex differences, highlighting the scientific bias of primarily publishing studies with positive results. In a more recent analysis, Chesterton et al6 summarized 16 studies, 10 that suggested that women may be more sensitive to pressure pain, and another 6 that did not find any sex differences. Additional studies not reported by Chesterton found that men had a higher pain tolerance than women,5 while another found a difference, but only to the temporal summation of pressure pain.46
Further complicating research on sex differences in pain is the nature of pain report, which combines both a sensory response to a noxious stimuli and a response bias, the willingness to report that response. Thus there is difficulty in ascertaining the extent to which these differences are primarily due to physiological (sensory) or subjective effects. This distinction is important because the treatments may vary if the sex differences are caused by sensory (ie, pain pathways), as opposed to nonsensory variables (ie, subjective ratings influenced by social expectancies). Studies have shown that women generally report both a greater number of painful symptoms and greater severity of pain in the clinical setting.45,49 It is widely believed that social factors contribute to the lower pain reports by men,49 and even contribute to the lower likelihood of men seeking medical attention.11 When examined via experimental pain procedures, social factors, such as the sex of the experimenter, are known to affect the results,28 with men reporting less pain to female experimenters compared to male experimenters. Other nonsensory factors that have been shown to affect individual differences in pain report are the presence of anxiety25 and pain catastrophizing (the belief that pain will be unbearable or extremely awful).14,16,48 Since these factors have been shown to be more prevalent in women,14,16 they could all have an impact upon sex differences in pain report. On the other hand, sensory factors may also play a role in pain report. These factors are presumably controlled by biological variables, such as a past history of chronic pain21,45 and genetics.34 For example, some rodent strains demonstrate a higher level of basal-pain sensitivity in females, others demonstrate no differences, and still others demonstrate a higher level of basal-pain sensitivity in males.34 These sex differences in pain responsivity as a function of genetics have also been demonstrated in humans.35
All of these factors that lead to the pain response make the study of pain more complicated. Pain is often thought of as a sensory process by neuroscientists, yet there are clearly social and emotional aspects to pain. Thus, when one group reports more pain than another, it may not be that one group is experiencing increased pain signals neuronally; it may just be that that group is more willing to report pain.14,37 In an attempt to separate the sensory and response bias components of pain report, the present study employed a version of statistical decision-making theory to generate 2 dependent variables. P(A) is a measure of the ability to distinguish among various stimulus intensities, and thus can be thought of as a measure of sensory sensitivity to differences in painful stimuli. B is a measure of stoicism or willingness to report pain, and thus can be thought of as a measure of the subjective aspect of pain report.
Changes in gonadal hormones during the menstrual cycle are another factor that should be assessed when examining sex differences in pain report. Estrogens have been linked to pain processing through modulatory effects on gamma-aminobutyric acid receptors, μ-opioid receptors, and nerve growth factor receptors in the dorsal root ganglion.1 In their review of this topic, Fillingim and Ness18 highlighted a trend for pain sensitivity to be greater during the postmenstrual compared to the premenstrual period. However, many studies described in the review found no effects of menstrual-cycle phase. In addition, the method by which the menstrual cycle was defined differed from one study to the next, and only one of the studies actually verified hormone levels. In a meta-analysis, only small effect sizes could be found for the effect of menstrual cycle phase on pain report.44
When specifically examining pressure pain across the menstrual cycle, results have been mixed. In 2 studies examining pressure-pain threshold in the masseter muscle and temporalis muscle in either the thumb or finger, one study showed that pain threshold was lower during the perimenstrual phase compared to the follicular and luteal phases of the menstrual cycle,23 while the other found that pain threshold was higher during the perimenstrual compared to the follicular phase.15 Two other studies examining pressure-pain thresholds across the menstrual cycle found no differences,2,24 while another found lower thresholds during the periovulatory phase.7 A study examining pressure pain by the number of tender points (the number of points on the body particularly sensitive to pressure) found that there were more tender points during the follicular as opposed to the luteal phase of the menstrual cycle.22 When examining pressure pain, differences between studies could also be caused by differences in the locations that pressure is placed. In addition, none of these studies verified blood hormone levels, and all but one,7 which used an ultrasonic screening, had no method for verifying menstrual-cycle phase outside of self-report. The inability to verify menstrual-cycle phase, the lack of a common method for defining phases, and the lack of confirmatory hormonal data makes interpretation of the results of these studies difficult.
The present study sought to examine the effects of sex and gonadal hormones on response to pain induced by mechanical pressure. The menstrual cycle was prospectively monitored, and divided into 5 phases verified with blood hormone levels and ovulation kits. In addition to men and normally menstruating women, the present study also included a group of women who were maintained on monophasic oral contraceptives to control for the effects of hormone fluctuations in women.
Methods
Participants
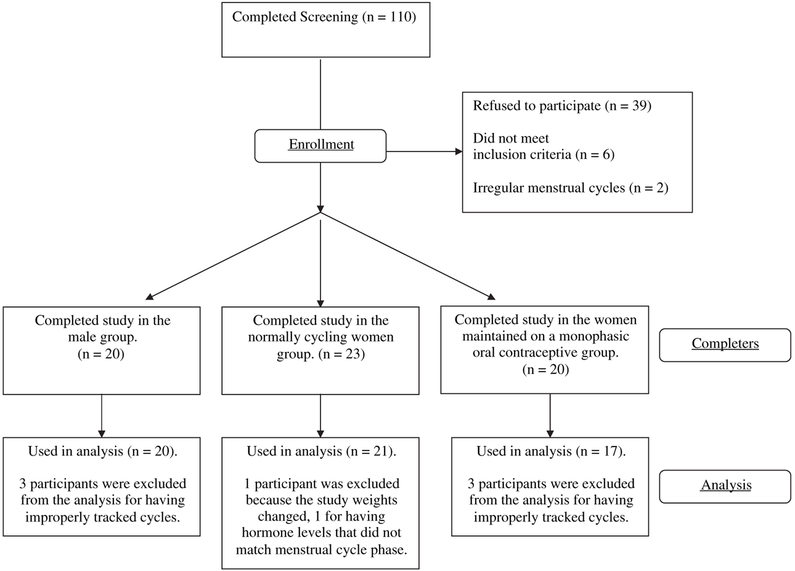
Normal, healthy individuals, aged 21 to 45 years, were recruited from the New York City area via newspaper advertisements. In total, 110 participants signed the study consent form, and 63 participants completed the study (Fig 1). Of the 47 participants who did not complete the study, 16 had scheduling conflicts, 12 found employment, 7 were lost to contact, 5 had low pain tolerance, 4 left for personal reasons unrelated to the study, 2 were discontinued by the investigators because of irregular menstrual cycles, and 1 was discontinued for elevated blood pressure. Participants were accepted into the study if they were physically healthy, as determined by urine and blood analyses, electrocardiogram, and physical examination. Participants were excluded from the study if they met DSM IV-TR criteria for substance abuse or dependence, had current or past Axis I or significant Axis II psychopathology, were pregnant or nursing, were taking prescription psychotropic medications, or had current chronic pain, or a history of chronic pain. Women with irregular menstrual cycles, or who were dysmenorrheic, amenorrheic, menopausal, or suffering from Premenstrual Dysphoric Disorder, were also excluded from the study. All participants had body mass indexes between 17.5 and 27.5, and reported no, or infrequent use of, over-the-counter analgesics. Participants completed several questionnaires in the screening process. Two were analyzed as data in response to factors believed to influence sex differences in pain report.20 One was the Previous Pain Experiences Questionnaire (Clark and Kuhl, personal communication), which asked participants if they had experienced a variety of painful events, and how painful they were. The participant answered, never experienced, of mild discomfort, moderate discomfort, and severe discomfort to a list of 47 painful experiences such as toothache, hit finger with hammer, stub toe, appendicitis, etc. Data also were obtained and analyzed for the Trait portion of the State-Trait Anxiety Inventory.47 The other questionnaires completed during screening were demographic in nature or used solely to determine inclusion/exclusion criteria.
Figure 1.
Flow diagram for enrollment and analysis of participants in the present study.
Participants, matched according to ethnicity and age, belonged to one of the following 3 groups: men, normally menstruating women, and women taking oral contraceptives. Only women using monophasic oral contraceptives were accepted into the latter group because these pills maintain relatively constant hormone levels across the cycle. By contrast, hormone levels fluctuate across days with the use of other oral contraceptives (eg, Ortho Tri-cyclen). Specific oral contraceptives used were: LoOvral (n = 5; Wyeth Pharmaceuticals, Philadelphia, PA), Ortho-Cyclen (n = 3; Ortho-McNeil Pharmaceutical Inc, Raritan, NJ), Alesse-28 (n = 3; Wyeth Pharmaceuticals), Desogen (n = 1; Organon USA Inc, Roseland, NJ), Levelen-ED (n = 1; Berlex Laboratories, Wayne, NJ), Low Ogestrel – 28 (n = 1; Watson Laboratories Inc, Corona, CA), Ortho-Novum 1/35 (n = 1; Ortho-McNeil Pharmaceutical Inc), Orcon (n = 1; Phaarmasia, Limited Hyderabad, India), and Ortho-Cept 28 (n = 1; Ortho-McNeil Pharmaceutical Inc).
Data were not analyzed for 5 participants who completed the study. Data from 1 normally menstruating woman were not used because of a change in study procedures, and data from the other normally menstruating woman were not used because her estradiol and progesterone levels were not consistent with her verbal report of menstrual-cycle phases. Data provided by 1 oral-contraceptive woman were not used because of a complete lack of menstrual bleeding and data provided by the 2 other oral-contraceptive women were not used because of incongruent self-reports of menstruation and predicted first day of menses by pill pack. Ultimately, data were analyzed from 21 normally menstruating women, 17 oral-contraceptive women, and 20 men. Ethnic backgrounds and ages of the participants can be found in Table 1.
Table 1.
Summary of the Ethnicities and Ages of the Participants in Each Group. Also Reported are Average Scores on the Pain Experiences Questionnaire and the Trait Portion of the State-Trait Anxiety Inventory
| Ethnicity |
||||||||
|---|---|---|---|---|---|---|---|---|
| Asian | Black | Caucasian | Hispanic | Other | Mean Age (years) | Pain exp. (avg) | Trait Anxiety | |
| GROUP | ||||||||
| NMW | 1 | 6 | 11 | 2 | 1 | 29.21 | 1.6 | 33.41 |
| OCW | 0 | 4 | 11 | 2 | 0 | 26 | 1.6 | 36.05 |
| Men | 1 | 4 | 10 | 3 | 2 | 28.31 | 1.3 | 33.25 |
The Institutional Review Board of the New York State Psychiatric Institute approved all experimental procedures and informed consent was obtained from all participants prior to initiation of the experimental procedures.
A mixed within- and between-participants design was used for this study, with group (normally menstruating women, oral-contraceptive women, and men) as the between-participants variable and menstrual-cycle phase as the within-participants variable of interest. Each participant completed 10 sessions. Two sessions were completed during each of 5 menstrual cycle phases. The cycle phases were verified in 3 ways: blood samples were collected at the beginning of each session day to measure estradiol and progesterone plasma levels, urine ovulation kits (measuring the surge in luteinizing hormone) were used in normally menstruating women during each monthly cycle, and a Daily Rating Form was completed each evening for the entire study that queried about physical and mood changes as well as the onset of menses. Men completed a comparable questionnaire. Normally menstruating women were tested during the menstrual phase (2 to 5 days after the onset of menstruation), the follicular phase (6 to 10 days after the onset of menstruation), the ovulatory phase (within 3 days after the ovulation kit turned positive, generally 13 to 15 days after the onset of menstruation), the luteal phase (within 7 to 12 days after the ovulation kit turned positive, generally 19 to 24 days after the onset of menstruation), and the late luteal phase (within 13 to 17 days after the ovulation kit turned positive, generally 25 to 28 days after the onset of menstruation). Oral-contraceptive women were tested on days corresponding to the menstrual (days 2 to 5), follicular (days 6 to 10), ovulatory (days 13 to 15), luteal (days 19 to 24) and late luteal (days 25 to 28) phases as described above for normally menstruating women, with their first day of menstruation being day 1. In order to maintain consistency across groups with respect to the number of sessions and the intersession interval, men completed 10 sessions based on a 28-day calendar with the first day of each month corresponding to day 1 of the menstrual cycle. When comparing men as well as oral-contraceptive women to normally menstruating women, it was necessary to conduct sessions in intervals that would correspond to normally menstruating women as pain report can change with repeated administration.27 The menstrual phase during which the first session was conducted was counterbalanced across participants. Prior to testing, participants completed a practice session to familiarize them with the mechanical-pressure test and questionnaires used during an actual session day.
Relevant Hormone Changes
Normally menstruating women have changes in hormone levels that may affect pain report. During the menstrual and follicular phases, levels of estradiol and progesterone are low.20 Then there is a spike of estradiol immediately preceding ovulation, followed by a decline and gradual rise of both estradiol and progesterone through the luteal phase.20 In the late luteal phase, these elevated levels of progesterone and estradiol decline, leading to the cycle beginning again.20 When women are maintained on a monophasic oral contraceptive, their levels of estradiol and progesterone are held low, indistinguishable from the levels found in men,27 through 3 weeks of exogenous administration of hormones. In the 4th week of oral-contraceptive treatment, placebo pills are taken and the levels of estradiol and progesterone drop even further than their already low levels, leading to menstruation. Hormone levels for this study were normal and reported in an earlier paper27 and thus are not reported directly here.
Experimental Session Day
Participants arrived between 9:00 and 9:30 AM on all session days. The experimenter conducting the session was of the same sex as the participant and conducted all measures with the participant individually. All participants were asked to refrain from drinking alcohol or taking over-the-counter analgesics within 12 hours of a session. Urine pregnancy tests were performed for all female participants on a monthly basis. Breath alcohol levels and urine drug toxicologies were obtained prior to beginning the session. If the test was negative, the experimental session was conducted. Sessions were canceled for 3 participants (2 normally menstruating women and 1 man) because of positive marijuana drug screens. All 3 participants returned after urine drug screens were negative and finished the study. No participant had more than 1 such incident. Each experimental day, venous blood samples (approximately 6 mL) for estradiol and progesterone were drawn into tubes containing SST gel and clot activator. Samples were centrifuged within 30 minutes of collection, yielding approximately 3 ml of plasma, and stored frozen until the time of analysis. Estradiol and progesterone levels were determined by Dr. Michel Ferin at the College of Physicians and Surgeons of Columbia University, Department of Obstetrics and Gynecology (New York, NY). Estradiol and progesterone were measured by a commercial solidphase, chemiluminescent immunoassay (Immulite; Diagnostic Products Co, DPC, Los Angeles, CA). For estradiol, the assay sensitivity was 20 pg/ml and the intra- and inter-assay coefficients of variation were 9.3% and 10.5%. For progesterone, the assay sensitivity was .2 ng/ml and the intra- and inter-assay coefficients of variation were 6.6% and 7.9%.
Participants received a standard breakfast every morning that remained constant across all sessions to control for the possible influence of macronutrient content. At the beginning and end of each session, blood pressure and heart rate were measured. Self-reported depression and anxiety symptoms were also assessed during each session, using the Beck Depression Inventory II (BDI-II)3 and the State-Trait Anxiety Inventory (STAI).47 The BDI-II was completed at the beginning and end of each session day while the State portion of the STAI was completed at the beginning and end of each session and during the pain task. Participants also completed the Multidimensional Affect and Pain Survey (101-MAPS)9 after the first trial of each of the pain tests. The 101-MAPS is a questionnaire designed to examine both the affective state of the participant as well as the level of pain they are feeling. The 3 Superclusters (sensory pain, emotional suffering, and well being) containing 30 subclusters were based on a dendrogram obtained by cluster analysis. Two experimentally induced pain procedures (the cold-pressor test (CPT) and mechanical-pressure test (MPT)) were used during each session. Each test was completed twice per session in alternating order, with twenty-minute rest periods between each test. Half of the participants were tested with the cold-pressor test first (CPT-MPT-CPT-MPT), and half the participants were tested with the mechanical-pressure test first (MPT-CPT-MPT-CPT). Results from the cold-pressor test, hormone levels, daily rating forms, STAI, and the Beck Depression Inventory, were reported in a previous paper.27
Mechanical-Pressure Test
The mechanical pressure test was modeled after that of previous research.19,29 Participants were seated on one side of the mechanical-pressure apparatus, separated from view of the research assistant by a curtain. Four different weights (440 g [4.31 N], 600 g [5.88 N], 780 g [7.64 N], or 955 g [9.359 N]) were placed 1 at a time upon the second and third phalanx of the left and right hands. The tip of the weights was 4 mm in diameter. Due to the 4-mm diameter tip, the pressure, in Pascals, for the stimuli were 682.3, 935.9, 1216.0, and 1489.6 Pa, respectively. Before each trial began, participants were read the following statement:
To test your sensitivity to mechanical pressure, several weights will be placed on your fingers for 30 seconds. You can ask me to remove the weight from your finger at any time if it becomes too painful—just say “stop,” but please try to keep the weight on your finger for the whole 30 seconds, if possible. There will be no damage to your fingers as a result of this test.
We will be switching between corresponding fingers from the right hand to the left hand. After each matching pair, I will ask you to compare the weights. During each 30-second period that a weight is on your finger, at 5-, 10-, 20-, and 30-second intervals, I will ask you to rate the sensation that you are feeling at that moment. I will say “rate,” and you will choose and tell me a sensation from the list in front of you. We will be testing the tops and the bottoms of your fingers.
On the side of the curtain facing the participant, a rating scale was shown that included the following statements: “not noticeable,” “feel pressure,” “slight discomfort,” “distinctly uncomfortable,” “faint pain,” “painful,” “definitely painful,” “extremely painful,” “excruciatingly painful,” and “worst possible pain.” The research assistant entered into a spreadsheet numbers corresponding to each rating (“not noticeable” = 1; “faint pain” = 5; “worst possible pain” = 10). These numbers are the inverse of the response bias measure (or B) in the statistical decision-making model. If the participant asked that the weight be taken off of his or her finger, the time was recorded and each missed rating was recorded as a 10. After each pair of stimulus intensities (on corresponding fingers of the right and left hand) was completed, the participant was asked which of the 2 weights was heavier and which of the 2 weights was more painful. The percentage of correct responses was calculated. Vital signs were measured at both the beginning and end of the mechanical-pressure test.
Screening Data
Based on a recent consensus report on methods for studying sex differences in pain,20 data on anxiety and history of pain were analyzed in the present study. Trait scores on the State-Trait Anxiety Inventory were examined for possible baseline group differences in anxiety levels. In addition, a locally developed questionnaire was used to examine pain (Previous Pain Experiences Questionnaire, Clark and Kuhl, personal communication). Participants were asked about 47 specific painful experiences. Possible answers were: never experienced, mild discomfort, moderate discomfort, or severe discomfort (coded 0, 1, 2, and 3, respectively). The experiences ranged from everyday occurrences such as “banged shin” to medical conditions such as “kidney stones.” A sum score was created to analyze pain-history data. Responses regarding childbirth and menstrual pain were removed from the analysis as only women could have experienced those events, which could have skewed the results. The pain experiences questionnaire also asked 2 additional questions about relative pain tolerance. Specifically, the questions were: “Other people say that you are generally (circle: much more, more, less, much less) sensitive to pain than others.” and “You would say that you are generally (circle: much more, more, less, much less) sensitive to pain than others.”
Data Analysis
The data-analysis plan was to conduct mixed between-and within-groups analyses, using a group (3 levels: normally menstruating women; oral-contraceptive women; men) by phase (5 levels: menstrual; follicular; ovulatory; luteal; late luteal), by day (2 levels: first session completed in a phase; second session completed in a phase), by trial (2 levels: first MPT completed in the day; second MPT completed in the day), by stimulus-intensity pair (3 levels: light [440 g and 600 g]; medium [600 g and 780 g]; heavy [780 g and 955 g]). Analyses were completed using the last time point data was collected (when differences are theorized to be at their maximum) as well as utilizing all time points. Within-groups repeated-measures analyses of variance for menstrual-cycle phase were conducted using SuperANOVA software (Abacus Concepts, Inc, Berkeley, CA, 1991). Planned pairwise comparisons were then conducted between each of the menstrual-cycle phases (menstrual vs follicular, menstrual vs ovulatory, follicular vs ovulatory, etc.). An ANOVA was conducted to examine between group differences. Separate ANOVAs were used to investigate group differences within each menstrual cycle phase. Group analyses were conducted using Statistica MANCOVA software (StatSoft Inc, Tulsa, OK) and SPSS software (SPSS Inc, Chicago, IL).
Data were analyzed for the mechanical-pressure test using statistical decision-making theory procedures.8,10,19,32 Statistical decision-making theory at its most basic form assumes that when a target stimulus, or signal, is presented, background noise is always present and varies randomly over time.8,10,32 In the statistical decision-making theory model, stimuli are presented randomly with respectto intensity, with a target stimulus being presented with and without a noise stimulus. In the present study, the target stimulus was the high-intensity stimulus, while the noise stimulus was the low-intensity stimulus. There were 4 possible decision outcomes: a report of pain to a high-intensity stimulus (hit); a report of pain to a low-intensity stimulus (false positive); a report of no pain to a high-intensity stimulus (miss); and a report of no pain to a low-intensity stimulus (correct rejection). In the present study, stimulus-intensity pairs represented target stimulus and noise pairs, such that when the very heavyweight (955 g) was the signal stimulus, the heavy weight (780 g) was the noise stimulus (heavy-stimulus intensities); when the heavy weight (780 g) was the signal stimulus, the medium weight (600 g) was the noise stimulus (medium-stimulus intensities); and when the medium weight (600 g) was the signal stimulus, the light weight (440 g) was the noise stimulus (light-stimulus intensities).
Two indexes are derived from the participant’s responses: an index of discriminability P(A), and the index of response bias, B. P(A) represents the participants’ ability to distinguish among stimuli of varying intensity.8,10,32 The scores for P(A) range from complete chance (.5) to perfect discrimination (1). P(A) is derived using the receiver operator characteristics (ROC) curve, a plot of hit rate (on the ordinate) and false positives (on the abscissa). P(A) was examined in individual stimulus-intensity pairs, averaged across all stimulus intensities, and at the 30-second time point within the mechanical-pressure test. The response bias index, or B, is the median point of the response scale (so that half the responses are above and half the responses are below), and represents the participants’ willingness to report pain. The scores for B range between 0 and 10. A low B value indicates that the participant is highly willing to report pain, while a high B value indicates that the participant is unwilling to report pain. This index is correlated with the sum of hit and false-positive rates, and is relatively independent from ability to discriminate. For more detailed accounts of statistical decision theory and its relation to pain, please see reference 8.
Correlations were completed using SPSS software (SPSS Inc). T-tests were also performed on STAI trait data and the pain-experiences questionnaire using SPSS software. Due to the number of tests run on each variable, a P value of .01 was used to indicate statistical significance for the mixed within- and between-groups ANOVAs and all findings with P values between .01 and .05 were reported as trend data.
Effect-size analyses for the menstrual-cycle phases were based on 1-way repeated-measures contrasts. Effect-size analyses for between-groups effects were based on a 1-way analysis of variance. A significance level of .01 was used for all effect-size analyses. Calculations of the number of subjects necessary to show significant differences were based on a power of 80%. Effect-size analyses were conducted by using nQuery software (Statistical Solutions, Saugus, MA).
Results
Demographic Data
As shown in Table 1, there were no significant differences between groups in ethnicity or age. In addition, the STAI revealed no differences between groups in trait anxiety, although the pain-experiences questionnaire did yield between-group differences. Normally menstruating women reported significantly more events as being moderately discomforting than men (12.2 compared to 5.8 in normally menstruating women and men, respectively, t = 3.5, P ≤ .001). The average score reported on the Previous Pain Experiences Questionnaire (Clark and Kuhl, personal communication) was higher for normally menstruating women than men (1.6 compared to 1.3 in normally menstruating women and men, respectively, t = 2.9, P ≤ .01, (maximum score 3). Oral-contraceptive women also reported a significantly higher average score than men (1.6 and 1.3 in oral contraceptive women and men, respectively, t = 2.7, P ≤ .01).
Response Criterion, B
All reports of response criterion, B, trends or significance are for the 30-second time point (final pain report) unless otherwise noted.
Within Groups
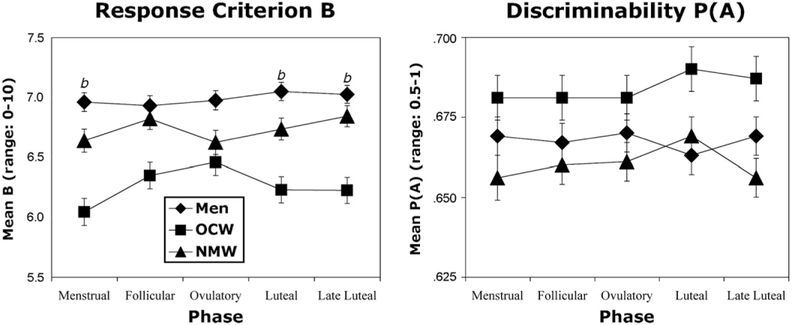
Fig 2 (left panel) shows that normally menstruating women had trends for higher response criterion B values during the follicular compared to the menstrual phase for the light-stimulus intensities (440 and 600 g; F(1,20) = 4.4, P ≤ .05). Normally menstruating women also had trends for higher response criterion B values during the late luteal compared to the ovulatory phase for the medium-stimulus intensities (600 and 780 g; F(1,20) = 4.4, P ≤ .05). No other effects of menstrual cycle phase were found for normally menstruating women.
Figure 2.
Mean values for response criterion B and P(A), discriminability, across the menstrual phases. Error bars represent ±1 SEM. Oral-contraceptive women (OCW) had significantly lower values for response criterion B in the menstrual phase compared to the follicular and ovulatory phases. There was a trend for normally menstruating women (NMW) to have higher values for P(A) in the luteal phase as compared to the menstrual and late luteal phases b represents a trend for a difference between oral contraceptive women and men. All trends are P ≤ .05.
Fig 2 (left panel) also shows that in the oral-contraceptive women group, participants had significantly higher criterion B values across the full pain report in the ovulatory (F(1,16) = 7.7, P ≤ .01) phase compared to the menstrual phase and trends for higher response criterion B values during the follicular (F(1,16) = 4.1, P ≤ .05) phase compared to the menstrual phase. Oral-contraceptive women had higher response criterion B values during the ovulatory (F(1,16) = 6.7, P ≤ .01) phase compared to the menstrual phase and a trend for higher response criterion B values during the follicular (F(1,16) = 4, P ≤ .05) compared to the menstrual phase. Oral-contraceptive women had higher response criterion B values during the ovulatory (F(1,16) = 6.7, P ≤ .01) phase compared to the menstrual phase for the light stimulus intensities (440 and 600 g) and trends were found for response criterion B values to be higher during the follicular (F(1,16) = 5.3, P ≤ .05) phase compared to the menstrual phase for the light stimulus intensities (440 and 600 g). Oral contraceptive women had higher response criterion B values during the ovulatory (F(1,16) = 6.6, P ≤ .01) phase compared to the menstrual phase for the medium-stimulus intensities (600 and 780 g) and trends for higher response criterion B values were found for the follicular (F(1,16) = 4.6, P ≤ .05) phase compared to the menstrual phase for the medium-stimulus intensities (600 and 780 g). Oral-contraceptive women tended to have higher response criterion B values during the ovulatory phase compared to the menstrual (F(1,16) = 5, P ≤ .05) and luteal (F(1,16) = 4.3, P ≤ .05) phases forthe heavy-stimulus intensities (780 and 955 g).
As expected, there were no statistically significant differences in or trends for phase effects for men (Fig 2, left panel).
Between Groups
Fig 2 (left panel) also shows that there were trends indicating that men had higher response criterion B values when averaged over the entire pain report than oral-contraceptive women during the menstrual (F(1,55) = 5.3, P ≤ .05), luteal (F(1,55) = 4.9, P ≤.05) and late luteal (F(1,55) = 4.8, P ≤.05) phases. There were also trends for higher B values for men compared to oral-contraceptive women for the medium- (600 and 780 g) (F(1,55) = 4.2, P ≤ .05) and heavy-(780 and 955 g) stimulus intensities (F(1,55) = 4.3, P ≤ .05) of weights. There were trends for men to have higher response criterion B values during the menstrual (F(1,55) = 4.6, P ≤ .05) and luteal (F(1,55) = 4.5, P ≤ .05) phases. Response criterion B values for the medium-stimulus intensities (600 and 780 g) tended to be higher for men compared to oral-contraceptive women during the menstrual (F(1,55) = 5.4, P ≤ .05), luteal (F(1,55 = 5.1, P ≤ .05), and late luteal (F(1,55) = 4, P ≤ .05) phases. There were also trends for men to have higher response criterion B values than oral-contraceptive women for the heavy-stimulus intensities (780 and 955 g) for the menstrual (F(1,55) = 4, P ≤ .05) and the luteal (F(1,55) = 4.7, P ≤ .05) phases.
There were no significant differences in response criterion B values between men and normally menstruating women, or normally menstruating women and oral-contraceptive women.
P(A): Discriminability
All reports of response criterion, B, trends or significance are for the 30-second time point (final pain report) unless otherwise noted.
Within Groups.
Fig 2 (right panel) shows that normally menstruating women tended to have higher P(A) values, indicating greater ability to discriminate between stimulus intensities, during the luteal phase compared to the menstrual (F(1,20) = 4.1, P ≤ .05) and late luteal (F(1,20) = 4.2, P ≤.05) phases. Examining just the medium-stimulus intensities (600 and 780 g), normally menstruating women showed a trend for a main effect of menstrual-cycle phase (F(1,20) = 2.8, P ≤.05), as well as having significantly higher discriminability values during the luteal compared to the late luteal (F(1,20) = 11.1, P ≤ .01) phase and a trend for higher P(A) values in the luteal as compared to the menstrual phase (F(1,20) = 4.2, P ≤ .05). Normally menstruating women also had higher discriminability P(A) values across the entire pain report for the heavy-stimulus intensities (780 and 955 g) during the late luteal phase compared to the ovulatory phase (F(1,20) = 4.3, P ≤ .05). Neither men nor oral-contraceptive women showed any significant effects or trends of phase for discriminability.
Between Groups.
Fig 2 (right panel) also shows that discriminability P(A) values across the entire pain report tended to be higher for oral contraceptive women compared to men for the medium-stimulus intensities (600 and 780 g) (F(1,55) = 4.4, P ≤ .05), and there was a trend for a sex by stimulus intensities interaction (F(4,110) = 2.5, P ≤ .05; Fig 3). Oral-contraceptive women tended to have higher values for discriminability than normally menstruating women for the light- (F(1,55) = 5.2, P ≤ .05) and medium- (F(1,55) = 5.4, P ≤ 0.05) stimulus intensities. Men also tended to have greater discriminability values than normally menstruating women for the medium-stimulus intensities (600 and 780 g) (F(1,55) = 4.5, P ≤ .05).
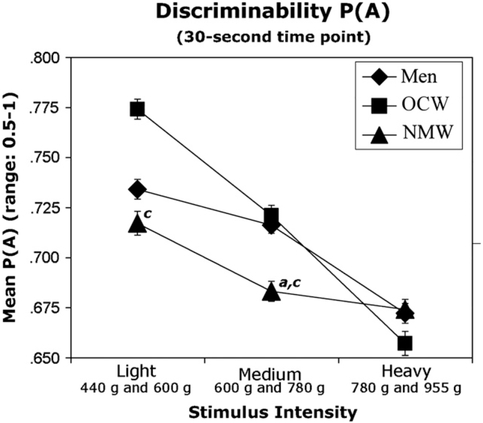
Figure 3.
Values of P(A), ability to discriminate among the stimulus-intensity pairs of the MPT, using just the 30-second time point. The sex by stimulus intensity pair interaction was significant. Error bars represent ±1 SEM. There was a trend for P(A) values for normally menstruating women (NMW) to be higher for the light-stimulus intensity pair as compared to the heavystimulus intensity pair. P(A) values for men were significantly higher for the light-stimulus intensity pair and medium-stimulus intensity pair as compared to the heavy-stimulus intensity pair. There was a trend for P(A) values to be higher for oral-contraceptive women (OCW) with the light-stimulus intensity pair and medium-stimulus intensity pair as compared to the heavystimulus intensity pair, and the light-stimulus intensity pair as compared to the medium-stimulus intensity pair. a represents a trend for a difference between normally menstruating women and men. c represents a trend for a difference between normally menstruating women and oral contraceptive women. All values are P ≤ .05.
Fig 3 shows that when examining individual stimulus intensities at the 30-second time point, oral-contraceptive women tended to have higher discriminability values than normally menstruating women (F(1,55) = 5.2, P ≤ .05) for the light-stimulus intensities (440 and 600 g). Oral-contraceptive women tended to have higher discriminability values than normally menstruating women during the menstrual (F(1,55) = 6, P ≤ .05), follicular (F(1,55) = 3.9, P ≤ .05), luteal (F(1,55) = 4.7, P ≤ .05), and late luteal (F(1,55) = 4.7, P ≤ .05) phases. The main effect of group showed a trend toward significance for the medium-stimulus intensities (600 and 780 g) (F(2,55) = 3.4, P ≤ .05). Oral-contraceptive women tended to have higher discriminability values for the medium-stimulus intensities (600 and 780 g) when compared to normally menstruating women (F(1,54) = 5.4, P ≤ .05) during the menstrual (F(1,55) = 3.9, P ≤ .05) and late luteal phases (F(1,55) = 9, P ≤ .01). Men also tended to have higher discriminability values for the medium-stimulus intensities (600 and 780 g) than did normally menstruating women (F(1,55) = 4.5, P ≤ .05). This trend was found during the ovulatory phase (F(1,55) = 4, P ≤ .05) and was significant in the late luteal phase (F(1,55) =11.73, P ≤ .001).
Percent of Stimuli Correctly Distinguished
Within Groups.
There were no significant effects or trends of phase in percentage correct with regards to participants answering the question which of the weights is heavier?″
Between Groups.
There was a trend for oral contraceptive women to be correct regarding which weight was heavier a higher percentage of the time than normally menstruating women (F(1,55) = 5.4, P ≤ .05). Oral-contraceptive women’s higher percentage of correct answers was significant for the light-stimulus intensities (440 and 600 g; F(1,55) = 7.5, P ≤ .01). This effect was found as a trend for the medium-stimulus intensities (600 and 780 g; F(1,55) = 5.5, P ≤ .05) and during the ovulatory (F(1,55) = 6.3, P ≤ .05) and late luteal (F(1,55) = 3.9, P ≤ .05) phases. There was a trend for men to be correct a higher percentage of time than normally menstruating women for the medium stimulus intensities (600 and 780 g; F((1,55) = 5.6, P ≤ .05) and a trend for oral-contraceptive women to be correct a higher percentage of time than men for the light-stimulus intensities (440 and 600 g; F(1,55) = 6.2, P ≤ .05).
101-MAPS
Within Groups.
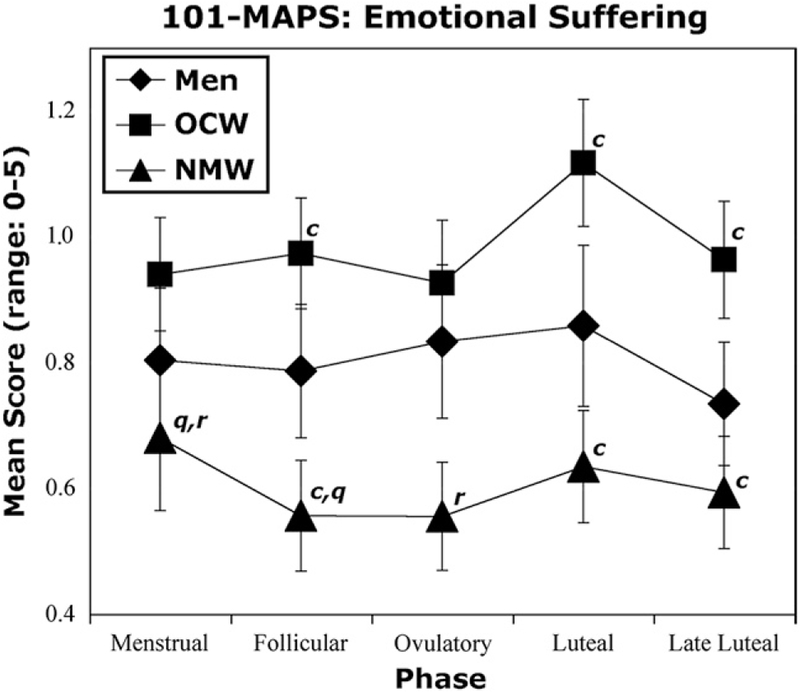
Fig 4 shows that for normally menstruating women, there was a trend for scores on the MAPS for emotional suffering to be greater during the menstrual phase as compared to the follicular (F(1,20) = 4.8, P ≤ .05) and the ovulatory phases (F(1,20) = 4.9, P ≤ .05; Fig 4). For oral-contraceptive women, there was a trend for scores for sensory pain to be greater during the luteal phase than during the ovulatory (F(1,16) = 4, P ≤ .05) and late luteal phases (F(1,16) = 4, P ≤ .05). Oral-contraceptive women also had a trend for greater well-being scores during the follicular as opposed to the menstrual (F(1,16) = 4.4, P ≤ .05) and luteal (F(1,16) = 5.3, P ≤ .05) phases. Men had a trend for greater well-being scores during the luteal phase (dates 19 to 24 of the calendar month) than during the menstrual (dates 1 to 5; F(1,19) = 4.5, P ≤ .05) and the late luteal phases (dates 25 to 28; F(1,19) = 5.2, P ≤.05). No other significant effects or trends were found.
Figure 4.
Mean scores for emotional suffering as measured by the M-MAPS. Scores for normally menstruating women (NMW) were significantly greater than for oral-contraceptive women (OCW). Error bars represent ± 1SEM. c represents a significant difference between normally menstruating women and oral-contraceptive women. q represents a trend for a difference between the menstrual and follicular phases. r represents a trend for a difference between the menstrual and ovulatory phases. All trend values are P ≤ .05.
Between Groups.
Fig 4 shows that there was a trend for oral-contraceptive women to report experiencing more emotional suffering as measured by the MAPS than normally menstruating women (F(1,55) = 4.2, P ≤ .05). This trend was true for the follicular (F(1,55) = 5.1, P ≤.05), luteal (F(1,55) = 5.3, P ≤.05), and late luteal phases (F(1,55) = 4.1, P ≤ .05).
Correlations Between Hormone Levels and Pain Report Variables
There was a trend for estradiol levels (values reported in Kowalczyk et al27) for normally menstruating women to be correlated with response criterion B values at the 30-second time point for the light-stimulus intensities (440 and 600 g; n = 21, r2 = .16, P ≤ .05; data not shown). In addition, there were trends for estradiol levels in normally menstruating women to be correlated with discriminability, P(A) scores, for the heavystimulus intensities (780 and 955 g; n = 21, r2 = .16, P ≤ .05). There was also a trend for progesterone levels (values reported in Kowalczyk et al27) in normally menstruating women to be correlated with discriminability P(A), across stimulus intensities (n = 21, r2 = .15, P ≤ .05), and for the light-stimulus intensities (440 and 600 g) (n = 21, r2 = .16, P ≤ .05).
Correlational analyses could not be performed for either the men or oral-contraceptive women due to floor effects resulting from the limit of detection for estradiol and progesterone.
Effect Size Analyses
Effect sizes for the between-groups analyses for meanresponse criterion B and mean discriminability P(A) were .128 and .032 respectively. In order to have a significant main effect of group, the sample sizes needed to obtain a P of .01 would be 114 for response criterion B and 437 for discriminability as measured by P(A).
Menstrual-cycle effects are presented in Table 2. In normally menstruating women, effect sizes for mean-response criterion B ranged from .024 for the comparison of the menstrual and ovulatory phases to .399 when comparing the menstrual and follicular phases. The number of normally menstruating women needed for significance for the measurement of response criterion B at the .01 level range from 20,185 when comparing the menstrual and ovulatory phases to 75 for the comparison of the menstrual and follicular phases. In normally menstruating women, effect sizes for discriminability, P(A), range from nil for the comparison of the menstrual and late luteal phases to .380 when comparing the menstrual and luteal phases. The fewest normally menstruating women needed to show significance in mean discriminability, P(A), at the .01 level is 82 when comparing the menstrual and luteal phases. In oral-contraceptive women, effect sizes for mean response criterion B ranged from .159 when comparing the follicular to late luteal phases, to .432 when comparing the menstrual to the late luteal phases. The number of oral-contraceptive women needed to show significance in mean response criterion B ranged from 464 when comparing the follicular and late luteal phases to 64 for the menstrual to late luteal comparison. Effect sizes found in oral-contraceptive women for mean discriminability, P(A), ranged from nil, in several conditions, to .219 when comparing the follicular and luteal phases. The lowest number of oral contraceptive women needed to show significance at the .01 level was 246 for the comparison of the follicular and luteal phases.
Table 2.
Effect Sizes and Necessary Sample Sizes for Normally Menstruating Women and Oral-Contraceptive Women to Reach Significance at the .01 Level for Menstrual Cycle Phase Contrasts Are Presented for Mean Response Criterion, B, and Mean Discriminitability, P(A). Target Power for the Sample Size Calculation Was 80%
| Phase Contrasts | Normally Menstruating Women |
Oral Contraceptive Women |
||||||
|---|---|---|---|---|---|---|---|---|
| Response Criterion, B |
Discriminability P(A) |
Response Criterion, B |
Discriminability P(A) |
|||||
| Effect Size |
Sample Size Needed |
Effect Size |
Sample Size Needed |
Effect Size |
Sample Size Needed |
Effect Size |
Sample Size Needed |
|
| Menstrual vs follicular | .399 | 75 | .063 | 2,935 | .319 | 116 | n/a | n/a |
| Menstrual vs ovulatory | .024 | 20,185 | .099 | 1,204 | .389 | 78 | n/a | n/a |
| Menstrual vs luteal | .154 | 495 | .380 | 82 | .209 | 270 | .114 | 897 |
| Menstrual vs late luteal | .292 | 138 | n/a | n/a | .432 | 64 | .088 | 1,521 |
| Follicular vs ovulatory | .325 | 112 | .025 | 19,032 | .207 | 274 | n/a | n/a |
| Follicular vs luteal | .122 | 787 | .150 | 519 | .175 | 384 | .219 | 246 |
| Follicular vs late luteal | .035 | 9,707 | .073 | 2,198 | .159 | 464 | .085 | 1,612 |
| Ovulatory vs luteal | .185 | 342 | .184 | 348 | .340 | 102 | .142 | 585 |
| Ovulatory vs late luteal | .322 | 114 | .141 | 590 | .271 | 160 | .067 | 2,613 |
| Luteal vs late luteal | .207 | 273 | .366 | 88 | n/a | n/a | .053 | 4,220 |
Discussion
The present study found few differences in pain report between normally menstruating women and men. When examining just one specific set of stimulus intensities at one time point, with the heavy weight as the signal and medium weight as the noise, men tended to be better able to discriminate the weights, as measured by P(A), than normally menstruating women. These results stand in contrast to a number of previous studies showing that women are better able to discriminate between painful stimuli than men. From much of the literature, normally menstruating women are more sensitive to pressure pain than men,6,42 so this effect is in the opposite direction as expected. The idea that the normally menstruating women in this study differ from normally menstruating women in other studies is an important one in terms of the clinical relevance of pain differences between men and women. That is, the variability in pain response when comparing individuals within the same sex may be greater than the variability in pain response when comparing between the sexes. This study may have included a particularly nonresponsive group of normally menstruating women but this is an argument in itself for the treatment of pain conditions on an individual basis regardless of sex.
The lack of difference in response criterion B (commonly thought of as a measure of stoicism) between normally menstruating women and men is intriguing as well. It is common for women to report more pain than men,20,49 as they (both oral-contraceptive women and normally menstruating women) did on the Previous Pain Experiences Questionnaire. The results on the Previous Pain Experiences Questionnaire illustrate the fact that our normally menstruating women were willing to report more pain in the past, yet did not do so in response to the administration of experimental pain. While there are many differences between the real life pains described in the Previous Pain Experiences Questionnaire (higher emotional quality, possible longer duration, reporting on past as opposed to present pain), this argues that the differences between men and normally menstruating women in pain report are subtle and can vary from condition to condition.
In normally menstruating women, trends for menstrual-cycle effects were found for discriminability (as measured by P(A) scores) between the luteal and the perimenstrual phases. There was a trend for the ability to discriminate, P(A), between 2 stimuli that was greater during the luteal phase compared to both the late luteal and menstrual phases. It is unlikely that this effect was merely due to sensory masking due to menstrual pain as there was not a significant effect between these menstrual phases in baseline State sum scores or on the State item, “I feel comfortable.” Additionally, participants were not run on their first day of menses. This finding could be considered consistent with the notion that elevated levels of estradiol may mediate increases in pain responsivity,18 as in this sample estradiol and progesterone levels were highest in the luteal phase.27 This effect, however, was not accompanied by a correlation between estradiol or progesterone levels. There were no consistent menstrual-cycle effects on the measure of stoicism, B, in normally menstruating women, as only 2 contrasts reached trend level only, and both of those were only for one set of stimulus intensities at one time point.
The effect sizes for normally menstruating women across different phases of the menstrual cycle obtained with this data were slightly lower than values found in a meta-analysis of pressure-pain data across the menstrual cycle.43 However, the effect sizes reported in previous work have been reported for tolerance and threshold studies as opposed to studies of statistical decision-making theory which could account for some of the differences in effect sizes.
While few effects were found between men and normally menstruating women in pain report, there were more consistent trends and results found when examining the group of oral-contraceptive women. For example, in several conditions examined, oral-contraceptive women tended to be better able to distinguish among painful stimuli than either men or normally menstruating women. This finding is a little perplexing given the assumption that hormone levels are involved in responsivity to pain. One might expect the group of oral-contraceptive women, which is comprised of females who have estradiol and progesterone levels that are barely distinguishable from men, to have pain perceptions that are intermediate between men and normally menstruating women. However, the trends and differences found in this study have been observed elsewhere. Drobek et al15 found that oral-contraceptive women had lower logtransformed pain-pressure thresholds than normally menstruating women. This effect reached significance only for the follicular phase. In an earlier study conducted by Raoet al,40 oral-contraceptive women had lower painpressure thresholds than normal control women during days 1 to 5 and days 15 to 18 of the menstrual cycle. Oral-contraceptive women also reported significantly more joint pain than normally menstruating women.22 In all 8 visual-analog scales measuring pain reported in this study,22 ratings of pain by oral-contraceptive women were higher, but no statistical analyses of these differences were reported. Using a different pain modality—cold pain—oral-contraceptive women were less able to adapt to pain than normally menstruating women.22 These studies suggest the possibility that oral-contraceptive women may be more sensitive to pain, though future studies would need to be conducted to specifically examine this possibility and its clinical relevance. Whenever oral contraceptive women are examined, it should be noted that they are a self-selected group, and there may be preexisting differences between oral-contraceptive women and normally menstruating women prior to oral-contraceptive use. The question of whether oral contraceptives themselves are causing these changes could be answered by examining a group both before and after starting oral-contraceptive use.
When examining the measure of willingness to report pain, B, oral-contraceptive women had higher values than men in several conditions examined. This effect is concordant with other studies showing sex differences in pain responsivity.17,42 In contrast to these results was the finding that normally menstruating women were not different from either men or oral-contraceptive women. Because there was no significant difference between normally menstruating women and either of the other groups, this result supports the notion that sex differences in pain report are not likely to be clinically relevant for normally menstruating women.
Another interesting development was that menstrual-cycle effects on response to mechanical-pressure pain were most clear for oral-contraceptive women. During the menstrual phase, oral-contraceptive women tended to have lower response criterion B values, indicating less stoicism, than during the follicular and ovulatory phases. Similar effects were demonstrated by Iselée et al,24 who found that for the masseter muscles, the temporalis muscle and the thumb, oral-contraceptive women had significantly lower thresholds for pressure pain during the perimenstrual phase (days 27-1) than during either the follicular phase (days 8 to 12) or the luteal phase (days 19 to 26). Rao et al41 reported that pain threshold to a mechanical stimulus was lower during the menstrual phase (days 1 to 5) compared to days 15 to 18 or days 25 to 30. The results of the current study, in conjunction with these previous studies, suggest that something other than changes in hormone levels during the menstrual cycle may be affecting pain report. Perhaps the physical discomfort of menstruation makes women more likely to report discomfort in general. Supporting this idea is the fact that effect sizes for response criterion B were found to be at moderate levels when comparing the menstrual phase to other phases in oral-contraceptive women.
Some might expect emotional suffering as measured by the 101-MAPS to be greater during the late luteal phase, as this is one of the defining characteristics of premenstrual dysphoric disorder.13,30 Other research has shown that for a large sample of women, a greater number of symptoms were reported during both the late luteal and menstrual phases, according to DSM-IV definitions of PMDD33 and the Moos Menstrual Distress Questionnaire.31 This finding suggests that the symptoms normally considered to be associated with the late luteal phase persist through menstruation. Our finding of increased emotional suffering by normally menstruating women during the menstrual phase compared to the follicular and ovulatory phases is consistent with the idea that symptoms normally associated with the premenstrual period persist during menstruation.
The higher level of emotional suffering in the 101-MAPS by oral-contraceptive women as compared with normally menstruating women found in the present study adds to a cluttered literature on mood and affect differences between oral-contraceptive women and normally menstruating women. Studies examining mood and affect across the menstrual cycle in oral-contraceptive women and normally menstruating women often find differences, but the direction of those differences and the phase of the menstrual cycle in which those differences are found is variable across studies.39 One study suggests that women on monophasic contraceptives have more negative affect during the menstrual phase compared to control women,50 while others suggest the opposite,40,51 and still others report no difference in negative affect.33,38 The results of the current study further complicate this literature by suggesting that when a painful stimuli, such as the MPT, is added, normally menstruating women are more adaptable to the painful stressor. Studies have shown that oral-contraceptive women have a decreased cortisol response to a psychological stressor26 and a stress-related increase in proinflammatory cytokine (IL-6) reactivity in comparison to normally menstruating women.44 This stress response of increased proinflammatory cytokines could also explain some of the differences seen in P(A) scores for oral-contraceptive women compared to normally menstruating women, as IL-6 has been shown to increase sensitivity to painful stimuli.12 This is conjecture, however, as stress responses were not directly examined in the present study.
The present data suggest that the differences in pain responsivity across the menstrual cycle and between men and normally cycling women are not clinically relevant, which is consistent with some previous studies.4,27 The differences in pain responsivity between oral contraceptive women and normally menstruating women shown in this study are particularly interesting. In 2002, 19% of women in America aged 15 to 44 used oral contraceptives as their primary form of birth control, and over 80% of sexually active women used oral contraceptives at some point in their lives.36 Given the large number of women taking oral contraceptives, it makes the possibility of increases in pain sensitivity caused by hormonal birth control a possibly important area for future study. If this effect is replicable, future studies should be conducted to determine if these effects resolve after the oral contraceptives are discontinued and if there is a difference among monophasic and triphasic birth control pills and implantable forms of hormonal birth control.
Acknowledgments
The assistance of Dr. John Kuhl, Dr. Crawford Clark, Katherine Strutynski, Daniel Kroch, Anastasia Wermert, Kimberly Blauner, Irina Brouda, Christy Hall, Jose Mora, Mabel Torres, and Suleman Bhana is gratefully acknowledged.
Supported by the National Institute of Dental and Craniofacial Research (R01 DE12763)
References
- 1.Aloisi AM: Gonadal hormones and sex differences in pain reactivity. Clin J Pain 19:168–174, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Amodei N, Nelson-Gray RO: Reactionsofdysmenorrheicand nondysmenorrheic women to experimentally induced pain throughout the menstrual cycle. J Behav Med 12:373–385, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Beck AT, Steer RA, Brown GK: BDI-II Beck Depression Inventory Manual, The Psychological Corp, 2nd Edition. San Antonio, TX, Harcourt Brace & Company, 1996 [Google Scholar]
- 4.Berkley KJ: Sex differences in pain. Behav Brain Sci 20: 371–380, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Carter LE, McNeil DW, Vowles KE, Sorrell JT, Turk CL, Ries BJ, Hopko DR: Effects of emotion on pain reports, tolerance and physiology. Pain Res Manag 7:21–30, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Chesterton LS, Barlas P, Foster NE, Baxter GD, Wright CC: Gender differences in pressure pain threshold in healthy humans. Pain 101:259–266, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cimino R, Farella M, Michelotti A, Pugliese R, Martina R: Does the ovarian cycle influence the pressure-pain threshold of the masticatory muscles in symptom-free women? J Orofac Pain 14:105–111, 2000 [PubMed] [Google Scholar]
- 8.Clark WC: Somatosensory and Pain Measurement by Statistical and Sensory Decision Theory, in Adelman G, Smith B (eds): Encyclopedia of Neuroscience, (3rd ed). Amsterdam. Elsevier, 2003 [Google Scholar]
- 9.Clark WC, Kuhl JP, Keohan ML, Knotkova H, Winer RT, Griswold GA: Factor analysis validates the cluster structure of the dendrogram underlying the Multidimensional Affect and Pain Survey (MAPS) and challenges the a priori classification of the descriptors in the McGill Pain Questionnaire (MPQ). Pain 106:357–363, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Clark WC: Applications of Statistical Decision Theory in Pain Assessment, in Schmidt RF, Willis WD (eds): Encyclopedic Reference of Pain. Heidelberg, Springer-Verlag, 2006 [Google Scholar]
- 11.Courtenay WH: Constructions of masculinity and their influence on men’s well-being: Atheory gender and health. Soc Sci Med 50:1385–1401, 2000 [DOI] [PubMed] [Google Scholar]
- 12.De Jongh RF, Vissers KC, Meert TF, Booij LH, De Deyne CS, Heylen RJ: The role of interleukin-6 in nociception and pain. Anesth Analg 96:1096–1103, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC, American Psychiatric Association, 2000 [Google Scholar]
- 14. Dixon KE, Thorn BE, Ward LC: An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain 112:188–196, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Drobek W, Schoenaers J, De Laat A: Hormone-dependent fluctuations of pressure pain threshold and tactile threshold of the temporalis and masseter muscle. J Oral Rehabil 29:1042–1051, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Edwards RR, Haythornthwaite JA, Sullivan MJ, Fillingim RB: Catastrophizing as a mediator of sex differences in pain: Differential effects for daily pain versus laboratory-induced pain. Pain 111:335–341, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Fillingim RB, Maxiner W: Gender differences in the responses to noxious stimuli. Pain Forum 4:308–221, 1995 [Google Scholar]
- 18.Fillingim RB, Ness TJ: Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev 24:485–501, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Gil KM, Wilson JJ, Edens JL, Webster DA, Abrams MA, Orringer E, Grant M, Clark WC, Janal MN: Effects of cognitive coping skills training on coping strategies and experimental pain sensitivity in African American adults with sickle cell disease. Health Psychol 15:3–10, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ: Consensus Working Group of the Sex, Gender, and Pain SIG of the IASP: Studying sex and gender differences in pain and analgesia: A consensus report. Pain 132(Suppl 1): 26–45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hapidou EG, De Catanzaro D: Sensitivity to cold pressor pain in dysmenorrheic and non-dysmenorrheic women as a function of menstrual cycle phase. Pain 34:277–283, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Hapidou EG, Rollman GB: Menstrual cycle modulation of tender points. Pain 77:151–161, 1988 [DOI] [PubMed] [Google Scholar]
- 23.Isselee H, De Laat A, Bogaerts K, Lysens R: Long-term fluctuations of pressure pain thresholds in healthy men, normally menstruating women and oral contraceptive users. Eur J Pain 5:27–37, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Isselee H, De Laat A, De Mot B, Lysens R: Pressure-pain threshold variation in temporomandibular disorder myalgia over the course of the menstrual cycle. J Orofac Pain 16: 105–117, 2002 [PubMed] [Google Scholar]
- 25.Jones A, Zachariae R: Investigation of the interactive effects of gender and psychological factors on pain response. Br J Health Psychol 9:405–418, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Pirke KM, Hellhammer DH: Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology 20:509–514, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Kowalczyk WJ, Evans SM, Bisaga AM, Sullivan MA, Comer SD: Sex differences and hormonal influences on response to cold pressor pain in humans. J Pain Mar 7: 151–160, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Levine FM, De Simone LL: The effects of experimenter gender on pain report in male and female subjects. Pain 44:69–72, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Malow RM, West JA, Sutker PB: Anxiety and pain response changes across treatment: Sensory decision analysis. Pain 38:35–44, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Man MS, MacMillan I, Scott J, Young AH: Mood, neuropsychological function and cognitions in premenstrual dysphoric disorder. Psychol Med 29:727–733, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Marriott A, Faragher EB: An assessment of psychological state associated with the menstrual cycle in users of oral contraception. J Psychosom Res 30:41–47, 1986 [DOI] [PubMed] [Google Scholar]
- 32.McNicol D: A Primer of Signal Detection Theory, First Edition. London, George Allen & Unwin, 1972 [Google Scholar]
- 33.Meaden PM, Hartlage SA, Cook-Karr J: Timing and severity of symptoms associated with the menstrual cycle in a community-based sample in the Midwestern United States. Psychiatry Res 30:27–36, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Mogil JS, Chesler EJ, Wilson SG, Juraska JM, Sternberg WF: Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neurosci Biobehav Rev 24:375–389, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Mogil JS, Ritchie J, Smith SB, Strasburg K, Kaplan L, Wallace MR, Romberg RR, Bijl H, Sarton EY, Fillingim RB, Dahan A: Melanocortin-1 receptor gene variants affect pain and mu-opioid analgesia in mice and humans. J Med Genet 42:583–587, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ: Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data 10:1–36, 2004 [PubMed] [Google Scholar]
- 37.Nicholson K, Martelli MF: The problem of pain. J Head Trauma Rehabil 19:2–9, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Oinonen KA: Mazmanian D: Effects of oral contraceptives on daily self-ratings of positive and negative affect. J Psychosom Res 51:647–658, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Oinonen KA, Mazmanian D: To what extent do oral contraceptives influence mood and affect? J Affect Disord 70: 229–240, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Paige KE: Effects of oral contraceptives on affective fluctuations associated with the menstrual cycle. Psychosom Med 33:515–537, 1971 [DOI] [PubMed] [Google Scholar]
- 41.Rao SS, Ranganekar AG, Saifi AQ: Pain threshold in relation to sex hormones. Indian J Physiol Pharmacol 31:250–254, 1987 [PubMed] [Google Scholar]
- 42.Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB: Sex differences in the perception of noxious experimental stimuli: A meta-analysis. Pain 74:181–187, 1998 [DOI] [PubMed] [Google Scholar]
- 43.Riley JL 3rd, Robinson ME, Wise EA, Price DD: A meta-analytic review of pain perception across the menstrual cycle. Pain 81:225–235, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Rohleder N, Wolf JM, Piel M, Kirschbaum C: Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology 28:261–273, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Rollman GB, Lautenbacher S: Sex differences in musculoskeletal pain. Clin J Pain 17:20–24, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Sarlani E, Grace EG, Reynolds MA, Greenspan JD: Sex differences in temporal summation of pain and after sensations following repetitive noxious mechanical stimulation. Pain 109:115–123, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Spielberger CD, Gorsuch RL, Lushene RE: STAI-Manual for the State-Trait Anxiety Inventory, First Edition. Palo Alto, CA, Consulting Psychologists Press, 1970 [Google Scholar]
- 48.Thorn BE, Clements KL, Ward LC, Dixon KE, Kersh BC, Boothby JL, Chaplin WF: Personality factors in the explanation of sex differences in pain catastrophizing and response to experimental pain. Clin J Pain 20:275–282, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Unruh AM: Gender variations in clinical pain experience. Pain 65:123–167, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Walker A, Bancroft J: Relationship between premenstrual symptoms and oral contraceptive use: A controlled study. Psychosom Med 52:86–96, 1990 [DOI] [PubMed] [Google Scholar]
- 51.Wilcoxon LA, Schrader SL, Sherif CW: Daily self-reports on activities, life events, moods, and somatic changes during the menstrual cycle. Psychosom Med 38:399–417, 1976 [DOI] [PubMed] [Google Scholar]