MINI-ABSTRACT
We determine whether endovascular or open revascularization provides an advantageous approach in treatment of symptomatic peripheral arterial disease for propensity-score matched cohorts of Medicare beneficiaries. We demonstrate that an endovascular approach is associated with improved long-term amputation free survival with only a modest relative increased risk of subsequent intervention.
Abstract
Objective
To determine whether endovascular or open revascularization provides an advantageous approach to symptomatic peripheral arterial disease (PAD) over the longer term.
Summary Background Data:
The optimal revascularization strategy for symptomatic lower extremity PAD is not established.
Methods
We evaluated amputation free survival, overall survival and relative rate of subsequent vascular intervention after endovascular or open lower extremity revascularization for propensity-score matched cohorts of Medicare beneficiaries with PAD from 2006 through 2009.
Results
Among 14,685 eligible patients, 5,928 endovascular and 5,928 open revascularization patients were included in matched analysis. Patients undergoing endovascular repair had improved amputation free survival compared to open repair at 30-days (7.4 vs. 8.9%, p=0.002). This benefit persisted over the long-term: At 4-years, 49% of endovascular patients had died or received major amputation compared to 54% of open patients (p<0.001). An endovascular procedure was associated with a risk-adjusted 16% decreased risk of amputation or death compared to open over the study period (hazard ratio: 0.84; 95% confidence interval, 0.79–0.89; p<0.001). The amputation free survival benefit associated with an endovascular revascularization was more pronounced in patients with congestive heart failure or ischemic heart disease than in those without (p=0.021 for interaction term). The rate of subsequent intervention at 30-days was 7.4% greater for the endovascular versus the open revascularization cohort. At 4-years, this difference remained stable at 8.6%.
Conclusions
Using population-based data, we demonstrate that an endovascular approach is associated with improved amputation free survival over the long-term with only a modest relative increased risk of subsequent intervention.
INTRODUCTION
Lower extremity peripheral arterial disease (PAD) is the most under-diagnosed cardiovascular disorder in the United States and affects over 12 million individuals.1–5 Patients with symptomatic PAD classically experience intermittent claudication (i.e. exertional muscular calf or thigh pain during walking that resolves with rest), with progression to critical limb ischemia (i.e. rest pain, ulceration, gangrene necrosis) in up to 25% of patients.6 Treatment for symptomatic patients who fail conservative management has traditionally been surgical revascularization. However beginning in the 1980’s, patients are more often offered a less invasive endovascular approach which is now widely utilized.7,8
The optimal revascularization strategy for symptomatic lower extremity PAD is not well established. Existing randomized controlled trials comparing endovascular to open revascularization for lower extremity PAD have been limited by patient selection and the inability to generalize findings beyond specialized centers.9–16 Given the lack of high-quality comparative data, two additional randomized controlled trials comparing open to endovascular intervention for lower extremity PAD have recently begun enrolling subjects.17,18 However, because endovascular therapy has become so prominent, these trials may have difficulty enrolling patients because patients’ prefer “less invasive” options making the establishment of clinical equipoise challenging.19 Moreover, these studies may take they several years to complete and thus the eventual results may not reflect contemporary clinical practice.
In this evaluation, our objective is to conduct a comparative evaluation of open versus endovascular treatment of lower extremity revascularization using nationally representative Medicare data. We examine immediate as well as long-term outcomes including rates of subsequent intervention to determine whether the relative benefit of these interventions changes over time.
METHODS
Data acquisition and cohort selection
We analyzed data from the Centers for Medicare & Medicaid Services Chronic Conditions Warehouse for 2006 to 2009, a longitudinal 5% sample representative of Medicare beneficiaries nationally.20 Records include patient demographics, clinical characteristics, Medicare enrollment data, and facility and provider claims. We selected all inpatients with a diagnosis for PAD using International Classification of Disease, ninth revision (ICD-9) codes, as previously described.21,22 We selected patients over age 65 who underwent an open or endovascular lower extremity revascularization procedure (see Table 1S, Supplemental Digital Content 1, which displays respective codes).22–24 We excluded patients who had incomplete data from enrollment in Medicare Part A and Part B, coverage by a Medicare health maintenance organization (HMO), railroad benefits in the year prior to surgery and trauma-related revascularization.22
Primary Outcome
Our primary outcome was the incidence of major amputation or death. We classified amputation as major when performed at the transtibial level or above as previously described.7,24,25 We did not include amputations at the metatarsal level or below as these do not represent failures of limb salvage.7 Because the purpose of revascularization is to preserve limb and life, any major amputation or death represents a failure of the procedure consistent with existing literature.9
Variables
Explanatory variables are listed in Table 1. We identified patients with diabetes using validated methodology published by Hebert et al.26 Severity of lower extremity PAD was dichotomized into claudication or critical limb ischemia based upon the diagnosis on admission (see Table 1S, Supplemental Digital Content 1, which displays respective codes).27 We examined validated Elixhauser comorbidities and defined cancer as a composite variable of lymphoma and metastatic cancer.28 Because the rate of endovascular interventions for PAD increased systematically over the study period, analysis adjusted for year of procedure. Patient’s residence and hospital location were classified using Rural-Urban Commuting Area (RUCA) codes.29,30 Origin of admission included: Referral (i.e. referral from physician, clinic, or HMO), transfer (i.e. transfer from hospital, skilled nursing facility, or another health care facility) or the emergency department.
Table 1:
Patient demographics, preoperative characteristics, and perioperative factors before and after propensity score matching
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristic | Endo (n=8,206) |
Open (n=6,479) |
Standardized Difference, % | Endo (n=5,928) |
Open (n=5,928) |
Standardized Difference, % |
| Age, years (SD) | 77.7 (6.9) | 77.2 (6.8) | 7 | 77.5 (6.9) | 77.4 (6.8) | 0 |
| Proportion male, % | 43.7 | 50.4 | 13 | 48.5 | 48.3 | 1 |
| Race, % | ||||||
| White | 83.2 | 86.2 | −8 | 85.7 | 85.9 | 0 |
| Black | 12.2 | 10.6 | 0 | 10.5 | 10.6 | 0 |
| Other | 4.7 | 3.2 | 8 | 3.7 | 3.5 | 0 |
| RUCA, % | ||||||
| Urban | 73.6 | 73.3 | 1 | 73.1 | 73.3 | 0 |
| Large Town | 13.1 | 13.8 | −2 | 13.7 | 13.7 | 0 |
| Rural | 13.2 | 12.9 | 1 | 13.3 | 13.0 | 1 |
| Medicaid, % | 22.5 | 19.6 | 7 | 19.8 | 20.0 | 1 |
| Hospital RUCA, % | ||||||
| Urban | 92.0 | 91.9 | 0 | 91.4 | 91.8 | −1 |
| Non-Urban | 8.0 | 8.1 | 0 | 8.6 | 8.2 | 1 |
| Admitted from, % | ||||||
| On referral | 76.1 | 75.1 | 2 | 75.5 | 75.7 | −1 |
| Emergency Department | 18.7 | 19.8 | −3 | 19.2 | 19.0 | 1 |
| On transfer | 5.3 | 5.1 | 1 | 5.3 | 5.3 | 0 |
| Teaching Hospital, % | 50.8 | 53.5 | −5 | 51.6 | 52.4 | 0 |
| Extent of Disease, % | ||||||
| Claudication | 33.5 | 24.1 | 21 | 26.5 | 25.7 | 1 |
| Critical Limb Ischemia | 66.5 | 75.9 | −21 | 73.5 | 74.3 | −1 |
| Myocardial Infarction, % | 11.6 | 9.8 | 6 | 10.4 | 10.2 | 0 |
| CHF / Ischemic Heart Disease, % | 77.2 | 73.7 | 8 | 74.6 | 74.9 | 0 |
| Stroke, % | 15.1 | 14.3 | 2 | 15.0 | 14.8 | 0 |
| Eye Disease, % | 1.2 | 0.9 | 3 | 0.8 | 0.9 | 0 |
| Diabetes, % | 51.3 | 44.2 | 14 | 46.2 | 46.0 | 0 |
| Electrolyte disorder, % | 35.0 | 31.5 | 8 | 32.5 | 32.4 | 2 |
| Renal Failure, % | 27.3 | 21.4 | 14 | 22.6 | 22.5 | 1 |
| Deficiency anemia, % | 37.5 | 33.3 | 9 | 33.9 | 34.0 | 1 |
| Chronic blood loss anemia, % | 5.5 | 5.6 | 0 | 5.6 | 5.5 | 1 |
| Coagulopathy, % | 7.4 | 7.2 | −1 | 7.1 | 7.2 | 0 |
| RA or collagen vascular disease, % | 6.8 | 6.2 | 3 | 6.1 | 6.2 | 0 |
| Chronic pulmonary disease, % | 38.7 | 40.2 | −3 | 39.0 | 39.2 | 1 |
| Hypertension, complicated, % | 77.2 | 77.2 | 0 | 76.8 | 77.0 | 0 |
| Obesity, % | 5.4 | 7.5 | 9 | 5.9 | 5.7 | 1 |
| Paralysis, % | 5.2 | 4.8 | 2 | 4.9 | 4.6 | 1 |
| Neurological disorder, % | 11.1 | 9.6 | 5 | 10.0 | 9.7 | −1 |
| Dementia, % | 4.8 | 5.0 | −1 | 5.0 | 5.0 | −2 |
| Pulmonary circulation disorder, % | 6.5 | 5.6 | 4 | 5.8 | 5.9 | 1 |
| Weight loss, % | 8.0 | 7.9 | 0 | 7.8 | 7.6 | −2 |
| Benign tumor, % | 10.1 | 11.9 | −6 | 11.6 | 11.3 | 0 |
| Cancer, % | 2.8 | 3.0 | −1 | 2.9 | 2.9 | 0 |
| Year, % | ||||||
| 2006 | 30.3 | 32.0 | −4 | 30.9 | 31.2 | 0 |
| 2007 | 26.7 | 25.3 | 3 | 26.0 | 25.9 | 0 |
| 2008 | 23.2 | 22.4 | 2 | 23.1 | 22.8 | 1 |
| 2009 | 19.8 | 20.3 | −1 | 20.0 | 20.1 | −1 |
The standardized differences are reported as percentages; a difference of less than 10% indicates a relatively small imbalance. Endo, endovascular; SD, standard deviation; RUCA, rural urban commuting area; RA, rheumatoid arthritis
Propensity Score
Given the differences in baseline characteristics between groups receiving endovascular and open procedures, we used propensity score matching to ensure that patient cohorts had similar covariate distribution. We first estimated the propensity score as the probability of receiving an endovascular intervention, conditioned on all preoperative characteristics and their interactions with renal failure, diabetes, and presence of critical limb ischemia.31 Matching was performed using a 1:1 protocol without replacement (nearest neighbor approach) with a caliper width equal to 0.25 of the standard deviation of the propensity score.32 We assessed balance between the groups before and after matching using standardized differences; values less than 10% for a given variable denote a relatively small imbalance.33 We present descriptive statistics for both unmatched and matched cohorts; all subsequent analyses are on matched cohorts.
Statistical Analysis
Chi-square tests evaluate unadjusted 30-day post-operative amputation and mortality rates between patient groups. Kaplan Meier curves depict overall survival and amputation free survival by procedure type, and log-rank tests identify differences between groups. We censored patients without incidence of amputation or death on December 31, 2009. We evaluated predictors of amputation free survival using a multivariable Cox proportional hazards model. Hazard ratios and 95% confidence intervals (95% CI) are presented with 2-sided p-values (alpha=0.05).
Interactions
We examined interactions between procedure type (endovascular and open) and common morbidities in the PAD population (extent of disease, diabetes, congestive heart failure/ischemic heart disease (CHF/IHD) and renal failure)34 to assess whether a significant disease burden moderates the association between procedure type and outcome. We also examined selected interactions between diabetes and comorbid conditions - history of myocardial infarction (MI), CHF/IHD, stroke, and eye disease - to explore if diabetes was associated with diminished amputation free survival.
Secondary interventions
We identified endovascular (i.e., angiogram, thrombolysis, angioplasty, stent-placement, atherectomy) and open (i.e., thrombectomy, open-bypass) interventions that occurred subsequent to the qualifying procedure (see Table 2S, Supplemental Digital Content 1, which displays respective codes) and determined the time to first intervention (Kaplan-Meier life tables) and the frequency of subsequent interventions. The data do not indicate laterality of secondary procedures. Assuming the frequency of subsequent intervention in the contralateral leg was equivalent in both the open and endovascular cohorts, we used the difference in intervention rates between the groups as a proxy measure of reintervention in the ipsilateral limb.
Sensitivity Analysis
To determine if our estimation of the treatment effect is valid using propensity score matching, we repeated the analysis using (1) propensity score standardized mortality ratio (SMR) weighting estimation and (2) propensity score stratification by quintiles, and compared these results to those obtained by matching.35
RESULTS
Study Sample
A total of 14,685 patients met sample inclusion criteria. Of these, 8,206 (55.9%) underwent endovascular and 6,479 (44.1%) underwent open revascularization. In the unmatched cohort, patients who underwent endovascular treatment were more often female, more frequently had comorbid conditions such as diabetes and renal failure, and less frequently had severe vascular disease manifested by critical limb ischemia. The numeric differences between cohorts with regard to these factors were small, suggesting that similar patients were treated with both techniques (Table 1). In the matched cohort, standardized differences in measured variables were well below 10%. The C-statistic for the propensity score logistic regression model was 0.627. The mean follow-up time for patients in the matched cohort was 618 days for endovascular and 597 days for open intervention.
Short-term Outcomes
Table 2 shows the 30-day post-operative amputation and mortality rates after lower extremity revascularization in propensity score matched cohorts. Patients undergoing an endovascular procedure experienced early amputation free survival advantage (7.4 vs. 8.9%, p=0.002) driven by a diminished mortality (5.3% mortality vs. 6.7%, p=0.001); there were no significant differences in early amputation rates between the endovascular and open cohorts. Stratified by extent of vascular disease, the amputation free survival benefit associated with an endovascular approach persisted for patients with critical limb ischemia, but not claudication.
Table 2:
30-day post-operative amputation and mortality rates after lower extremity revascularization in propensity score matched cohorts
| All Patients | Endo (n=5,928) |
Open (n=5,928) |
P-value |
| Amputation or Mortality, % | 7.4 | 8.9 | 0.002 |
| Amputation, % | 2.5 | 2.7 | 0.416 |
| Mortality, % | 5.3 | 6.7 | 0.001 |
| Patients with Claudication | Endo (n=1,572) |
Open (n=1,524) |
P-value |
| Amputation or Mortality, % | 1.8 | 2.5 | 0.215 |
| Amputation, % | 0.1 | 0.3 | 0.239 |
| Mortality, % | 1.7 | 2.2 | 0.366 |
| Patients with CLI | Endo (n=4,356) |
Open (n=4,404) |
P-value |
| Amputation or Mortality, % | 9.3 | 11.2 | 0.005 |
| Amputation, % | 3.3 | 3.5 | 0.580 |
| Mortality, % | 6.5 | 8.3 | 0.001 |
Percentages are compared using Chi-squared comparisons of proportions. Endo, endovascular; CLI, critical limb ischemia
Long-term Outcomes
Figure 1 shows 4-year amputation free survival by procedure (panel A) and 4-year survival by procedure (panel B) using matched cohorts. Within 1 year, an estimated 24.8% of endovascular and 29.4% of open patients either died or underwent amputation. This 5% differential persisted for the study duration; at 4-years an estimated 48.6% of endovascular and 54.0% of open patients experienced amputation or death (p<0.001).
Figure 1.
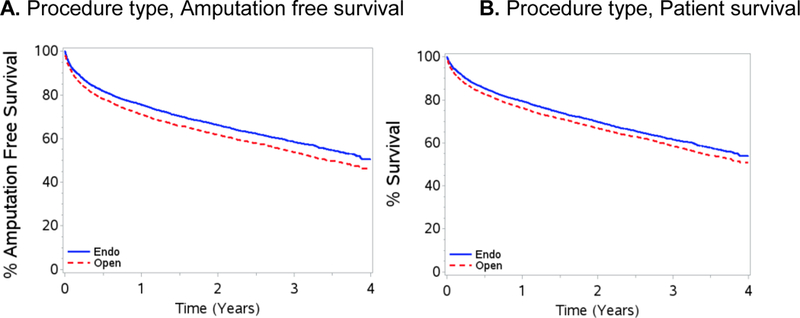
A, 4-year amputation free survival by procedure type and B, 4-year survival by procedure type. Endo, endovascular
Comparing amputation free (Figure 1, panel A) to overall survival (Figure 1, panel B), the majority of events are attributable to death rather than amputation; at 4 years, only an estimated 3.1% of endovascular patients versus 4.2% of open patients underwent amputation.
Amputation Free Survival
Table 3 shows the results of the multivariable Cox proportional hazards model predicting amputation free survival in the matched cohorts. After controlling for baseline characteristics, an endovascular procedure conferred a 16% lower risk of amputation or death compared to an open procedure over the study period (hazard ratio: 0.84, 95% CI: 0.79–0.89).
Table 3:
Cox proportional hazard model predicting amputation or death in propensity score matched groups
| Characteristic | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|
| Procedure type | |||
| Open | Ref | . | . |
| Endo | 0.84 | 0.79–0.89 | <0.001 |
| Age, years | 1.05 | 1.04–1.05 | <0.001 |
| Male sex | 1.12 | 1.06–1.19 | <0.001 |
| Extent of Disease | |||
| Claudication | Ref | . | . |
| Critical Limb Ischemia | 1.93 | 1.78–2.08 | <0.001 |
| Diabetes | 1.18 | 1.11–1.35 | <0.001 |
| Renal Failure | 1.47 | 1.38–1.57 | <0.001 |
| CHF / Ischemic Heart Disease | 1.05 | 0.98–1.13 | 0.156 |
| Myocardial Infarction | 1.24 | 1.15–1.34 | <0.001 |
| Stroke | 1.11 | 1.03–1.20 | 0.006 |
| Electrolyte disorder | 1.21 | 1.13–1.30 | <0.001 |
| Deficiency anemia | 1.19 | 1.11–1.27 | <0.001 |
| Coagulopathy | 1.15 | 1.05–1.26 | 0.003 |
| Chronic pulmonary disease | 1.16 | 1.10–1.23 | <0.001 |
| Neurological disorder | 1.42 | 1.31–1.53 | <0.001 |
| Dementia | 1.19 | 1.07–1.31 | 0.001 |
| Pulmonary circulation disorder | 1.22 | 1.11–1.35 | <0.001 |
| Weight loss | 1.23 | 1.12–1.34 | <0.001 |
| Cancer | 1.34 | 1.16–1.54 | <0.001 |
The model is additionally adjusted for race, eye disease, chronic blood loss anemia, rheumatoid arthritis or collagen vascular disease, complicated hypertension, obesity, benign tumor, Medicaid eligibility ever, hospital and patient rural urban commuting area, hospital type, origin of admission and year of procedure. Endo, endovascular; CHF, congestive heart failure.
Major risk factors associated with amputation or death included: diagnosis of critical limb ischemia vs. claudication (HR: 1.93; 95% CI: 1.78–2.08), renal failure (HR: 1.47; 95% CI: 1.38–1.57), history of MI (HR: 1.24; 95% CI: 1.15–1.34) and diabetes (HR: 1.18; 95% CI: 1.11–1.35).
Interactions
The majority of comorbidities did not influence relative outcomes (endovascular conferring a slight advantage, regardless of diabetes, renal failure, or critical limb ischemia) except for CHF or IHD. Specifically, in patients with CHF or IHD, the advantage of an endovascular intervention was enhanced (hazard ratio: 0.80, 95% CI: 0.75–0.85) (see Figure 1S, Supplemental Digital Content 1, which displays survival curves for interactions). Additionally, tested interactions between diabetes with history of myocardial infarction, stroke, and eye disease were not significant (p>0.05).
Subsequent Interventions
Secondary interventions were relatively more common after endovascular than open repair in matched patients (Table 4). During the first 30-days, patients who had an initial endovascular revascularization underwent subsequent intervention 7.4% more often than open patients. The relative rate of subsequent intervention did not further increase over time; at 4-years the rate of subsequent intervention was 8.6% higher for endovascular versus open patients. Absolute rates of subsequent interventions (unadjusted for contralateral interventions) are provided in Table 3S, Supplemental Digital Content, for reference. Interestingly, the majority of patients who underwent a subsequent intervention following either procedure did so only once (endovascular cohort 60.4%, open cohort: 58.8%).
Table 4:
Relative rate of undergoing a subsequent intervention after primary endovascular or open lower extremity revascularization in matched cohorts
| Relative rate: Endovascular vs. Open, % (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Subsequent Intervention | 30-days | Year 1 | Year 2 | Year 3 | Year 4 |
| Any | +7.4 (6.3–8.5) | +7.6 (5.7–9.5) | +7.0 (4.9–9.1) | +6.3 (3.9–8.7) | +8.6 (5.5–11.7) |
Absolute subsequent intervention rates (shown in supplemental appendix table 3) are cumulative over time.
Sensitivity Analysis
We found similar results after repeating the multivariable Cox proportional hazards model analysis predicting amputation free survival using SMR weighting (endovascular versus open revascularization hazard ratio: 0.84; 95% CI: 0.80–0.88) and propensity score stratification by quintiles (endovascular versus open revascularization hazard ratio: 0.84; 95% CI: 0.74–0.95) (see Table 4S and 5S, Supplemental Digital Content 1, which displays results of Cox proportional hazards model analyses and propensity score quintiles respectively).
DISCUSSION
Our results indicate that in a cohort of Medicare beneficiaries with symptomatic lower extremity PAD, endovascular treatment is associated with improved amputation free survival compared to an open surgery, particularly among patients with congestive heart failure or ischemic heart disease. This benefit is pronounced in the short-term, persists over the long-term, and is largely driven by an early differential in mortality. We also examined the frequency of subsequent interventions and found that they are somewhat more frequent after endovascular interventions during the first year, but thereafter the differential between the two cohorts remains constant. We used propensity score matching to equalize the patient cohorts.
We found that in the first year, patients who underwent an endovascular procedure had lower risk of amputation or death compared to open patients (24.8 vs. 29.4%). Furthermore, we found that the early amputation free survival advantage in the endovascular procedure persisted at 4-years. Our findings contrast with those reported in the BASIL trial, the most prominent randomized controlled trial to date.9 In BASIL, there was no difference in amputation free survival overall, and when investigators examined patients two years beyond randomization, they found that open surgery was associated with a decreased risk of amputation or death.9 Three phenomena may explain the contrast between our results and those observed in BASIL. First, endovascular technology and surgical technique have improved over time, and the BASIL trial (1999–2004) predates our study period. Second, we studied patients with claudication and critical limb ischemia, whereas the BASIL trial included only patients with severe limb ischemia albeit our findings in the limb threat cohort revealed an advantage of endovascular intervention.7,36,37 Third, distinct study populations may explain the difference: The BASIL trial was conducted in the United Kingdom with patients meeting specific inclusion criteria and intense follow-up, and our dataset is comprised of older adults treated as part of usual practice in the United States.
We hypothesized that patients with common, systemic comorbidity and severe vascular disease would be less likely to tolerate an open procedure than those without comorbidity. We also hypothesized that the relative outcome of the two interventions might be influenced by conditions such as diabetes or renal failure because of their effect on disease anatomy such as increased calcification, or that the differential in outcomes would be altered in patients with limb threat versus claudication related to extent of disease. To test this, we evaluated interactions between procedure type and select comorbidity burden in predicting outcomes. These hypotheses were largely unsupported, although we found partially consistent results for patients with CHF/IHD. Specifically, patients with CHF/IHD had decreased risk of amputation or death after an endovascular procedure compared to an open procedure; patients without CHF/IHD did not experience the same benefit from an endovascular approach. Remarkably, the lack of significance for the remainder of tested interactions shows that comorbidity did not alter the benefit associated with an endovascular approach. It appears that, although these comorbid conditions are associated with worse overall amputation free survival, the advantage of an endovascular procedure is consistent across patient populations.
Historically, and consistent with our findings, long-term mortality in patients with symptomatic PAD, particularly in patients with critical limb ischemia, is very high ranging from 40–70% at 5-years.10,36,38–41 However over the short-term, we found a strikingly high perioperative mortality rate after revascularization, regardless of the intervention used. This was particularly so in patients with critical limb ischemia: 6.5% and 8.3% of patients died within 30-days of an endovascular or open procedure, respectively. These findings contradict the common belief that minimally invasive approaches are associated with insignificant morbidity and mortality. Our results highlight that in a real-world setting, the prognosis after lower extremity revascularization for symptomatic PAD is poor, regardless of approach.
A common criticism of endovascular repair is that it may be less durable, requiring additional interventions to maintain limb salvage.7,15,42,43 We did identify an increased rate of subsequent intervention for endovascular procedures over the first 30 days. However the differential between endo and open during this time period was only 7%. Moreover, beyond 30-days the rate of subsequent intervention was identical for the two cohorts. Thus, at least over a four year period, the reintervention rate for endovascular procedures is only slightly increased compared to open revascularization.
Our results should be interpreted in the context of several limitations. First, anatomical characteristics are not available in this data set. Nevertheless, extant literature has demonstrated that clinical phenotypes for PAD correlate closely with the prevalence of specific patient comorbidities such as diabetes,44,45 renal failure,46,47 and critical limb ischemia.48,49 By matching these factors in our model, we feel that we have succeeded in matching patient anatomy. We are also unable to determine laterality of procedures, subsequent amputations, or subsequent interventions. We presume some percentage of amputations or subsequent procedures occurred in the contralateral extremity. Given the high likelihood that both groups had subsequent interventions on the contralateral extremity at approximately the same rate, the relative difference in the rates of subsequent interventions likely reflects the true discrepancy in reintervention rates.42 Furthermore, undergoing a major amputation, regardless of side, indicates a failure of the procedure concurrent with the rationale previously described.9 Finally, the generalizability of our analysis is limited to Medicare inpatients (although these represent the majority of patients undergoing lower extremity revascularization);50 and is also limited to the population represented by endovascular patients that were matched. In performing a sensitivity analysis using alternative propensity score techniques, we found almost identical results, suggesting that our estimates of treatment effect on the population studied are valid and reproducible.
CONCLUSION
Determining an optimal revascularization strategy for patients with symptomatic lower extremity PAD requires integration of surgeon and patient preferences, available resources, patient comorbidity and relevant anatomy. Given availability of both endovascular and open revascularization options, we show that an endovascular approach is associated with improved long-term amputation free survival. There is initially an increased risk of subsequent intervention after an endovascular procedure; however, this risk is modest and confined to the first 30 days after primary intervention. As always, the selection of interventions cannot be generalized and should be individualized for each patient based upon multiple factors and local expertise.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING AND CONFLICTS OF INTEREST
Jason T. Wiseman is supported by an NIH research training grant (T32 HL110853). K. Craig Kent and Sara Fernandes-Taylor are supported by an AHRQ research grant (R21 HS023395). The project described was supported by the Clinical and Translational Science Award program, through grant UL1TR000427 from the NIH National Center for Advancing Translational Sciences. Support was also provided by the Health Innovation Program and the University of Wisconsin School of Medicine and Public Health Wisconsin Partnership Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Mcdermott MM. The magnitude of the problem of peripheral arterial disease: Epidemiology and clinical significance. Cleve Clin J Med 2006; 73: S2–S7. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 33: S1–S75. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Murphy TP, Lovell MB, et al. Gaps in public knowledge of peripheral arterial disease: the first national PAD public awareness survey. Circulation 2007; 116: 2086–2094. [DOI] [PubMed] [Google Scholar]
- 4.Goodney PP, Tarulli M, Faerber A, et al. Fifteen-Year Trends in Lower Limb Amputation, Revascularization, and Preventive Measures Among Medicare Patients. JAMA Surg 2014; 150: 84–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malas MB, Qazi U, Glebova N, et al. Design of the Revascularization With Open Bypass vs Angioplasty and Stenting of the Lower Extremity Trial (ROBUST): a randomized clinical trial. JAMA Surg 2014; 149: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 6.Andreozzi GM, Martini R. The fate of the claudicant limb. European Heart Journal-Supplements 2002; 4: 41–45. [Google Scholar]
- 7.Goodney PP, Beck AW, Nagle J, et al. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg 2009; 50 :54–60. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PL, Gelijns A, Moskowitz A, et al. Understanding trends in inpatient surgical volume: Vascular interventions, 1980–2000. J Vasc Surg 2004; 39: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: An intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010; 51: 5S–17S. [DOI] [PubMed] [Google Scholar]
- 10.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366: 1925–1934. [DOI] [PubMed] [Google Scholar]
- 11.McQuade K, Gable D, Hohman S, et al. Randomized comparison of ePTFE/nitinol self-expanding stent graft vs prosthetic femoral-popliteal bypass in the treatment of superficial femoral artery occlusive disease. J Vasc Surg 2009; 49: 109–116. [DOI] [PubMed] [Google Scholar]
- 12.Van der Zaag ES, Legemate DA, Prins MH, et al. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. Eur J Vasc Endovasc Surg 2004; 28: 132–137. [DOI] [PubMed] [Google Scholar]
- 13.Kedora J, Hohmann S, Garrett W, et al. Randomized comparison of percutaneous Viabahn stent grafts vs prosthetic femoral-popliteal bypass in the treatment of superficial femoral arterial occlusive disease. J Vasc Surg 2007; 45: 10–16. [DOI] [PubMed] [Google Scholar]
- 14.Forbes JF, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Health-related quality of life outcomes, resource utilization, and cost-effectiveness analysis. J Vasc Surg 2010; 51: 43S–51S. [DOI] [PubMed] [Google Scholar]
- 15.Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: Analysis of amputation free and overall survival by treatment received. J Vasc Surg 2010; 51: 18S–31S. [DOI] [PubMed] [Google Scholar]
- 16.Bradbury AW, Bell J, Lee AJ, et al. Bypass or Angioplasty for Severe Limb Ischaemia? A Delphi Consensus Study. Eur J Vasc Endovasc Surg 2002; 24: 411–416. [DOI] [PubMed] [Google Scholar]
- 17.Best Endovascular vs. Best Surgical Therapy in Patients With Critical Limb Ischemia (BEST-CLI) [Clinicaltrials.gov website]. Available at: https://clinicaltrials.gov/ct2/show/NCT02060630. Accessed May 19, 2015.
- 18.Revascularization With Open Bypass Versus Angioplasty and STenting of the Lower Extremity Trial (ROBUST) [Clinicaltrials.gov website]. Available at: https://clinicaltrials.gov/ct2/show/NCT01602159. Accessed May 19, 2015. [DOI] [PubMed]
- 19.Mills N, Blazeby JM, Hamdy FC, et al. Training recruiters to randomized trials to facilitate recruitment and informed consent by exploring patients’ treatment preferences. Trials 2014; 15: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CMS Chronic Conditions Warehouse [Chronic Conditions Data Warehouse website] Available at: http://www.ccwdata.org/. Accessed March 1, 2015.
- 21.Vogel TR, Kruse RL. Risk factors for readmission after lower extremity procedures for peripheral artery disease. J Vasc Surg 2013; 58: 90–97. [DOI] [PubMed] [Google Scholar]
- 22.Holman KH, Henke PK, Dimick JB, et al. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg 2011; 54: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vogel TR, Dombrovskiy VY, Galiñanes EL, et al. Preoperative statins and limb salvage after lower extremity revascularization in the Medicare population. Circ Cardiovasc Interv 2013; 6: 694–700. [DOI] [PubMed] [Google Scholar]
- 24.Goodney PP, Holman K, Henke PK, et al. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg 2013; 57: 1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from U.S. Medicare 2000–2008. J Am Coll Cardiol 2012; 60: 2230–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hebert P, Geiss L, Tierney E, et al. Identifying Persons with Diabetes Using Medicare Claims Data. Am J Med Qual 1999;14: 270–277. [DOI] [PubMed] [Google Scholar]
- 27.Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc Health Risk Manag 2007; 3: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elixhauser A, Steiner C, Harris DR, et al. Measures for Use with Administrative Data Comorbidity. Med Care 1998; 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 29.United States Department of Agriculture Economic Research Service; Rural Urban Commuting Area Codes [Website]. June 2, 2014. Available from: http://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx#.Ujsj0F8o5aQ. Accessed March 14, 2015.
- 30.Mell MW, Bartels C, Kind A, et al. Superior outcomes for rural patients after abdominal aortic aneurysm repair supports a systematic regional approach to abdominal aortic aneurysm care. J Vasc Surg 2012; 56: 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubin DB. Using propensity scores to help design observational studies: Application to the tobacco litigation. Health Serv Outcomes Res Methodol 2002; 2: 169–188. [Google Scholar]
- 32.Rosenbaum PR, Rubin DB. Constructing a Control Group Using Multivariate Matched Sampling Methods that Incorporate the Propensity Score. Am Stat 1985; 39: 33–38. [Google Scholar]
- 33.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: A systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 2007; 134: 1128–1135. [DOI] [PubMed] [Google Scholar]
- 34.Goodney PP, Nolan BW, Schanzer A, et al. Factors associated with death 1 year after lower extremity bypass in Northern New England. J Vasc Surg 2010; 51: 71–78. [DOI] [PubMed] [Google Scholar]
- 35.Brookhart MA, Wyss R, Layton JB, et al. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6: 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel TR, Su LT, Symons RG, et al. Lower extremity angioplasty for claudication: a population-level analysis of 30-day outcomes. J Vasc Surg 2007; 45: 762–767. [DOI] [PubMed] [Google Scholar]
- 37.Sachs T, Pomposelli F, Hamden A, et al. Trends in the national outcomes and costs for claudication and limb threatening ischemia: Angioplasty vs bypass graft. J Vasc Surg 2011; 54: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 38.Dormandy J, Heeck L, Vig S. The fate of patients with critical leg ischemia. Semin Vasc Surg 1999; 12: 142–147. [PubMed] [Google Scholar]
- 39.Raghunathan A, Rapp JH, Littooy F, et al. Postoperative outcomes for patients undergoing elective revascularization for critical limb ischemia and intermittent claudication: A subanalysis of the Coronary Artery Revascularization Prophylaxis (CARP) trial. J Vasc Surg 2006; 43: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 40.Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg 2006; 43: 742–752. [DOI] [PubMed] [Google Scholar]
- 41.Nowygrod R, Egorova N, Greco G, et al. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg 2006; 43: 205–216. [DOI] [PubMed] [Google Scholar]
- 42.Schermerhorn ML, O’Malley AJ, Jhaveri A, et al. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med 2008; 358: 464–474. [DOI] [PubMed] [Google Scholar]
- 43.Faries P, Morrissey NJ, Teodorescu V, et al. Recent advances in peripheral angioplasty and stenting. Angiology 2002; 53: 617–626. [DOI] [PubMed] [Google Scholar]
- 44.American Diabetes Association Peripheral Arterial Disease in People with Diabetes American. J Am Podiatr Med Assoc 2005; 95: 309–319. [Google Scholar]
- 45.Jude E, Chalmers N, Oyibo S, et al. Peripheral Arterial Disease in Diabetic and Nondiabetic Patients. Diabetes Care 2001; 24: 1433–1437. [DOI] [PubMed] [Google Scholar]
- 46.Sigrist M, Bungay P, Taal MW, et al. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant 2006; 21: 707–714. [DOI] [PubMed] [Google Scholar]
- 47.Wasmuth S, Baumgartner I, Do DD, et al. Renal insufficiency is independently associated with a distal distribution pattern of symptomatic lower-limb atherosclerosis. Eur J Vasc Endovasc Surg 2010; 39: 591–596. [DOI] [PubMed] [Google Scholar]
- 48.Rueda CA, Nehler MR, Perry DJ, et al. Patterns of artery disease in 450 patients undergoing revascularization for critical limb ischemia: implications for clinical trial design. J Vasc Surg 2008; 47: 995–1000. [DOI] [PubMed] [Google Scholar]
- 49.Diehm N, Shang A, Silvestro A, et al. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg 2006; 31: 59–63. [DOI] [PubMed] [Google Scholar]
- 50.Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg 2007; 45: 55–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


