Abstract
Primary hypothyroidism is associated with oxidative stress and insufficient antioxidant capacity. This study was conducted to evaluate the effects of levothyroxine replacement therapy on paraoxonase 1 (PON-1) serum levels in a patients with primary hypothyroidism. Thirty-one patients with primary hypothyroidism compared to 20 healthy controls were recruited from. A venous blood sample were taken after an overnight fasting for biochemical parameters, before and after starting levothyroxine therapy (100 μ g/day) for 3 months duration. The biochemical variables were PON-1 serum levels, lipid profiles, triiodothyronine (T3), thyroxin (T4), and thyroid stimulating hormone (TSH) serum levels. Levothyroxine replacement therapy leads to a significant amelioration of thyroid functions, lipid profile, cardiometabolic measures P < 0.05 in patients with primary hypothyroidism. Levothyroxine leads to significant elevation in PON-1 serum levels from 188.42 ± 19.81 (U/mL) to 361.23 ± 33.62 (U/mL) P < 0.0001. This study concluded that levothyroxine replacement therapy significantly increases PON-1 serum levels in patients with primary hypothyroidism and attenuating hypothyroidism-induced oxidative stress.
Keywords: Hypothyroidism, levothyroxine, PON-1
INTRODUCTION
Primary hypothyroidism is an endocrine and metabolic disorder characterized by nonspecific and subtle clinical signs and symptoms.[1] The initial biochemical change in hypothyroidism is elevation in the thyroid stimulating hormone serum levels (TSH) with normal thyroxine (T4) and triiodothyronine (T3) levels, which called subclinical hypothyroidism, but when T4 and T3 sera levels decline, this called overt hypothyroidism.[2] The incidence of primary hypothyroidism is 1%–2%, which is more in females than males; the causes of this clinical condition are autoimmune thyroiditis, thyroid surgery, radioiodine therapy, and malignancy.[3] Primary hypothyroidism elevates the risk of ischemic heart disease due to the reduction of nitric oxide that leads to endothelial dysfunction and vascular complications.[4] In hypothyroidism, the basal metabolic rate is reduced that decrease the production of free radicals production. Recently, a significant association between hypothyroidism and augmentation of free radical productions are occurring leading to the detrimental effects on the endogenous antioxidant enzymes, which is not same in different tissues.[5,6] Human body antioxidant defence mechanisms against oxidative stress include non-enzymatic and enzymatic pathways. Paraoxonase-1 (PON-1) is one of the enzymatic pathways that found on high-density lipoprotein (HDL) which concerned with hydrolysis of oxidized lipoproteins.[7] PON genotype forms are PON-1, PON-2, and PON-3 which are encoded by genes located on chromosome-7, the differences in them are related to the activity and main location.[8] PON-1 is synthesized by the liver and transported closely with HDL in the plasma; it inhibits low-density lipoprotein (LDL) oxidation, superoxide productions, and HDL peroxidation.[9] Therefore, it regarded as potential antioxidant leads to cytoprotection against lipid peroxidations. Consequently, PON-1 activity is decline in oxidative stress of different etiology as in ischemic heart disease and hemolytic anemia.[10] Torun et al., study demonstrated that increased oxidative stress in primary hypothyroidism may be due to insufficient antioxidant capacity and alterations in lipid metabolism.[11]
Levothyroxine is a synthetic hormone similar to thyroxin used in treatment of primary hypothyroidism, which is peripherally converted into an active form T3 and binds the nuclear receptors, absorbed orally and affected by food and 99% of it bind the plasma proteins (inactive) while the free form is regarded as active form, levothyroxine elimination half-life is 7 days in healthy controls and 10 days in hypothyroid patients.[12]
Therefore, the present study was designed to evaluate the effects of levothyroxine therapy on PON-1 serum levels in patients with idiopathic primary hypothyroidism.
PATIENTS AND METHODS
In this study, 31 patients (17 females and 14 males) with primary hypothyroidism were selected according to the criteria of the British Thyroid Association Executive Committee,[13] with a mean age of 45.87 ± 11.89 compared to 20 healthy controls. The patients were recruited from the Iraqi Endocrinology Center; Baghdad-Iraq during August 2017, this study was done in cooperation with the Department of Clinical Pharmacology, College of Medicine, Al-Mustansiriyia University. This study was approved by the Ethics Committee and Scientific Medical Board at College of Medicine, Al-Mustansiriyia University according to the Declaration of Helsinki 2008.[14] Learned and informed consent was taken from all enrolled patients before starting the study.
Study design
Thirty-one patients, a newly diagnosed primary hypothyroidism were selected; 10 ml of venous blood sample were taken at morning after an overnight fasting for baseline biochemical parameters that regarded as baseline (before starting levothyroxine therapy (100 μg tablet, Merck Darmstadt, Germany SIN 9741P) daily for 3 consecutive months’ duration then second blood samples were taken, which regarded as posttreatment effects. Blood samples were stored at −20°C until the time of biochemical assays.
Biochemical assays
The measurement of PON-1 levels was done by ELISA method (ELISA Kit SK00141-01, AVISCERA BIOSCIENCE INC). T3, T4, and TSH levels were measured by Enzyme Immunoassay, Colorimetric (Accu Bind VAST KITS).
Measurement of lipid profiles
Triglyceride (TG) (TG ELISA Kit Wako Chemicals USA, Inc.,) total cholesterol (TC) (Cell Biolab, Inc.) and HDL (Cell Biolab, Inc.,) were assessed by specific ELISA kits; from this profile, we can measure the followings: LDL = (TC)-(HDL)-(TG)/5, very LDL = TG/5.[15]
Anthropometric and cardio-metabolic measures
Body mass index (BMI) = body weight (kg)/height (m2).[16]
Atherogenic coefficient (AC) = (TC-HDL)/HDL.[17]
Atherogenic index = log (TG/HDL), when TG and HDL measured in mmol/l.[18]
Cardiac risk ratio (CRR) = TC/HDL.[19]
Statistical analysis
SPSS 19.0 for Windows (SPSS Inc., Chicago, USA) was used for data analysis. Data were presented as mean ± standard deviation, paired and unpaired Student's t-test, were used for evaluation of differences regarding P < 0.05 as the lowest limits of significance.
RESULTS
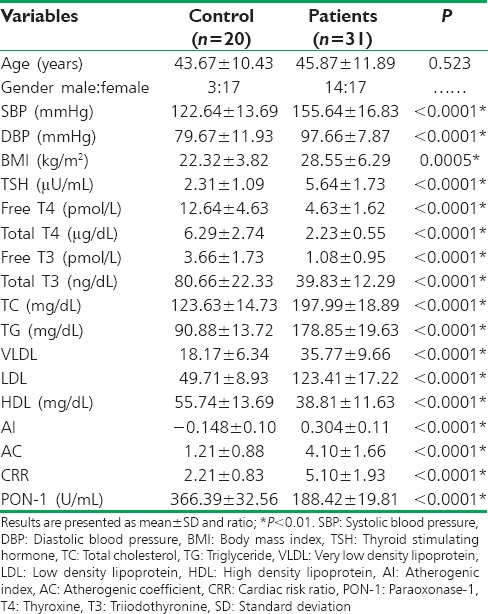
A total number of 51 out of 55 individuals completed the study; four patients were excluded because they not met the selected criteria [Figure 1]. The enrolled individuals (31 patients and 20 healthy controls) were presented with specific characteristics [Table 1]. Patients with primary hypothyroidism before starting the treatment were significantly differed from controls in biochemical and anthropometric parameters P < 0.05 except at the age were they do not differ significantly P = 0.523, serum PON-1 serum levels in patients with primary hypothyroidism were 188.42 ± 19.81 (U/mL) that differ significantly from normal healthy controls PON-1 serum levels 366.39 ± 32.56 (U/mL), P < 0.0001.
Figure 1.
Consort flow diagram of the present study
Table 1.
Characteristics of the study
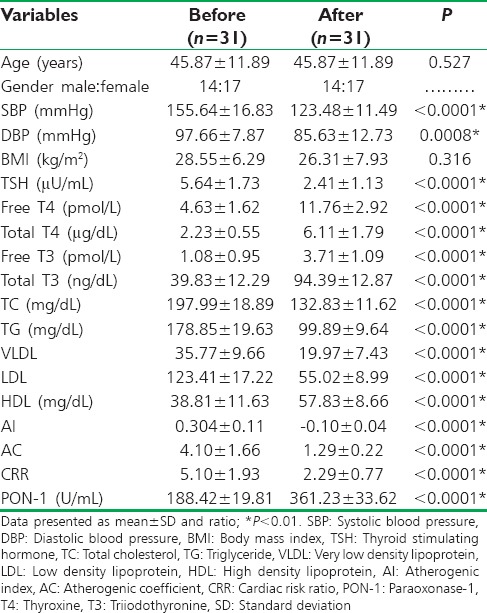
Levothyroxine treatment led to a significant amelioration in thyroid functions, lipid profile, cardiometabolic measures, and serum PON-1 serum level P < 0.05 in patients with primary hypothyroidism, without significant effect on BMI P = 0.316. Indeed, results of the present study demonstrated minor significant differences between controls and patients at posttreatment period in BMI P = 0.0477, total T 3P= 0.023, TC P = 0.033, and TG P = 0.020, but there was a high significant of difference in diastolic blood pressure P < 0.0001 [Table 2].
Table 2.
Baseline biochemical measures, cardiometabolic profiles and serum paraoxonase-1 serum levels in patients with primary hypothyroidism compared to healthy subjects
Moreover, levothyroxine (100 μg/day) for 2 months duration led to highly significant amelioration in all biochemical variables and blood pressure changes as well as rising in PON-1 serum levels P < 0.01, but there was insignificant effect on BMI P = 0.316 [Table 3].
Table 3.
Biochemical measures, cardiometabolic profiles and serum paraoxonase-1 serum levels in patients with primary hypothyroidism before and after treatment with levothyroxine (100 μg/day)
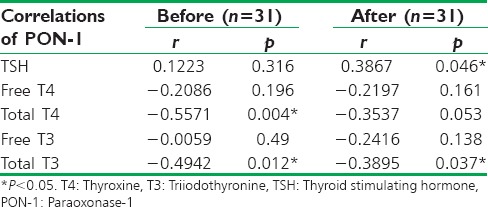
At pretreatment period, PON-1 serum levels were positively correlated with TSH serum levels (r = 0.1223) and negatively correlated with free T4 (r = −0.2086, P = 0.196), total T4 (r = −0.5571, P = 0.004), Free T3 (r = −0.0059, P = 0.49), and total T3 (r = −0.4942, P = 0. 012). While, at posttreatment period PON-1 serum levels were positively correlated with TSH serum levels (r = 0.3867, P = 0.046), negatively correlated with free T4 (r = −0.2197, P = 0.161), total T4 (r = −0.3537, P = 0.053), free T3 (r = −0.2416, P = 0.138) and Total T3 (r = −0.3895, P = 0.037). Thus, PON-1 serum levels were positively correlated with TSH serum levels and negatively correlated with T4 and T3 [Table 4].
Table 4.
Correlations between paraoxonase-1 serum level and thyroid profile before and after treatment
DISCUSSION
Thyroid hormones control and regulate oxidative metabolism, body's antioxidant, free radical generations, and basal metabolic rate.[20] Hypothyroidism is associated with a reduction of antioxidant productions.[21] Sarandol et al., study revealed the augmentation of oxidative stress in overt hypothyroidism due to dysfunction in mitochondrial respiratory chain which control oxidative and antioxidant balance.[22]
The present study showed significant detrimental effects of overt hypothyroidism on lipid profile, cardio-metabolics profile and the antioxidant PON-1 serum levels compared to normal healthy controls these findings correspond with a recent study that demonstrated an association between hypothyroidism with significant elevations in the inflammatory biomarkers that predispose to cardiovascular complications.[23]
Patients with overt primary hypothyroidism in the current study were associated with significant reduction in PON-1 serum levels, as supported by Azizi et al., study that demonstrated a reduction in the PON-1 serum level is linked to primary hypothyroidism,[24] while Milionis et al. revealed insignificant differences in PON-1 serum levels in patients with primary hypothyroidism compared to control.[25]
Posttreatment effects of levothyroxine led to significant amelioration in biochemical and cardiometabolic profile with elevation in PON-1 serum levels these compatible with Sigal et al., study that showed significant dyslipidemia and reduction in PON-1 serum levels that are reversed by levothyroxine replacement therapy[26] while Kebapcilar et al. showed insignificant elevation in PON-1 serum levels after levothyroxine replacement therapy.[27]
In addition, levothyroxine therapy improves atherogenic index, AC, blood pressure, and CRR in patients with primary hypothyroidism since; experimental hypercholesterolemia in hypothyroidism lead to cardiomyocyte damage and endothelial dysfunction with significant elevations in cardiac risk scores and atherogenic index, which per se explain the beneficial effects of levothyroxine on CRR[28] as revealed in our study.
Moreover, Baskol et al., study showed a higher oxidative markers and lower PON-1 activity in hypothyroidism lead to lipid peroxidation and prooxidant status that provoked the reduction of PON-1 antioxidant activity.[29] This explains the potential therapeutic benefits of levothyroxine in rising PON-1 serum levels and consequently, improvement in antioxidant system at physiological levels. The linking between primary hypothyroidism and oxidative stress remain unknown, antioxidant deficiency in hypothyroidism lead to failure in neutralizing the intrinsic and extrinsic oxidant factors which contribute to oxidative stress and cell damage.[30] Therefore, levothyroxine act as protective hormone against oxidative stress through activation of antioxidant defense and reduction in lipid peroxidation.[31]
In reality, the present study exhibited a positive correlation between PON-1 serum levels and TSH levels with negative correlations with free and total T3 and T4 as supported by Yavuz et al., study that exposed a negative correlation between PON-1 activity and thyroid hormones with positive correlation with TSH in TSH-suppressed goiter.[32]
In addition, low PON-1 serum levels in our patients may be due to high BMI and dyslipidemia as supported by previous studies that illustrated a link between low-HDL-PON activity and membrane peroxidation in obese and dyslipidemic patients.[33]
CONCLUSION
Levothyroxine pharmacotherapy significantly increases PON-1 serum levels in patients with overt primary hypothyroidism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors kindly acknowledge Professor Sadiq M. Al-Hamash head of Al-Mustansiriyia University.
REFERENCES
- 1.Toft Kristensen T, Larsen J, Pedersen PL, Feldthusen AD, Ellervik C, Jelstrup S, et al. Weight gain and serum TSH increase within the reference range after hemithyroidectomy indicate lowered thyroid function. J Thyroid Res 2014. 2014:892573. doi: 10.1155/2014/892573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Källén B, Norstedt Wikner B. Maternal hypothyroidism in early pregnancy and infant structural congenital malformations. J Thyroid Res 2014. 2014:160780. doi: 10.1155/2014/160780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malekpour B, Mehrafshan A, Saki F, Malekmohammadi Z, Saki N. Effect of posttraumatic serum thyroid hormone levels on severity and mortality of patients with severe traumatic brain injury. Acta Med Iran. 2012;50:113–6. [PubMed] [Google Scholar]
- 4.Kc R, Khatiwada S, Deo Mehta K, Pandey P, Lamsal M, Majhi S, et al. Cardiovascular risk factors in subclinical hypothyroidism: A Case control study in Nepalese population. J Thyroid Res 2015. 2015:305241. doi: 10.1155/2015/305241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolini G, Pitto L, Kusmic C, Balzan S, Sabatino L, Iervasi G, et al. New insights into mechanisms of cardioprotection mediated by thyroid hormones. J Thyroid Res 2013. 2013:264387. doi: 10.1155/2013/264387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mourouzis I, Politi E, Pantos C. Thyroid hormone and tissue repair: New tricks for an old hormone? J Thyroid Res 2013. 2013:312104. doi: 10.1155/2013/312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kayikcioglu M, Saygi S, Azarsiz E, Can LH, Kultursay H, Sözmen EY. Serum paraoxonase 1 activity and oxidative markers of LDL in patients with cardiac syndrome X. Acta Cardiol. 2007;62:245–9. doi: 10.2143/ac.62.3.2020812. [DOI] [PubMed] [Google Scholar]
- 8.Paltoglou G, Tavernarakis G, Christopoulos P, Vlassi M, Gazouli M, Deligeoroglou E, et al. PON1-108 TT and PON1-192 RR genotypes are more frequently encountered in Greek PCOS than non-PCOS women, and are associated with hyperandrogenaemia. Clin Endocrinol (Oxf) 2013;79:259–66. doi: 10.1111/cen.12139. [DOI] [PubMed] [Google Scholar]
- 9.Jiang H, Stabler SP, Allen RH, Maclean KN. Altered expression of ApoA-I, ApoA-IV and PON-1 activity in CBS deficient homocystinuria in the presence and absence of treatment: Possible implications for cardiovascular outcomes. Mol Genet Metab. 2012;107:55–65. doi: 10.1016/j.ymgme.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Erenler AK, Kocabaş R, Doğan T, Erdemli HK, Yetim M. Paraoxanase as an indicator of myocardial ischemia and its utility in determining extension of ischemia. Am J Emerg Med. 2015;21 doi: 10.1016/j.ajem.2015.09.008. pii: S0735-6757(15)00784-6. [DOI] [PubMed] [Google Scholar]
- 11.Torun AN, Kulaksizoglu S, Kulaksizoglu M, Pamuk BO, Isbilen E, Tutuncu NB. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin Endocrinol (Oxf) 2009;70:469–74. doi: 10.1111/j.1365-2265.2008.03348.x. [DOI] [PubMed] [Google Scholar]
- 12.Bolton S. Bioequivalence studies for levothyroxine. AAPS J. 2005;7:E47–53. doi: 10.1208/aapsj070106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okosieme O, Gilbert J, Abraham P, Boelaert K, Dayan C, Gurnell M, et al. Management of primary hypothyroidism: Statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf) 2016;84:799–808. doi: 10.1111/cen.12824. [DOI] [PubMed] [Google Scholar]
- 14.Puri KS, Suresh KR, Gogtay NJ, Thatte UM. Declaration of Helsinki, 2008: Implications for stakeholders in research. J Postgrad Med. 2009;55:131–4. doi: 10.4103/0022-3859.52846. [DOI] [PubMed] [Google Scholar]
- 15.Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadilly AK. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases. 2018;6 doi: 10.3390/diseases6010009. pii: E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Kuraishy HM, Al-Gareeb AI. Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: Role of statins. J Lab Physicians. 2017;9:163–9. doi: 10.4103/0974-2727.208263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8–14. doi: 10.4103/0976-0105.195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Kuraishy HM, Al-Gareeb AI. Effect of orlistat alone or in combination with Garcinia cambogia on visceral adiposity index in obese patients. J Intercult Ethnopharmacol. 2016;5:408–14. doi: 10.5455/jice.20160815080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkuraishy HM, Al-Gareeb AI. New insights into the role of metformin effects on serum omentin-1 levels in acute myocardial infarction: Cross-sectional study. Emerg Med Int 2015. 2015:283021. doi: 10.1155/2015/283021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarım Ö. Thyroid hormones and growth in health and disease. J Clin Res Pediatr Endocrinol. 2011;3:51–5. doi: 10.4274/jcrpe.v3i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alturfan AA, Zengin E, Dariyerli N, Alturfan EE, Gumustas MK, Aytac E, et al. Investigation of zinc and copper levels in methimazole-induced hypothyroidism: Relation with the oxidant-antioxidant status. Folia Biol (Praha) 2007;53:183–8. doi: 10.14712/fb2007053050183. [DOI] [PubMed] [Google Scholar]
- 22.SarandÖl E, Taş S, Dirican M, Serdar Z. Oxidative stress and serum paraoxonase activity in experimental hypothyroidism: Effect of vitamin E supplementation. Cell Biochem Funct. 2005;23:1–8. doi: 10.1002/cbf.1119. [DOI] [PubMed] [Google Scholar]
- 23.Aziz KM. Association of hypothyroidism with body mass index, systolic blood pressure and proteinuria in diabetic patients: Does treated hypothyroidism with thyroxine replacement therapy prevent nephropathy/Chronic renal disease? Curr Diabetes Rev. 2016;12:297–306. doi: 10.2174/1573399812666151029101622. [DOI] [PubMed] [Google Scholar]
- 24.Azizi F, Raiszadeh F, Solati M, Etemadi A, Rahmani M, Arabi M, et al. Serum paraoxonase 1 activity is decreased in thyroid dysfunction. J Endocrinol Invest. 2003;26:703–9. doi: 10.1007/BF03347350. [DOI] [PubMed] [Google Scholar]
- 25.Milionis HJ, Tambaki AP, Kanioglou CN, Elisaf MS, Tselepis AD, Tsatsoulis A. Thyroid substitution therapy induces high-density lipoprotein-associated platelet-activating factor-acetylhydrolase in patients with subclinical hypothyroidism: A potential antiatherogenic effect. Thyroid. 2005;15:455–60. doi: 10.1089/thy.2005.15.455. [DOI] [PubMed] [Google Scholar]
- 26.Sigal GA, Medeiros-Neto G, Vinagre JC, Diament J, Maranhão RC. Lipid metabolism in subclinical hypothyroidism: Plasma kinetics of triglyceride-rich lipoproteins and lipid transfers to high-density lipoprotein before and after levothyroxine treatment. Thyroid. 2011;21:347–53. doi: 10.1089/thy.2010.0313. [DOI] [PubMed] [Google Scholar]
- 27.Kebapcilar L, Comlekci A, Tuncel P, Solak A, Secil M, Gencel O, et al. Effect of levothyroxine replacement therapy on paraoxonase-1 and carotid intima-media thickness in subclinical hypothyroidism. Med Sci Monit. 2010;16:CR41–7. [PubMed] [Google Scholar]
- 28.Nepomnyashchikh LM, Lushnikova EL, Polyakov LP, Molodykh OP, Klinnikova MG, Russkikh GS, et al. Structural changes in the myocardium and serum lipid spectrum in experimental hypercholesterolemia and hypothyroidism. Bull Exp Biol Med. 2013;155:692–6. doi: 10.1007/s10517-013-2228-8. [DOI] [PubMed] [Google Scholar]
- 29.Baskol G, Atmaca H, Tanriverdi F, Baskol M, Kocer D, Bayram F. Oxidative stress and enzymatic antioxidant status in patients with hypothyroidism before and after treatment. Exp Clin Endocrinol Diabetes. 2007;115:522–6. doi: 10.1055/s-2007-981457. [DOI] [PubMed] [Google Scholar]
- 30.Campos C, Casado Á. Oxidative stress, thyroid dysfunction & down syndrome. Indian J Med Res. 2015;142:113–9. doi: 10.4103/0971-5916.164218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iervasi G, Nicolini G. Thyroid hormone and cardiovascular system: From basic concepts to clinical application. Intern Emerg Med. 2013;8(Suppl 1):S71–4. doi: 10.1007/s11739-013-0911-4. [DOI] [PubMed] [Google Scholar]
- 32.Yavuz DG, Yüksel M, Deyneli O, Ozen Y, Aydin H, Akalin S, et al. Association of serum paraoxonase activity with insulin sensitivity and oxidative stress in hyperthyroid and TSH-suppressed nodular goitre patients. Clin Endocrinol (Oxf) 2004;61:515–21. doi: 10.1111/j.1365-2265.2004.02123.x. [DOI] [PubMed] [Google Scholar]
- 33.Ferretti G, Bacchetti T, Masciangelo S, Bicchiega V. HDL-paraoxonase and membrane lipid peroxidation: A comparison between healthy and obese subjects. Obesity (Silver Spring) 2010;18:1079–84. doi: 10.1038/oby.2009.338. [DOI] [PubMed] [Google Scholar]


