Abstract
Type 2 diabetes mellitus (T2DM) is a metabolic disorder associated with abundant adipocytokine changes which may play an important role in the progression of insulin resistance and micro- and macro-vascular complications. Therefore, the objective of this study was to assess the differential effect of metformin alone or in combination with glyburide on apelin serum levels in patients with T2DM. In this case–control study, fifty patients with T2DM in the age range of 45–65 years and twenty-five healthy controls matched for age and body weight were recruited from single endocrinology center, subdivided according to the diabetic pharmacotherapy into: Group I: healthy controls (n = 25), Group II: T2DM patients on metformin (n = 15), Group III: T2DM patients on glyburide (n = 17), and Group IV: T2DM patients on metformin plus glyburide (n = 28). Biochemical and anthropometric variables in relation to apelin serum levels were estimated. Apelin serum levels were low in normal healthy controls compared to T2DM patients (P < 0.01). The differential effect of diabetic pharmacotherapy on apelin serum level was statistically significant (P < 0.01) compared to the controls, but insignificant when compared among used drugs (P > 0.05). Apelin level was high in T2DM compared to the controls; both metformin and glyburide might play a role in this elevation.
Keywords: Apelin, glyburide, metformin, type 2 diabetes mellitus
INTRODUCTION
In recent times, type 2 diabetes mellitus (T2DM) has become a serious and common public health problem, since there are about 415 million diabetic patients worldwide in 2015 that will progress to about 642 million diabetic patients by the year 2040.[1] T2DM is a metabolic disorder associated with numerous adipocytokine changes which may play an important role in the progression of insulin resistance and micro- and macro-vascular complications.[2] Most of the adipocytokines are synthesized and secreted by adipocytes such as leptin, omentin-1, and adiponectin[3] in patients with T2DM; there are significant changes in visceral adiposity and gluco-lipid metabolism that per se lead to adipocytokine dysregulation.[4]
Apelin is derived from preproapelin 77 amino that cleaved into apelin-36, apelin-17, apelin-13, and apelin-12, which is the active form that binds the apelin receptor. Apelin is found in different tissues such as central nervous system (CNS), heart, lung, mammary gland, and gastrointestinal tract. Apelin binds the apelin receptors which are highly expressed in pancreatic islet cells that are involved in the glucose metabolism and stimulation of insulin secretion.[5] Moreover, apelin is an important adipocytokine engaged in the energy metabolism since it improves insulin sensitivity through augmentation of skeletal muscle glucose uptake and provokes cardiac contractility and CNS stimulation.[6] Apelin is mainly produced and secreted from white adipose tissue; hence, apelin serum levels are high in obesity. In addition, apelin synthesis and secretion is stimulated by insulin, so apelin serum levels are elevated in patients with T2DM due to insulin resistance and visceral obesity.[7,8]
The relationship between apelin serum levels and T2DM is still in controversy; human and animal model studies showed apelin at low levels in patients with a newly diagnosed T2DM.[9] Other studies illustrated high apelin levels in obese diabetic patients. On the contrary, a study reported low apelin serum levels in obese diabetic patients.[10]
The differential effect of diabetic pharmacotherapy on apelin serum levels is lacking; some studies exposed the effect of metformin and dipeptidyl peptidase-4 inhibitors on apelin levels in patients with T2DM.[11]
Therefore, the objective of this study was to assess the differential effect of metformin alone or in combination with glyburide on apelin serum levels in patients with T2DM.
MATERIALS AND METHODS
In this case–control study, fifty patients with T2DM in the age range of 45–65 years and twenty-five healthy controls matched for age and body weight (BW) were recruited from a single endocrinology center. All of the enrolled patients and healthy controls gave an informed verbal and written consent for this study and agreement to use their samples in this study. The study was approved by the Scientific Committee Review Board. All measures and procedures were in harmony with the principled standards and Declaration of Helsinki.
Study design
In this study, the diabetic patients were selected according to the grading of American Diabetes Association criteria.[12] Fifty patients with T2DM (31 males + 19 females) and twenty-five healthy controls (13 females + 12 males) matched for age and BW were selected. T2DM patients and healthy controls were subdivided as follows:
Group I: Healthy controls (n = 25)
Group II: T2DM patients on metformin 500 mg tid (n = 15)
Group III: T2DM patients on glyburide 5 mg twice daily (n = 17)
Group IV: T2DM patients on metformin 500 mg tid plus glyburide 5 mg twice daily (n = 28).
Exclusion criteria
Patients with end-stage kidney disease, liver cirrhosis, malignancy, thyroid disorders, psychic and mental disorders, connective tissue diseases, sepsis, shock, and any T2DM not on metformin or glyburide were excluded from the study.
Sample processing
Ten ml of venous blood was drawn after an overnight fasting, 7 mL for glucose, lipid profile and other routine investigations; 3 mL of blood was transferred into specific tubes while containing ethylenediaminetetraacetic acid centrifugated at 3000 rpm for estimation of serum apelin and which fasting insulin by specific enzyme-linked immunosorbent assay (ELISA) kit methods.
Biochemical measurements
Insulin serum level was estimated by ELISA kit method (ACS-180 Mu/L, CIBA CORING DIAGNOSTIC, MEDIFIED, USA), and serum apelin was estimated by ELISA kit method (human apelin EIA, Q9ULZ1, EIA-APC-1, Ray Biotech, Inc., USA).
The Homeostasis Model Assessment-Insulin resistance (HOMA-IR) estimates steady-state β-cell function (β%) and insulin sensitivity (s%) as follows:
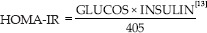
Insulin sensitivity was estimated by Quantitative Insulin Sensitivity Check Index (QUICKI equation) as follows:

Fasting glucose in mg/dL was measured by glucometer, while glycated hemoglobin (HbA1c) was measured by ELISA Kit, MB845, BIOVISION, China.
Lipid profile was measured according to the Friedewald formula as follows:[15]
Atherogenic index = log (triglyceride [TG]/high-density lipoprotein [HDL]); low risk <0.11, intermediate risk 0.11–0.21, and high risk >0.021.
Atherogenic coefficient = Total cholesterol − HDL/HDL.
Cardiac risk ratio = Total cholesterol/HDL and very low-density lipoprotein = TG/5.[16]
Anthropometric measurements
BW and height were measured by stadiometer and then body mass index (BMI) was calculated as BW (kg)/height (m2).[17] Blood pressure measurements were also determined at supine position during investigation period.
Statistical analysis
Statistical Package for the Social Sciences software (SPSS Inc., Chicago, IL, USA) was used. Data were presented as mean ± standard deviation, and the variables were tested by Pearson's correlation test and one-way ANOVA test followed by post hoc test regarding P < 0.05 as statistically significant.
RESULTS
Consort flow diagram of the present study illustrated that five patients were excluded because they do not meet the inclusion criteria, and thus fifty patients with T2DM and twenty-five controls continued this study [Figure 1].
Figure 1.
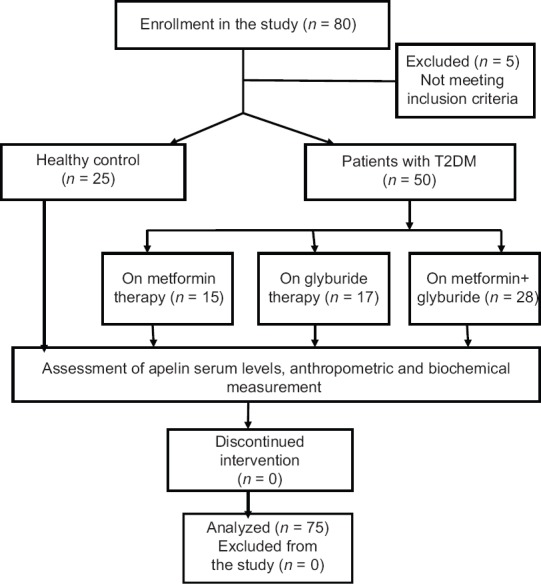
Consort flow diagram of the present study
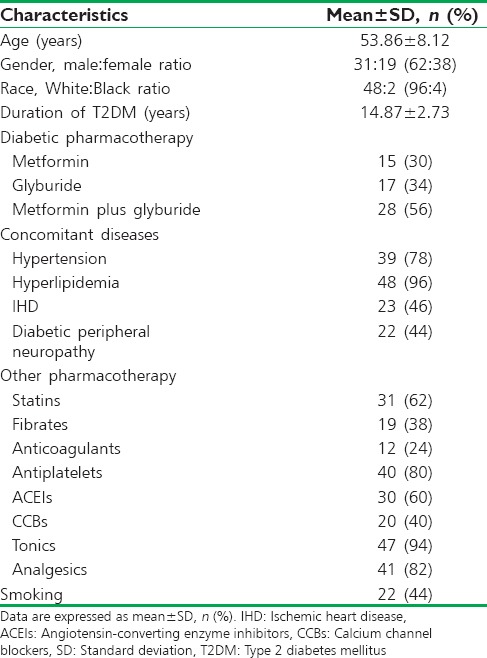
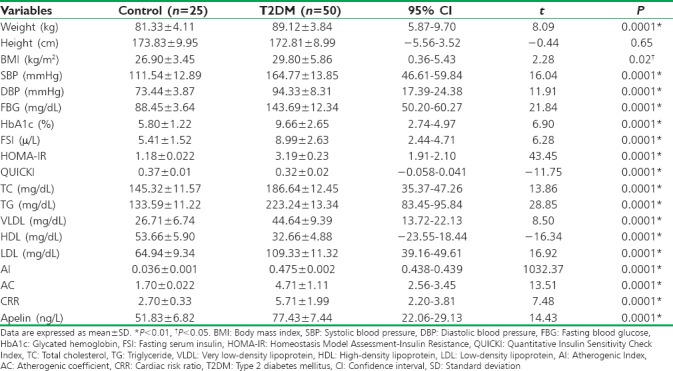
Characteristics of the recruited diabetic patients in the present study showed that age of patients was within 53.86 ± 8.12 years, 62% of them were males, and 38% were females, most of those patients were of White race (96%), and only 4% were of Black race. Regarding the current diabetic pharmacotherapy, 30% of them were on metformin, 34% on glyburide, and 56% on metformin plus glyburide. The associated diseases in those patients were hypertension (78%), hyperlipidemia (96%), ischemic heart disease (IHD) (46%), and diabetic peripheral neuropathy (44%); other characteristics are summarized in Table 1.
Table 1.
Metabolic and clinical characteristics of recruited type 2 diabetes mellitus patients
The differences between controls and T2DM patients were highly significant in all metabolic and anthropometric variables (P < 0.01) except of height (P > 0.05). BMI was 29.80 ± 5.86 in diabetic patients compared to controls (26.90 ± 3.45) (P = 0.02). Apelin serum levels were low (51.83 ± 6.82 ng/L) in normal healthy controls compared to 77.43 ± 7.44 ng/L in T2DM patients (P < 0.01) [Table 2].
Table 2.
Anthropometric and biochemical changes in relation to apelin serum levels in patients with type 2 diabetes mellitus compared to the healthy volunteers
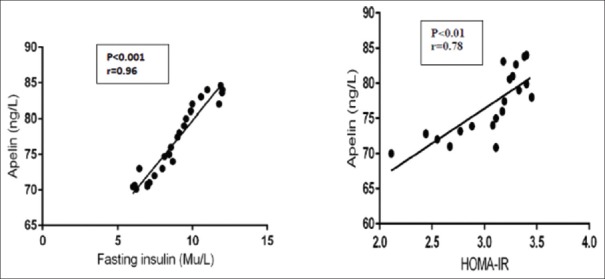
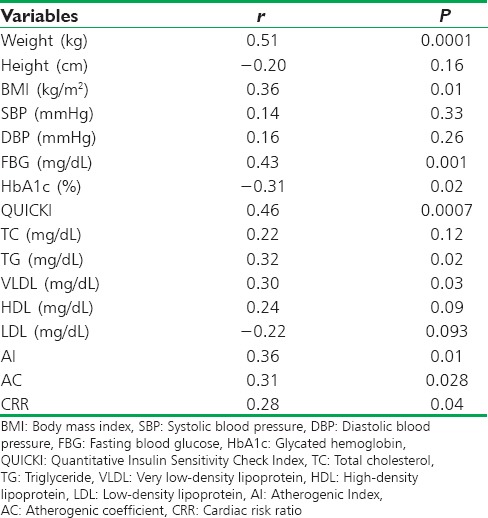
Apelin serum levels in patients with T2DM were highly correlated with fasting insulin levels (P < 0.001) and with HOMA-IR (P < 0.01) [Figures 2 and3]; also it showed significant correlations with the parameters of cardiac-metabolic profile [Table 3].
Figure 2.
Correlations of apelin levels with fasting insulin and Homeostasis Model Assessment-Insulin resistance in patients with type 2 diabetes mellitus
Figure 3.
Apelin serum levels in patients with type 2 diabetes mellitus compared to the controls *P < 0.01
Table 3.
Correlations of apelin with anthropometric and biochemical variables in patients with type 2 diabetes mellitus
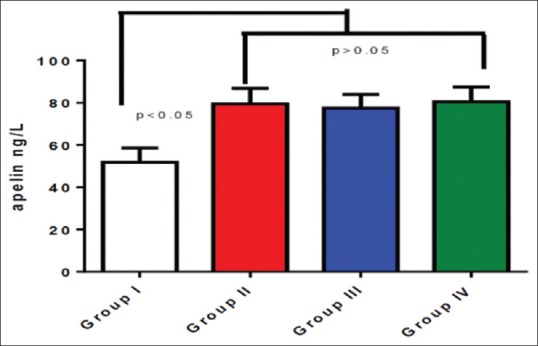
The differential effect of diabetic pharmacotherapy on apelin serum level was statistically significant (P < 0.01) compared to the controls, but insignificant when compared among used drugs (P > 0.05); the difference was high between controls and diabetic patients [Table 4].
Table 4.
The differences in apelin serum levels among patients with type 2 diabetes mellitus regarding the specific diabetic pharmacotherapy compared to the controls
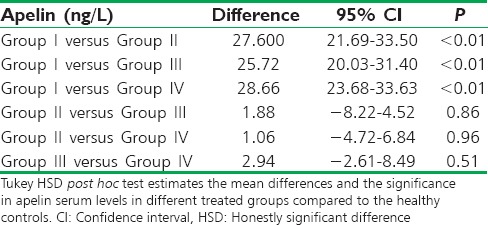
Thus, apelin serum levels were low in healthy controls compared to the diabetic patients (P < 0.01), whereas there were insignificant differences in apelin serum levels in diabetic patients regarding specific diabetic pharmacotherapy [Figure 3].
Moreover, the present study exposed insignificant differences in apelin serum levels in sex of both controls and diabetic patients [Figure 4].
Figure 4.
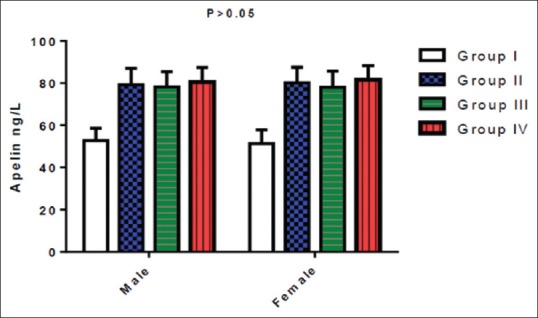
Gender differences in apelin serum levels
DISCUSSION
In T2DM, many adipokines participate in the control of blood glucose homeostasis and body energy; among them, a new peptide called apelin is widely expressed in human tissues. Apelin therapy leads to significant reduction of insulin resistance and improves insulin sensitivity.[18] Furthermore, apelin regulates adipose tissue lipolysis, since administration of apelin leads to significant reduction of adiposity. Apelin acts as a regulator of insulin secretion, but the remarkable point is the controversy about apelin serum levels in T2DM.[19]
The present study illustrated a high apelin serum level in patients with T2DM compared to the healthy controls as revealed by Dray et al's. study which showed elevated serum apelin in patients with T2DM.[20] On the contrary, Zhang et al's. study showed low level of serum apelin in T2DM,[21] while Telejko et al's. study confirmed normal apelin level in patients with T2DM.[22] Raised apelin serum levels in the present study might be due to the elevation of BMI and insulin resistance given that most of the recruited patients were obese with significant insulin resistance. These findings correspond with Ba et al's. study which revealed that high apelin serum levels were linked to obesity and level of insulin resistance, since apelin is positively correlated with BMI.[23] Moreover, low apelin serum level was observed following loss of BW as demonstrated by Khajeh et al., study illustrated a rapid reduction in apelin serum levels following partial gastric resection in patients with morbid obesity.[24] Cavallo et al's. cohort study observed that apelin was increased in relation to the level of insulin resistance in diabetic patients independent of BW since; 3 days following bariatric surgery for morbidly obese patients led to significant improvement in cardiometabolic profile and reduction in apelin serum levels independently from weight loss.[25] In addition, a study confirmed that apelin serum levels were increased more in type 1 DM than T2DM, suggesting that obesity is not the chief determinant of apelin levels.[26]
Similarly, apelin improves insulin sensitivity and increases muscle glucose uptake through inhibition of adenylate cyclise and stimulation of AMPK activity; thus, apelin is necessary for insulin sensitivity,[27] which coincided with the findings of the present study.
Our study also showed that elevated apelin serum levels were associated with hyperlipidemia, high atherogenic index, elevated cardiac risk ratio, and other cardiometabolic disturbances as supported by Akinci et al's. study that illustrated a significant association of apelin serum levels with cardiometabolic risk factors in gestational DM.[28]
In addition, apelin serum levels are significantly correlated with most of the cardiometabolic risk factors, serum insulin, and HOMA-IR, but they are negatively correlated with HbA1c; these findings might explain the compensatory elevation of apelin levels to overcome these disturbances. The negative association with HbA1c could indicate that apelin plays an important potential role in the glycemic balance.[29] Nearly 46% of the recruited patients are associated with IHD; it has been documented that apelin serum levels are reduced in IHD;[30] thus, elevated apelin serum levels in the current study are mainly predicted by the effect of T2DM than the effect of IHD.
In the current study, 44% of the recruited patients were associated with peripheral diabetic neuropathy which may explain high apelin serum levels since Bilir et al's. study confirmed that apelin levels were high in patients with diabetic neuropathy compared to the diabetic patients not associated with diabetic neuropathy; this elevation in apelin levels might be due to the microangiopathic complications.[31]
Regarding the effect of diabetic pharmacotherapy on apelin serum levels in patients with T2DM, metformin and/or glyburide significantly elevates apelin serum levels compared to the controls, but there were insignificant differences between the treated groups. This finding was supported by Fan et al's. study that illustrated the significant effect of metformin in the elevation of apelin serum levels compared to the baseline value due to activation of apelin secretion though 5 monophosphate-activated protein kinase activation.[11] In contrast, a study showed that metformin may play an important role in the reduction of apelin serum levels in women with polycystic ovarian syndrome.[32]
The apelin serum levels were high in patients with T2DM treated with glyburide, but no previous studies have documented these effects, since Araki et al's. study confirmed that glimepiride significantly raised adiponectin serum levels.[33] Besides, gliclazide improves adiponectin serum levels in patients with T2DM;[34] thus, glyburide might increase apelin serum levels through elevation of adiponectin, which is positively correlated with apelin serum levels. A study by Alexiadou et al. showed that exogenous insulin administration does not change the apelin serum levels in T2DM;[35] therefore, the positive effect of glyburide on apelin serum levels may not be mediated through provoking insulin secretion; unfortunately, adiponectin serum levels were not assessed in the present study.
Regarding other pharmacotherapy treatments received by patients with T2DM, 62% of the patients were on statins which may increase apelin levels as observed in a study by Kadoglou et al. that observed significant effect of atorvastatin in the elevation of apelin serum levels in patients with T2DM.[36]
The present study did not observe any gender differences in apelin serum levels in patients with T2DM, since all the recruited women in this study were at postmenopausal period as supported by Karakoc et al's. study that illustrated insignificant effect of gender in relation to apelin serum levels in patients with T2DM.[37]
Limitations
Limitations of the present study were small sample size, being single-center study, and not involved patients with a newly diagnosed T2DM. Furthermore, HOMA score was used in the present study (which is less accurate method) instead of euglycemic hyperinsulinemic clamp method (which is more accurate method). Indeed, it is extremely difficult to mention normal standard level of apelin due to the presence of different forms (apelin 12, 13, 18, and 36) in the circulation which may lead to cross-reactivity. Therefore, larger -scale studies are recommended to illustrate the effect of diabetic pharmacotherapy on specific apelin serum levels.
CONCLUSION
Apelin level was high in T2DM patients compared to normal healthy controls; both metformin and glyburide might play a role in this elevation.
Ethics committee approval
Ethics committee approval was established for this clinical study from the College of Medicine, Al-Mustansiriyia University (Decision Date/No: 02-01-2016/22).
Informed consent
Written informed consent was gained from all patients and healthy controls in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Alkuraishy HM, Hamada MT, Al-Samerraie AY. Effects of metformin on omentin levels in a newly diagnosed type II diabetes mellitus: Randomized, placebo controlled study. Mustansiriya Med J. 2016;15:49–55. [Google Scholar]
- 2.Alkuraishy HM, Al-Gareeb AI. New insights into the role of metformin effects on serum omentin-1 levels in acute myocardial infarction: Cross-sectional study. Emerg Med Int 2015. 2015:283021. doi: 10.1155/2015/283021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Kuraishy HM, Al-Gareeb AI. Effects of rosuvastatin alone or in combination with omega-3 fatty acid on adiponectin levels and cardiometabolic profile. J Basic Clin Pharm. 2016;8:8–14. doi: 10.4103/0976-0105.195080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Kuraishy HM, Al-Gareeb AI. Effect of orlistat alone or in combination with Garcinia cambogia on visceral adiposity index in obese patients. J Intercult Ethnopharmacol. 2016;5:408–14. doi: 10.5455/jice.20160815080732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen T, Liu N, Xu GM, Liu TJ, Liu Y, Zhou Y, et al. Apelin13/APJ promotes proliferation of colon carcinoma by activating notch3 signaling pathway. Oncotarget. 2017;8:101697–706. doi: 10.18632/oncotarget.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaganathan R, Ravindran R, Dhanasekaran S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can J Diabetes. 2017 doi: 10.1016/j.jcjd.2017.10.040. pii: S1499-2671(17)30334-9. [DOI] [PubMed] [Google Scholar]
- 7.Hwangbo C, Wu J, Papangeli I, Adachi T, Sharma B, Park S, et al. Endothelial APLNR regulates tissue fatty acid uptake and is essential for Apelin's glucose-lowering effects. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aad4000. pii: eaad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuzbashian E, Zarkesh M, Asghari G, Hedayati M, Safarian M, Mirmiran P, et al. Is apelin gene expression and concentration affected by dietary intakes? A systematic review. Crit Rev Food Sci Nutr. 2018;58:680–8. doi: 10.1080/10408398.2016.1262325. [DOI] [PubMed] [Google Scholar]
- 9.Yu S, Zhang Y, Li MZ, Xu H, Wang Q, Song J, et al. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J (Engl) 2012;125:3440–4. [PubMed] [Google Scholar]
- 10.Nagib AM, El-Diasty A, El Husseny MA, El-Gamal EM, Abbas MH, Refaie AF, et al. Apelin and new-onset diabetes after transplant in living kidney allograft recipients. Exp Clin Transplant. 2015;13:319–23. [PubMed] [Google Scholar]
- 11.Fan Y, Zhang Y, Li X, Zheng H, Song Y, Zhang N, et al. Treatment with metformin and a dipeptidyl peptidase-4 inhibitor elevates apelin levels in patients with type 2 diabetes mellitus. Drug Des Devel Ther. 2015;9:4679–83. doi: 10.2147/DDDT.S85740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hickner RC, Brunson MA, McCammon M, Mahar MT, Garry JP, Houmard JA, et al. Diabetic groups as defined by ADA and NDDG criteria have a similar aerobic capacity, blood pressure and body composition. American Diabetes Association and National Diabetes Data Group. Diabetologia. 2001;44:26–32. doi: 10.1007/s001250051576. [DOI] [PubMed] [Google Scholar]
- 13.Wu CZ, Lin JD, Hsia TL, Hsu CH, Hsieh CH, Chang JB, et al. Accurate method to estimate insulin resistance from multiple regression models using data of metabolic syndrome and oral glucose tolerance test. J Diabetes Investig. 2014;5:290–6. doi: 10.1111/jdi.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosi F, Bonora E, Moghetti P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: A comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod. 2017;32:2515–21. doi: 10.1093/humrep/dex308. [DOI] [PubMed] [Google Scholar]
- 15.Jialal I, Inn M, Siegel D, Devaraj S. Underestimation of low density lipoprotein-cholesterol with the friedewald equation versus a direct homogenous low density lipoprotein-cholesterol assay. Lab Med. 2017;48:220–4. doi: 10.1093/labmed/lmx023. [DOI] [PubMed] [Google Scholar]
- 16.Cheraghi M, Shahsavari G, Maleki A, Ahmadvand H. Paraoxonase 1 activity, lipid profile, and atherogenic indexes status in coronary heart disease. Rep Biochem Mol Biol. 2017;6:1–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Kuraishy HM, Al-Gareeb AI. Acylation-stimulating protein is a surrogate biomarker for acute myocardial infarction: Role of statins. J Lab Physicians. 2017;9:163–9. doi: 10.4103/0974-2727.208263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attané C, Foussal C, Le Gonidec S, Benani A, Daviaud D, Wanecq E, et al. Apelin treatment increases complete fatty acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes. 2012;61:310–20. doi: 10.2337/db11-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo M, Chen F, Lin T, Peng Y, Li W, Zhu X, et al. Apelin-13 decreases lipid storage in hypertrophic adipocytes in vitro through the upregulation of AQP7 expression by the PI3K signaling pathway. Med Sci Monit. 2014;20:1345–52. doi: 10.12659/MSM.890124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dray C, Debard C, Jager J, Disse E, Daviaud D, Martin P, et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am J Physiol Endocrinol Metab. 2010;298:E1161–9. doi: 10.1152/ajpendo.00598.2009. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Shen C, Li X, Ren G, Fan X, Ren F, et al. Low plasma apelin in newly diagnosed type 2 diabetes in Chinese people. Diabetes Care. 2009;32:e150. doi: 10.2337/dc09-1146. [DOI] [PubMed] [Google Scholar]
- 22.Telejko B, Kuzmicki M, Wawrusiewicz-Kurylonek N, Szamatowicz J, Nikolajuk A, Zonenberg A, et al. Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Res Clin Pract. 2010;87:176–83. doi: 10.1016/j.diabres.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 23.Ba HJ, Chen HS, Su Z, Du ML, Chen QL, Li YH, et al. Associations between serum apelin-12 levels and obesity-related markers in Chinese children. PLoS One. 2014;9:e86577. doi: 10.1371/journal.pone.0086577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khajeh E, Panahi N, Golpaie A, Shirvani SH, Afarideh M, Ghamarnejad O, et al. Plasma apelin and asymmetric dimethylarginine (ADMA) levels shortly after laparoscopic greater curvature plication. Obes Surg. 2017;27:1596–603. doi: 10.1007/s11695-016-2509-1. [DOI] [PubMed] [Google Scholar]
- 25.Cavallo MG, Sentinelli F, Barchetta I, Costantino C, Incani M, Perra L, et al. Altered glucose homeostasis is associated with increased serum apelin levels in type 2 diabetes mellitus. PLoS One. 2012;7:e51236. doi: 10.1371/journal.pone.0051236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Habchi M, Duvillard L, Cottet V, Brindisi MC, Bouillet B, Beacco M, et al. Circulating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic control. Clin Endocrinol (Oxf) 2014;81:696–701. doi: 10.1111/cen.12404. [DOI] [PubMed] [Google Scholar]
- 27.Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, et al. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E59–67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akinci B, Celtik A, Tunali S, Genc S, Yuksel F, Secil M, et al. Circulating apelin levels are associated with cardiometabolic risk factors in women with previous gestational diabetes. Arch Gynecol Obstet. 2014;289:787–93. doi: 10.1007/s00404-013-3070-y. [DOI] [PubMed] [Google Scholar]
- 29.Hashemi M, Rezaei H, Eskandari-Nasab E, Kaykhaei MA, Taheri M. Association between the apelin rs2235306 gene polymorphism and metabolic syndrome. Turk J Med Sci. 2014;44:775–80. doi: 10.3906/sag-1210-44. [DOI] [PubMed] [Google Scholar]
- 30.Kadoglou NP, Lampropoulos S, Kapelouzou A, Gkontopoulos A, Theofilogiannakos EK, Fotiadis G, et al. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease – KOZANI STUDY. Transl Res. 2010;155:238–46. doi: 10.1016/j.trsl.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Bilir B, Ekiz Bilir B, Yilmaz I, Soysal Atile N, Yildirim T, Kara SP, et al. Association of apelin, endoglin and endocan with diabetic peripheral neuropathy in type 2 diabetic patients. Eur Rev Med Pharmacol Sci. 2016;20:892–8. [PubMed] [Google Scholar]
- 32.Sun X, Wu X, Zhou Y, Yu X, Zhang W. Evaluation of apelin and insulin resistance in patients with PCOS and therapeutic effect of drospirenone-ethinylestradiol plus metformin. Med Sci Monit. 2015;21:2547–52. doi: 10.12659/MSM.894926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Araki T, Emoto M, Konishi T, Ikuno Y, Lee E, Teramura M, et al. Glimepiride increases high-density lipoprotein cholesterol via increasing adiponectin levels in type 2 diabetes mellitus. Metabolism. 2009;58:143–8. doi: 10.1016/j.metabol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Drzewoski J, Zurawska-Klis M. Effect of gliclazide modified release on adiponectin, interleukin-6, and tumor necrosis factor-alpha plasma levels in individuals with type 2 diabetes mellitus. Curr Med Res Opin. 2006;22:1921–6. doi: 10.1185/030079906X132424. [DOI] [PubMed] [Google Scholar]
- 35.Alexiadou K, Kokkinos A, Liatis S, Perrea D, Katsilambros N, Tentolouris N, et al. Differences in plasma apelin and visfatin levels between patients with type 1 diabetes mellitus and healthy subjects and response after acute hyperglycemia and insulin administration. Hormones (Athens) 2012;11:444–50. doi: 10.14310/horm.2002.1376. [DOI] [PubMed] [Google Scholar]
- 36.Kadoglou NP, Sailer N, Kapelouzou A, Lampropoulos S, Vitta I, Kostakis A, et al. Effects of atorvastatin on apelin, visfatin (nampt), ghrelin and early carotid atherosclerosis in patients with type 2 diabetes. Acta Diabetol. 2012;49:269–76. doi: 10.1007/s00592-011-0310-0. [DOI] [PubMed] [Google Scholar]
- 37.Karakoç A, Sahin A, Polat ES, Aliyev E, Yildirim A, Bakan N, et al. Serum apelin and ADMA levels in type 2 diabetics with and without vascular complications. Diabetes Metab Syndr. 2016;10:S106–9. doi: 10.1016/j.dsx.2016.03.005. [DOI] [PubMed] [Google Scholar]