Abstract
Introduction:
Children with posterior urethral valve (PUV) may develop urinary bladder (UB) dysfunction even after valve fulguration (VF). Using Urodynamics (UDS), we sought to identify whether age at VF and time elapsed since VF contributed to UB dysfunction.
Materials and Methods:
Between January 2009 and July 2016, 39 PUV patients referred to a tertiary care center for UDS were classified into Groups A and B (based on age if <2 or ≥2 years at VF) and subclassified into Group A1/A2 and B1/B2 depending on time duration after VF (TVU). A1 and B1 constituted TVU ≤4 years and A2 and B2 constituted TVU >4 years, respectively.
Results:
Median (range) ages at VF and UDS were 18 (1–108) months and 9 (1–19) years. Median (range) time between VF and UDS was 60 (6–164) months. Reduced compliance was seen in 67%, detrusor overactivity in 38.5%, and leak in 15.4% boys, respectively. Median (range) Qmax was 8 (0–28) ml/s and 25% boys had hypocontractile detrusor at voiding. Statistically significant reduction was found in compliance when comparing Group B versus Group A (P = 0.037) and in bladder capacity (P = 0.002) and compliance (P = 0.043) in Group A2 versus A1.
Conclusions:
Boys with VF at <2 years had better urodynamic profiles than those with fulguration over 2 years of age. As the time period since fulguration increased, there was a higher incidence of bladder dysfunction in both the groups.
INTRODUCTION
Posterior urethral valve (PUV) is the most common cause of lower urinary tract obstruction in male infants with an incidence of 1:5000–8000 male infants.[1] In a simplistic scenario, valve fulguration (VF) should cure the disease, but this does not necessarily happen, and children continue to have persistent problems with long-term morbidity including end-stage renal disease. While there is no control over developmental renal dysplasia, a better understanding of bladder behavior and its appropriate management can go a long way in maximizing long-term outcome in PUV patients, which centers on maintaining bladder function. We sought to study the urodynamic abnormalities in boys with PUV following fulguration to identify whether age at VF and time elapsed since fulguration is related to the development of urinary bladder (UB) dysfunction using urodynamic studies (UDS).
MATERIALS AND METHODS
This was a retrospective review of a UDS database from a tertiary care referral urology center in South India between January 2009 and July 2016. Out of the 260 pediatric (<18 years) UDS performed during this time frame, 49 boys had undergone VF for PUV. Baseline clinical, demographic, and urodynamic parameters were studied. The first UDS done following VF was taken for analysis. We excluded boys on bladder-directed management such as clean intermittent self-catheterization with or without nighttime continuous bladder drainage, recurrent VF, or other surgical procedures for PUV. Thirty-nine boys were considered for final analysis.
Brief procedure
UDS was done in a dedicated UDS suite using Andromeda Ellipse unit (Andromeda Medizinsche System, GmbH, Munich, Germany). A free flow uroflowmetry was performed before the UDS. Children <5 years of age were anesthetized for placement of urethral and rectal catheters for the purpose of vesical and abdominal pressure measurement. A 5F double lumen urethral catheter for bladder filling and vesical pressure measurement was placed after anesthetizing the urethra with 2% lignocaine jelly. A 5F open-ended catheter covered with balloon was used as abdominal transducer. A standard filling cystometrogram was performed after adequate zeroing of transducers. Bladder capacity (BC) was calculated based on standard formulae. For children up to 2 years, expected BC (EBC) (ml) was calculated by 38+ (2.5 × age in months).[2] In children older than 2 years, EBC (ml) was calculated by (age in years + 2) × 30.[3] Bladder filling was initiated at the slowest possible pump speed with 0.9% normal saline at 37°C based on age and EBC. Roughly, it was at one-tenth of the volume calculated. The minimum speed was 5 ml/s. When it exceeded 10 ml/, the speed was kept at 10 ml/s. For poor compliance during the initial filling phase, it was kept at 5 ml/s. Filling parameters assessed included bladder sensation, compliance, capacity, detrusor overactivity (DO), and leak on filling cystometrogram based on International Continence Society terminology.[4] During voiding phase, voided volume, maximal flow rate (Qmax), Detrusor pressure (Pdet) at Qmax (PdetQmax), abdominal straining at micturition, and detrusor hypocontractility were noted. Postvoid residual urine (RU) was measured with ultrasound. Maximal BC was calculated as the sum of voided volume and postvoid RU and compared with EBC.[2,3] There was no definite cutoff for reduced compliance. We carefully observed if the Pdet line rose during filling phase and filling was immediately stopped and restarted at a lower speed after Pdet reached baseline. When change in volume with Pdet was >30 cm/ml H2O, it was considered as poor compliance. All urodynamic tracings were reported by the senior author with two decades of experience in performing pediatric urodynamics and interpreting urodynamic tracings. Whenever children voided with bladder volume below 100 ml or due to poor compliance or DO, a second fill cycle was initiated at 5 ml/min.
Clinical data and complications
Clinical data assessed were age at VF, age at UDS, complications of PUV such as upper urinary tract changes (UUTCs), chronic kidney disease (CKD), and valve bladder syndrome (VBS). The estimated glomerular filtration rate (eGFR) was calculated using the formula, GFR = k × length (cm) × 88.5/serum creatinine (μmol/l), where k is an age and sex-specific constant (k = 0.33 for low birth weight infants; k = 0.45 for infants <1 year; k = 0.55 for children ≤12 years; k = 0.70 for boys >12 years of age).[5] CKD was defined as a sustained fall in eGFR to <60 ml/min/1.73 m2 for at least 3 months.[6] UUTCs were considered when hydroureteronephrosis and vesicoureteral reflux existed. VBS was defined as intrinsic UB dysfunction leading to high bladder volumes and poor compliance which prevents adequate drainage of UUT.[7] Incontinence was defined as involuntary loss of urine.[4] Altered BC was calculated based on percentage of EBC. Compliance was monitored throughout the filling phase. It was based on the change in bladder volume and detrusor pressures at the start of bladder filling and at the point before which detrusor contraction leading to significant leak or cystometric capacity is reached, whichever occurred earlier. DO was defined as involuntary detrusor contractions occurring during the filling phase.[4]
As we sought to identify if age at VF and time duration between VF and UDS (TVU) is related to UB dysfunction in PUV, we divided our patients into Groups A (<2 years) and Group B (≥2 years) based on age at VF. These groups were subdivided further into A1 and A2 and B1 and B2 based on TVU. While Groups A1 and B1 had an UDS done within 4 years of VF, the A2 and B2 groups had an UDS done after 4 years of VF. This classification was based on the median age at VF and TVU.
Statistical analysis
Statistical analysis was performed using SPSS version 20 (IBM Corp., Armonk, NY, USA) for Windows. The normality of the data was assessed using a Stem and Leaf Plot. Variables were summarized using mean with standard deviation, median with interquartile range, and percentages based on the characteristics of the variable. Independent samples t-test or Mann–Whitney U-test as appropriate was used. Chi-Square test was used for categorical variables. A P < 0.05 was considered statistically significant.
RESULTS
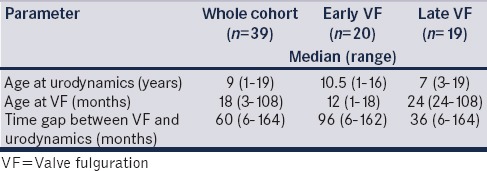
The baseline characteristics are depicted in Table 1. The median (range) TVU was 60 (6–144) months. Clinical presentation included incontinence (13; 33.3%), enuresis (9; 23%), recurrent urinary tract infections (5; 12.8%), and deranged renal parameters (8; 20.5%). Thirteen (33.3%) children had a poor urinary stream. Group A (early VF) had 20 (51.3%) patients and Group B (late VF) had 19 (48.7%) patients. Group A1 had 8 (20.5%), A2 had 12 (30.8%), B1 had 11 (28.2%), and B2 had 8 (20.5%) patients.
Table 1.
Baseline characteristics
Urodynamic study abnormalities and complications
Reduced compliance was seen in 20 (51.3%), reduced bladder sensation in 2 (5.13%), DO in 15 (38.46%), leak in 6 (15.4%), hypocontractile detrusor in 9 (23.1%), and acontractile detrusor in 6 (15.4%) children. Complications included CKD (8; 20.5%), UUTC (17; 43.6%), and VBS (5; 12.8%).
Early versus late valve fulguration (Group A vs. B)
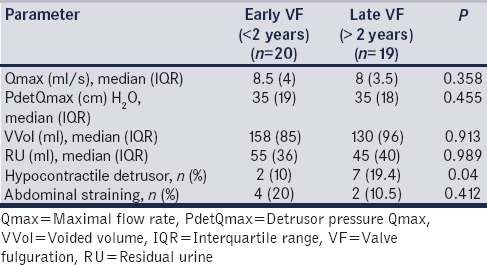
Reduced compliance (35% vs. 68.4%, P = 0.037), DO (25% vs. 52.6%; P = 0.043), and hypocontractile detrusor (10% vs. 19.4%; P = 0.04) had a higher incidence after late VF [Tables 2 and 3]. The estimated BC was similar in both groups of children [Table 2]. UUTC was more common in late VF (25% vs. 63%; P = 0.048).
Table 2.
Filling parameters: Comparison between early versus late valve fulguration
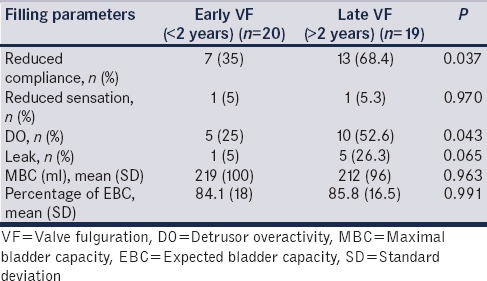
Table 3.
Voiding parameters: Comparison between early versus late valve fulguration
Early valve fulguration-TVU <4 years versus >4 years (Group A1 vs. A2)
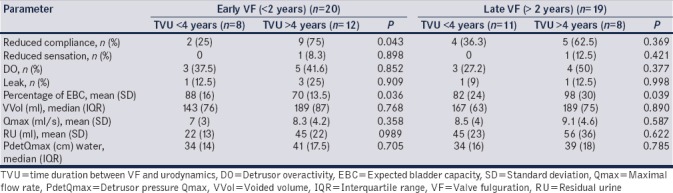
Among early VF, compliance (12.5% vs. 91.6%, P = 0.002) and UB capacity (25% vs. 75%, P = 0.043) were significantly abnormal in TVU > 4 years. They had a reduced EBC for age (88% vs. 70%; P = 0.036) [Table 4] and all children with CKD in early VF were TVU > 4 years (none vs. 41.6%; P = 0.03).
Table 4.
Early valve fulguration-comparison based on time duration between VF and urodynamics - <4 years versus >4 years
Late valve fulguration-TVU <4 years versus >4 years (Group B1 vs. B2)
Among late VF, 4 (50%) children who were TVU > 4 years of VF (B2) had large capacity bladder versus 9% in B1 (P = 0.05), larger percentage EBC (98% in B2 vs. 82% in B1; P = 0.039) [Table 4], and UUTC (7/8, 87.5% in B2 vs. 3/7, 42.8% in B1; P = 0.04).
SUMMARY: POOR URODYNAMIC STUDY OUTCOMES BASED ON THE TIME AT VALVE FULGURATION AND TVU
Late VF (VF >2 years) had an overall higher incidence of poor compliance, DO, hypocontractile detrusor, and UUTC. In early VF (VF <2 years), when TVU was >4 years (UDS done beyond 4 years after VF), children had a higher rate of poorer compliance and CKD. In late VF, higher rates of overdistended bladder and UUTC were seen in patients in TVU >4 years.
DISCUSSION
Various studies have suggested that proactive UDS-directed bladder management might improve long-term bladder outcomes. UDS is necessary to track changes and alter management in the first two decades.[7] UDS provides a useful tool to test the efficacy of treatment as well as determine any refinements in ongoing treatment.[5] We observed similar patterns with respect to capacity, compliance, and detrusor function in PUV patients post-VF as observed in previously published studies.[8,9,10,11] Altered capacity was seen in 48%–81% of patients in other studies, while we observed a 67% incidence. Altered compliance was seen in 36%–65% of patients, respectively, while we observed it in 52% of our patient population.
Detrusor abnormalities
DO was comparable to that observed by Emir et al. (38%).[10] Hypocontractile detrusor was seen in 36% in Lal et al. study, while we observed a 23% incidence.[9] In boys younger than 8 years of age, we observed a 50% incidence of DO comparable to 67% by Holmdahl et al. and 44% by De Gennaro et al.[11,12] Similarly, hypocontractile detrusor was seen in 20% below 8 years of age as against 31% observed by De Gennaro et al.[12] Beyond 8 years, hypocontactility incidence was 33%, while De Gennaro et al. observed it to be 50%.[12] The BC at puberty was twice in Holmdahl et al. study, while our population had a 1.2 times increased capacity.[11] These data suggest that the detrusor decompensates with time leading to poor tone and hypocontractility.
Complications
CKD was noticed in 17%–30% across previous studies and we observed it in 20% of our population. UUTC was seen in 45%–50% in other studies while our incidence of the same to be 43%.[11,12]
Age at valve fulguration
Youssif et al. have compared the clinical and urodynamic outcomes of neonatal VF and compared them with those who underwent fulguration after the age of 1 year.[13] They found that there was better resolution of hydroureteronephrosis and bladder function on UDS in the group with neonatal VF. We also found similar clinical and urodynamic outcomes in the group with early VF. Although our follow-up period was longer, we had no cases of neonatal VF. Our study group is unique in that most of our patients underwent VF later as compared to other published series.[11,12,13] This gives an insight into the lack of early detection and intervention and reflects the delayed referral and management as is commonly seen in developing countries. In addition, it also gives us a chance to study whether acceptable outcomes are achieved with later VF and also to study the natural history of children with PUV.
Bladder-directed management
It is important to recognize that management of bladder dysfunction is the only modifiable factor to avoid renal failure.[13] The importance of bladder dysfunction in patients with PUV suggests the possible role of UDS evaluation yielding a higher incidence of abnormal bladder more so when there are clinical symptoms.[14] It is also prudent to understand that normal urodynamic findings in the PUV patients do not rule out renal deterioration. Progressive renal tubular damage during childhood leads to inability to conserve sodium and free water and can lead to nephrogenic diabetes with large urine volumes. These exaggerated urine volumes can overwhelm the functional ability of the bladder, especially in one with limited functional potential.[15]
Limitations
This is a retrospective analysis. None of the patients underwent VF in the neonatal age group. This reflects the referral pattern, lack of antenatal diagnosis, and frequent late diagnosis in the patient strata referred to us. As this is from a UDS database with multihospital referral pattern and was mostly for symptomatic patients, this cohort represents only a subset of patients who underwent VF and not all patients who underwent VF. As the baseline clinical renal function was not retrievable for all children, this study could not identify the implications of urodynamic outcomes of PUV boys in renal functional outcomes. Furthermore, the division of patients into early and late fulguration with 2 years as cutoff point has no precedent but enabled equitable distribution of patients for analysis and was also the point at which we saw statistically differing outcomes.
CONCLUSIONS
Boys with VF at <2 years have better urodynamic profiles than those with fulguration over 2 years of age. As the time period since fulguration increases, there is a higher incidence of bladder and renal dysfunction irrespective of age at fulguration.
Footnotes
Financial support and sponsorship: Nil.
Conflicts of interest: There are no conflicts of interest.
REFERENCES
- 1.Kumar S, Fisk NM. Distal urinary obstruction. Clin Perinatol. 2003;30:507–19. doi: 10.1016/s0095-5108(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 2.Holmdahl G, Hanson E, Hanson M, Hellström AL, Hjälmås K, Sillén U, et al. Four-hour voiding observation in healthy infants. J Urol. 1996;156:1809–12. [PubMed] [Google Scholar]
- 3.Koff SA. Estimating bladder capacity in children. Urology. 1983;21:248. doi: 10.1016/0090-4295(83)90079-1. [DOI] [PubMed] [Google Scholar]
- 4.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the international continence society. Neurourol Urodyn. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GJ, Furth SL. Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol. 2007;22:1839–48. doi: 10.1007/s00467-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 6.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, et al. National kidney foundation's kidney disease outcomes quality initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics. 2003;111:1416–21. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 7.Glassberg KI. The valve bladder syndrome: 20 years later. J Urol. 2001;166:1406–14. [PubMed] [Google Scholar]
- 8.Wen JG, Li Y, Wang QW. Urodynamic investigation of valve bladder syndrome in children. J Pediatr Urol. 2007;3:118–21. doi: 10.1016/j.jpurol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Lal R, Bhatnagar V, Agarwala S, Grover VP, Mitra DK. Urodynamic evaluation in boys treated for posterior urethral valves. Pediatr Surg Int. 1999;15:358–62. doi: 10.1007/s003830050599. [DOI] [PubMed] [Google Scholar]
- 10.Emir H, Eroğlu E, Tekant G, Büyükünal C, Danişmend N, Söylet Y, et al. Urodynamic findings of posterior urethral valve patients. Eur J Pediatr Surg. 2002;12:38–41. doi: 10.1055/s-2002-25093. [DOI] [PubMed] [Google Scholar]
- 11.Holmdahl G, Sillén U, Hanson E, Hermansson G, Hjälmås K. Bladder dysfunction in boys with posterior urethral valves before and after puberty. J Urol. 1996;155:694–8. [PubMed] [Google Scholar]
- 12.De Gennaro M, Mosiello G, Capitanucci ML, Silveri M, Capozza N, Caione P, et al. Early detection of bladder dysfunction following posterior urethral valves ablation. Eur J Pediatr Surg. 1996;6:163–5. doi: 10.1055/s-2008-1066497. [DOI] [PubMed] [Google Scholar]
- 13.Youssif M, Dawood W, Shabaan S, Mokhless I, Hanno A. Early valve ablation can decrease the incidence of bladder dysfunction in boys with posterior urethral valves. J Urol. 2009;182:1765–8. doi: 10.1016/j.juro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Hale JM, Wood DN, Hoh IM, Neild GH, Bomanji JB, Chu A, et al. Stabilization of renal deterioration caused by bladder volume dependent obstruction. J Urol. 2009;182:1973–7. doi: 10.1016/j.juro.2009.05.104. [DOI] [PubMed] [Google Scholar]
- 15.Woodhouse CR. Adolescent urology: A challenge for adult urologists. Indian J Urol. 2007;23:340–6. doi: 10.4103/0970-1591.35049. [DOI] [PMC free article] [PubMed] [Google Scholar]


