Abstract
Aims
To understand the paradox of an increased fracture risk despite increased bone mineral density (BMD) in persons with type 2 diabetes (DM2).
Patients and Methods
We studied 80 old persons with DM2. Mineral metabolism, parathyroid hormone (PTH), 25-hydroxyvitamin D (25OHD), bone turnover – osteocalcin, procollagen type 1 N-terminal propeptide (P1NP) and C-terminal telopeptide of type 1 collagen (CTX) – were measured and BMD was assessed at the lumbar spine (LS) and femoral neck (FN). Data was analyzed with the Statistical Package for the Social Sciences Program.
Results
Low levels of 25OHD (84%) and high values of PTH (20%) were found. Osteocalcin was directly related to CTX, p < 0.001, with increased bone formation and increased BMD (z-score) at LS and FN. PTH was directly related to osteocalcin and CTX and inversely related to BMD at the FN, p < 0.05. Patients with dyslipidemia presented higher P1NP, p < 0.05 and patients with hypertension presented higher BMD at LS and FN, p < 0.01.
Conclusion
Old type 2 diabetics present increased bone formation, PTH-driven. Low grade secondary hyperparathyroidism may explain the paradox of an increased fracture risk despite increased BMD.
Keywords: Diabetes Mellitus, Old age, Bone mineral density, Bone turnover, Parathyroid hormone
Introduction
Vertebral fractures typically occur 15–20 years after menopause, in relation to everyday routine tasks and may result in chronic back pain, height loss, kyphosis and reduced lung capacity. They double the risk for femoral fractures. Hip fractures occur later in the 75–79 years age group, in relation to falls, and generally result in definitive major motility limitations including institutionalization, with marked mortality (15–25%) within the first year [1], [2].
The social impact is enormous, including the economic costs, estimated in Europe at about 3–4% of total health costs [1], [2].
Diabetes Mellitus presents an increasing prevalence with aging (>25% after age 65) when osteoporosis becomes more common [3]. The relation between osteoporosis and diabetes may go deeper than their coincidence in the older age groups; insulin and adipose tissue hormones like leptin and adiponectin, deeply affect bone remodeling while osteoblast and osteoclast hormones like osteocalcin and sclerostin modulate insulin resistance and insulin secretion; a basic relation between bone mass, energy metabolism and fertility is emerging [4].
Diabetes Mellitus is a chronic systemic disease with widespread biochemical abnormalities like protein glycation and oxidative stress, prevalent micro- and macrovascular disease and with long term use of multiple drugs – insulin and oral or parenteric hypoglycemic agents, anti-hypertensive medications, hypolipidemic drugs and anti-platelet agents. All these factors may adversely affect mineral metabolism, bone turnover, bone density, microarchitecture and resilience [5], [6]. Furthermore persons with diabetes may be at an increased risk of falls and everyday trauma, because of hypoglycemia, diabetic retinopathy, autonomic and peripheral neuropathy [3], [5].
We and others have previously shown that persons with diabetes commonly present mineral metabolism abnormalities including hyper- and hypocalcemia (mainly due to insulin or diuretic use) and secondary hyperparathyroidism due both to diabetic nephropathy and to deficiency/insufficient vitamin D. Low 25-hydroxyvitamin D (25OHD) is almost universal in persons with diabetes and is related to metabolic control and to diabetic nephropathy. Serum parathyroid hormone (PTH) but not 25OHD was related to serum calcium, phosphate and magnesium and higher PTH levels were found in persons with diabetes and micro- or macrovascular disease [7], [8].
Assessment of Bone Mineral Density (BMD) and fracture risk is therefore relevant in diabetic care [9]. However while BMD is generally reported to be decreased in persons with type 1 diabetes, it is generally increased in the much larger group of those with type 2 diabetes; type 1 but not type 2 diabetes is now considered a form of secondary osteoporosis [10], [11], [12]. Despite this an increased fracture risk has been consistently reported in persons with both type 1 and type 2 diabetes, suggesting decreased bone quality, although the responsible mechanisms remain speculative [10], [11], [12]. The situation is indeed similar at several levels to that of patients with chronic kidney disease (CKD) where BMD assessment is not routinely recommended since it not predictive of fracture risk or to senile osteoporosis where the fracture risk is greater than would be predicted from BMD data [13], [14].
We evaluated mineral metabolism, bone turnover and bone mineral density in old persons with type 2 diabetes and related these to common clinical parameters of diabetes, like time since diagnosis, metabolic control, the presence or absence of micro- and macrovascular disease and classes of drugs used. We hoped this might shed light regarding the relevance of the problem and the mechanisms of bone disease in diabetes mellitus, namely in regard to previously documented low levels of 25OHD and increased serum PTH levels.
Subjects and methods
We included in this study all persons older than 65 years, with type 2 diabetes assisted by one of us, at a tertiary public center.
Standard clinical care [15] was provided and the following clinical indexes were retrieved: sex and actual age, years since diagnosis, height and actual weight (body mass index was computed accordingly [weight(kg)/height(m)2, BMI]), last available glycated hemoglobin (HbA1c) and serum C-peptide levels; microvascular disease as defined: retinopathy – no retinopathy, retinopathy without laser therapy and retinopathy with previous laser therapy (grouped when indicated as retinopathy yes or no); nephropathy – no nephropathy, microalbuminuria > 30 mg/24 h and renal failure with serum creatinine > 1.5 mg/dL (grouped when indicated as nephropathy yes or no); peripheral neuropathy – yes or no on clinical questioning. Hypertension (yes/no) and dyslipidemia (yes/no); drugs being used for diabetes mellitus, hypertension, dyslipidemia and anti-platelet agents; drugs or supplements (D&S) that include anti-osteoporotic agents or calcium or vitamin D.
Serum calcium, phosphate, magnesium, parathyroid hormone (PTH), 25-hidroxyvitamin D (25OHD), osteocalcin, procollagen type 1 N-terminal propeptide (P1NP) and C-terminal telopeptide of type 1 collagen (CTX) and C-peptide were obtained at the last appointment in a venous sample drawn in the morning with no fasting required.
Analytical measurements were performed at the Chemical Pathology Department of the hospital using standardized commercially available methods – chemical colorimetric methods for serum calcium, phosphate and magnesium – and chemiluminescence immunoassay (CLIA) methods for serum parathyroid, osteocalcin, P1NP, CTX and C-peptide (Diagnostics Product Corporation, Los Angeles, California) or radioimmunoassay (RIA) methods for 25-hydroxyvitamin D-25-Hydroxyvitamin D (Dia Sorin Inc., Stillwater). Glycated hemoglobin (HbA1c) was measured using HPLC. Intra- and interassay variation coefficients were always below 10%. Units and reference values are presented in Table 1. Reference values were obtained for general population 20–79 years unless otherwise indicated and are periodically updated to support clinical decisions.
Table 1.
Clinical characteristics of the patients.
| mean ± sd | % | |
|---|---|---|
| Sex Female/Male | 46/54 | |
| Age (years) | 73 ± 7 | |
| Years since diagnosis | 21 ± 11 | |
| BMI (kg/m2) | 28.8 ± 4.6 | |
| IT/OT | 63/37 | |
| Last HbA1c (%) [*RV-4.0-6.0] | 7.8 ± 1.6 | |
| C-Peptide (ng/mL) [RV-1.1-5.0] | 1.1–5.0 | |
| Retinopathy (1/2/3) | 53/15/32 | |
| Nephropathy (1/2/3) | 57/28/15 | |
| Peripheral neuropathy (1/2) | 52/48 | |
| Hypertension (1/2) | 8/92 | |
| Dyslipidemia (1/2) | 29/71 | |
| IHD (1/2/3) | 85/7/9 | |
| PVD (1/2/3) | 84/15/1 | |
| Anti-hypertensive drugs (1/2) | 7/93 | |
| Hypolipidemic drugs (1/2) | 31/69 | |
| Anti-platelet drugs (1/2) | 18/82 | |
| D&S (1/2) | 90/10 |
RV – Reference values; for IFCC (mmol/mol) [RV-20-42] 62 ± 13.
BMD was assessed by dual energy x-ray absorptiometry at the Rheumatology Department. Measurements (g/cm2) were obtained at the lumbar spine L1-L4 (LS) and femoral neck (FN) using the scanner Lunar ProdigyTM, GE Medical Systems and the Encore Software version 16.0 according to the manufacturer instructions. The coefficient of variation was below 2% at both sites.
Data was entered in a Statistical Package for the Social Sciences (IBM SPSS, 24th version) database and the same program was used for statistical analysis. Results are presented as the mean ± standard deviation or as percent as appropriate. The Kolmogorov-Smirnov test was used to verify the normal distribution of continuous variables and those not presenting that distribution were log transformed prior to analysis; for the sake of simplicity however, when no significant differences were found, results regarding non transformed variables are reported. Analysis used the chi-squared test or Anova with the Least Significant Difference (LSD) for pos hoc comparisons between defined variables, as appropriate, and multiple regression analysis for exploring the relation between continuous variables. The limit of significance is 0.05 [16].
Results
We included in this report 80 persons with type 2 Diabetes Mellitus aged 65 years or older assisted by one of us at the outpatient diabetic department of a public central hospital.
The general clinical characteristics of these patients are presented in Table 1. Patients are both sexes, old, with long standing diabetes and most are using insulin because of β cell failure. They are only overweight, not obese and present a fair metabolic control that is within the target recommended for this age group [15]. Almost half present microvascular disease – retinopathy, nephropathy or peripheral neuropathy. Most present risk factors for macrovascular disease – hypertension and dyslipidemia – although only a few have clinical evidence for macrovascular disease – ischemic heart disease, peripheral vascular disease or cerebrovascular disease. Most are using anti-hypertensive drugs and/or hypolipidemic drugs and/or anti-platelet agents but the use of drugs containing calcium, vitamin D or anti-osteoporotic agents was only marginal.
Parameters of mineral metabolism and bone turnover are presented in Table 2. Regarding calcium, phosphate and magnesium, only isolated and mild abnormalities were found - four cases of isolated mild hypercalcemia and two cases of isolated mild hypomagnesemia (6 out of 80 patients, 8%). Reasons for these abnormalities are not further explored since they were the subject of another report [7] and furthermore on the whole mean values are well within the reference range.
Table 2.
Parameters of Mineral Metabolism, Bone Turnover and Bone Mineral Density (BMD).
| Reference range | mean ± sd | |||
|---|---|---|---|---|
| Calcium (mg/dL) | 8.6–10.2 | 9.6 ± 0.4 | ||
| Phosphate (mg/dL) | 2.4–5.1 | 3.5 ± 0.5 | ||
| Magnesium (mg/dL) | 1.3–2.7 | 1.9 ± 0.3 | ||
| PTH (pg/mL) | 14–72 | 57 ± 42 | ||
| 25OHD (ng/mL) | 30–80 | 21 ± 10 | ||
| Osteocalcin (ng/mL) | 3–14 | 18 ± 10 | ||
| P1NP (ng/mL) | 15–37 | 36 ± 18 | ||
| CTX (ng/mL) | 0.1–0.6 | 0.3 ± 0.2 | ||
| T-score | Range | Z-score | Range | |
| Lumbar Spine (LS) | −0.4 ± 1.7 | −4.1–5.1 | 0.7 ± 1.6 | −2.1–5.7 |
| Femoral neck (FN) | −1.0 ± 1.3 | −4.0–2.2 | 0.5 ± 1.1 | −1.7–3.5 |
| Normal1 | Osteopenia2 | Osteoporosis3 | ||
| Lumbar Spine (LS)(%) | 64 | 29 | 7 | |
| Femoral Neck (FN)(%) | 43 | 45 | 12 | |
for BMD (T-score) ≥ −1.0.
for BMD (T-score) < −1.0 and > −2.5.
for BMD (T-score) ≤ −2.5.
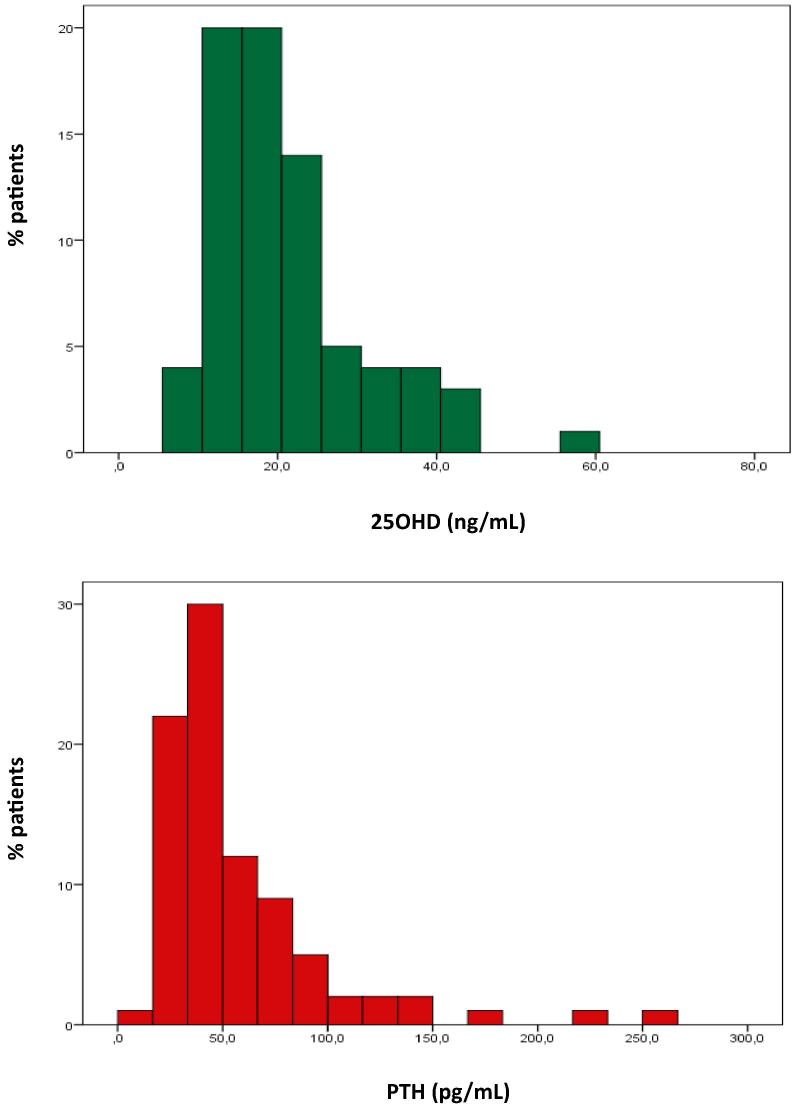
Low levels of 25OHD are almost universal (84%) – 56% with deficient levels (<20 ng/mL) and 28% with insufficient levels (20–29 ng/mL) so that only 16% presented adequate 25OHD levels (30–80 ng/mL) with no case of toxicity (Fig. 1) [8]. As noted there were no cases with low serum calcium levels. Globally 23% of the patients with low levels of 25OH presented increased serum PTH levels, 24% of the patients with very low 250HD levels (<20 ng/mL) and 9% of the patients with low 25OHD levels (20–29 ng/mL). However if we exclude patients with nephropathy, only 16% of the patients with low 25OHD levels present high serum PTH levels.
Fig. 1.
Distribution of 25OHD and PTH values. 25OHD (top) reference range 30–80 ng/mL; PTH (bottom) reference range 14–72 pg/mL.
High values of serum PTH are also common 20%, with half of them above 100 pg/mL (Fig. 1); increased serum PTH meets the criteria for secondary hyperparathyroidism, since calcium and phosphate levels are always normal [17]. In fact in all cases of serum PTH > 100 pg/mL patients presented both nephropathy and low 25OHD levels, as did half of those with PTH levels between 72 and 99 pg/mL, and the remainder had only low levels of 25OHD. 13% of those without nephropathy present high serum PTH levels against 21% of those with microalbuminuria and 55% of those with increased serum creatinine levels. Interestingly enough nephropathy by itself seems to be associated with low levels of 25OHD: 78% of the patients without nephropathy present low levels of 25OHD against 81% of those with microalbuminuria and 100% of those with increased serum creatinine (χ2 = 5.488, p < 0.05 for patients with and without nephropathy).
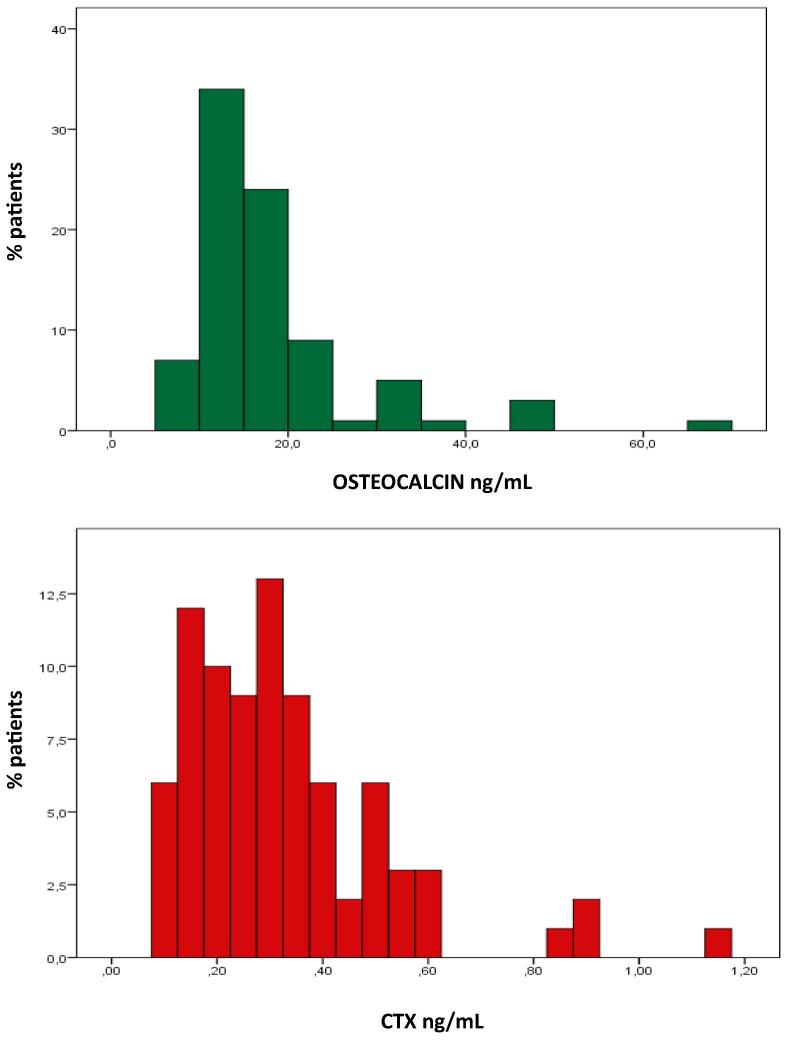
Results regarding osteocalcin and P1NP are concordant – both are significantly related r = 0.652, p < 0.001 – and suggest increased bone formation in old type 2 diabetic patients. The mean value for osteocalcin is above the reference interval, there are no abnormally low osteocalcin values and 52% of the patients present values above the upper limit of the reference range (Fig. 2). The man value for P1NP is near the upper limit of the reference interval, there are only 2 low values of P1NP but 32% high values of P1NP. By contrast there is no evidence for increased bone resorption; the mean value for CTX is exactly in the middle of the reference interval, there are no low levels of CTX but also only four high values (Fig. 2). However bone resorption and bone formation remain strongly related – osteocalcin vs. CTX r = 0.877, p < 0.001, P1NP vs. CTX r = 0.665, p < 0.001.
Fig. 2.
Distribution of osteocalcin and CTX values. Osteocalcin (top) reference range 3–14 ng/mL; CTX (bottom) reference range 01–06 ng/mL.
Serum PTH was directly related to osteocalcin – r = 0.639, p < 0.001 – P1NP – r = 0.186, p < 0.1 – and CTX – r = 0.542, p < 0.001. 25OHD was only weakly and inversely related to P1NP – r = 0.222, p < 0.07. Furthermore when both PTH and 25OHD levels were simultaneously considered, only PTH remained a significant factor. Age was also a direct and significant factor for osteocalcin – r = 0.271, p < 0.05 – but not for P1NP and also for CTX – r = 0.289, p < 0.01, but again when both age and PTH were simultaneously considered only PTH remained significant.
The biomarker of bone resorption (but not those of bone formation) was significantly higher in females than in males – CTX 0.39 ± 0.23 ng/mL vs. 0.28 ± 0.14 ng/mL, t = 2.675, p < 0.01. Neither the use of insulin vs. the use of oral agents, time since diagnosis, metabolic control (HbA1c), C-peptide levels, nor the presence/absence of retinopathy, nephropathy, peripheral neuropathy or hypertension were significantly related to markers of bone turnover. However patients with dyslipidemia presented higher values of bone turnover markers – osteocalcin 19 ± 12 ng/mL vs. 15 ± 5 ng/mL, t = 1.859, p < 0.07, P1NP 38 ± 20 ng/mL vs. 29 ± 8 ng/mL, t = 2.146, p < 0.05 and CTX 0.36 ± 0.21 ng/mL vs. 0.27 ± 0.13, t = 1,829, p < 0.08.
Results regarding bone mineral density are presented in Table 2. Taken as a group, and when adjusted for sex and age (z-score), these patients present a slightly increased bone mineral density both at LS (0.7 ± 1.6) and at FN (0.5 ± 1.1). The differences are mild – less than one standard deviation regarding the mean. As noted by the large standard deviation, there is a large variability (−2.1 to + 5.7 for the LS and −1.7 to +3.5 for the FN). As expected data regarding the femoral neck seems more uniform while that regarding the lumbar spine is more heterogeneous probably due to osteoarthritis artefacts. However BMD (t-scores) at both sites are strongly and directly correlated – LS vs. FN r = 0.712, p < 0.001.
Since bone mineral density is increased when sex- and age-adjusted, it is not surprising that when bone mineral density is compared to controls at age 20 (the t-score), persons with diabetes present decreased bone mineral density (−0.4 ± 1.7 for the lumbar spine and −1.0 ± 1.3 for the femoral neck) although as noted, the decrease is less than would be expected for age.
Age was inversely related to BMD (t-score) at the FN – r = −0.309, p < 0.005 – but not at LS. BMD (t-score at the FN) was inversely related to osteocalcin – r = −0.294, p < 0.01 – but not to P1NP – and also inversely related to CTX – r = −0.330, p < 0.005. PTH is also inversely related to BMD at the femoral neck – r = −0.273, p < 0.05. In fact when age and PTH are simultaneously considered both remain significant, while when PTH and osteocalcin or PTH and CTX are simultaneously considered only markers of bone turnover remain significant.
BMD (t-score) is significantly higher in males at both sites considered – lumbar spine 0.2 ± 1.7 vs. −0.9 ± 1.5, t = 3.134, p < 0.005 and femoral neck −0.7 ± 1.2 vs. −1.5 ± 1.3, t = 3.016, p < 0.005. However BMD (t-score) at the femoral neck was not significantly different in those using insulin or oral agents (although lower values at all sites were found in those using insulin), or in those with or without retinopathy, nephropathy, or peripheral neuropathy (although at all sites, patients with microvascular disease presented lower values). However patients with hypertension presented significantly higher values at both sites – lumbar spine −0.2 ± 1.7 vs. −1.8 ± 1.0, t = 2.459, p < 0.05 and femoral neck −0.9 ± 1.3vs. −2.4 ± 0.8 t = 2.613, p < 0.01, while there was no difference between patients with or without dyslipidemia. BMD was not significantly related to time since diagnosis, body mass index or last HbA1c, but was significantly and inversely related to cholesterol (but not to triglycerides or HDLc) and to systolic (direct) (but not to diastolic blood pressure), inversely related to magnesium, and was not significantly related to C-peptide.. Stepwise regression analysis for clinical and routine analytical parameters, to minerals, hormones affecting mineral metabolism, and bone markers selects as the best predictive model the one that includes cholesterol (8%), systolic blood pressure (11%), magnesium (21%) and osteocalcin or CTX or PTH (24% in every case) with the progressive adjusted r2 indicated within brackets.
Discussion
Fuller Albright was among the first to describe the association of poorly controlled DM and osteoporosis in 1948 [2].
Results however are conflicting and not easy to interpret.
An increased risk of fractures has been documented in both type 1 and type 2 DM [10], [11], [12], the risk being increased by 5–10 fold regarding hip fractures, even when the increased risk of falls because of retinopathy, peripheral neuropathy, hypoglycemia and postural hypotension is accounted for [18], [19], [20].
However, bone mineral density (BMD) the primary diagnostic marker of osteoporosis and an important predictive factor for fracture risk is decreased in type 1 DM (between 20 and 40%) [21] and increased in type 2 DM when age and gender are considered [18], [19], [20]. Type 1 DM, but not type 2 DM, is considered a secondary cause of osteoporosis by the World Health Organization [2]. Theoretically, the increased BMD in subjects with type 2 diabetes should result in a reduction of prevalent osteopenia and osteoporosis and therefore the need for osteoporotic treatment and the average 0.3 increase in hip BMD should translate in a 10% reduction of fracture risk in general and a 20% reduction of hip fractures in particularly [22]. BMD in persons with type 2 diabetes has been related to HbA1c [18], [19], to insulin resistance [23] and less clearly to advanced glycation end-products (AGEs) like urinary pentosidine [24].
Two unsolved problems may then be stated: 1) why is the BMD of type 2 patients increased contrasting with type 1 patients; relevant factors must not be found in diabetes per se, but rather should be specific for type 1 or 2 diabetes; 2) why is the fracture risk increased despite the increased BMD; relevant factors probably affecting bone microarchitecture and resilience may be the same for type 1 and type 2 patients.
To address these problems we selected a sample of old persons with type 2 diabetes that reflects the real conditions of the diabetic practice. Its small size restricts conclusions to those where the effect is of enough magnitude to reach significance for the small size that defines individual practice.
Patients in this sample are old, with long standing disease and marked β-cell failure that requires the use of insulin. However they are only overweight not obese and maintain a fair metabolic control. Also even if common, micro- and macrovascular disease are less prevalent than could be expected, namely at the clinical level for this age group. These data may suggest that old persons with type 2 diabetes are probably a special group of persons with diabetes, selected survivors [25]. However the sample is probably representative of old type 2 diabetic patients assisted everywhere in the developed world.
In these patients, mineral metabolism is generally normal with only isolated mild abnormalities that were the subject of another report, and are related to insulin and diuretic use and nephropathy [7]. As noted vitamin D deficiency is extremely common (84%) but its significance remains unclear, since calcium levels are always normal and only rarely (16%) it is associated with increased serum PTH levels [8], [26]. Secondary hyperparathyroidism is much less common (20%) and is related both to the development of diabetic nephropathy and to low levels of 25OHD with severe nephropathy with increased serum creatinine levels being the dominant factor [8], [27], [28]. Since mineral metabolism is grossly normal, factors relevant to understand BMD and fracture risk in diabetes must be found in hormones modulating bone formation and resorption. Low 25OHD levels and increased serum PTH are obvious candidates since additionally these abnormalities are common in subjects with diabetes. Also if culprits do not reside in 25OHD and PTH either directly or indirectly, data regarding BMD and fracture risk in diabetic patients should be explained otherwise and despite these abnormalities.
In this sample of old persons with type 2 diabetes, there is evidence for increased bone formation regarding bone resorption [29]. The data are consistent, with increased bone formation suggested by either osteocalcin or P1NP that are strongly interrelated. Bone resorption and bone formation remain coupled as suggested by the strong relation of either osteocalcin or P1NP with CTX and no inference can be made regarding the intensity of the remodeling process. Other studies have suggest decreased bone turnover in subjects with diabetes that would favor the use of anabolic agents like the parathyroid hormone analogs in relation to anti-resorptive agents like bisphosphonates, strontium ranelate or denosumab; however no specific experimental studies have been developed to address this issue [10], [21]. Two specific caveats may be considered: bone resorption and bone formation remain obviously coupled but that does not mean the processes are symmetrical as is indicated by osteoporosis itself and by differences regarding anabolic or anti-resorptive agents; and reference values for bone turnover markers are off course gender and age specific, but both genders were equally included in this study, and since bone formation decreases with ageing and only old persons were included, differences would be even more marked.
The increased bone formation in regard to bone resorption is directly associated to serum PTH, not to age, vitamin D or factors related to diabetes itself, namely time since diagnosis or the quality of metabolic control. Furthermore, as noted, the relation of bone remodeling to serum PTH is evident and uniform for the whole range of PTH values. It is most interesting the clear association of hyperlipidemia, generally hypercholesterolemia with increased bone remodeling that has already been reported by other groups, as well as the association between BMD and systolic blood pressure [30], [31]. Both these factors may contribute to differences regarding young persons with type 1 diabetes and old persons with type 2 diabetes.
Data regarding bone mineral density agrees with the literature and is not surprising [10], [11], [12], [18], [19], [20]. Taken as a group, and when adjusted for sex and age (Z-score), these patients present indeed a slightly increased bone mineral density both at the lumbar spine and at the femoral neck. The differences are mild – less than one standard deviation. At the clinical level this would translate in an increased fracture risk at about 2× the general population but less than would be expected for age. So old persons with diabetes have an increased fracture risk because they are old but less so than the general old population.
To begin with, the data seems coherent: patients have increased bone formation and therefore not surprisingly increased BMD. Bone remodeling with increased bone formation is related in a continuous way to serum PTH. However bone formation, bone resorption and serum PTH are inversely related to BMD. Multiple regression analysis reveals markers of bone remodeling to be the significant factor – when entered with serum PTH, either osteocalcin, P1NP or CTX, serum PTH is no longer significant and either marker remains significant, but not both markers of bone formation and resorption. This suggests that while increased bone formation may be involved in the increased BMD in the end, the intensity of the remodeling process is associated with decreased BMD and a more porous brittle bone will result.
These somehow surprising conclusions lead us to consider other similar conditions. In senile osteoporosis and CKD pathogenic factors include decreased vitamin D levels and increased serum PTH levels and fracture risk is greater than would be expected by the BMD data [13], [14], [27], [28], [32]. Primary hyperparathyroidism is now generally diagnosed in asymptomatic subjects, and normal results regarding BMD have been reported but again with an increased fracture risk [33], [34], [35], [36]. Curiously enough, teriparatide (1–34 PTH) increases bone formation, but while intermittent low dose administration is anabolic, prolonged high dose administration increases bone resorption [37], [38], [39].
BMD as measured is in fact apparent density because non bone tissue like bone marrow is included and areal not volumetric measurements are obtained. It is also an imperfect static measurement that underscores bone microarchitecture and resilience [40]. Furthermore, half of the subjects with fragility fractures present normal or only slightly decreased BMD values in the osteopenic range [1], [2]. FRAX and other algorithms loose much of their validity in diabetic patients [10], [11], [12], [41]. Calcaneal quantitative ultrasound that is considered to specifically evaluate bone microarchitecture and quality is not a significant predictor of bone fractures, more specifically vertebral fractures in diabetic patients [42]. High-resolution peripheral quantitative computed tomography (HR-pQCT) may better distinguish trabecular from cortical bone, while the trabecular bone score (TBS) offers a three dimensional measure of trabecular bone microarchitecture [43], [44] and consistent lower values have been found in diabetic subjects [23], [45]. Also the important role of vitamin D on bone structure and function was not apparent in this work [46]. However despite these well recognized limitations our data offer a possible explanation why old type 2 diabetic subjects may present increased BMD and despite this an increased fracture risk.
This report also presents common limitations of clinical studies: the sample size is small, the study is retrospective, FRAX scores were not computed, actual fractures were not assessed, other endocrine factors were not measured and statistical relations do not imply causality. However it is reasonable to further explore the role of PTH in bone status now that the paradigm is changing with the increasing recognition of low grade secondary hyperparathyroidism and the recent use of teriparatide in intermittent albeit daily administration.
In short we found that old persons with type 2 diabetes, probably selected survivors, present indeed mildly increased BMD both at the lumbar spine and femoral neck. The increased BMD may be related to the observed increased bone formation regarding bone resorption that is clearly PTH driven. PTH is mildly increased because of low 25OHD levels and diabetic nephropathy. We suggest that mild PTH increase such that found in senile osteoporosis, early stages of chronic kidney disease and asymptomatic and incidental primary hyperparathyroidism, may be responsible for the increased BMD in old type 2 diabetic, and despite this because of increased porosity an increased fracture risk results. Additionally we confirm other previous reports that bone remodeling is increased in patients with dyslipidemia and that BMD is higher in patients with higher systolic blood pressure and these factors may contribute to differentiate persons with type 1 and type 2 diabetes on what relates to BMD.
Declarations of interest
None.
Funding
No funding was obtained either public or private for this work that results from the clinical and academic duties of the authors working in a public academic hospital.
Research on human subjects
This report only includes anonymously retrieved clinical and analytical data of patients assisted at a public academic hospital according to the Standards of Cinical Practice, with no additional intervention. This report fully complies with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Individual author contributions
João Martin Martins: assisted the patients, defined the research protocol, defined the database and retrieved the data, analyzed the data and wrote the report.
Patrícia Aranha: defined the research protocol, defined the database and retrieved the data, analyzed the data and wrote the report.
Acknowledgement
Authors wish to thank Rheumatology Colleagues José Carlos Romeu and Inês Seixas, for the many valuable discussions.
References
- 1.Pisani P., Renna M.D., Conversano F., Casciaro E., Di Paola M., Quarta E. Major osteoporotic fragility fractures: risk factor updates and societal impact. World J Orthopedics. 2016;7(3):171–181. doi: 10.5312/wjo.v7.i3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapurlat R.D., Genant H.K. Osteoporosis. In: Jameson J.L., De Grot L.J., de Kretser D.M., Giudice L.C., Grossman A.B., Melmed S., Potts J.T., Weir G.C., editors. Endocrinology – adult and pediatric. 7th ed. Elsevier/Saunders; Philadelphia, PA: 2016. pp. 1184–1213. [Google Scholar]
- 3.Polonsky K.S., Burant C.F. Type 2 diabetes mellitus. In: Melmed S., Polonsky K.S., Larsen P.E., Kronenberg H.M., editors. Williams textbook of endocrinology. 13th ed. Elsevier; Philadelphia, PA: 2016. pp. 1386–1450. [Google Scholar]
- 4.de Paula F.J.A., Horowitz M.C., Rosen C.J. Novel insights into the relationship between diabetes and osteoporosis. Diabetes Metab Res. 2010;26(8):622–630. doi: 10.1002/dmrr.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cagliero E. Diabetes and long-term complications. In: Jameson J.L., De Grot L.J., de Kretser D.M., Giudice L.C., Grossman A.B., Melmed S., Potts J.T., Weir G.C., editors. Endocrinology – adult and pediatric. 7th ed. Elsevier/Saunders; Philadelphia, PA: 2016. pp. 898–906. [Google Scholar]
- 6.Gilbert M.P., Pratley R.E. The impact of diabetes and diabetes medications on bone health. End Rev. 2015;36(2):194–213. doi: 10.1210/er.2012-1042. [DOI] [PubMed] [Google Scholar]
- 7.Martins J.M., Aranha P. Abnormal mineral metabolism in diabetic patients – relevance to micro- and macrovascular disease and to bone metabolic disorder. J Diabetes Treat. 2018;1:JDBT-142. [Google Scholar]
- 8.Katrinaki M., Kampa M., Margioris A., Castanas E., Malliaraki N. Vitamin D levels in a large Mediterranean cohort: reconsidering normal cut-off values. Hormones. 2016;15(2):205–223. doi: 10.14310/horm.2002.1674. doi: 10.14310 /horm.2002.1674. [DOI] [PubMed] [Google Scholar]
- 9.National Osteoporosis Guideline Group. Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK (Updated March 2014). www.shef.ac.uk/NOOG (accessed 30.03.2018).
- 10.Oei L., Rivadeneira F., Zillikens M.C., Oei E.-H.G. Diabetes, diabetic complications, and fracture risk. Curr Osteoporos Rep. 2015;13:106–115. doi: 10.1007/s11914-015-0260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackuliak P., Payer J. Osteoporosis, fractures and diabetes. Int J Endocrinol. 2014;ID:820615. doi: 10.1155/2014/820615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starup-Linde J., Vestergaard P. Diabetes and osteoporosis: cause for concern? Eur J Endocrinol. 2015;173:R93–R99. doi: 10.1530/EJE-15-0155. [DOI] [PubMed] [Google Scholar]
- 13.Lundquist A.L., Nigwekar S.U. Optimal management of bone mineral disorders in chronic kidney disease and ESRD. Curr Opin Nephrol Hypertens. 2016;25(2):120–126. doi: 10.1097/MNH.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stathopoulos I.P., Ballas E.G., Lampropoulou-Adamidou K., Trovas G. A review on osteoporosis in men. Hormones. 2014;13(4):441–457. doi: 10.14310/horm.2002.1550. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Standards of Medical Care in Diabetes – 2016 Position Statement. Diabetes Care. 2016;39(Suppl 1):S6–S106. [Google Scholar]
- 16.Norman G.R., Streiner D.L. Mosby Year Book Inc; St. Louis: 1994. Biostatistics. The bare Essentials. [Google Scholar]
- 17.Bringhurst F.R., Demay M.B., Kronenberg H.M. Hormones and disorders of mineral metabolism. In: Melmed S., Polonsky K.S., Larsen P.E., Kronenberg H.M., editors. Williams textbook of endocrinology. 13th ed. Elsevier; Philadelphia, PA: 2016. pp. 1254–1322. [Google Scholar]
- 18.Schwartz A.V., Sellmeyer D.E., Ensrud K.E., Cauley J.A., Tabor H.K., Schreiner P.J. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 19.Bonds D.E., Larson J.C., Scwartz A.V., Strotmeyer E.S., Strotmeyer E.S., Robbins J. Risk of fracture in women with type 2 diabetes: the women’s health initiative observational study. J Clin Endocrinol Metab. 2006;91(9):3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 20.Ishii S., Cauley J.A., Crandall C.J., Srikanthan P., Greendale G.A., Huang M.H. Diabetes and femoral neck strength: findings from the hip strength across the menopausal transition study. J Clin Endocrinol Metab. 2012;97(1):190–197. doi: 10.1210/jc.2011-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hough F.S., Pierroz D.D., Coooper C., Ferrari S.L. IOF CSA Bone and Diabetes Working Group. Mechanisms and evaluation of bone fragility in type 1 diabetes mellitus. Eur J Endocrinol. 2016;174 doi: 10.1530/EJE-15-0820. R127-R38. [DOI] [PubMed] [Google Scholar]
- 22.Melton L.J., Riggs L., Leibson C.L., Achenbach S.J., Camp J.J., Bouxsein M.L. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab. 2008;93(12):4804–4809. doi: 10.1210/jc.2008-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J.H., Choi H.J., Ku E.J., Kim K.M., Kim S.W., Cho N.H. Trabecular bone score as an indicator for skeletal deterioration in diabetes. J Clin Endocrinol Metab. 2015;100(2):475–482. doi: 10.1210/jc.2014-2047. [DOI] [PubMed] [Google Scholar]
- 24.Schwatz A.V., Garnero P., Hillier T.A., Sellmeyer D.E., Stotmeyer E.S., Feingold K.R. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–2386. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohan V., Rani C.S.S., Amutha A., Dhulipala S., Anjana R.M., Parathasarathy B. Clinical profile of long-term survivors and nonsurvivors with type 2 diabetes. Diabetes Care. 2013;36(8):2190–2197. doi: 10.2337/dc12-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson J.E., Brannon P.M., Rosen C.J., Taylor C.L. Vitamin D deficiency – is there really a pandemic? N Engl J Med. 2016;375(19):1817–1820. doi: 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 27.Perry K.W., Salusky I.B. Chronic kidney disease mineral and bone disorder. In: Jameson J.L., De Grot L.J., de Kretser D.M., Giudice L.C., Grossman A.B., Melmed S., Potts J.T., Weir G.C., editors. Endocrinology – adult and pediatric. 7th ed. Elsevier/Saunders; Philadelphia, PA: 2016. pp. 1214–1229. [Google Scholar]
- 28.Yenchek R.H., Ix J.H., Shlipak M.G., Bauer D.C., Rianon N.J., Kritchevsky S.B. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130–1136. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delmas P.D., Eastell R., Garnero P., Seibel M.J., Stepan J. for the Committee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int. 2000;Suppl 6:S2–S17. doi: 10.1007/s001980070002. [DOI] [PubMed] [Google Scholar]
- 30.Mandal C.C. High cholesterol deteriorates bone health: new insights into molecular mechanisms. Front Endocrinol. 2015;5:1–11. doi: 10.3389/fendo.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazici S., Yazici M., Korkmaz U., Engin Erkan M., Erdem Baki A., Erden I. Relationship between blood pressure levels and bone mineral density in postmenopausal Turkish women. Arch Med Sci. 2011;7(2):264–270. doi: 10.5114/aoms.2011.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D’Amelio P., Isaia G.C. Male osteoporosis in the elderly. Int J Endocrinol. 2015 doi: 10.1155/2015/907689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverberg S.J., Bilezikian J.P. Primary hyperparathyroidism. In: Jameson J.L., De Grot L.J., de Kretser D.M., Giudice L.C., Grossman A.B., Melmed S., Potts J.T., Weir G.C., editors. Endocrinology – adult and pediatric. 7th ed. Elsevier/Saunders; Philadelphia, PA: 2016. pp. 1105–1124. [Google Scholar]
- 34.Khan A.A., Hanley D.A., Rizzoli R., Bollersley J., Young J.E., Reinmark L. Primary hyperparathyroidism: review and recommendations on evaluation, diagnosis, and management. A Canadian and international consensus. Osteoporosis Int. 2017;28:1–19. doi: 10.1007/s00198-016-3716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandeira F., Cusano N.E., Silva B.C., Cassibba S., Almeida C.B., Machado V.C.C. Bone disease in primary hyperparathyroidism. Arq Bras Endocrinol Metab. 2014;58(5):553–561. doi: 10.1590/0004-2730000003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa A.G., Bilezikian J.P. Bone turnover markers in primary hyperparathyroidism. J Clin Densitom. 2013;16(1):22–27. doi: 10.1016/j.jocd.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen S., Hauge E.M., Jensen J.-E.B., Brixen K. Differing effects of PTH 1–34, PTH 1–84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQT. J Bone Mineral Res. 2013;28(4):736–745. doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]
- 38.Lindsay R., Krege J.H., Marin F., Jin L., Stepan J.J. Teriparatide for osteoporosis: importance of the full course. Osteoporos Int. 2016;27:2395–2410. doi: 10.1007/s00198-016-3534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burge R.T., Disch D.P., Gelwicks S., Zhang X., Krege J.H. Hip and other fragility fracture inicidence in real-world teriparatide-treated patients in the United States. Osteoporos Int. 2017;28:799–809. doi: 10.1007/s00198-016-3888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oei L., Koromani F., Rivadeneira F., Zilikens M.C., Oei E.H.G. Quantitative imaging methods in osteoporosis. Quant Imaging Med Surg. 2016;6:680–698. doi: 10.21037/qims.2016.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto M. Insights into bone fragility in diabetes: the crucial role of bone quality on skeletal strength. Endocrine J. 2015;62(4):299–308. doi: 10.1507/endocrj.EJ15-0129. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T., Sugimoto T. Bone metabolism and fracture risk in type 2 diabetes mellitus. Endocr J. 2011;58(8):613–624. doi: 10.1507/endocrj.ej11-0063. [DOI] [PubMed] [Google Scholar]
- 43.Burghardt A.J., Link T.M., Majumdar S. High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res. 2011;469:2179–2193. doi: 10.1007/s11999-010-1766-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harvey N.C., Gkluer C.C., Binkley N., McCloskey E.V., Brandi M.L., Cooper C. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone. 2015;78:216–224. doi: 10.1016/j.bone.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leslie W.D., Aubry-Rozier B., Lamy O., Hans D. the Manitoba Bone Density Program. TBS (Trabecular Bone Score) and diabetes-related fracture risk. J Clin Endocrinol Metab. 2013;98(2):602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 46.Williamson L., Hayes A., Hanson E.D., Pivonka P., Sims N.A., Gooi J.H. High dose dietary vitamin D3 increases bone mass and strength in mice. Bone Rep. 2017;6:44–50. doi: 10.1016/j.bonr.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]