Abstract
Background
Disseminated tuberculosis (TB) or miliary TB is defined as lymphohematogenous dissemination of Mycobacterium tuberculosis bacilli, which may then affect virtually any organ system. The multiple organ involvement in disseminated TB can mimic metastatic cancer and can make the diagnosis challenging. False negatives are common therefore repeating microbiologic and histologic samples is essential.
Case report
We report the case of a young immunocompetent patient presenting with multiple atypical extra-pulmonary TB involvement. The patient presented with pulmonary, pleural, bilateral testicular and multiple bone involvement including calcanerium abscesses. These lesions were initially described as metastasis by the radiologist. Therefore lymphoma and metastatic testicular cancer as well as TB were high on the differential in this young foreign-born male. Pleural, broncho-alveolar lavage, bone marrow and cerebrospinal fluid acid-fast bacilli smear and microbiologic culture were all negative. However the histologic examination of the trans-bronchial biopsy and pleural biopsy showed necrotizing granuloma and helped to narrow down the diagnosis. The patient improved with RIPE therapy.
Conclusion
This case illustrates the diagnostic difficulty of disseminated TB with atypical organ involvement. Culture is the gold standard for diagnosing TB but is a long process and with 23% of culture negative TB in the United-States, the diagnosis sometimes relies on thoroughly ruling-out differential diagnosis and histologic examination.
Keywords: Disseminated tuberculosis, Extra-pulmonary tuberculosis, Testicular tuberculosis, Skeletal tuberculosis
1. Introduction
In the United States, the prevalence of tuberculosis (TB) remains relatively low, and a majority of cases occur in foreign-born patients [1]. Disseminated TB or miliary TB is defined as lymphohematogenous dissemination of Mycobacterium tuberculosis bacilli, which may then affect virtually any organ system. It is estimated that disseminated TB accounts for 1–2% of all tuberculosis cases [2]. The multiple organ involvement of disseminated TB can make the diagnosis challenging. Here we report the case of a foreign-born immunocompetent patient presenting to a hospital in the United States who was ultimately diagnosed with miliary tuberculosis with profoundly disseminated multi-system involvement mimicking metastatic cancer.
2. Case presentation
A 25-year-old Indian male without any significant past medical history presented to a hospital in the United States with progressively worsening systemic symptoms over the course of three months. Symptoms included fatigue, night sweats, and fever, as well as a non-productive cough. He reported unintentional weight loss of 30 pounds over three months, as well as ten days of worsening low back pain that radiated to his thighs. Notably, the patient had immigrated to the United States from India two-and-a-half years prior to this admission and had briefly visited India one year prior. He had no history of tuberculosis, and he denied any known exposures to tuberculosis.
In the emergency department, he was febrile to 103.1°F and tachycardic to 160 beats per minute. However, he was normotensive and breathing comfortably on room air with an oxygen saturation of 97%. On general examination, he appeared thin but was awake and alert in no acute distress. On pulmonary examination, he was found to have decreased breath sounds and dullness to percussion over the right middle and lower lung fields, as well as mild clubbing of the digits. The remainder of the physical examination was unremarkable.
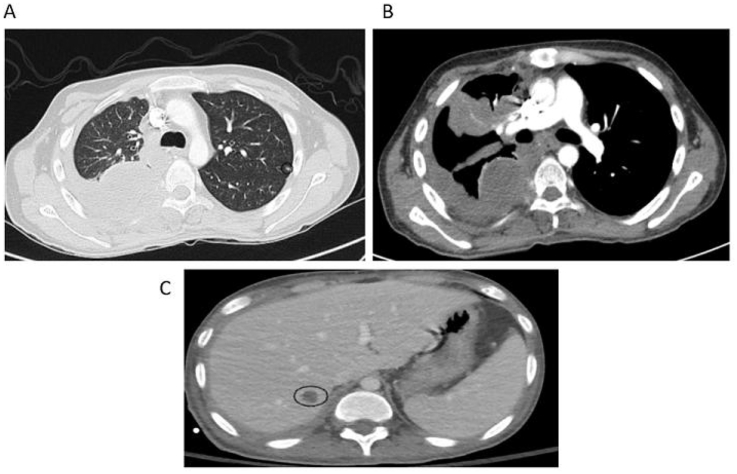
He received a chest x-ray and subsequent CT angiogram of the chest, which demonstrated a large right loculated pleural effusion, right upper lobe density, right-sided compressive atelectasis, multiple bilateral subcentimeter nodules, and a 1.5 cm right hepatic lesion (Fig. 1).
Fig. 1.
CTA Chest and CT abdomen. CT of the chest demonstrated (A) multiple bilateral subcentimeter nodules (A and B) a large right loculated pleural effusion, and (C) a nonspecific 1.5 cm right hepatic lesion.
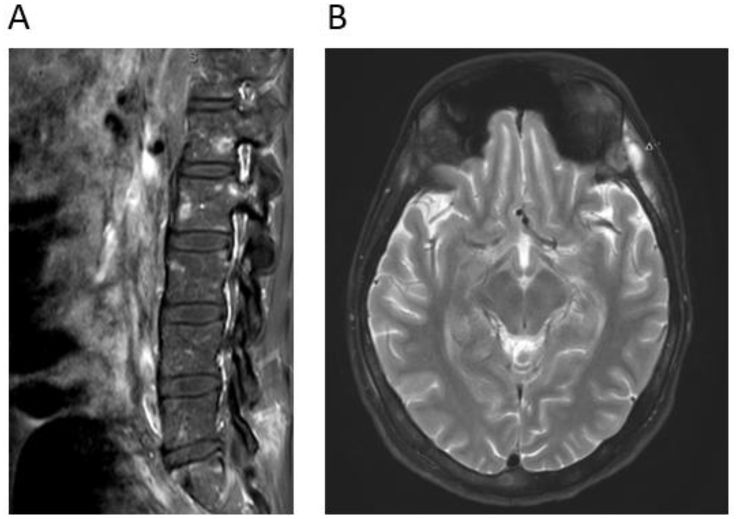
To evaluate his reported back pain, an MRI of the lumbar spine was ordered, and the results showed multiple poorly defined bony lesions consistent with metastatic disease in the vertebral bodies (Fig. 2A).
Fig. 2.
MRI spine (T1 post-contrast) and brain (T2-weighted). (A) Multiple poorly defined bony lesions were identified on MRI of the spine, which were higher signal on T1 after contrast, consistent with bony metastatic lesions involving the vertebral bodies and posterior elements. (B) Subsequent MRI of the brain found diffuse heterogeneous calvarial marrow signal, representing infiltrative disease of the bone marrow. There are also multiple calcanerium abscesses and diffuse thickening of the dura.
In an attempt to identify the primary source of the metastases, an ultrasound of the testes was then performed, which demonstrated bilateral masses suspicious for additional metastases, though primary testicular malignancy could not be excluded by imaging alone. Follow-up serum alpha fetoprotein (AFP) and beta-hCG levels were within normal limits.
Initial hematologic studies revealed a mild normocytic anemia, leukocytosis, and mild thrombocytosis. A metabolic panel revealed hyponatremia (127 mmol/dL) and elevated alkaline phosphatase (201 IU/L), and hypoalbuminemia.
Thoracentesis was performed upon admission, which showed a lymphocyte-predominant exudative effusion. No acid fast bacteria were isolated from the pleural fluid, and an adenosine deaminase (ADA) level was normal.
The patient subsequently underwent bronchoscopy, and the culture was negative for acid fast bacilli after six weeks. However, transbronchial biopsy demonstrated necrotizing granulomas in the lung and fibrous tissue. Stains for mycobacteria and fungi were negative. This was followed by pleural biopsy, which was also negative for mycobacterial culture but demonstrated focal granulomatous inflammation.
Interferon gamma release assay (QuantiFERON Gold) and tuberculin skin test were both positive.
The patient was started on antibiotic therapy of rifampin, isoniazid, pyrazinamide, and ethambutol. After a week of treatment, the patient continued spiking fevers, we proceeded to ruling out central nervous system involvement. An MRI of the brain was done and demonstrated calcanerium abscesses, infiltration of the bone marrow, and thickening of the dura (Fig. 2B). Given these findings, a bone marrow biopsy and lumbar puncture were performed, which were both normal with once again negative acid fast bacilli staining and culture.
The patient continued with standard antituberculous therapy and gradually improved. After his fevers resolved and mental status normalized, the patient was discharged.
3. Discussion
TB is a worldwide leading cause of infectious morbidity and mortality. Disseminated TB is defined as tuberculous infection involving the blood stream, bone marrow, liver, or two or more noncontiguous sites, or miliary TB [3]. This case report underlies many rare and unique manifestations of disseminated TB, and the diagnosis dilemma it engenders. The various extra-pulmonary TB (EP-TB) involvement, as well as the multiple negative microbiologic testing in this immunocompetent patient led to a wide array of differential diagnoses including: sarcoidosis, metastatic testicular cancer, lymphoma and fungal infection.
Although pulmonary TB is the most common form of the disease, EP-TB manifestations occur in only 15–20% of patients and disseminated TB in 2–5% of patients [1,3]. Genito-urinary TB (GU-TB) comprises 4% of EP-TB cases, with 3% of these GU-TB cases being testicular TB [4]. Cases of bilateral testicular involvement are even rarer. Middle aged males are the most commonly affected demographic, and the main differential diagnoses considered for testicular involvement in this population are testicular cancer, lymphoma, genito-urinary sarcoidosis, hydrocele and epididymo-orchitis. Due to these diagnostic challenges, these patients face an increased risk of misdiagnosis and delay of treatment [5,6]. Ultrasound can facilitate the diagnosis and show testicular hypoechoic nodules [4]. Pleural and bone involvement are more common manifestations of EP-TB [7,8]. However multifocal skeletal TB is rare and can be difficult to differentiate from bony metastasis on CT and MRI [9]. In case of persisting fever despite treatment, central nervous system involvement should be ruled out with a lumbar puncture and a brain MRI [10]. As in our patient, rare cases of calvarial tuberculosis have been described [11]. Brain MRI can show various signs of TB including basal meningeal enhancement, parenchymal tuberculoma and TB abscesses [12,13]. Patients with disseminated TB can also exhibit abnormal laboratory findings such as hypoalbuminemia, hyponatremia, elevated alkaline phosphatase and γ-glutamyl transferase and anemia [3].
Per consensus guidelines, the diagnosis of active tuberculosis should include smear and culture of sputum samples for acid-fast bacilli (AFB), as well as nucleic acid amplification testing. When induced sputum samples are unobtainable, flexible bronchoscopic sampling is recommended and should include transbronchial biopsy as the yield for bronchoalveolar lavage is unknown. Additionally, suspected extrapulmonary sites should be sampled for acid-fast bacilli smear, mycobacterial culture, nucleic acid amplification testing, and histologic examination [14]. False negative AFB smear are common and should not exclude the diagnosis. Culture is the gold standard microbiologic test for diagnosing TB, however it is time consuming (4–6 weeks) and has variable sensitivity (50–100%) depending on time of collection and culture media type (liquid, solid media) [15]. Culture negative TB was reported in 23% of TB cases in the United-States in 2014 [1]. In a study by Wang et al. the yield of pleural culture and pleural biopsy was found to be respectively 55% and 61% [3]. In another study focusing on patients with disseminated TB, the yield of pleural biopsy (77%) was higher than the yield of AFB staining (43%), PCR (70%) or culture (72%) [16]. In an instance of negative culture, such as in our case, biopsy can help increase the yield and decrease the time of diagnosis.
Treatment of EP-TB follows the same recommendations as pulmonary TB, with an intensive phase consisting of two months of quadritherapy (Rifampin, Isoniazid, Pyrazinamide, and Ethambutol), followed by a continuation phase consisting of four months of dual therapy (Rifampin, Isoniazid). In culture-negative TB the total duration of treatment can be reduced to four months (continuation phase of two months). Some experts recommend extending therapy to a nine months regimen when there is skeletal involvement and to a twelve months regimen in cases of CNS involvement [17].
4. Conclusion
Due to the diversity of possible presentations, disseminated TB can imitate many other diseases and definitive diagnosis can prove elusive. In addition misdiagnosis and delay in treatment increases mortality. False negatives are frequent, but diagnosis can be improved by obtaining multiple culture samples as well as biopsies for histopathologic examination. Differential diagnoses should be thoroughly ruled out.
References
- 1.US Department of Health and Human Services . 2017. Centers for Disease Control and Prevention. Reported Tuberculosis in the United States, 2016. [Google Scholar]
- 2.Sharma S.K., Mohan A., Sharma A., Mitra D.K. Miliary tuberculosis: new insights into an old disease. Lancet Infect. Dis. 2005 Jul;5(7):415–430. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang J.-Y., Hsueh P.-R., Wang S.-K., Jan I.-S., Lee L.-N., Liaw Y.-S. Disseminated tuberculosis: a 10-year experience in a medical center. Medicine (Baltim.) 2007 Jan;86(1):39–46. doi: 10.1097/MD.0b013e318030b605. [DOI] [PubMed] [Google Scholar]
- 4.Das A., Batabyal S., Bhattacharjee S., Sengupta A. A rare case of isolated testicular tuberculosis and review of literature. J. Fam. Med. Prim. Care. 2016 Jun;5(2):468–470. doi: 10.4103/2249-4863.192334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson R.W., Yu H., Dahl D.M., Hurtado R.M., Sajed D.P. Case 10-2018: an 84-year-old man with painless unilateral testicular swelling. N. Engl. J. Med. 2018 Mar 28;378(13):1233–1240. doi: 10.1056/NEJMcpc1712224. [DOI] [PubMed] [Google Scholar]
- 6.Cho Y.S., Joo K.J., Kwon C.H., Park H.J. Tuberculosis of testis and prostate that mimicked testicular cancer in young male soccer player. J. Exerc. Rehabil. 2013 Jun 30;9(3):389–393. doi: 10.12965/jer.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barman B., Tiewsoh I., Lynrah K.G., Wankhar B., Beyong T., Issar N.K. Miliary tuberculosis with pulmonary and extrapulmonary component complicated with acute respiratory distress syndrome. J. Fam. Med. Prim. Care. 2017;6(3):688–690. doi: 10.4103/2249-4863.222031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali Chaudhry L., Al-Solaiman S. Multifocal tuberculosis: many faces of an old menace. Int. J. Mycobacteriol. 2013 Mar 1;2(1):58–69. doi: 10.1016/j.ijmyco.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Marudanayagam A., Gnanadoss J.J. Multifocal skeletal tuberculosis: a report of three cases. Iowa Orthop. J. 2006;26:151–153. [PMC free article] [PubMed] [Google Scholar]
- 10.Chou P.-S., Liu C.-K., Lin R.-T., Lai C.-L., Chao A.-C. Central nervous system tuberculosis: a forgotten diagnosis. Neurol. 2012 Jul;18(4):219–222. doi: 10.1097/NRL.0b013e3182610347. [DOI] [PubMed] [Google Scholar]
- 11.Sridharan S., Balasubramanian D. Primary calvarial tuberculosis. Surg. Neurol. Int. 2017;8:126. doi: 10.4103/sni.sni_495_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernaerts A., Vanhoenacker F.M., Parizel P.M., Van Goethem J.W.M., van Altena R., Laridon A. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur. Radiol. 2003 Aug 1;13(8):1876–1890. doi: 10.1007/s00330-002-1608-7. [DOI] [PubMed] [Google Scholar]
- 13.Hwang J.H., Lee K.M., Park J.E., Kim H.-G., Kim E.J., Choi W.S. Atypical cerebral manifestations of disseminated Mycobacterium tuberculosis. Front. Neurol. 2017;8:462. doi: 10.3389/fneur.2017.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewinsohn D.M., Leonard M.K., LoBue P.A., Cohn D.L., Daley C.L., Desmond E. Official American thoracic society/infectious diseases society of America/centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin. Infect. Dis. 2017 Jan 15;64(2):111–115. doi: 10.1093/cid/ciw778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrrell F.C., Budnick G.E., Elliott T., Gillim-Ross L., Hildred M.V., Mahlmeister P. Probability of negative mycobacterium tuberculosis complex cultures based on time to detection of positive cultures: a multicenter evaluation of commercial-broth-based culture systems. J. Clin. Microbiol. 2012 Oct;50(10):3275–3282. doi: 10.1128/JCM.01225-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S.N., Jung J., Kim Y.-K., Lee J.Y., Kim S.-M., Park S.J. Diagnostic usefulness of IFN-gamma releasing assays compared with conventional tests in patients with disseminated tuberculosis. Medicine (Baltim.) 2015 Jul;94(28):e1094. doi: 10.1097/MD.0000000000001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nahid P., Dorman S.E., Alipanah N., Barry P.M., Brozek J.L., Cattamanchi A. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016 Oct 1;63(7):e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]