Abstract
The concurrence of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) and anti-glomerular basement membrane (GBM) disease, known as double-positive disease, is rare, but it occurs at a much higher frequency than expected by chance. Double-positive disease has an aggressive clinical course, with no optimal treatment strategy. Here we describe a patient with steroid-refractory double-positive disease who was treated successfully with the addition of plasma exchange (PE) and cyclophosphamide (CPA). A 78-year-old Japanese woman who was diagnosed with diffuse alveolar hemorrhage and rapidly progressive glomerulonephritis received two cycles of pulse steroid therapy. However, her respiratory and renal condition deteriorated. She was found to be positive for both myeloperoxidase-ANCA and anti-GBM antibodies. The combination of PE and CPA improved her systemic condition. This is the first case report of a patient with steroid-refractory double-positive disease who was successfully treated with the addition of PE and CPA. The marked contrast in therapeutic response to corticosteroids alone and the addition of PE and CPA in this case strongly implies that earlier induction of combination therapy aimed at rapid removal of pathogenic autoantibodies and prevention of ongoing antibody production might improve the outcome of this life-threatening disease.
Keywords: Anti-glomerular basement membrane disease, Antineutrophil cytoplasmic antibody-associated vasculitis, Double-positive disease, Plasma exchange, Immunosuppression, Relapse
1. Introduction
Anti-glomerular basement membrane (GBM) disease and antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) are each clinically associated with the development of rapidly progressive glomerulonephritis (RPGN) and diffuse alveolar hemorrhage (DAH) [1,2]. These diseases are rare, but are associated with an increased risk of morbidity and mortality from renal or respiratory failure. Anti-GBM disease is an immune complex small vessel vasculitis affecting glomerular and pulmonary capillary beds with anti-GBM antibody deposition in the basement membrane. AAV is a necrotizing vasculitis with few or no immune deposits, predominantly affecting small vessels associated with ANCAs specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA) [[1], [2], [3]]. The concurrence of ANCA and anti-GBM disease, known as double-positive disease, is an extremely rare condition, but it is recognized to occur at a much higher frequency than expected by chance. Patients who are double-positive account for approximately half of all patients with anti-GBM disease. Approximately 10% of patients with AAV are reported to also have anti-GBM antibodies [[4], [5], [6]]. The relatively high concurrence rate for ANCA and anti-GBM disease and worse prognosis of patients with double-positive disease compared to those of patients with either ANCA or anti-GBM alone [7] suggest that these two conditions might synergistically impact disease initiation and progression. At present, there is no optimal treatment strategy for double-positive disease.
Here we report a patient with steroid-refractory double-positive disease treated successfully with a combination of plasma exchange (PE) and cyclophosphamide (CPA). The distinct therapeutic responses to corticosteroids and PE/CPA in the present case might provide important information about therapeutic strategies for this life-threatening disorder as well as better understanding of the pathogenesis of this complex autoimmune disease.
2. Clinical report
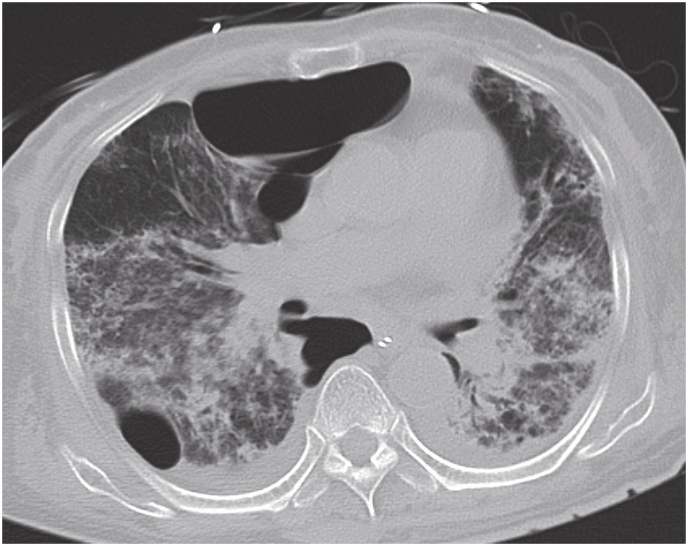
A previously healthy 78-year-old Japanese woman with a 3-month history of hemoptysis visited a primary care physician. She had never smoked and had no history of alcohol use. She did not take any medications or dietary supplements routinely. Laboratory data included a white blood cell count of 12,500/μL. Her chest radiograph showed infiltrative shadows in the lower lung fields bilaterally. She was initially treated with oral azithromycin and intravenous meropenem for 7 days; however, the infiltrative shadows expanded and she developed acute respiratory failure. Chest computed tomography (CT) showed diffusely distributed ground-glass opacities in both lung fields (Fig. 1). Bronchoscopy revealed active bleeding from the superior segmental bronchus of both lungs. She was diagnosed with DAH and treated with 2 cycles of pulse methylprednisolone therapy (1000 mg IV daily for 3 consecutive days). However, on hospital day 11, her respiratory and renal condition deteriorated. She was referred to our hospital.
Fig. 1.
Computed tomography on arrival at our hospital showed diffusely distributed ground-glass opacities in both lung fields.
Physical examination at the time of transfer showed that her body temperature was 37.3 °C, blood pressure was 144/63 mmHg, pulse was 60 beats per minute, oxygen saturation was 95% with oxygen supplementation at 12 L/min via a mask with reservoir bag, and respiratory rate was 32 breaths per minute. Her Glassgow Coma Scale score was 14 (4 for eyes, 4 for verbal, 6 for motor score). Lung auscultation revealed decreased breath sounds in the right lower lung field. The abdominal and cardiac examinations were normal. Laboratory data included an urine protein level 1+, uric blood level 3+, a white blood cell count of 21,400/μL with 97.8% neutrophils, hemoglobin of 10.3 g/dL, blood urea nitrogen of 48.3 mg/dL, creatinine of 1.11 mg/dL, and lactate dehydrogenase of 337 U/L. Arterial blood gas analysis showed severe type 1 respiratory failure. In addition, she was positive for both MPO-ANCA and anti-GBM antibodies (52.7 U/mL and 13.5 U/mL, respectively). Other data are shown in Table 1.
Table 1.
The laboratory data on admission.
| Hematology | Biochemistry | Serology | |||
|---|---|---|---|---|---|
| WBC | 21.4 × 103/μL | TP | 5.05 g/dL | β-D glucan | 6.0pg/mL |
| Neut. | 97.8% | Alb | 2.15 g/dL | Anti-nuclear antibody | 20 |
| Lymph. | 1.8% | BUN | 48.3 mg/dL | Rheumatoid factor | 4.1IU/mL |
| Mono. | 0.3% | Cre | 1.11 mg/dL | Anti-SS-A antibody | 1.0U/mL |
| Eo. | 0.0% | UA | 3.9 mg/dL | Anti-SS-B antibody | 1.0U/mL |
| RBC | 3.56 × 106/μL | Na | 140 mmol/L | PR3-ANCA | 1.0EU/mL |
| Hb | 10.3g/dL | K | 3.9 mmol/L | MPO-ANCA | 52.7EU/mL |
| Hct | 29.8% | Cl | 104mmol/L | Anti-Scl-70 antibody | 1.0U/mL |
| Plt. | 33.9 × 104/μL | Ca | 7.4 mg/dL | Anti-Jo1 antibody | 1.0U/mL |
| Coagulation | IP | 2.6 mg/dL | Anti-GBM antibody | 13.5EU/mL | |
| PT-INR | 1.19 | LD | 337 U/L | Anti-ARS antibody | <5.0 |
| Fibrinogen | 449mg/dL | AST | 13 U/L | Anti-ribonucleoprotein antibody | 2.0ng/mL |
| D-dimer | 1.96μg/mL | ALT | 10 U/L | Anti-smith antibody | 1.0U/mL |
| Blood gas analysis (O2 12L/min, mask reservoir) | CRP | 5.94mg/dL | Matrix metalloproteinase-3 | 307U/mL | |
| pH | 7.478 | Procalcitonin | 0.10 ng/dL | Bacteriological examinations | |
| PaO2 | 48.9 Torr | Fe | 14μg/dL | Blood culture | Negative |
| PaCO2 | 32.3 Torr | BNP | 61.1pg/mL | Sputum culture | Negative |
| HCO3− | 23.7mmol/L | Urinalysis | Urine culture | Negative | |
| RBC | 30–49/HPF | ||||
| WBC | 1–4/HPF | ||||
We could not perform any histological examinations because of her poor systemic condition. Her renal function deteriorated rapidly, with proteinuria and hematuria. We clinically diagnosed RPGN. Given the context of DAH, RPGN, and double-positivity for MPO-ANCA and anti-GBM antibodies, she was diagnosed with double-positive disease.
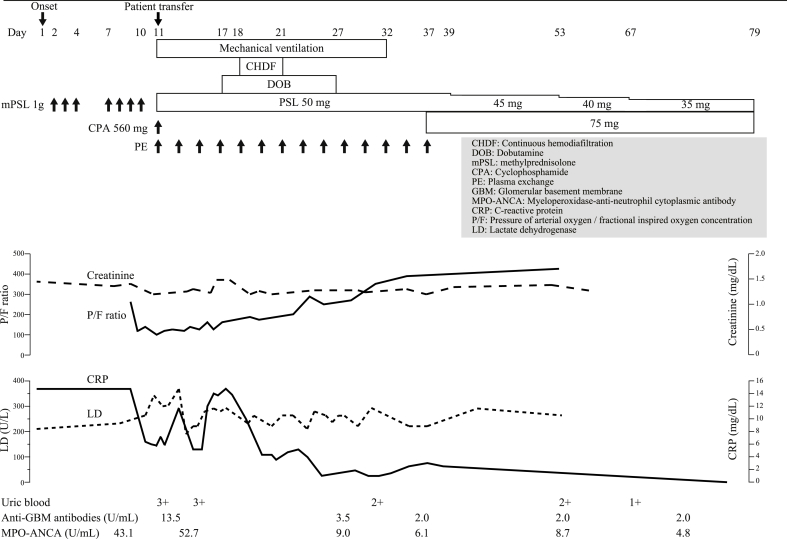
The patient underwent mechanical ventilation, vasopressor therapy, and continuous hemodiafiltration. Based on the diagnosis of double-positive disease, we added intravenous CPA (560 mg/day, single injection, then changed to oral administration on day 37, 75 mg/day) and PE (7 times every 2 weeks, total 14 times). She also received prednisolone, tapered from a maximum starting dose of 50 mg/day. Afterwards, her systemic condition gradually improved (Fig. 2). She had an uncomplicated hospital course and was successfully weaned from mechanical ventilation on day 32. MPO-ANCA levels decreased from 52.7 U/mL on day 11 to 6.1 U/mL on day 35, and anti-GBM antibody levels normalized on day 35. She improved clinically and was transferred to her previous doctor for rehabilitation on day 79. Chest CT 52 days after discharge showed substantial resolution of the ground-glass opacities. At 96 weeks after discharge with oral prednisolone therapy (4 mg/day), she was asymptomatic with normal serum levels of anti-GBM and MPO-ANCA antibodies.
Fig. 2.
Summary of the patient's clinical course.
3. Discussion
This is the first case report of a patient with steroid-refractory double-positive disease who was successfully treated with the addition of PE and CPA. The marked contrast in therapeutic response between corticosteroids alone and the addition of PE and CPA in this case strongly implies that earlier induction of combination therapy in double-positive disease aimed at rapid removal of pathogenic autoantibodies and prevention of ongoing antibody production might improve outcomes.
Anti-GBM antibodies play a major role in the pathogenesis of anti-GBM disease. They target the noncollagenous domain 1 of the α3 chain of type IV collagen [α3(IV)NC1], which has two major epitopes, EA and EB, found in the basement membrane specific to the glomerulus and alveolus [8,9]. With regards to the pathogenesis of AAV, B cell–derived ANCAs cause neutrophil activation and subsequent endothelial damage [10]. B cell depletion is associated with lower circulating ANCA levels and clinical remission in the majority of patients with AAV, strongly implicating that ANCAs are involved in the pathogenesis of AAV. Co-presentation with both anti-GBM antibodies and ANCAs appears more commonly than expected, as described above, although the mechanisms underlying the association between ANCAs and anti-GBM antibodies and the possible common pathogenic mechanisms remain unclear. No cross-reactions have been demonstrated between ANCAs and anti-GBM antibodies at the B-cell level; these two antibodies are antigenically distinct [11,12]. Several lines of evidence indicate that double-positive disease exhibits a more aggressive clinical course than either anti-GBM or AAV alone. Patients with double-positive disease require renal replacement therapy more frequently than patients with either anti-GBM disease or AAV alone [7,13]. In addition, serum creatinine levels in double-positive disease are higher than in either anti-GBM disease or AAV alone [6,7]. Our patient required renal replacement therapy at presentation. Furthermore, in one study, none of the double-positive patients with severe renal failure at presentation recovered renal function despite immunosuppression with or without PE [5]. This is in stark contrast to patients with pure AAV who present with a need for dialysis, of whom 75% recover renal function [14]. These findings suggest that double-positive disease is truly a hybrid disease phenotype and the aggressive features of double-positive disease may reflect more severe glomerular and alveolar damage due to an anti-GBM–mediated mechanism, in addition to ANCA-mediated endothelial disruption.
With regards to the mechanisms that initiate double-positive disease, almost all patients with anti-GBM disease have detectable levels of MPO-ANCA or PR3-ANCA before disease onset [15]. In addition, as seen in the present case, the duration of symptoms before diagnosis is similar for AAV and double-positive disease (median, 10–12 weeks), but is significantly longer than for single-positive anti-GBM disease (median, 2 weeks) [7]. Furthermore, double-positive patients have more features of chronicity on renal biopsy compared with patients with single-positive anti-GBM disease [7]. These findings suggest that ANCAs trigger endothelial injury and patients develop anti-GBM responses through the modification or exposure of originally sequestered disease epitopes in the GBM. This would explain why double-positive patients have anti-GBM antibodies with broader reactivity [16]. However, if so, it is unusual that only 10% of patients with AAV have anti-GBM disease. At present, it is not fully understood whether AAV predisposes to the development of anti-GBM disease or whether ANCA production occurs during the progression of anti-GBM disease. Further investigations are needed to elucidate the precise pathogenesis of double-positive disease.
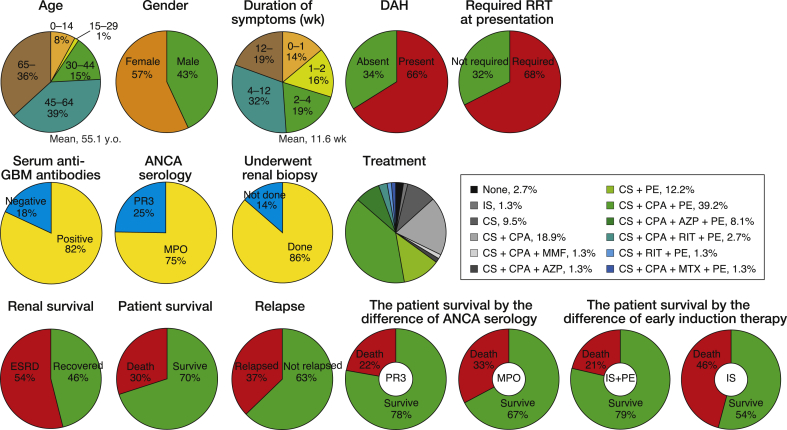
Based on our literature search, as of August 31, 2018, the 74 cases of double-positive disease have been described in 65 published articles (Fig. 3, Table S1). Overall, these case reports indicate that 75% (55 of 73) of patients have MPO-ANCA and 25% (18 of 73) of patients have PR3-ANCA. The comparative over-representation of MPO-ANCA in double-positive disease is a universally accepted finding in most retrospective studies [[5], [6], [7],13]. Prior cases of double-positive disease have demonstrated that double-positive patients with MPO-ANCA positivity have worse prognosis than double-positive patients with PR3-ANCA positivity: the mortality of double-positive patients with MPO-ANCA and PR3-ANCA are 33% (18 of 55) and 22% (4 of 18), respectively. Of note, a recent study showed that the majority (63%, 42 of 67) of patients with anti-GBM disease are positive for autoantibodies against the linear fragment of the MPO heavy chain N-terminus, whereas only 24% (16 of 67) of them were positive for intact MPO [17]. Furthermore, several autoantibodies against the MPO linear fragment are associated with disease severity, including high serum creatinine levels and high probability of progression to end-stage renal disease. The authors hypothesized that intermolecular and intramolecular epitope spreading from linear MPO peptides to α3(IV)NC1 and conformational MPO epitopes might occur during the initiation and progression of double-positive disease. Taken together, these findings suggest that MPO-ANCA, especially autoantibodies against the linear fragment of MPO, might be specifically involved in the development of double-positive disease.
Fig. 3.
Summary of 74 double-positive cases in the published articles. ANCA, antineutrophil cytoplasmic antibody; AZP, azathioprine; CPA, cyclophosphamide; CS, corticosteroids; DAH, diffuse alveolar hemorrhage; ESRD; end-stage renal disease; GBM, glomerular basement membrane; IS, immunosuppression; MMF, mycophenolate mofetil; MPO, myeloperoxidase; MTX, methotrexate; PE, plasma exchange; PR3, proteinase 3; RIT, rituximab; RRT, renal replacement therapy; wk, week.
In the present case, 2 cycles of pulse methylprednisolone therapy had no effect at all, whereas addition of PE and CPA improved the patient's systemic condition. The use of PE in association with corticosteroids and CPA is the gold-standard treatment for anti-GBM disease [18,19]. In generalized organ-threatening AAV, pulse CPA and corticosteroids are recommended to induce remission [1]. The use of PE for less severe renal disease and DAH in AAV remains controversial [1]. On the other hand, the optimal treatment strategy against double-positive disease has not been defined because of a lack of randomized trials for this rare disease. The outcomes of 52 double-positive patients with MPO-ANCA positivity identified in our literature search showed that the survival rate of patients who received both immunosuppressive drugs and PE is 74% (26 of 35), whereas the survival rate of patients who received immunosuppressive drugs is only 53% (9 of 17). The high survival rate associated with combination therapy suggests that the essentiality this therapeutic strategy for the treatment of double-positive disease. With regards to the mechanism why adding both CPA and PE were highly effective for improving systemic condition in the present double-positive patient, multidirectional machineries are considered to contribute to the disease remission. PE rapidly removes pathogenic autoantibodies (i.e., MPO-ANCA and anti-GBM antibodies). PE might also inhibit disease development through the rapid removal of autoantibodies against the linear fragment of MPO, which influence intermolecular and intramolecular epitope spreading. Furthermore, sufficient immunosuppression might repress disease progression through the prevention of ongoing antibody production as well as amelioration of end-organ inflammation.
There are four findings in the present case that support double-positive disease being a truly hybrid disease phenotype. First, as reported in several retrospective studies of double-positive disease [[5], [6], [7],13,20,21], the present patient was older than most patients with anti-GBM disease alone. Second, the duration of symptoms before diagnosis in the present patient was relatively long compared to that of patients with anti-GBM disease alone. Third, the present case demonstrated severe manifestations (DAH and need for dialysis) at presentation, which are prevalent in patients with anti-GBM disease but less common in patients with AAV [7]. Fourth and most importantly, the present patient did not respond to immunosuppressive therapy alone, but had a good clinical response after the induction of PE and CPA. Intriguingly, because of the nature of the AAV component, patients with double-positive disease are more vulnerable to relapse than patients with anti-GBM disease, which is generally considered a monophasic non-relapsing condition. In one study, 22% (2 of 9) of double-positive patients relapsed, compared with 0 of 34 cases of anti-GBM disease alone [13]. Another study reported that approximately half of patients with AAV and double-positive patients have recurrent disease, whereas none with anti-GBM disease alone experienced relapse during a median follow-up of 4.8 years [7]. Thus, in addition to early diagnosis, careful long-term follow-up and monitoring are crucial in the management of double-positive disease, including the consideration for maintenance immunosuppression. Over 96 weeks of follow-up after discharge, our patient did not have recurrence while on continuous administration of low-dose prednisolone.
In summary, we described a case of steroid-refractory double-positive disease successfully treated with the addition of PE and CPA. Patients with either AAV or anti-GBM disease should be tested for second antibody to screen for double-positive disease. Since double-positive disease might be a truly hybrid disease phenotype, early intensive combination immunosuppression and PE therapy, and careful long-term follow-up and maintenance immunosuppression are vital for achieving an excellent outcome.
Declarations of interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2018.09.016.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Bosch X., Guilabert A., Espinosa G., Mirapeix E. Treatment of antineutrophil cytoplasmic antibody associated vasculitis: a systematic review. J. Am. Med. Assoc. 2007;298:655–669. doi: 10.1001/jama.298.6.655. [DOI] [PubMed] [Google Scholar]
- 2.McAdoo S.P., Pusey C.D. Anti-glomerular basement membrane disease. Clin. J. Am. Soc. Nephrol. 2017;12:1162–1172. doi: 10.2215/CJN.01380217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennette J.C., Falk R.J., Bacon P.A., Basu N., Cid M.C., Ferrario F., Flores-Suarez L.F., Gross W.L., Guillevin L., Hagen E.C., Hoffman G.S., Jayne D.R., Kallenberg C.G., Lamprecht P., Langford C.A., Luqmani R.A., Mahr A.D., Matteson E.L., Merkel P.A., Ozen S., Pusey C.D., Rasmussen N., Rees A.J., Scott D.G., Specks U., Stone J.H., Takahashi K., Watts R.A. 2012 revised international Chapel hill Consensus Conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 4.Jayne D.R., Marshall P.D., Jones S.J., Lockwood C.M. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990;37:965–970. doi: 10.1038/ki.1990.72. [DOI] [PubMed] [Google Scholar]
- 5.Levy J.B., Hammad T., Coulthart A., Dougan T., Pusey C.D. Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 2004;66:1535–1540. doi: 10.1111/j.1523-1755.2004.00917.x. [DOI] [PubMed] [Google Scholar]
- 6.Rutgers A., Slot M., van Paassen P., van Breda Vriesman P., Heeringa P., Tervaert T.W. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ANCAs in crescentic glomerulonephritis. Am. J. Kidney Dis. 2005;46:253–262. doi: 10.1053/j.ajkd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.McAdoo S.P., Tanna A., Hrušková Z., Holm L., Weiner M., Arulkumaran N., Kang A., Satrapová V., Levy J., Ohlsson S., Tesar V., Segelmark M., Pusey C.D. Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 2017;92:693–702. doi: 10.1016/j.kint.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedchenko V., Bondar O., Fogo A.B., Vanacore R., Voziyan P., Kitching A.R., Wieslander J., Kashtan C., Borza D.B., Neilson E.G., Wilson C.B., Hudson B.G. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N. Engl. J. Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanacore R., Ham A.J., Voehler M., Sanders C.R., Conrads T.P., Veenstra T.D., Sharpless K.B., Dawson P.E., Hudson B.G. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–1234. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McClure M., Gopaluni S., Jayne D., Jones R. B cell therapy in ANCA-associated vasculitis: current and emerging treatment options. Nat. Rev. Rheumatol. 2018;14:580–591. doi: 10.1038/s41584-018-0065-x. [DOI] [PubMed] [Google Scholar]
- 11.Cui Z., Zhao M.H., Segelmark M., Hellmark T. Natural autoantibodies to myeloperoxidase, proteinase 3, and the glomerular basement membrane are present in normal individuals. Kidney Int. 2010;78:590–597. doi: 10.1038/ki.2010.198. [DOI] [PubMed] [Google Scholar]
- 12.Hellmark T., Niles J.L., Collins A.B., McCluskey R.T., Brunmark C. Comparison of anti-GBM antibodies in sera with or without ANCA. J. Am. Soc. Nephrol. 1997;8:376–385. doi: 10.1681/ASN.V83376. [DOI] [PubMed] [Google Scholar]
- 13.Alchi B., Griffiths M., Sivalingam M., Jayne D., Farrington K. Predictors of renal and patient outcomes in anti-GBM disease: clinicopathologic analysis of a two-centre cohort. Nephrol. Dial. Transplant. 2015;30:814–821. doi: 10.1093/ndt/gfu399. [DOI] [PubMed] [Google Scholar]
- 14.Jayne D., Rasmussen N., Andrassy K., Bacon P., Tervaert J.W., Dadoniené J., Ekstrand A., Gaskin G., Gregorini G., de Groot K., Gross W., Hagen E.C., Mirapeix E., Pettersson E., Siegert C., Sinico A., Tesar V., Westman K., Pusey C. European Vasculitis Study Group, A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N. Engl. J. Med. 2003;349:36–44. doi: 10.1056/NEJMoa020286. [DOI] [PubMed] [Google Scholar]
- 15.Olson S.W., Arbogast C.B., Baker T.P., Owshalimpur D., Oliver D.K., Abbott K.C., Yuan C.M. Asymptomatic autoantibodies associate with future anti-glomerular basement membrane disease. J. Am. Soc. Nephrol. 2011;22:1946–1952. doi: 10.1681/ASN.2010090928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang R., Hellmark T., Zhao J., Cui Z., Segelmark M., Zhao M.H., Wang H.Y. Antigen and epitope specificity of anti-glomerular basement membrane antibodies in patients with goodpasture disease with or without anti-neutrophil cytoplasmic antibodies. J. Am. Soc. Nephrol. 2007;18:1338–1343. doi: 10.1681/ASN.2006111210. [DOI] [PubMed] [Google Scholar]
- 17.Li J.N., Cui Z., Wang J., Hu S.Y., Jia X.Y., Guan Z., Chen M., Xie C., Zhao M.H. Autoantibodies against linear epitopes of myeloperoxidase in anti-glomerular basement membrane disease. Clin. J. Am. Soc. Nephrol. 2016;11:568–575. doi: 10.2215/CJN.05270515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson J.P., Whitman W., Briggs W.A., Wilson C.B. Plasmapheresis and immunosuppressive agents in antibasement membrane antibody-induced Goodpasture's syndrome. Am. J. Med. 1978;64:354–359. doi: 10.1016/0002-9343(78)90065-7. [DOI] [PubMed] [Google Scholar]
- 19.Savage C.O., Pusey C.D., Bowman C., Rees A.J., Lockwood C.M. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980-4. Br. Med. J. (Clin. Res. Ed.) 1986;292:301–304. doi: 10.1136/bmj.292.6516.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosch X., Mirapeix E., Font J., Borrellas X., Rodríguez R., López-Soto A., Ingelmo M., Revert L. Prognostic implication of anti-neutrophil cytoplasmic autoantibodies with myeloperoxidase specificity in anti-glomerular basement membrane disease. Clin. Nephrol. 1991;36:107–113. [PubMed] [Google Scholar]
- 21.Segelmark M., Hellmark T., Wieslander J. The prognostic significance in Goodpasture's disease of specificity, titre and affinity of anti-glomerular-basement-membrane antibodies. Nephron Clin. Pract. 2003;94:c59–c68. doi: 10.1159/000072022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.