Abstract
Purpose
To show the utility of MRI and histology in diagnosing rare cases of trigeminal hypertrophic interstitial neuropathy (HIN).
Observations
A 57-year-old African-American woman presented with a 4-year history of right eye proptosis with tearing, headaches, and worsening right-sided trigeminal neuralgia symptoms and jaw pain. HIV and diabetes tests were negative and thyroid function was normal. MRI identified abnormal thickening of all trigeminal nerve divisions and proptosis secondary to right trigeminal nerve V1 division enlargement. The excised tissue contained S-100 positive Schwann cells in an onion-bulb pattern. Headaches resolved, but proptosis and mild trigeminal neuralgia remained 1 year post-surgery.
Conclusions and importance
Trigeminal HIN is very rare, but presents as chronic progressive ocular symptoms with trigeminal neuralgia. Trigeminal nerve hypertrophy is identified by MRI and confirmed histopathologically by detection of Schwann cells in an onion bulb formation.
Keywords: Exophthalmos, Onion bulb, Schwann cell, Trigeminal nerve, Cranial nerve
1. Introduction
The list of differential diagnoses for unilateral proptosis is extensive. The most common cause of proptosis in adults is thyroid orbitopathy, but other etiologies should be considered including inflammatory orbital disorders, vascular orbital disorders, intraorbital neoplasms, metastatic orbital tumors, retrobulbar hemorrhage, Cushing's syndrome, and orbital fracture.1
Unilateral proptosis caused by hypertrophic interstitial neuropathy (HIN) of a retro-orbital nerve has not been reported, to our knowledge. Thirty-seven years ago the first case of focal trigeminal HIN presenting with facial trigeminal analgesia with no ocular involvement was reported.2 The first histologically documented case of focal trigeminal HIN with ocular symptoms was reported a little over 25 years ago.3 Neuroimaging utilizing CT and MRI clearly displayed the unilateral hypertrophic trigeminal nerve and the neuro-pathological evaluation of the excised tissue was significant for concentric rings of hypertrophic Schwann cells in an “onion bulb” formation; pathognomonic for HIN.4, 5, 6 Thus, we present the third case of focal trigeminal HIN, and the second case to present with ocular symptoms, be detected by MRI, and be confirmed by histopathological detection of Schwann cells in an onion bulb formation. Based upon current cases, the diagnosis of trigeminal HIN in a patient with unilateral proptosis can be aided significantly by careful medical history and a comprehensive neuro-exam with MRI studies.
2. Case report
A 57-year-old African-American woman presented to the Eye Clinic at Louisiana State University Health Sciences Center in Shreveport, LA in late February 2015 with a 4-year history of right eye proptosis and excessive tearing. She complained of generalized headaches and was seen by neurology, diagnosed with migraine headaches, and given topiramate without improvement of symptoms. A careful review of systems revealed pain triggered by chewing, speaking, and brushing her teeth, as well as numbness, burning, and tingling in the trigeminal V1 and V2 dermatomal distributions. She reported right-sided facial muscle spasms and hyperesthesia to external stimuli. There was no history of periorbital lesions, herpetic keratitis, trauma to the head, or infection. Her past medical history was negative for diabetes, thyroid disease, cancer, oral herpes, recent infection, and recent head trauma. Her family history was negative for autoimmune disorders and neurological disease.
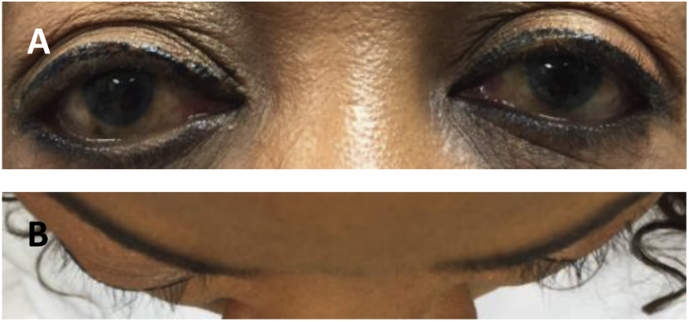
On physical examination, her pupils were equally round and reactive to light, extraocular muscles were intact, and the dilated fundus exam was normal. The right eye was actively tearing (Fig. 1A) and visibly proptotic (Fig. 1B). Hertel exophthalmometer measurement of the right eye showed a 25 mm proptosis compared to a 21 mm proptosis of the left eye. The patient had a mild right eye ptosis with dermatochalasis that was more pronounced in appearance due to the proptosis of the other eye. Right eyelid closure weakness was noted. There were no other neurological abnormalities. The remainder of the physical exam was within normal limits.
Fig. 1.
Ocular photos. (A) Frontal and (B) overhead photos, taken at initial presentation, demonstrating 4 mm right eye proptosis.
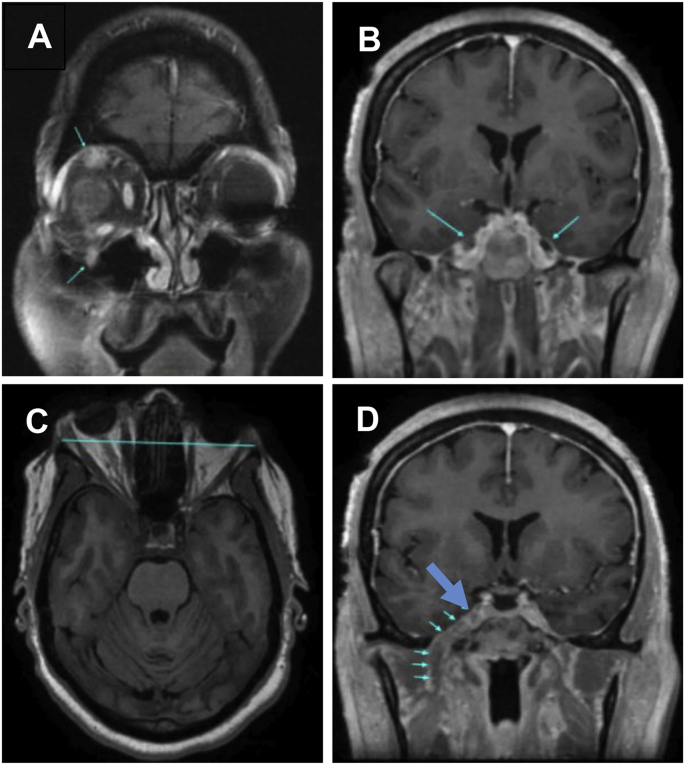
Screening tests for diabetes and thyroid disease were within normal limits and HIV testing was negative. MRI of the orbit, face, and neck with and without contrast performed a month after presentation showed enlargement and enhancement of the trigeminal nerve in the right cavernous sinus continuing through the right superior orbital fissure, foramen rotundum, and foramen ovale (Fig. 2). The right eye proptosis appeared to be due to the thickened nerve pushing forward on the orbit. A 17 × 16 mm, well-circumscribed, solid and cystic mass arising from the V2 division of the trigeminal nerve was situated in the right pterygopalatine space. A nasal endoscopy performed 2 months after initial presentation showed a left mid-septal deviation/spur, but was otherwise a normal nasopharynx.
Fig. 2.
MRI scans at one month post presentation. (A) Post contrast coronal T1 with fat saturation. The upper blue arrow demonstrates the enlarged ophthalmic nerve (V1) and the lower blue arrow shows the enlarged maxillary nerve (V2) as they exit the orbit. (B) Post contrast coronal T1 with fat saturation. The blue arrows point to bilateral Meckel's cave. The right Meckel's cave (large blue arrow) enhances with contrast and is larger than the left Meckel's cave, which is normal and does not enhance with contrast. (C) Axial T1W. The blue line represents the interzygomatic line and demonstrates the proptosis of the right eye. (D) Post contrast coronal T1 weighted images with fat saturation. The bottom five small arrows demonstrate the abnormally thickened and contrast enhancing mandibular nerve V3 as it exits through the foramen ovale. The top large blue arrow points to the two upper branches of the trigeminal nerve, V1 and V2. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A right frontotemporal craniotomy was performed 5 months post-presentation for biopsy and de-bulking of the known right pterygopalatine mass. Dissection of the extradural middle fossa revealed the 2nd and 3rd divisions of the trigeminal nerve. A mass was detected that extended through the foramen rotundum into the pterygopalatine fossa. The mass was dissected away from the middle fossa, the foramen rotundum was enlarged, and the tumor was dissected and removed along the distribution of V2 through the foramen rotundum and in the pterygopalatine fossa. Multiple excised fragments of soft tan colored tissue were submitted for complete pathological analysis. Analysis of an intraoperative frozen section yielded a preliminary diagnosis of spindle cell neoplasm consistent with schwannoma. The patient tolerated the procedure well with no intra- or post-operative complications.
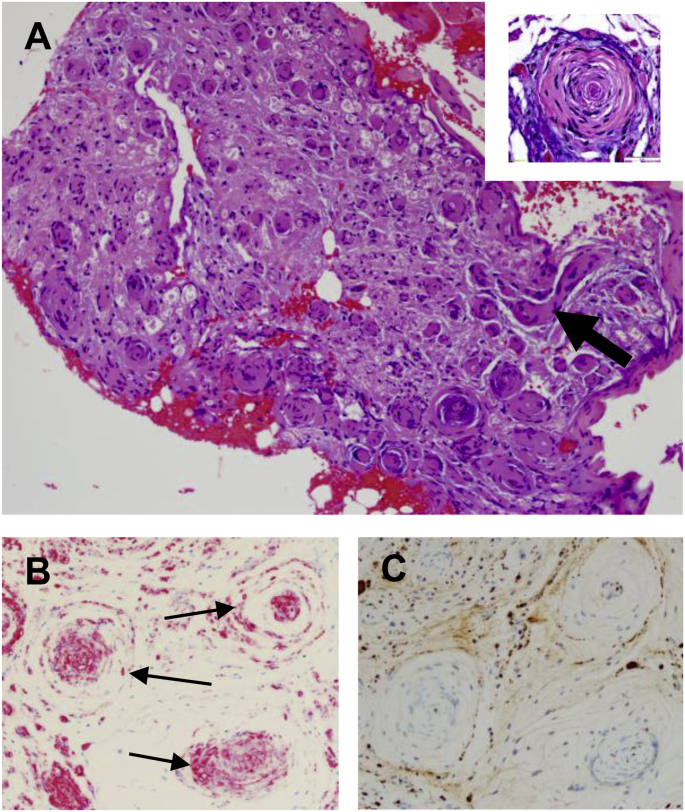
Sections of the resected tissue fragments were of myelinated nerve and ganglion tissue (Fig. 3A). Microscopic evaluation revealed fibrotic endoneurium containing copious amounts of dense collagen. Myxoid debris and collagen were found in varying amounts scattered throughout the stroma. Examination of the endoneurium showed proliferation of S-100 positive (Fig. 3B) and neurofilament positive axons (Fig. 3C), CD34 (tumor antigen) negative (not shown), and narrow spindle cells with wavy nuclei consistent with the appearance of Schwann cells.7,8 The Schwann cells were arranged both haphazardly in the endoneurium and in concentric rings, i.e., onion bulb formations, of varying sizes around axons (Fig. 3A).
Fig. 3.
Histopathology of excised tissue. (A) Low power (10X) of the hematoxylin and eosin stained excised trigeminal nerve tissue containing numerous hypertrophic endoneurial cells in fibrotic stroma. Multiple “onion bulb” structures are shown in cross-section. Note the longitudinal section of one affected axon (large arrow). Insert: a high power (40X) magnification of central axon surrounded by layers of cells forming the concentric rings or “onion bulb” pattern. (B) S-100 positive staining (pink) of the “onion bulbs” (arrows) is consistent with Schwann cell origin. (C) Neurofilament staining of central axons (brown dots at center of onion bulbs) shows normal axon size, ruling out neurofibromatosis. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The patient's headaches resolved following surgery in July 2015, but a feeling of right-sided eye pressure remained. The patient continued to have a measurable proptosis of the right eye, but no visual complaints. Her 1-year follow-up MRI showed a decrease in lesion size in comparison to the preoperative MRI consistent with the tumor debulking procedure. Unfortunately, there was not a significant resolution of her trigeminal neuralgia symptoms. However, she reports having learned to avoid many of her known triggers and is satisfied with her course of treatment thus far. Based upon her persistent symptoms of trigeminal neuralgia, a neurology consult was requested to evaluation her for therapy to gain further symptomatic relief.
A review of the clinical presentation, diagnosis, treatment and clinical outcomes of the reported2,3,6 and current trigeminal HIN cases (Table 1) revealed a diverse demographics that includes a 41 year-old Native American male, a 61 year-old Hispanic female, and a 57 year-old African-American female. Notably, all cases presented with a >3 year past medical history of unilateral facial neuralgia and pain. Two cases presented with 4–5 year history of orbital symptoms; unilateral ptosis with diplopia or unilateral proptosis, ptosis, and tearing. The results of neuroimaging studies (CT and MRI) supported a preliminary diagnosis of hypertrophic neuropathy involving the trigeminal nerve. All cases were confirmed as trigeminal HIN by the detection of Schwann cell “onion bulb” formations in the pathology sections of the resected hypertrophic trigeminal nerve tissue. Symptoms persisted in 2 cases post surgery, while one died from surgical complications.
Table 1.
Review of trigeminal HIN cases.
| Age | Race | Gender | Presentation | Imaging & Pathology | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 412 | American Indian | Male | 3 yr history of OS facial pain. Paroxysmal attacks of OS facial pain triggered by pressure to upper OS lip. Dx: Trigeminal neuralgia |
CT scan prompted decompression surgery. Biopsied trigeminal nerve. Pathology; Schwann cells in “onion bulb” formation. | Previously, 2 antral sinus operations Left suboccipital craniectomy |
Facial pain resolved, moderate OS persistent hyperesthesia |
| 613,6 | Hispanic | Female | 3 yr history of diplopia, OS temporal and intraorbital pain, 5 yr history of progressive OS ptosis. Partial 3rd & 6th nerve palsy. Dx: Cranial tumor | MRI showed thickening of the OS trigeminal nerve 3rd division. Biopsied trigeminal nerve. Pathology; Schwann cells in “onion bulb” formation. |
Left-sided pterional craniotomy | Surgical complications caused the patient's death |
| 57 | African-American | Female | 4 yr history of non-painful OD proptosis (4 mm) with excessive tearing and medically unresponsive migraine headaches; trigeminal facial neuralgia and muscle spasms triggered by external stimuli. Dx: Trigeminal neuralgia |
MRI showed thickening of the OD trigeminal nerve. Biopsied trigeminal nerve. Pathology; Schwann cells in “onion bulb” formation. |
Right frontotemporal craniotomy with partial removal of maxillary/pterogomaxillary mass | Headaches resolved, proptosis persisted, partial right V1 and complete right V2 numbness. |
3. Discussion
Similarities were noted regarding the clinical course, neuroimaging, and pathologic findings in the cases of HIN of the trigeminal nerve. Each patient reported a history of trigeminal neuralgia and chronic facial pain unresponsive to pain control therapy, 2 patients described a trigeminal neuralgia triggered by external stimuli, and 2 reported 3–5 year progression of ocular symptoms. Trigeminal nerve thickening was revealed by neuroimaging studies. MRI with and without contrast scans yields more lesion detail than CT images.2,3,6 In this regard, the true incidence of HIN is unknown since HIN cases may be under reported based on the CT scan imaging. In cases 2 and 3 (Table 1), the extent of unilateral trigeminal nerve enlargement and involvement was distinguished definitively by MRI scans. The histopathology of the excised tissues was consistent with benign lesions composed of concentric layers of Schwann cell processes and collagen around an axon forming an “onion bulb” pattern. The spindle-shaped cells forming the “onion bulb” structures were strongly S-100 positive and CD34 negative consistent with proliferating Schwann cells, rather than perineuronal cells.6, 7, 8
Multiple pathologic insults have been linked to hypertrophic mononeuropathies including nerve entrapment, compression, trauma, inflammation, perineurial barrier defect, and increased blood vessel permeability.4, 5, 6 No history of head trauma and none of the aforementioned pathologic entities were identified in our patient (or the previously reported cases).
The molecular pathogenesis of hypertrophic neuropathy in general, and HIN of the trigeminal nerve in particular, is not clearly defined; some investigational studies point to a reactive/traumatic etiology2,6,9, 10, 11 and others favor a neoplastic process.12, 13, 14 Several cellular mechanisms can cause nerve enlargement as reviewed by Guibord et al.15 They include, but are not limited to, intense endoneurial inflammation and edema (e.g., leprosy), marked onion bulb formation by Schwann cells (e.g., hereditary motor and sensory neuropathy type 1), reactive perineurial cell proliferation (e.g, localized hypertrophic neuropathy), and neoplastic Schwann cell proliferation (e.g., neurofibromatosis).
The unique neuro-cytopathology characteristically found in hypertrophic mononeuropathies has earned it the alternate name of onion-bulb neuropathy or Schwann cell onion-bulb tumor.2 The onion bulb pattern is thought to be due to repeated episodes of demyelination and remyelination seen in a variety of different hereditary and chronic diseases including, diabetic polyneuropathy,16 chronic inflammatory polyneuropathy,17 Refsum's disease,18 globoid cell leukodystrophy,19 Recklinghausen's disease (neurofibromatosis type I).20,21
The dilemma in this case was the disparity between the presenting symptoms of unilateral proptosis with tearing and the history of medically unresponsive headaches. The MRI provided the basis for a presumptive diagnosis of hypertrophic neuropathy involving the trigeminal nerve. Armed with this insight, a more careful history and neurological examination affirmed a history of chronic trigeminal neuralgia. Reduction in the HIN mass resulted in only a partial reduction in symptoms.
4. Conclusions
Clinicians evaluating patients with chronic unilateral mild proptosis or ptosis with trigeminal neuralgia should consider HIN as one of the differentials. The patient's workup should include MRI scan, being more specific than CT, to elucidate the pathology and post-surgical confirmation of the diagnosis by cytopathology.
Patient consent
This case study was conducted in compliance with HIPPA regulations. The IRB waiver and patient's consent to publish de-identified clinical photographs and radiological images are on file.
Acknowledgement and disclosures
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: ERD, JP, KTTD, MPL, MAS, GP, WAB.
Authorship
All authors attest they meet current ICMJE criteria for Authorship.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2018.09.010.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Kamminga N., Jansonius N.M., Pott J.W., Links T.P. Unilateral proptosis: the role of medical history. Br J Ophthalmol. 2003;87(3):370–371. doi: 10.1136/bjo.87.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baskin D.S., Townsend J.J., Wilson C.B. Isolated hypertrophic interstitial neuropathy of the trigeminal nerve associated with trigeminal neuralgia. Case report of an entity not previously described. J Neurosurg. 1981;55:987–990. doi: 10.3171/jns.1981.55.6.0987. [DOI] [PubMed] [Google Scholar]
- 3.Jordan J.E., Lane B., Marks M., Chang Y., Weinberger M. Cranial hypertrophic interstitial neuropathy. Am J Neuroradiol. 1992;13:1552–1554. [PMC free article] [PubMed] [Google Scholar]
- 4.Dyck P.J. Experimental hypertrophic neuropathy. Pathogenesis of onion-bulb formations produced by repeated tourniquet applications. Arch Neurol. 1969;21(1):73–95. doi: 10.1001/archneur.1969.00480130087010. [DOI] [PubMed] [Google Scholar]
- 5.McLeod J.G., Prineas J.W., Walsh J.C. Onion bulb formations in chronic polyneuropathies. Proc Aust Assoc Neurol. 1971;8:125–129. [PubMed] [Google Scholar]
- 6.Chang Y., Horoupian D.S., Jordan J., Steinberg G. Localized hypertrophic mononeuropathy of the trigeminal nerve. Arch Pathol Lab Med. 1993;117(2):170–176. [PubMed] [Google Scholar]
- 7.Sarlomo-Rikala M., Miettinen M. Gastric schwannoma--a clinicopathological analysis of six cases. Histopathology. 1995;27(4):355–360. doi: 10.1111/j.1365-2559.1995.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 8.Perentes E., Nakagawa Y., Ross G.W., Stanton C., Rubinstein L.J. Expression of epithelial membrane antigen in perineurial cells and their derivatives. An immunohistochemical study with multiple markers. Acta Neuropathol. 1987;75(2):160–165. doi: 10.1007/BF00687077. [DOI] [PubMed] [Google Scholar]
- 9.Iyer A., Hassan E., Newman W., Kneen R. Congenital trigeminal anaesthesia: a rare preventable cause of visual loss in children. BMJ Case Rep. 2012 doi: 10.1136/bcr.03.2012.5955. 2012 Jul 3. pii: bcr0320125955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson L.N., Krohel G.B., Allen S.D., Mozayeni R. Recurrent herpes labialis as a potential risk factor for nonarteritic anterior ischemic optic neuropathy. J Natl Med Assoc. 1996;88(6):369–373. [PMC free article] [PubMed] [Google Scholar]
- 11.Yassini P.R., Sauter K., Schochet S.S., Kaufman H.H., Bloomfield S.M. Localized hypertrophic mononeuropathy involving spinal roots and associated with sacral meningocele. Case report. J Neurosurg. 1993;79:774–778. doi: 10.3171/jns.1993.79.5.0774. [DOI] [PubMed] [Google Scholar]
- 12.Bilbao J.M., Khoury N.J., Hudson A.R., Briggs S.J. Perineurioma (localized hypertrophic neuropathy) Arch Pathol Lab Med. 1984;108:557–560. [PubMed] [Google Scholar]
- 13.Emory T.S., Scheithauer B.W., Hirose T., Wood M., Onofrio B.M., Jenkins R.B. Intraneural perineurioma. A clonal neoplasm associated with abnormalities of chromosome 22. Am J Clin Pathol. 1995;103:696–704. doi: 10.1093/ajcp/103.6.696. [DOI] [PubMed] [Google Scholar]
- 14.LaPoint S.F., Powers J.M., Woodruff J.M. Schwann cell-onion bulb tumor of the trigeminal nerve: hyperplasia, dysplasia or neoplasia? Acta Neuropathol. 2000;99(1):67–72. doi: 10.1007/pl00007408. [DOI] [PubMed] [Google Scholar]
- 15.Guibord N., Chalk C., Wein F., Richardson J., Snipes G.J., Del Carpio R. Trigeminal nerve hypertrophy in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1998;51(5):1459–1462. doi: 10.1212/wnl.51.5.1459. [DOI] [PubMed] [Google Scholar]
- 16.Ballin R.H., Thomas P.K. Hypertrophic changes in diabetic neuropathy. Acta Neuropathol. 1968;11(2):93–102. doi: 10.1007/BF00690213. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda M., Ikeda S., Sakurai S., Nezu A., Yanagisawa N., Inuzuka T. Hypertrophic neuritis due to chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): a postmortem pathological study. Muscle Nerve. 1996;19(2):163–169. doi: 10.1002/(SICI)1097-4598(199602)19:2<163::AID-MUS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Fardeau M., Engel W.K. Ultrastructural study of a peripheral nerve biopsy in Refsum's disease. J Neuropathol Exp Neurol. 1969;28(2):278–294. [PubMed] [Google Scholar]
- 19.Suzuki K., Grover W.D. Krabbe's leukocystrophy (globoid cell leukodystrophy). An ultrastructural study. Am J Obstet Gynecol. 1970;106(7):385–396. [PubMed] [Google Scholar]
- 20.Onishi A., Nada O. Ultrastructure of the onion bulb-like lamellated structure observed in the sural nerve in a case of von Recklinghausen's disease. Acta Neuropathol. 1972;20(3):258–263. doi: 10.1007/BF00686907. [DOI] [PubMed] [Google Scholar]
- 21.Thomas P.K., King R.H., Chiang T.R., Scaravilli F., Sharma A.K., Downie A.W. Neurofibromatous neuropathy. Muscle Nerve. 1990;13(2):93–101. doi: 10.1002/mus.880130202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.