Abstract
Diabetic myonecrosis (DMN) is an under-diagnosed complication of long-standing poorly controlled diabetes mellitus. It presents as abrupt pain and swelling of the extremity, mostly lower limbs. Diagnosis is often delayed as it mimics a number of clinical entities such as deep vein thrombosis (DVT), cellulitis, necrotizing fasciitis and malignancy. Failure to properly identify this condition can result in increased morbidity through exposure to unnecessary tests and biopsy. A 56-year-old male with a history of complicated type 2 diabetes mellitus, hypertension presented to emergency with gradually worsening left calf pain for last 2 weeks. A lower-extremity venous Doppler was negative for DVT. Magnetic resonance imaging (MRI) was suggestive of muscle edema likely of inflammatory etiology. Muscle biopsy revealed myonecrosis with ischemic myopathy and was negative for vasculitis or inflammatory myopathy. He was managed conservatively and his symptoms resolved in 4 weeks. After 6 months he had recurrence in right thigh which was managed conservatively too. Given these findings, a diagnosis of recurrent diabetic myonecrosis was made. Myonecrosis is a less known microvascular complications of diabetes and should always be keep in mind when evaluating a diabetic patient with muscle pain. Diagnosis can be made on MRI in appropriate clinical settings. The clinical course is usually self-limiting and patients respond well to supportive medical therapy that involves bed rest, strict glycemic control along with analgesic.
Keywords: Diabetic myonecrosis, Painful limb, Diabetes
Highlights
-
•
Diabetic myonecrosis remains under diagnosed entity.
-
•
High index of suspicion is required in diabetic patient with muscle pain.
-
•
MRI is modality of choice.
1. Introduction
Recently, diabetes has emerged as global problem, affecting more than 400 million populations worldwide [1]. Macrovascular and microvascular complications related to diabetes are well known problems that contribute to significant morbidity, disability and mortality among diabetic population. Little is known in literature regarding diabetic myonecrosis (DMN), also known as diabetic muscle infarction that can lead to significant morbidity in patients with long standing diabetes. Pathogenesis of this rare entity is poorly understood. Literature reports role of microangiopathy, atherosclerotic plaquing of microvessels, superimposed vasculitis, ischemic-reperfusion injury and thrombosis of microvasculature, along with causative role of coagulation fibrinolytic cascade with hypercoagulable state secondary to low antithrombin-III levels [[2], [3], [4]].
The diagnosis of DMN is often very challenging and difficult. The diagnosis is often missed or the condition is misdiagnosed unless physician or radiologist is well aware of the clinical presentation and diagnostic clues [Table 1]. Physical exam reveals swollen and tender muscle, mimicking deep venous thrombosis (DVT) and almost all the time presumptive diagnosis of DVT is made and often examination remains suboptimal due to fear of throwing thrombus. However, if examination done properly, muscle may feel indurated that can suggest muscular etiology. At time diagnosis of lymphangitis/cellulitis is considered due to presence of subcutaneous edema and fluid. Other differential include abscess, necrotizing fasciitis, compartment syndrome, osteomyelitis, polymyositis, and dermatomyositis, drug induced myositis and superficial thrombophlebitis. However, absence of overlying erythema and classic constitutional symptoms should make physician to consider diagnosis of DMN in long standing poorly controlled diabetes with leg pain [2]. Range of motion of surrounding joints may be limited because of the pain. Acute compartment syndrome can be easily excluded clinically in the absence of history of recent trauma or surgery or from chronic compartment syndrome in the absence of chronic exercise. Rarely, tumors like lymphoma are considered in a patient with history of lymphoma. Other common tumor in extremity in this age group is soft tissue sarcoma, however presence of abrupt pain and rapid development of swelling does not favor sarcoma. Aim of the laboratory testing and imaging studies is to narrow the differentials. Blood work classically shows normal to mildly increased WBC, normal to elevated inflammatory markers (ESR, CRP), normal or mild elevation of muscle enzymes, like CPK [5]. Other laboratory test that are performed to exclude autoimmune causes of muscle pain includes rheumatologic panel including ANA, ds DNA, antiphospholipid antibody, septic screen to rue out infective conditions and mannometry to rule out compartment syndrome.
Table 1.
Clinical and imaging mimics of Diabetic Myonecrosis.
| Disease condition | Major cause | Clinical signs and symptoms | Imaging |
|
|---|---|---|---|---|
| US | Cross-sectional | |||
| Diabetic Myonecrosis | Long standing uncontrolled diabetes | Swollen and tender muscle without overlying erythema | Non-specific muscle edema, subfascial and interfascial fluid | MRI T1: subtle loss of intermuscular septae T2: subcutaneous and intramuscular edema in a reticulated pattern as well as subfascial and interfascial fluid collections T1 Post Gad: heterogeneous enhancement corresponding to T2 signal changes |
| DVT | Immobility, hypercoagulability | Swollen and tender muscle with overlying erythema | Clot in deep veins of involved extremities | |
| Superficial thrombophlebitis | Recent IV, infection, pregnancy, obesity, hypercoagulability | Swollen extremity, tender erythematous skin and increased temperature | Clot in the superficial veins of involved extremities | |
| Cellulitis/lymphangitis | Congenital or acquired lymphedema with superimposed infection/inflammation | Swollen extremity, tender erythematous skin and increased temperature | Subcutaneous edema and extrafascial fluid | MRI: T2: subcutaneous edema in a reticulated pattern and extrafascial fluid collections |
| Abscess | Trauma, surgery | Swollen and tender muscle with or without overlying erythema, increased temperature | Localized fluid collection within subcutaneous soft tissue and/or muscles with surrounding soft tissue edema and hyperemia, = /-septae, sediment, or gas within the central fluid collection |
MRI T1 Post gad: Peripherally enhancing fluid collection with extensive surrounding edema, DWI: restricted diffusion Post contrast CT: Peripherally enhancing fluid collection with extensive surrounding edema + soft tissue air |
| Acute or chronic Compartment syndrome | Acute- high velocity deceleration trauma Chronic exertional – exercise related |
Swollen extremity, pain, paresthesia, paresis, pain, decreased pulse and pale skin | Acute- muscle edema + soft tissue hematoma, Chronic-non-specific muscle edema and subfascial edema |
MRI Acute- features similar to diabetic myonecrosis + High T1 signal correspond to hematoma Chronic- features similar to diabetic myonecrosis |
| Necrotizing Fasciitis | Rapidly progressive muscle and subcutaneous tissue infection, most commonly polymicrobial | Swollen extremity, local pain and tenderness with systemic signs of infection-fever, chills, nausea, weakness | Non-specific muscle edema, subfascial fluid, limited by soft tissue air, if present suggestive of diagnosis | MRI features similar to diabetic myonecrosis Post contrast CT: soft tissue swelling + heterogeneously enhancing muscles and subcutaneous tissue ± abscess + soft tissue air |
| Inflammatory myositis | Polymyositis- multi-compartment, recurrent increased serum markers, dermatomyositis-skin involvement, drug induced myositis-history of predisposing drug intake | Swollen and tender muscle with or without overlying erythema, +/− increased temperature | Non-specific muscle edema, | MRI features similar to diabetic myonecrosis |
| Osteomyelitis | Bone infection | Swollen extremity, local pain and tenderness with systemic signs of infection-fever, chills, nausea, weakness | Non-specific soft tissue edema | MRI STIR: Bone marrow edema and surrounding soft tissue edema T1 Post Gad: heterogeneous enhancement corresponding to STIR signal changes |
| Tumors | Lymphoma or soft tissue sarcoma | Progressive swelling and pain of involved extremity, prior history in case of Lymphoma | Soft tissue mass with internal vascularity with surrounding edema | MRI T1 Post Gad: hydrogenous enhancing mass ± surrounding soft tissue edema Lymphoma: restricted diffusion on DWI |
Ultrasonography (US) is the initial imaging study that should be performed to rule out venous thrombosis, superficial thrombophlebitis, underlying abscess or localized fluid collection and necrotizing fasciitis [5]. Magnetic resonance imaging (MRI) is next modality of choice with sensitivity of T2-weighted MRI approaches 90% for picking up active muscle disease however specificity for muscle infarction only 43% [6].
With the increasing prevalence of diabetes, cases of DMN are expected to increase. It is important for physician to be aware of this under diagnosed condition to catch the diagnosis at the earliest and prevent iatrogenic morbidity with unnecessary invasive diagnostic interventions.
2. Case presentation
A 56 years old male with complicated type-2 diabetes mellitus (DM) with end stage renal disease on peritoneal dialysis and diabetic retinopathy presented to emergency department with gradually worsening left calf pain for last 2 weeks. Pain was described as sharp, constant, 10/10 in intensity, localized to back side of left calf, no radiation, no relation with exertion and minimally relieved with analgesic medication. He denied history of trauma, surgery, or injection in the area. No history of fever or chills, though he reported swelling of left calf with no overlying redness. He was able to ambulate with difficulty because of excruciating pain. Physical examination of left lower extremity showed swelling over left side of back of left calf. No overlying erythema. On palpation, area was tender, warm and indurated. Range of motion of left knee was limited secondary to pain. Motor strength was intact and patient had no neurovascular deficits. Initial laboratory testing showed white blood cell count of 4400 cells/mm3, HBA1c level of 12.1%, glucose level of 360 mg/dl, erythrocyte sedimentation rate (ESR) of 120 mm/h, and C-reactive protein (CRP) level of 22 mg/L, serum creatine kinase of 426U/L (normal value 60–110U/L). Other electrolytes were within normal limits. The rheumatologic work up; coagulation profile and septic screen were negative. US color Doppler showed no evidence of deep venous thrombosis. However, it showed non-specific muscle edema in lateral and medial head of gastrocnemius [Fig. 1]. Vascular and general surgery recommended conservative treatment, as there were no sign of compartment syndrome. However, due to persistent pain, MRI was considered which demonstrated muscle enlargement, extensive high T2 muscle signal and corresponding heterogeneous enhancement in the muscles of left calf as well as subcutaneous edema and subfascial fluid [Fig. 2]. Differential diagnosis of infective/inflammatory myosistis was made. To confirm the diagnosis and to rule out other causes, muscle biopsy was performed by surgeon without complication. Patient tolerated the procedure well. Biopsy showed coagulative necrosis with thrombosis of small vessels, surrounding inflammation and areas of early fibrosis in skeletal muscle [Fig. 3]. Diagnosis of diabetic myonecrosis was made on the basis of clinical presentation, imaging and biopsy results. He was managed with bed rest, analgesics, and better glycemic control with optimization of insulin regimen. He followed up in clinic with better glucose control and complete resolution of symptoms in 4weeks. However, he had a recurrence of similar pain after 8 months, but this time pain was localized in the right thigh. He was non-compliant with his insulin regimen over last 1 month and laboratory testing showed poor glucose control. On US color Doppler, there was no evidence of deep venous thrombosis, however, non-specific muscle edema in lateral and medial head of gastrocnemius was noted [Fig. 4]. MRI of right thigh was done which showed enlargement and extensive high T2 muscle signal with corresponding heterogeneous enhancement in the right thigh anterior compartment muscles group as well as subcutaneus and subfascial edema and fluid [Fig. 5]. Presumptive diagnosis of recurrent diabetic myonecrois was made. Patient was again managed by supportive therapy consisting of bed rest; analgesic, strict blood glucose control with complete resolution of symptoms in 6 weeks. This case report has been reported in line with the SCARE criteria [7].
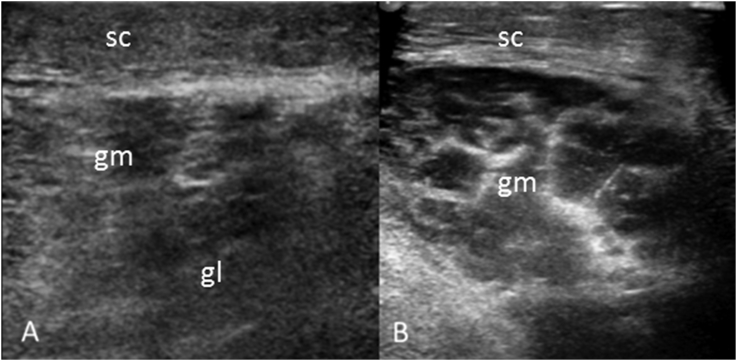
Fig. 1.
US left calf (A) longitudinal and (B) transverse demonstrating edema in the subcutaneous tissue and in the lateral head (gl) and medial head (gm) of gastrocnemius.
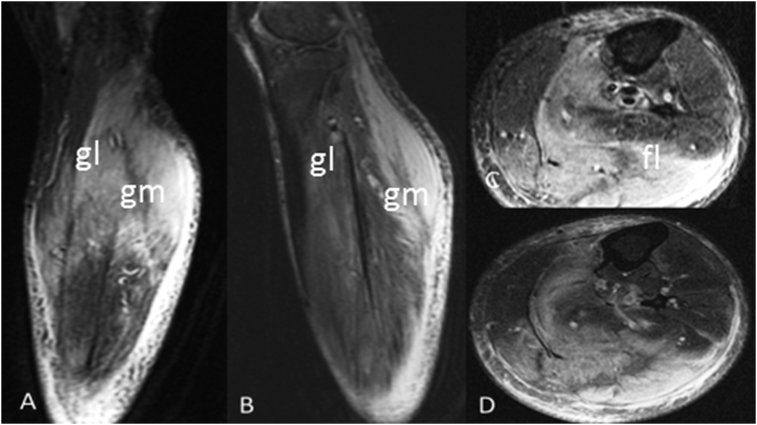
Fig. 2.
Myonecrosis left calf. Extensive high T2 muscle signal and corresponding enhancement in the medial head (gm) and lateral head (gl) of the gastrocnemius muscle, and to a lesser extent within the medial part of soleus muscle and popliteus as well as well as subcutaneous edema and subfascial fluid (fl). (A): Coronal-T2WI, (B): Coronal- Post Gad T1WI, (C): Axial- T2WI, (D): Axial-Post Gad TI1WI.
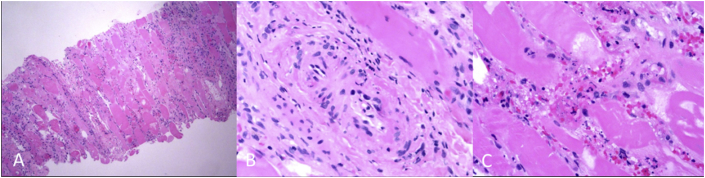
Fig. 3.
Histology (H & E stain) of left calf showing skeletal muscle with areas of confluent myonecrosis with surrounding edema, fibrosis, and inflammatory cell infiltration. (A)×10 magnification, (B)×100 magnification (C)×400 magnification.
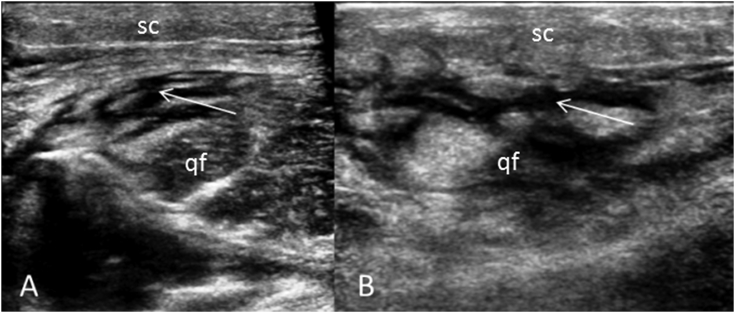
Fig. 4.
US right thigh (A) transverse and (B) longitudinal demonstrating edema in the subcutaneous tissue and quadriceps femoris muscle (qf). Note sub-fascial fluid and mildly displaced or partial loss of normal fatty intermuscular septae (arrows).
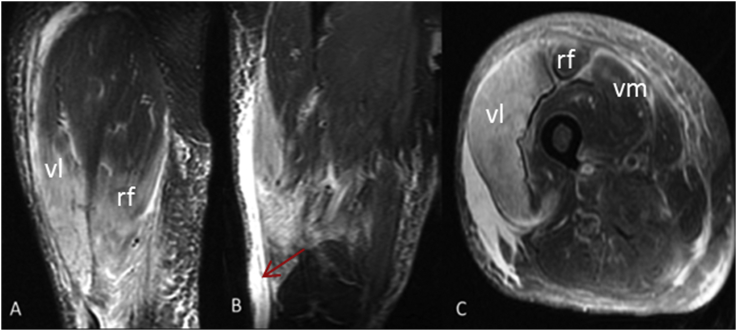
Fig. 5.
Myonecrosis right thigh. Extensive high T2 muscle signal and corresponding enhancement in the RIGHT thigh is most prominent within the vastus lateralis muscle (vl), and to a lesser extent within the adjacent fibers of the rectus femoris (rf) and possibly within the superficial fibers of the vastus medialis (vm) as well as subcutaneous edema and subfascial fluid (red arrow). (A): Coronal- Post Gad T1WI, (B): Coronal- T2WI, (C): Axial-Post Gad TI1WI. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3. Discussion
DMN is a rare and underrated complication of poorly controlled and long-standing diabetes. Often, the diagnosis is missed or misdiagnosed on the initial patient encounter leading of increased morbidity. It is reported to be more common in type −1 diabetes but can also occur in type-2 diabetes with high incidence among females [8]. Our patient had known complicated Type 2 diabetes, diagnosed more than 15 years back. Patients typically already have known macro and micro vascular complications of diabetes [9]. Classically the patient presents with acute onset of pain, tenderness, and edema of the affected muscles group [8,9]. Quadriceps is the most affected muscle (as in our case), reported in 60–65% of the cases followed by hip adductors in 13%, hamstrings in 8% and hip flexors in only 2% [2]. Involvement of the flexors and abductors of hip and muscles of upper limb is rare [2]. Bilateral disease is reported in about 10% of the patients [3,8]. Recurrence in the same or opposite limb is reported in about 50% of cases [3], our patient had recurrence in opposite limb.
Based on clinical symptoms and physical findings of muscle tenderness, similar to most reported cases [5,8], DVT was the presumptive diagnosis in our case. However, it is important to emphasize that the use of anticoagulation should be avoided while waiting to make diagnosis as anticoagulation can worsen the condition. Also, there should be low threshold to order urgent MRI of extremities after ruling out DVT in diabetic patients. Consistent with the findings of our patient, typical findings on MRI T1-weighted image includes diffuse edema of involved muscles groups with mildly displaced or partial loss of normal fatty intermuscular septa. There is diffuse high intensity signal on T2 weighted images and corresponding heterogeneous enhancement of the affected muscles group, which represents edema and inflammation, and a focal area of low intensity signal suggestive of muscle necrosis and ischemia [10]. These features specifically help in differentiating DMN from neoplasm, which appear more heterogeneous and completely disrupt fascial planes. Absence of bone marrow edema and enhancement helps rule out osteomyelitis. Presence of subfasicial edema and fluid on T2WI comfortably exclude lymphangitis/cellulitis [11]. MRI cannot help differentiate various muscles pathologies associated with edema and enhancement; however, it is important for physicians and radiologists to have high index of clinical suspicion of DMN in appropriate clinical settings.
Early recognition is necessary to avoid unnecessary diagnostic testing. Diagnostic tests for thrombosis and muscle necrosis will be positive and therefore are unhelpful. Treatment is usually consists of conservative therapy, comprising of bed rest, analgesics to control pain and aggressive control of diabetes mellitus. Although most patients have an excellent short-term recovery with complete resolution of symptoms in 4–6 weeks, high 5-year mortality is reported secondary to underlying diabetic complications [[3], [4], [5]].
Muscle biopsy should be avoided unless diagnosis is unclear and patient is deteriorating with conservative measurements. Muscle biopsy is often associated with iatrogenic risk such as delayed tissue healing and complications such as hematoma and superimposed infection in patients with poorly controlled diabetes [5]. Typical findings on a biopsy include necrosis, lymphocytic infiltration, and thrombosis of microvasculature and areas of surrounding fibrosis [9]. Our patient underwent muscle biopsy on his first admission due to low level of suspicion of DMN, however second time, diagnosis of recurrent DMN was made only on clinical and MRI findings.
4. Conclusion
Diabetic myonecros is an under-diagnosed complication as diagnosis can be challenging.
Clinicians need to have a high index of suspicion when evaluating a diabetic patient with muscle pain and should have low threshold to use urgent MRI to make the diagnosis of DMN. Current recommendations include making the diagnosis on the basis of clinical and radiological findings alone and avoidance of anticoagulation and unnecessary invasive investigation such as open muscle biopsy due to associated complications. Findings described in this report can be helpful in providing the clinicians useful information in making the diagnosis of DMN.
Patient consent
The informed consent was obtained from the patient included in the study.
The study protocol is consistent with the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee.
Provenance and peer review
Not commissioned externally, peer reviewed.
Grant support
None.
Ethical approval
Does not apply.
Patient consent was obtained.
Sources of funding
None.
Author contribution
Sonali Gupta: Writing paper.
Pradeep Goyal: Writing paper, reviewing images.
Pranav Sharma: Writing paper.
Priti Soni: Interpreting pathology slides, reviewing paper.
Puneet S Kochar: Reviewing paper, editing.
Conflicts of interest
Authors declare no conflicts of interest.
Research registry number
Do not appy
Guarantor
Sonali Gupta.
Contributor Information
Sonali Gupta, Email: gupta.sonali2706@gmail.com.
Pradeep Goyal, Email: pradeepgoyal78@gmail.com.
Pranav Sharma, Email: drpranavsharma29@gmail.com.
Priti Soin, Email: drpritisoin1980@gmail.com.
Puneet S. Kochar, Email: drpuneetkochar@gmail.com.
References
- 1.World Health Organization . World Health Organization; 2016. Global Report on Diabetes. [Google Scholar]
- 2.Mukhopadhyay P., Barai R., Philips C.A., Ghosh J., Saha S. An unusual case of myonecrosis. Case Rep. Endocrinol. 2011 Jul 28;2011 doi: 10.1155/2011/624020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastogi A., Bhadada S., Saikia U., Bhansali A. Recurrent diabetic myonecrosis: a rare complication of a common disease. Indian J. Med. Sci. 2011 Jul 1;65(7):311. [PubMed] [Google Scholar]
- 4.Palmer G.W., Greco T.P. Diabetic thigh muscle infarction in association with antiphospholipid antibodies. Semin. Arthritis Rheum. 2001 Feb 28;Vol.30(4):272–280. doi: 10.1053/sarh.2001.19961. (WB Saunders) [DOI] [PubMed] [Google Scholar]
- 5.Nagdev A., Murphy M., Sisson C. Bedside ultrasound for the detection of diabetic myonecrosis. Am. J. Emerg. Med. 2008 Oct 1;26(8) doi: 10.1016/j.ajem.2008.02.017. 969-e3. [DOI] [PubMed] [Google Scholar]
- 6.Morcuende J.A., Dobbs M.B., Buckwalter J.A., Crawford H. Diabetic muscle infarction. Iowa Orthop. J. 2000;20:65. [PMC free article] [PubMed] [Google Scholar]
- 7.Agha R.A., Fowlwer A.J., Saeta A., Barai I., Rajmohan S., Orgill D.P., Afifi R., Al-Ahmadi R., Albrecht J., Alsawadi A., Aronson J. The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016 Oct 1;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Choudhury B.K., Saikia U.K., Sarma D., Saikia M., Choudhury S.D., Bhuyan D. Diabetic myonecrosis: an underreported complication of diabetes mellitus. Indian J. Endocrinol. Metabol. 2011 Jul;15(Suppl1):S58. doi: 10.4103/2230-8210.83052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhasin R., Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J. Community Hosp. Intern. Med. Perspect. 2013 Jan 1;3(1):20494. doi: 10.3402/jchimp.v3i1.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jelinek J.S., Murphey M.D., Aboulafia A.J., Dussault R.G., Kaplan P.A., Snearly W.N. Muscle infarction in patients with diabetes mellitus: MR imaging findings. Radiology. 1999 Apr;211(1):241–247. doi: 10.1148/radiology.211.1.r99ap44241. [DOI] [PubMed] [Google Scholar]
- 11.Goyal P., Chaudry G., Alomari A.I. Lymphedema. Springer International Publishing; 2015. Conventional imaging modalities for the diagnosis of lymphedema; pp. 149–155. [Google Scholar]