Abstract
The lifetime risk for diabetic patients to develop a diabetic foot ulcer (DFU) is 25%. In these patients, the risk of amputation is increased and the outcome deteriorates.
More than 50% of non-traumatic lower-extremity amputations are related to DFU infections and 85% of all lower-extremity amputations in patients with diabetes are preceded by an ulcer; up to 70% of diabetic patients with a DFU-related amputation die within five years of their amputation.
Optimal management of patients with DFUs must include clinical awareness, adequate blood glucose control, periodic foot inspection, custom therapeutic footwear, off-loading in high-risk patients, local wound care, diagnosis and control of osteomyelitis and ischaemia.
Cite this article: EFORT Open Rev 2018;3:513-525. DOI: 10.1302/2058-5241.3.180010
Keywords: diabetic foot ulcers, infection, osteomyelitis, revascularization, wound dressings
Introduction
Diabetic foot ulcer (DFU) is a localized injury to the skin and/or underlying tissue of the foot of patients with diabetes mellitus; the occurrence of foot problems increases the risk of amputation and death of these patients.1-16 Half of DFUs occur in the plantar surface and the other half in other areas of the foot. Neuropathy, peripheral artery disease (PAD), deformities of the foot related to motor neuropathy and minor foot trauma, infection and osteomyelitis are major threats relating to DFU.1,3,10 Eradication of the infection is difficult and recurrences are common, leading to purulent ulcers, functional distortion of the foot and the need for amputation.9 The lifetime risk of diabetic patients developing a DFU is 25%;1,2 > 50% of non-traumatic lower-extremity amputations are related to DFU infections and 85% of all lower-extremity amputations in diabetic patients are preceded by a DFU.1,2,7,9 Up to 70% of diabetic patients with a DFU-related amputation die within five years of their amputation;2 mortality increases with the level of amputation.7 Inevitably, DFUs have a significant financial cost;2,6 this cost is estimated at > $1 billion annually in the United States,6 approximately £650 million annually in the UK and > €10 billion annually in Europe.2
Optimal management of patients with DFUs includes clinical awareness, adequate blood glucose control, periodic foot inspection, custom therapeutic footwear, off-loading, local wound care, diagnosis and control of osteomyelitis and ischaemia.7-9 However, most of these patients will end with an above-ankle amputation. This article aims to summarize the current knowledge for the diagnosis and management of patients with DFUs and to increase the awareness of the treating physicians for their prevention, early diagnosis and prompt treatment.
Pathophysiology
DFUs can be divided into neuropathic, ischaemic and neuro-ischaemic.1,3,10 Sensorimotor and sympathetic diabetic neuropathy are major risk factors for DFUs.10,12 Sensory neuropathy leads to loss of pain, pressure and temperature sensation; in this setting, trauma, even minor, ulceration or infection is perceived less or not at all by the patient.13 Motor neuropathy leads to muscle weakness and atrophy of the lower foot and ankle, resulting in abnormal loading of the plantar aspect of the foot.10,11 Foot deformities such as hammer toes and claw foot develop secondary to motor neuropathy, leading to focal areas of increased pressure and formation of calluses and ulcers. Sympathetic neuropathy results in reduced sweating, skin dryness with cracks and fissures, and increased blood flow to the foot with arteriovenous shunting.13 PAD presents in approximately 50% of patients with DFUs.14,15 Macrovascular and microvascular PAD leads to reduced skin blood flow, thickening of basement membrane and endothelial capillary swelling,10 and is associated with poor ulcer healing, need for amputation and poor outcome.14,15
Evaluation
Evaluation of DFU requires a multidisciplinary foot care team.17 It should include the patient’s medical history, laboratory values, dermatological, musculoskeletal, neurological and vascular status.18 Past medical history should include blood glucose values, previous ulcers or amputations, vascular symptoms, surgeries and angioplasties, smoking habit, neuropathic symptoms, renal and retinal function, and co-morbitities.18-25 Physical examination should determine the size, depth, colour and position of the DFU, neuropathy, ischaemia or neuro-ischaemia of the foot, bone exposed, necrosis, infection, and the colour and consistency of exudates. Musculoskeletal examination should include foot deformities, joint mobility, muscle wasting and presence of calluses.1 Muscles, cartilage, tendons and ligament function will be altered due to motor neuropathy, which will culminate in a limitation of foot mobility and an abnormal walking pattern. Common diabetic foot deformities are claw toes (metatarsophalangeal joint hyperextension with interphalangeal flexion), hammer toes (distal phalangeal extension), prominent metatarsal heads, pes cavus and Charcot arthropathy.18 Calluses develop at increased pressure sites, which progressively thicken, haemorrhage underneath and eventually ulcerate.25 Charcot arthropathy occurs secondary to neuropathy and most often affects the midfoot. It is characterized by acute inflammation with collapse of the foot and/or the ankle. The patient initially sustains an unperceived injury but continues to walk until a severe inflammatory process leads to osteopenia, distention of joints, and foot and/or ankle dislocation. In late stages, the foot develops a ‘rocker bottom’ appearance.26 Charcot arthropathy in diabetic patients is a more complex entity with a higher mortality compared with Charcot arthropathy in non-diabetic patients.26
Neurological evaluation
Neurological evaluation should include the protective sensation with the Semmes-Weinstein 10-g monofilament, the vibratory sensation with a 128-Hz tuning fork and the cold-warm discrimination.19-22 The 10-g monofilament is used for evaluation of protective sensation.20 Protective pain can be established by application of the 10-g monofilament across several sites of the foot including the first, third and fifth metatarsal head and the plantar surface of the distal phalanx of the hallux.19 A positive test is characterized by the inability of the patient to feel the monofilament when it is pressed against the foot with enough force to bend the filament, and it is associated with clinically significant large-fibre peripheral neuropathy.21 The 128-Hz tuning fork is used for evaluation of vibratory sensation. It should be tested over the tip of the hallux bilaterally.19 The response is abnormal when the patient loses vibratory sensation while the examiner still perceives vibration.21 Absence of cold-warm discrimination identifies patients with small nerve fibre damage; these patients experience a burning or electric shock type pain that is worse at night.22
Vascular evaluation and tissue microcirculation
Vascular evaluation should include palpation of the femoral, popliteal, posterior tibial and dorsalis pedis artery pulses (characterized as present or absent).19 Patients with absent pulses should undergo measurement of the ankle-brachial index (ABI). ABI is a measure of perfusion at the level of the foot; a portable Doppler ultrasound probe (frequency range 5 to 8 MHz) and sphygmomanometer cuff are used to measure systolic pressures on the arm, the ankle and pedal circulation (posterior tibial, dorsalis pedis and, occasionally, peroneal arteries) on both legs. The ratio of the pressures in the distal circulation to the lower value obtained from the brachial arteries yields an ABI. An ABI < 0.9 indicates impaired arterial blood flow. However, evidence of a normal ABI in a person with diabetes is not reliable as increased arterial stiffness may reduce distal flow, and medial arterial calcification may result in incompressible vessels leading to falsely elevated pressures (ABI > 1.1).23 Local tissue perfusion can also be measured by toe pressure, Doppler ultrasound or transcutaneous oxygen tension. A toe pressure of < 50 mmHg in the presence of ulceration indicates severe limb ischaemia. Venous refilling of > 5 seconds or delayed discoloration may indicate poor arterial perfusion on a pink and relatively warm foot.24 Any diabetic patient with DFU and limb ischaemia should be referred immediately to acute services as it is a limb-threatening, and possibly a life-threatening, condition.2
A significant issue in patients with diabetic angiopathy and critical limb ischaemia with tissue loss is to assess the tissue viability before the revascularization or before determining the level of amputation. Identifying tissue viability among patients with DFUs is important, given its association with failure to heal. In addition, failure to successfully determine the level of amputation adds the subsequent risk of perioperative cardiovascular events that can lead to death. Standard Doppler arterial waveforms, ABI measurement, toe pressures, transcutaneous tissue oxygenation and thermal mapping have been traditionally used to potentially provide regional perfusion information and also to predict amputation levels.17,27,28 However, a significant variability in foot and limb salvage has been observed in clinical practice. Although all these techniques provide useful information in the assessment of tissue and foot perfusion there is no widely accepted standard for the evaluation of tissue microcirculation and the prediction of wound healing.
When ABI measurements are difficult in patients with PAD, simple alternatives are the toe-to-brachial index (TBI)29 or the pole test.30 For management of PAD, the recommended imaging techniques are Duplex ultrasonography (DUS), CT and MRI against a gold standard of contrast angiography that uses an intra-arterial injection of contrast media and biplanar radiographs. Nephrogenic systemic fibrosis and exposure to radiation are the main limitations of CT and MR angiography in patients with impaired renal function. DUS offers easy non-invasive two-dimensional (2D) colour images and haemodynamic data using Doppler shift frequency analysis of the arterial tree to the level of the pedal vessels. It is the easiest of vascular imaging modalities, though its availability may be limited by the expertise of the operator.31 Compared with contrast angiography, CT offers far superior resolution; however, CT uses more contrast medium which is a limitation in patients with deteriorating renal function. MR angiography is relatively non-invasive when compared with CT and has better overall diagnostic accuracy compared with CT and DUS. There is very limited evidence that suggests that DUS is comparable with contrast angiography in planning clinical management.32 For the evaluation of healing cutaneous wounds, there is a need for a functional microcirculation. A functional microcirculation is essential to maintain tissue viability. In current practice, non-invasive technology assessments of tissue viability may be achieved by measuring the TBI or transcutaneous oxygen tension (TcPO2). TBI is measured using an optical sensor to detect arterial flow and a cuff connected to a sphygmomanometer in the same way as blood pressure is measured. TcPO2 is measured using skin surface sensors at 43 °C to 45 °C; this is a non-invasive technique signifying local tissue nutrition. TcPO2 measurements are affected by capillary density and are influenced by oedema as well as skin thickness.33 In patients with ankle pressure < 60 mmHg, TcPO2 and TBI measurements are recommended as markers of tissue microcirculation.32,34 TBI and TcPO2 indicate the likelihood of wound healing on account of their sensitivity to the microcirculation and should be used in preference to ABI.34-36 TcPO2 > 40 mmHg and TBI > 20 mmHg are associated with decreasing probabilities of amputation and, therefore, increasing probability of wound healing.37
New techniques using a laser scanner to detect perfusion38 and an optical scanner to measure tissue oxygen saturation39 have the potential to improve the predictive values of current techniques of assessing tissue viability around wounds.
Infection evaluation
DFU infection should be recognized, classified and treated promptly.40,41 Risk factors for DFU infection include: 1) a positive probe-to-bone test result; 2) wound chronicity (DFU present for > 30 days); 3) history of recurrent DFUs; 4) traumatic foot wound; 5) PAD in the affected limb; 6) previous lower extremity amputation; 7) neuropathy with loss of protective sensation; 8) renal insufficiency; and 9) history of walking barefoot.41
DFU infection should be defined clinically by ≥ 2 classic findings of inflammation (redness, warmth, swelling, tenderness or pain) or purulence, and should be classified by severity into mild (superficial and limited in size and depth), moderate (deeper or more extensive) or severe (accompanied by systemic signs or metabolic perturbations).40-42 Patients with neuropathy may not manifest the typical signs of inflammation. Secondary signs suggestive of infection include wound undermining, friable or discoloured granulation tissue, malodour or wound exudates.
DFU cultures should be obtained for microbiological examination. Ideally, soft-tissue or bone-tissue cultures should be obtained from the base of the debrided wound and sent for cultures.2 Alternatively, a deep wound swab or aspiration of purulent secretions may provide a diagnostic sample. Cultures should be obtained from clinically infected DFUs; cultures should not be obtained from clinically non-infected wounds, as all ulcers are contaminated.2
The most common pathogens in DFU infections are Gram-positive cocci – mainly Staphylococcus aureus and Staphylococcus epidermidis.42 The most common Gram-negative pathogens are Escherichia coli, Klebsiella pneumonia, Proteus species and Pseudomonas aeruginosa. Chronic, severe infections or infections occurring after antibiotic treatment are often polymicrobial by Gram-negative bacilli and Gram-positive cocci. Anaerobic pathogens are more commonly isolated in necrotic wounds and infections of ischaemic feet.40
Osteomyelitis is a serious complication of DFU infection and increases the risk of treatment failure and need for amputation. Osteomyelitis should be suspected when an ulcer lies over a bony prominence, especially when it fails to heal despite adequate off-loading, or when a toe is erythematous and indurated (sausage toe). Probe-to-bone test is a useful clinical diagnostic tool for osteomyelitis; if a blunt sterile metal probe gently inserted through a wound strikes bone, this substantially increases the likelihood that the patient has osteomyelitis.43 Plain radiographs of the foot have relatively low sensitivity and specificity for documentation or exclusion of osteomyelitis (Fig. 1). MRI is the imaging modality of choice for the diagnosis of osteomyelitis. When MRI is unavailable or contraindicated, leukocyte or antigranulocyte bone scans may be performed.41 The definitive method for diagnosing osteomyelitis is a bone-tissue biopsy with histological sections showing inflammation and infection or a positive result on bone culture.40
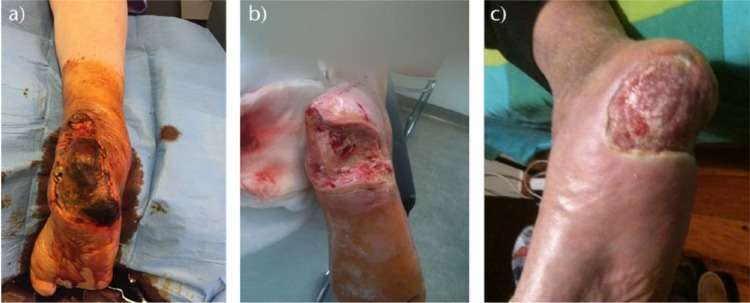
Fig. 1.
a, b) Photographs of the right foot of a 69-year-old diabetic woman with a heel and a medial malleolus purulent DFU. c) Anteroposterior and d) lateral radiographs of the right leg show complete distortion of the ankle and talar joints and osteolysis at the distal tibia and fibula. She was treated with a below-knee amputation, intravenous antibiotics and blood glucose control.
Treatment
Revascularization, surgical and conservative wound debridement, and eradication of the infection are the principal aims of DFU treatment. The essential steps to preserve a moist, non-infected wound are to: 1) treat the underlying cause; 2) control ischaemia; 3) control infection; 4) debride the wound; 5) dress the wound; and 6) off-load the foot.2 Achieving metabolic control and treatment of comorbidities should involve optimal diabetes control, managing risk factors such as increased blood pressure and dyslipidaemia, quitting smoking, management of oedemas and correction of malnutrition. The patients’ footwear should be examined for proper fit and wear; patients should be educated that any foreign bodies may ulcerate the foot.43 Local wound care requires tissue debridement, control of inflammation and infection, moisture balance and epithelial edge advancement.
Revascularization
Acute limb ischaemia is a clinical emergency and may increase the risk of death or amputation if not managed early and effectively. Impaired circulation or ischaemia is an indication for revascularization in order to achieve and maintain DFU healing and avoid or delay amputation.2 Angiography helps to determine the feasibility of and approach to arterial revascularization. An endovascular-first approach in appropriately selected patients is often advocated based on a lower procedural risk.44,45 Among the emerging imaging modalities are hyperspectral imaging and indocyanine green fluorescent angiography.17,27,28,32,46 Diabetic angiopathy predominantly affects the infrapopliteal vessels. A new concept which has been recently investigated in order to achieve a better outcome after revascularization procedures is the angiosome concept. The angiosome concept is based on the hypothesis that the lower extremity surface is supplied by infrapopliteal arteries consistently corresponding to regions of the foot.47 There is limited and conflicting evidence suggesting that angiosome-directed revascularization procedures improve wound healing and limb salvage.48-51 It seems reasonable to target the angiosome when feasible. However, it should be mentioned that angiosome-directed revascularization is not always feasible, often due to the occlusive disease affecting the corresponding feeding artery. Indirect revascularization through collateral vessels plays a crucial role in the treatment of ischaemic foot and can enhance the healing of ulcers and decrease the amputation rate in patients with critical limb ischaemia with tissue loss. However, direct revascularization in concordance with the angiosome hypothesis does not correlate with the results of all studies.47
Debridement of all necrotic, callus and fibrous tissue is paramount for DFU healing. Debridement can be surgical, autolytic, larval, hydrosurgical and ultrasonic; the choice depends on the available expertise, patient preferences, clinical context and cost.52,53 Debridement may be performed in a single stage or it may need to be repeated for maintenance of the wound bed. During debridements and at wound dressing changes, the size, depth and characteristics of the wound must be evaluated; ischaemia, infection or inflammation may impair wound healing, and when these occur, the treatment plan must be modified.8
Surgical debridement
The role of surgery is important as it has been shown to be more effective in DFU healing compared with other debridement options.54-62 However, it has been based more on clinical judgment and less on structured evidence. In the surgical debridement of DFUs, the clinical presentation, infection and severity of the ulcer, anatomic concepts of the foot and timing for surgery should be considered.60,61
Surgical debridement involves cutting away dead and infected tissues followed by daily application of saline-moistened cotton gauze. Excision of infected tissue and non-viable tissue should be done until a bleeding healthy base is obtained (Fig. 2).60-62 If new necrotic tissue continues to form, wounds should be debrided as often as necessary.54 It is important to debride the wound margins as well as the centre of the wound to prevent the ‘edge effect’,55 that is to turn a chronic ulcer into an acute one. Surgical debridement should be repeated no less frequently than on a weekly basis as it has been associated with more rapid healing of ulcers.56 Healthy tissue loss should be minimized, foot function should be preserved and deformities which can precipitate recurrence of ulcers should be prevented or corrected.57 Metatarsal head resection is a common treatment for metatarsal head osteomyelitis in DFUs. However, a recent study concluded that metatarsal head resection is associated with a high risk of DFU recurrences and development of new DFUs because of inadequate bone resection and development of foot deformities.60-62 Therefore, careful pre-operative planning of the amount of bone to be resected is necessary.61,62
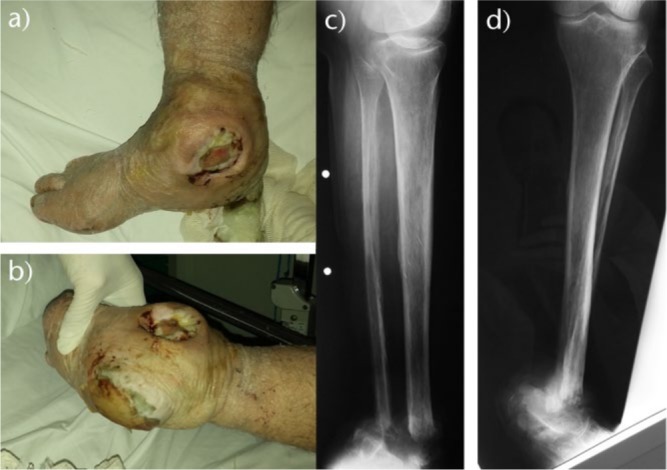
Fig. 2.
a) Photograph of the right foot of a 72-year-old diabetic man shows a DFU at the heel with soft-tissue necrosis. b) Surgical debridement in healthy viable tissue was done and tissue cultures were obtained. Post-operatively, he was administered per os antibiotics for three months and was educated for blood glucose control and wound dressing changes once per day with silver-impregnated dressings. c) Photograph of the foot five months post-operatively showing wound healing with granulation tissue, without evidence of infection.
Conservative debridement
Autolytic debridement is a method to liquefy a necrotic tissue with moist wound dressing. A wound covered with an occlusive dressing allows accumulation of tissue fluids containing macrophages, neutrophils and enzymes, which remove bacteria and digest necrotic tissues. Autolytic debridement is not recommended for infected DFUs or in the presence of ischaemia and/or dry gangrene (Fig. 3).56,59
Fig. 3.
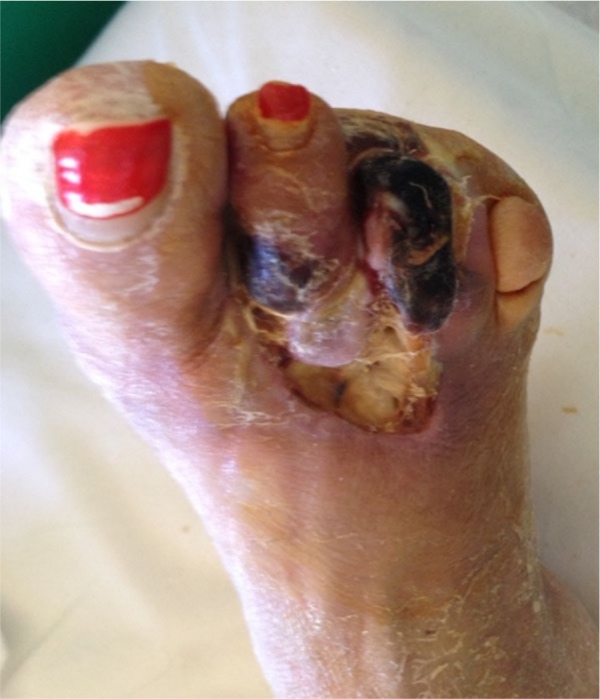
Photograph of the right foot of a 53-year-old diabetic woman shows a DFU at the dorsum of the foot and dry gangrene of the second and third toes. She was treated with third ray amputation, wound debridement, intravenous antibiotics and blood glucose control.
Mechanical debridement involves wound irrigation and dressing changes for the removal of unhealthy tissue without damaging healthy tissues. The wet gauze dressing is applied to the wound bed and kept to dry.57 The necrotic debris embedded in the gauze is mechanically stripped from the wound bed on removal of the dressing and wound irrigation. Mechanical debridement is used in the management of surgical wounds and venous leg ulcers.56 Its drawbacks are time and financial cost.
Enzymatic debridement involves debridement of necrotic tissue by topical enzymes such as streptokinases, trypsin, papain, fibrinolysin-DNase, collagenase, papainurea and streptodornase. These agents are usually applied once daily. It is recommended for sloughy, infected, necrotic wounds where surgical debridement is contraindicated.56,57
Maggot debridement is considered a biological debridement option using maggots or fly larva that are raised in a sterile environment. The most commonly used fly is Lucilia sericata. They can be used in humans when conventional treatments have failed. Maggots are applied to the wound and wrapped with secondary dressing.58 Larvae secrete is a powerful autolytic enzyme that liquefies necrotic tissue, stimulates the healing process and destroys bacterial biofilms. It is indicated for open wounds and ulcers that contain gangrenous or necrotic tissues with or without infection.56 Larval therapy has been shown to be safe and effective and can significantly diminish wound odour and bacterial count, including MRSA.
Novel devices operating on biological, physical, electrical, mechanical, electromechanical, optical and MR signals have been developed and tried on DFUs, with varying results. Topical negative pressure wound dressing changes63,64 and ultrasound therapy have been used with promising results.65
Infection control
The management of infected DFUs requires proper classification,41 appropriate antimicrobial therapy and debridement, preferably surgical.66 The Infectious Diseases Society of America (IDSA) and International Working Group on the Diabetic Foot (IWGDF) recommend classifying infected DFUs by severity and do not recommend the administration of antibiotics for the management of clinically uninfected DFUs (Table 1). Patients with mild infection are highly unlikely to require hospitalization, develop osteomyelitis or undergo amputation. In contrast, a more aggressive treatment is necessary for patients with moderate or severe DFU infection.66
Table 1.
Classification and grading of DFU infections
| Clinical manifestations of infection | IWGDF grade40
IDSA classification40 |
|---|---|
| No systemic or local signs of infection | 1 (uninfected) |
| Local infection* involving only the skin or subcutaneous tissue (without involvement of deeper tissues and without signs of a systemic inflammatory response syndrome†); any erythema present extends 0.5 to 2 cm around the wound | 2 (mild infection) |
| Local infection* with erythema > 2 cm around the wound, or involving structures deeper than skin and subcutaneous tissues (e.g. abscess, osteomyelitis, septic arthritis, fasciitis) and no signs of a systemic inflammatory response syndrome† | 3 (moderate infection) |
| Local infection* with signs of a systemic inflammatory response syndrome† | 4 (severe infection) |
Local infection is defined as the presence of at least two of the following: local swelling or induration; erythema > 0.5 cm around the ulcer in any direction; local tenderness or pain; local warmth; and purulent discharge. Other causes of inflammatory response of the skin (e.g. trauma, gout, acute Charcot neuroarthropathy, fracture, thrombosis, venous stasis) should be excluded
Systemic inflammatory response syndrome is defined as the presence of at least two of the following: temperature > 100.4 °F (38 °C) or < 96.8 °F (36 °C); heart rate > 90 beats per minute; respiratory rate > 20 breaths per minute or partial pressure of arterial carbon dioxide < 32 mmHg; white blood cell count > 12 000 per μL (12.00 × 109per L) or < 4000 per μL (4.00 × 109per L) or ≥ 10% immature band forms
For mild (superficial) DFU infections in patients who have not recently received antibiotics, appropriate per os antibiotics for a duration of one to two weeks is usually sufficient. If the wound does not respond to treatment, further tissue cultures should be obtained.67 Topical antimicrobial therapy may be used for some mild superficial infections, in DFUs with reduced antibiotic tissue penetration because of poor vascular supply, and in non-healing wounds with an absence of signs and symptoms but with a clinical suspicion of increased bacterial bioburden.68 However, the use of topical antimicrobial therapy is not recommended because of paucity of high-quality evidence.67-69 Antiseptics such as cadexomer iodine and silver-based dressings may be preferable to topical antibiotics because of decreased rates of bacterial resistance and contact sensitivity.69
For moderate and severe DFU infections, an empiric antibiotic regimen with activity against Gram-positive and Gram-negative organisms, including anaerobic bacteria, must be offered; empiric regimens should consider the most likely pathogens and the local epidemiology.2 When culture and sensitivity results are available, empiric therapy should be switched to definitive appropriate treatment.2,70
Severe DFU infection should be treated initially with parenteral antibiotics.70 Depending on the tissue involved, adequacy of debridement, type of soft-tissue wound cover and wound vascularity, parenteral antibiotics should be administered for a duration of two to four weeks. For osteomyelitis, at least four to six weeks of parenteral antibiotic agents with adequate penetration to bone is required; a shorter duration of two to three weeks can be offered if the entire infected bone has been surgically debrided. Antibiotics should be continued until there is evidence of resolution of the infection, even if the wound has not healed.67,70 At present, no specific antibiotic duration regimen has shown superiority for the treatment of DFU osteomyelitis. In general, long antibiotic duration is necessary (several weeks to months), depending on bacterial isolates, use or not of concomitant surgery and other factors.71 If inflammation does not improve after one or two antibiotic courses, antibiotics should be discontinued and further tissue cultures should be obtained; cultures should ideally be repeated two to three weeks after antibiotics cessation.67
Early surgical consultation should be obtained for patients with a rapidly deteriorating wound, exposed or necrotic bones and large sequestra, DFUs that do not respond to therapy, DFU osteomyelitis, limbs with critical ischaemia, and life- or limb-threatening infections, such as those presenting with necrotizing fasciitis, gas gangrene (Fig. 4), extensive soft-tissue loss or evidence of compartment syndrome.2,67-71 In the surgical management of DFU osteomyelitis, local antibiotic delivery systems can be used in an attempt to provide high local antibiotic concentrations.71 Several biodegradable and non-biodegradable local antibiotic delivery systems have been reported for the management of DFU osteomyelitis by local elution of antibiotics (mostly gentamicin, tobramycin and vancomycin).71 These include polymethylmethacrylate (PMMA) antibiotic-loaded bone cement in beads or spacers, calcium sulfate cement, combined calcium sulfate and hydroxyapatite beads, impregnated sponges and pellets, and bone graft substitutes.71 However, the literature is sparse and randomized controlled trials are required to guide treatment decisions in DFU osteomyelitis.71
Fig. 4.
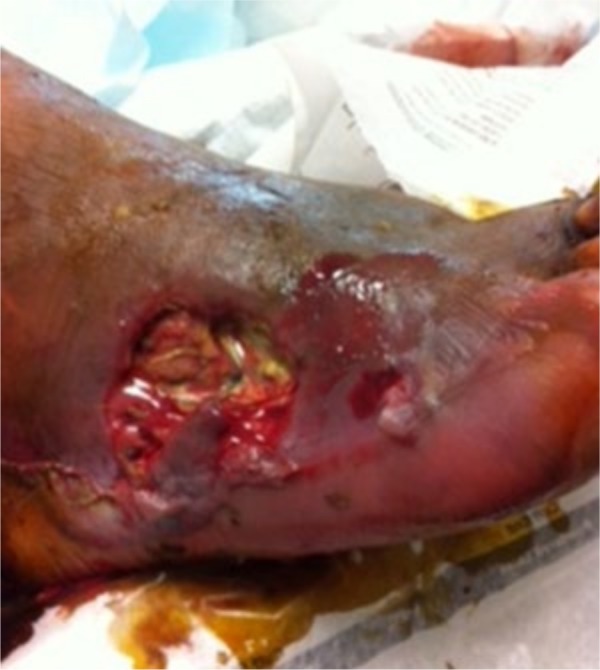
Photograph of the right foot of a 74-year-old diabetic man shows a DFU at the lateral side of the surface of the foot with wet gangrene and gas accumulation at the soft tissue. He was treated with multiple surgical debridements, intravenous antibiotics and blood glucose control; however, because of PAD he ended up with a below-knee amputation.
Biofilm formation is an important predictor for the failure of DFUs to heal.70 Biofilm-associated bacterial colonies are often multispecies, have low metabolic activity, are encased with a glycocalyx matrix making them resistant to antibiotics, and cannot be detected by routine cultures.54 The biofilm-forming ability of bacteria has been associated with increased antibiotic resistance. The mechanism of multidrug resistance in biofilm-forming organisms is believed to be a direct result of close cell–cell contact in the biofilm. The mechanisms for tolerance are: 1) antibiotics whose mechanism of action depends on the division of cells, are inactive against microbes in a biofilm, which are in a slow-growing, dormant state; 2) drug permeation is hindered by the polysaccharide matrix of the biofilm; and 3) drug efficacy is altered in the micro-environment of the biofilm (pH and osmotic variations).72 Therefore, treatment should aim to disrupt a biofilm that is already formed; this can be done with debridement using physical methods and/or application of topical agents. Debridement not only removes the bacteria and biofilm but also removes colonized necrotic tissue.73 Reformation of the biofilm is prevented with antimicrobial dressings.
Wound dressings
There is little evidence to support the use of any single dressing product over another in promoting a moist wound bed for DFU.2,8,53,56,57,69,74-88 Studies of impregnated dressings such as silver, hydrofibre and collagen found no statistically significant difference in wound healing compared with basic dressings, but they found a benefit for the peri-wound skin; however, these findings were limited by lack of high-quality data, lack of continuity in measured outcomes and small sample size.69,74 In any case, wounds should be cleansed at each dressing change and after debridement with a wound-cleansing solution or saline. Cleaning helps remove devitalized tissue, rebalance the bioburden, reduce exudate for the wound bed to heal and may also help to remove biofilms.2,8,69
The primary goal of dressings in DFUs is to create a moist occlusive wound environment that prevents infection and further trauma, absorbs chronic wound fluid, promotes granulation and provides autolytic debridement.2,8 Non-adherent dressings that protect the wound bed are standard treatment for most wounds. Adverse effects such as maceration, infection or further loss of tissue should prompt a change in wound dressing type. Dressings that have longer wearing times and do not require trained personnel for application, maintain adherence to the skin but non-adherence to the wound bed, are comfortable and can be acquired with the lowest cost appropriate to the clinical circumstances are recommended.8
Currently available dressings for DFUs include simple saline, silver- or heparan sulphate-impregnated, iodine, films, foams, hydrogels, hydrocolloids, alginates and honey-impregnated;2,8,53,56,57,69,74-88 growth factors and platelet-rich plasma dressing have also been reported to promote healing of DFUs.76-87 Large exudative wounds should be treated with dressings with high absorbency; in contrast, dry wounds may need the addition of moisture through hydrogels, hydrocolloids or non-absorbent dressings. More advanced dressings containing collagen and other extracellular matrix proteins are sometimes required to reduce the effects of an exaggerated inflammatory state that has been associated with chronic wounds.69
Wet-to-dry or simple saline dressings have a good mechanical debriding action and help in wound-bed preparation. They are absorptive as well as adherent, and inexpensive; however, they require frequent dressing changes (two to three times per day) depending on the type and severity of the wound. At dressing changes, a gentle cleanser such as normal saline or a neutral-pH cleanser is recommended to minimize wound irritation and patients’ discomfort.57,75
Silver-impregnated dressings are available in various formulations and have been associated with antimicrobial properties. They maintain a moist wound environment and absorb large amounts of exudates.53,75 Silver-impregnated dressings have a prolonged wear time but a high cost.56
Iodine is toxic to human cells as well as bacteria and fungi at high doses.53 It should not be used on granulating or epithelizing wounds because of its cytotoxicity to keratinocytes and fibroblasts, and slows down the healing process. In contrast, povidone iodine solution-impregnated dressings or soaked gauges are very effective in healing sutured wounds and hyper-granulating wounds to suppress or hamper further granulation, and for dry gangrene to accelerate demarcation of the gangrene.53
Polyurethane films often form the outer layer of other dressings such as hydrocolloids, foams, hydrogel sheets and composite dressings. The vapour-permeable films allow the diffusion of gases and water vapour, which help to maintain a moist wound-healing environment. They are comfortable and transparent and allow for wound inspection without removing the dressing. They can be used for low exudating wounds;75 however, they are not suitable for infected wounds, and if exudates collect under the film they must be drained or replaced.56,57
Polyurethane foam dressings contain hydrophilic polyurethane foam. They are very absorbent, thereby preventing maceration, non-adherent and have a semi-permeable backing that allows moisture to escape. Polyurethane foam dressings loosen slough by creating a moist wound environment, assisting in proper wound-bed preparation and promoting the proliferative stage of wound healing. They maintain a moist wound environment and therefore can be easily removed without pain and do not cause wound sloughing or trauma on removal.57,75 They are also used as outer dressings after the application of topical antibiotics, such as metronidazole, or hydrogels. They can be left unchanged for up to seven days depending on the severity of the DFU.
Hydrogel dressings consist of cross-linked insoluble starch or carboxy-methylcellulose polymers and up to 96% water. Hydrogels donate fluid to dry necrotic and slough wounds and promote autolysis and debridement by rehydrating the wound. Hydrogel dressings are available as flat sheets, amorphous hydrogel or beads. They are the best choice for the treatment of dry wounds with necrotic eschar; the hydrogel reaches a 50% debridement level more quickly than wet-to-dry dressings, they are more cost-effective and also provide an analgesic effect.56,75 They should be avoided on plantar DFUs as they may cause maceration of the skin surrounding the wound.57
Hydrocolloid dressings absorb low to moderate levels of exudate and can be used to promote autolytic debridement of dry, sloughy or necrotic wounds. They are usually composed of a hydrocolloid matrix bonded onto a vapour-permeable film or foam backing. When in contact with the wound surface, this matrix forms a gel to provide a moist environment.56 Hydrocolloids have been shown to retain growth factors under the dressing and to promote granulation and epithelialization. Additionally, the low pH created by the hydrocolloid is effective for the treatment of wounds infected by Pseudomonas species.75 They should be avoided on plantar DFUs as they may cause maceration of the skin surrounding the wound.56
Alginate dressings (calcium alginate and calcium sodium alginate) are bacteriostatic, haemostatic and highly absorbent with the ability to absorb approximately 15 to 20 times their own weight in wound fluid; therefore, they could manage excessive wound exudates and can assist in granulating, epithelializing and cavity wounds.57,75 The alginate forms a gel when it comes into contact with the wound surface which can be lifted off with dressing removal or rinsed away with sterile saline.56,57,75 Honey-impregnated dressings have been associated in vitro with antimicrobial and anti-inflammatory properties. However, in vivo evidence is insufficient, particularly in comparison with silver-impregnated antimicrobial dressings.57,75
A novel heparan sulphate glycosaminoglycan mimetic product for local application to promote wound healing has recently become available. It is a biophysical therapeutic product comprising a polysaccharide as an innovative biomaterial to accomplish mechanical tissue engineering and skin regeneration in the site of ulceration. In a recent report, complete ulcer healing was accomplished in all type 2 diabetic patients with difficult-to-heal foot and lower-extremity ulcerations after a mean treatment duration of 4.92 months (2 to 12), without any complications.88
Platelet-derived growth factor beta (PDGF-b) has also been reported as a topical therapy for the management of non-infected DFUs.76,77 It is applied in the form of a once-daily gel along with debridement on a weekly basis. Becaplermin is a recombinant human-PDGF (rh-PDGF-BB) that has been shown to accelerate wound closure in DFUs that extend into the subcutaneous tissue or beyond and have adequate blood supply.77 However, increased incidence of cancer in patients treated with becaplermin, especially at high doses, has been reported; therefore, further studies are necessary to evaluate the risk-benefit and effectiveness of this therapy.76 Platelet-rich plasma (PRP), extracted from the patient’s plasma, includes a high platelet concentration in a fibrin clot that can be easily applied to the ulcer area.76 The fibrin clot is absorbed during wound healing within days to weeks following its application. A shorter closure time and higher healing percentage have been reported with PRP in DFUs.78-83
Subcutaneous administration of granulocyte colony-stimulating factor (GCFS) in infected DFUs has also been reported with variable results.84-87 Basic fibroblast growth factor (bFGF) is beneficial for the formation of granulation tissue and normal healing. Epidermal growth factor (EGF) acts on epithelial cells, fibroblasts and smooth muscle cells to promote healing. Evidence for the use of EGF in DFUs is limited, with only a small amount of data reporting a significantly higher rate of ulcer healing with EGF use compared with placebo.76,84-87
Pain encountered during dressing changes is a common complaint of patients with DFUs. Pain-free removal of dressings and prevention of further trauma to the wound and the peri-wound skin is important in wound care. Strong adhesive forces can cause skin stripping, especially where the skin is vulnerable. Additionally, pain, and the anticipation of pain, can cause stress for patients; it has been shown that this can delay wound healing. Dressings with soft silicone form a bond between the soft silicone interface and the skin surface that allows the dressing to be removed without causing trauma or pain.89 It is important to recognize that, despite the misconception that pain or discomfort does not occur in neuropathic or neuro-ischaemic foot ulceration, pain is a real issue for some patients suffering from DFUs, particularly during wound-dressing changes.90
Off-loading
Pressure relief under weight-bearing areas is important to heal plantar DFUs. Off-loading devices reduce pressure at the site of a wound by redistributing loading forces across the plantar surface of the foot and, in some cases, the leg as well, thereby preventing isolated excessive force at the DFU site.91 An ideal off-loading device must be patient-compliant, easy to apply, cost-effective, effective in wound healing and comfortable for ambulation. These include total contact casts (TCCs), walker air casts and removable cast walkers (RCWs), crutch-assisted walking, therapeutic shoes and non-removable knee-high devices with an appropriate foot–device interface.54,91-94
TCCs are probably the ideal method for off-loading plantar DFUs. In general, TCCs are indicated for ulcers associated with neuropathy, Charcot foot and post-operative off-loading of the foot. Weekly changing or modifying of the TCC is recommended because there can be volume changes in the affected extremity. TCCs can immobilize the ankle and reduce the stride length, which decelerates the foot and reduces the force applied to it. TCCs are contraindicated in the presence of untreated infection or osteomyelitis and in patients with severe PAD. Caution, patient education and compliance are necessary, as inappropriate application may result in new ulcerations and complications such as deformity of toenails, ischaemia, fungal infection and dermatitis, joint rigidity and muscular atrophy.92 It should be used with caution in deep or heavily draining wounds and in ataxic, blind or severely obese patients. Additionally, any advanced wound-healing adjunctive therapies that require daily applications may not be suitable for use with patients using an off-loading device such as the TCC.
RCWs are made from various rigid materials that provide similar whole-foot load reduction as the TCC but with a greater degree of flexibility to reduce the incidence of side effects. They can be removed for dressing changes. Therapeutic shoes have been produced using a variety of techniques and materials, including felted foam, rubber, cork and leather, with or without a rigid rocker-bottom sole. Half shoes only provide a rear foot platform or offer heel relief.93 Therapeutic shoes are more acceptable to patients. However, they should be examined thoroughly in all patients at every visit.94 Innovative methods of designing in-shoe orthoses,95 socks using nanotechnology96 and therapeutic MR97 offer potential value, as does the role of Internet-based telemonitoring for diagnosis, treatment and/or prevention.32
Amputation
Above-the-ankle amputation is often a complication or need for a patient with infected DFUs and PAD. The five-year mortality after a diabetes-related amputation is up to 60%, which is higher than for certain malignancies. Following a major amputation, 50% of patients will have their other limb amputated within four years and approximately 50% of them will die within five years of developing a DFU (Fig. 5).1,2 Lower-limb amputation may be indicated in the presence of PAD and progressing life-threatening infection, and in patients with poorly controlled diabetes with chronic ischaemia who have a failed angioplasty or bypass revascularization surgery.98,99 Patients at high risk for ulceration and those who have undergone an amputation for a DFU should be examined one to three times per month by a DFU specialist team. At each examination, the patients’ feet should be inspected and the need for vascular evaluation assessed.2
Fig. 5.
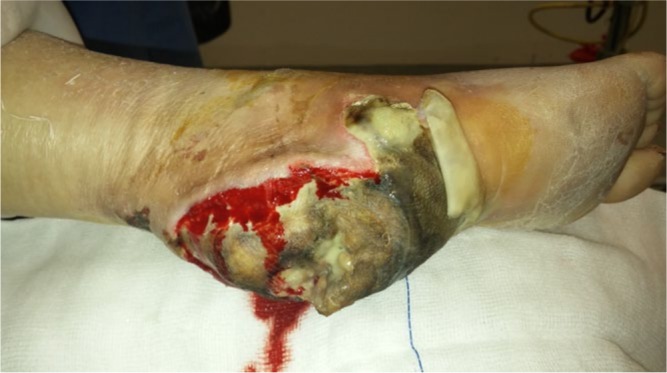
Photograph of the right foot of a 68-year-old diabetic woman shows a DFU at the heel of the foot with dry gangrene. She was treated with multiple surgical debridements, intravenous antibiotics and blood glucose control; however, because of persistent infection, osteomyelitis and PAD she ended up with a below-knee amputation. Post-operatively, she experienced acute heart and renal failure; she was admitted to the intensive care unit and died seven days later.
The rate of DFU-related amputations and related mortality remains high despite the awareness campaigns and reported increase in utilization of conservative and interventional therapeutic strategies. Possible explanations include the complexity and high burden of comorbidities in these patients and the low rate of invasive angiography and revascularization procedures before amputation. Therefore, DFU patients considered for an amputation should undergo a standard diagnostic work-up including angiography to consider the possibility of revascularization in case of ischaemia.98
Conclusion
A multidisciplinary foot care approach is necessary for the management of DFU. This should be done at experienced centres by physicians with specialist training in diabetic foot problems including orthopaedic and vascular surgeons, endocrinologists with expertise in diabetology, diabetes specialist nursing, biomechanics and orthotics, and physical medicine and rehabilitation doctors with access to rehabilitation, psychological and nutritional services.2 Education for patients regarding foot hygiene, nail care and proper footwear is important to reduce the risk of injury and DFU.16,40,100,101 Education alone may effectively reduce the incidence of DFUs and the rate of amputation.
Footnotes
ICMJE Conflict of interest statement: None declared.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Peter-Riesch B. The diabetic foot: the never-ending challenge. Endocr Dev 2016;31:108-134. [DOI] [PubMed] [Google Scholar]
- 2. National Institute for Health and Care Excellence. Diabetic foot problems: prevention and management (NG19). London: NICE, 2015. https://www.nice.org.uk/guidance/ng19 (date last accessed 9 February 2018). [PubMed] [Google Scholar]
- 3. Brownrigg JR, Davey J, Holt PJ, et al. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia 2012;55(11):2906-2912. [DOI] [PubMed] [Google Scholar]
- 4. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006;3(11):e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg 2016;33:149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995–2008: Possible impact of aggressive cardiovascular risk management. Diabetes Care 2008;31(11):2143-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hingorani A, LaMuraglia GM, Henke P, et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63(2)(suppl):3S-21S. [DOI] [PubMed] [Google Scholar]
- 8. Chadwick P, McCardle J. Assessing infected ulcers: a step-by-step guide. J Wound Care 2015;24(5)(suppl 2):15-19. [DOI] [PubMed] [Google Scholar]
- 9. Schaper NC, Van Netten JJ, Apelqvist J, et al. Prevention and management of foot problems in diabetes: a Summary guidance for daily practice 2015, based on the IWGDF guidance documents. Diabetes Metab Res Rev 2016;32(suppl 1):7-15. [DOI] [PubMed] [Google Scholar]
- 10. Andreassen CS, Jakobsen J, Andersen H. Muscle weakness: a progressive late complication in diabetic distal symmetric polyneuropathy. Diabetes 2006;55(3):806-812. [DOI] [PubMed] [Google Scholar]
- 11. Jude EB, Spittle M, Connor H, Boulton AJ. The diabetic foot 1998. Diabet Med 1999;16(2):170-172. [DOI] [PubMed] [Google Scholar]
- 12. Boulton AJ. What you can’t feel can hurt you. J Am Podiatr Med Assoc 2010;100(5):349-352. [DOI] [PubMed] [Google Scholar]
- 13. Wu SC, Driver VR, Wrobel JS, Armstrong DG. Vascular Foot ulcers in the diabetic patient, prevention and treatment. Health Risk Manag 2007;3(1):65-76. [PMC free article] [PubMed] [Google Scholar]
- 14. Forsythe RO, Hinchliffe RJ. Assessment of foot perfusion in patients with a diabetic foot ulcer. Diabetes Metab Res Rev 2016;32(suppl 1):232-238. [DOI] [PubMed] [Google Scholar]
- 15. Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ; International Working Group on the Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev 2016;32(suppl 1):2-6. [DOI] [PubMed] [Google Scholar]
- 16. Roberts P, Newton V. Assessment and management of diabetic foot ulcers. J Community Nurs 2011;16(10):485-486, 488-490. [DOI] [PubMed] [Google Scholar]
- 17. Boulton AJ, Armstrong DG, Albert SF, et al. ; American Diabetes Association. American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care 2008;31(8):1679-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mulder G, Armstrong D, Seaman S. Standard, appropriate, and advanced care and medical-legal considerations: part one - Diabetic foot ulcerations. Wounds. 2003;15(4):92-106. [Google Scholar]
- 19. Craig AB, Strauss MB, Daniller A, Miller SS. Foot sensation testing in the patient with diabetes: introduction of the quick & easy assessment tool. Wounds. 2014;26(8):221-231. [PubMed] [Google Scholar]
- 20. Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. JAMA 2005;293(2):217-228. [DOI] [PubMed] [Google Scholar]
- 21. Javed S, Petropoulos IN, Tavakoli M, Malik RA. Clinical and diagnostic features of small fiber damage in diabetic polyneuropathy. In: Zochodne DW, Malik RA, eds. Handbook of clinical neurology. Vol. 126 Cambridge, MA: Elsevier, 2014:275-290. [DOI] [PubMed] [Google Scholar]
- 22. Forsythe RO, Hinchliffe RJ. Assessment of foot perfusion in patients with a diabetic foot ulcer. Diabetes Metab Res Rev 2016;32(suppl 1):232-238. [DOI] [PubMed] [Google Scholar]
- 23. LoGerfo FW, Coffman JD. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N Engl J Med 1984;311(25):1615-1619. [DOI] [PubMed] [Google Scholar]
- 24. van Schie CH, Vermigli C, Carrington AL, Boulton A. Muscle weakness and foot deformities in diabetes: relationship to neuropathy and foot ulceration in Caucasian diabetic men. Diabetes Care 2004;27(7):1668-1673. [DOI] [PubMed] [Google Scholar]
- 25. La Fontaine J, Lavery L, Jude E. Current concepts of Charcot foot in diabetic patients. Foot 2016;26:7-14. [DOI] [PubMed] [Google Scholar]
- 26. Gemechu FW, Seemant F, Curley CA. Diabetic foot infections. Am Fam Physician 2013;88(3):177-184. [PubMed] [Google Scholar]
- 27. Ballard JL, Eke CC, Bunt TJ, Killeen JD. A prospective evaluation of transcutaneous oxygen measurements in the management of diabetic foot problems. J Vasc Surg 1995;22:485-492. [DOI] [PubMed] [Google Scholar]
- 28. Braun JD, Trinidad-Hernandez M, Perry D, et al. Early quantitative evaluation of indocyanine green angiography in patients with critical limb ischemia. J Vasc Surg 2013;57(5):1213-1218. [DOI] [PubMed] [Google Scholar]
- 29. European Stroke Organisation, Tendera M, Aboyans V, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the task force on the diagnosis and treatment of peripheral artery diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32(22):2851-2906. [DOI] [PubMed] [Google Scholar]
- 30. Smith FCT, Shearman CP, Simms MH, Gwynn BR. Falsely elevated ankle pressures in severe leg ischaemia: the pole test an alternative approach. Eur J Vasc Surg 1994;6:408-412. [DOI] [PubMed] [Google Scholar]
- 31. Collins R, Cranny G, Burch J, et al. A systematic review of duplex ultrasound, magnetic resonance angiography, and computed tomography angiography. Health Technol Assess 2007;11:iii-iv, xi-xiii, 1-184. [DOI] [PubMed] [Google Scholar]
- 32. Mani R, Margolis DJ, Shukla V, et al. Optimizing technology use for chronic lower-extremity wound healing: A consensus document. Int J Low Extrem Wounds 2016;15(2):102-119. [DOI] [PubMed] [Google Scholar]
- 33. Mani R. Transcutaneous measurements of oxygen tension in venous ulcer disease. Vasc Med Rev 1995;6:121-131. [Google Scholar]
- 34. Rerkasem K, Kosachunhanun N, Sony K, et al. Underrecognized peripheral arterial disease in patients with diabetes mellitus in Thailand: we must consider neuroischemic foot ulcers from this fallout. Int J Low Extrem Wounds 2015;14(2):132-135. [DOI] [PubMed] [Google Scholar]
- 35. Faglia E, Clerici G, Caminiti M, et al. Predictive value of transcutaneous oxygen tension for above the ankle amputation in diabetic patients with critical limb ischaemia. Eur J Vasc Endovasc Surg 2007;33:731-736. [DOI] [PubMed] [Google Scholar]
- 36. Arsenault KA, McDonald J, Devereaux PJ, et al. The use of transcutaneous oximetry to predict complications of chronic wound healing: a systematic review and meta-analysis. Wound Repair Regen 2011;19:657-663. [DOI] [PubMed] [Google Scholar]
- 37. Apelqvist JAP, Leplantalo MJA. The ulcerated leg: when to revascularise. Diabetes Metab Res Rev 2012;28(S1):30-35. [DOI] [PubMed] [Google Scholar]
- 38. Gurtner GL, Jones GE, Nelligan PC, et al. Intraoperative laser angiography using the SPY system: a review of the literature and recommendations for us. Ann Surg Innov Res 2013;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nouvang A, Hoogwerf B, Mohler E, et al. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhaemaglobin and deoxyhaemoglobin. Diabetes Care 2009;32:2036-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lipsky BA, Berendt AR, Cornia PB, et al. ; Infectious Diseases Society of America. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54(12):e132-e173. [DOI] [PubMed] [Google Scholar]
- 41. Vardakas KZ, Horianopoulou M, Falagas ME. Factors associated with treatment failure in patients with diabetic foot infections: an analysis of data from randomized controlled trials. Diabetes Res Clin Pract 2008;80(3):344-351. [DOI] [PubMed] [Google Scholar]
- 42. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. ; International Working Group on the Diabetic Foot. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016;32(suppl 1):45-74. [DOI] [PubMed] [Google Scholar]
- 43. Bakker K, Apelqvist J, Schaper NC; International Working Group on Diabetic Foot Editorial Board. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev 2012;28(suppl 1):225-231. [DOI] [PubMed] [Google Scholar]
- 44. Garg K, Kaszubski PA, Moridzadeh R, et al. Endovascular-first approach is not associated with worse amputation-free survival in appropriately selected patients with critical limb ischemia. J Vasc Surg 2014;59(2):392-399. [DOI] [PubMed] [Google Scholar]
- 45. Kinlay S. Management of critical limb ischemia. Circ Cardiovasc Interv 2016;9(2):e001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benitez E, Sumpio BJ, Chin J, Sumpio BE. Contemporary assessment of foot perfusion in patients with critical limb ischemia. Semin Vasc Surg 2014;27(1):3-15. [DOI] [PubMed] [Google Scholar]
- 47. McCallum JC, Lane JS., III Angiosome-directed revascularization for critical limb ischemia. Semin Vasc Surg 2014;27(1):32-37. [DOI] [PubMed] [Google Scholar]
- 48. Huang TY, Huang TS, Wang YC, et al. Direct revascularization with the angiosome concept for lower limb ischemia: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94(34):e1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Soares Rde A, Brochado Neto FC, Matielo MF, et al. Concept of angiosome does not affect limb salvage in infrapopliteal angioplasty. Ann Vasc Surg 2016;32:34-40. [DOI] [PubMed] [Google Scholar]
- 50. Špillerová K, Sörderström M, Albäck A, Venermo M. The feasibility of angiosome-targeted endovascular treatment in patients with critical limb ischemia and foot ulcer. Ann Vasc Surg 2016;30:270-276. [DOI] [PubMed] [Google Scholar]
- 51. Zheng XT, Zeng RC, Huang JY, et al. The use of the angiosome concept for treating infrapopliteal critical limb ischemia through interventional therapy and determining the clinical significance of collateral vessels. Ann Vasc Surg 2016;32:41-49. [DOI] [PubMed] [Google Scholar]
- 52. Elraiyah T, Domecq JP, Prutsky G, et al. A systematic review and meta-analysis of débridement methods for chronic diabetic foot ulcers. J Vasc Surg 2016;63(2 Suppl):37S-45S.e1-2. [DOI] [PubMed] [Google Scholar]
- 53. Ahmad J. The diabetic foot. Diabetes Metab Syndr 2016;10(1):48-60. [DOI] [PubMed] [Google Scholar]
- 54. Armstrong DG, Lavery LA, Nixon BP, Boulton AJ. It’s not what you put on, but what you take off: techniques for debriding and off-loading the diabetic foot wound. Clin Infect Dis 2004;39(suppl 2):S92-S99. [DOI] [PubMed] [Google Scholar]
- 55. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes 2015;6(1):37-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Amin N, Doupis J. Diabetic foot disease: from the evaluation of the “foot at risk” to the novel diabetic ulcer treatment modalities. World J Diabetes 2016;7(7):153-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kavitha KV, Tiwari S, Purandare VB, et al. Choice of wound care in diabetic foot ulcer: A practical approach. World J Diabetes 2014;5(4):546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fran G. The advantages and disadvantages of non-surgical management of the diabetic foot. Diabetes Metab Res Rev 2008;24(suppl 1):S72-S75. [DOI] [PubMed] [Google Scholar]
- 59. Lavery LA, Armstrong DG, Murdoch DP, et al. Validation of the Infectious Diseases Society of America’s diabetic foot infection classification system. Clin Infect Dis 2007;44(4):562-565. [DOI] [PubMed] [Google Scholar]
- 60. Aragón-Sánchez J. Seminar review: A review of the basis of surgical treatment of diabetic foot infections. Int J Low Extrem Wounds 2011;10(1):33-65. [DOI] [PubMed] [Google Scholar]
- 61. Aragón-Sánchez J, Lázaro-Martínez JL, Alvaro-Afonso FJ, Molinés-Barroso R. Conservative surgery of diabetic forefoot osteomyelitis: how can I operate on this patient without amputation? Int J Low Extrem Wounds 2015;14(2):108-131. [DOI] [PubMed] [Google Scholar]
- 62. Sanz-Corbalán I, Lázaro-Martínez JL, Aragón-Sánchez J, et al. Analysis of ulcer recurrences after metatarsal head resection in patients who underwent surgery to treat diabetic foot osteomyelitis. Int J Low Extrem Wounds 2015;14(2):154-159. [DOI] [PubMed] [Google Scholar]
- 63. Dumville JC, Hinchliffe RJ, Cullum N, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2013;(10):CD010318. [DOI] [PubMed] [Google Scholar]
- 64. Driver VR, Blume PA. Evaluation of wound care and health-care use costs in patients with diabetic foot ulcers treated with negative pressure wound therapy versus advanced moist wound therapy. J Am Podiatr Med Assoc 2014;104:147-153. [DOI] [PubMed] [Google Scholar]
- 65. Ennis WJ, Foremann P, Mozen N, et al. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double-blind, controlled, multi-center study. Ostomy Wound Manage 2005;51:24-39. [PubMed] [Google Scholar]
- 66. Lipsky BA, Berendt AR, Deery HG, et al. ; Infectious Diseases Society of America. Diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2004;39(7):885-910. [DOI] [PubMed] [Google Scholar]
- 67. Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009;49(10):1541-1549. [DOI] [PubMed] [Google Scholar]
- 68. Richmond NA, Vivas AC, Kirsner RS. Topical and biologic therapies for diabetic foot ulcers. Med Clin North Am 2013;97(5):883-898. [DOI] [PubMed] [Google Scholar]
- 69. Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother 2015;16(6):821-832. [DOI] [PubMed] [Google Scholar]
- 70. Banu A, Noorul Hassan MM, Rajkumar J, Srinivasa S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas Med J 2015;8(9):280-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Panagopoulos P, Drosos G, Maltezos E, Papanas N. Local antibiotic delivery systems in diabetic foot osteomyelitis: time for one step beyond? Int J Low Extrem Wounds 2015;14(1):87-91. [DOI] [PubMed] [Google Scholar]
- 72. Malik A, Mohammad Z, Ahmad J. The diabetic foot infections: biofilms and antimicrobial resistance. J Diabetes Metab Syndr 2013;7(2):101-107. [DOI] [PubMed] [Google Scholar]
- 73. Braun LR, Fisk WA, Lev-Tov H, et al. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol 2014;15(3):267-281. [DOI] [PubMed] [Google Scholar]
- 74. Kavitha KV, Tiwari S, Purandare VB, et al. Choice of wound care in diabetic foot ulcer: A practical approach. World J Diabetes 2014;5(4):546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Alexiadou K, Doupis J. Management of diabetic foot ulcers. Diabetes Ther 2012;3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22(5):569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dhatariya K, Gooday C, Franke B, et al. An open, non-comparative, multicentre evaluation of performance and safety using an antimicrobial exudate transfer dressing on diabetic foot ulcers: a case series. J Wound Care 2016;25(5):256-265. [DOI] [PubMed] [Google Scholar]
- 78. Deng W, Boey J, Chen B, et al. Platelet-rich plasma, bilayered acellular matrix grafting and negative pressure wound therapy in diabetic foot infection. J Wound Care 2016;25(7):393-397. [DOI] [PubMed] [Google Scholar]
- 79. Perez-Zabala E, Basterretxea A, Larrazabal A, et al. Biological approach for the management of non-healing diabetic foot ulcers. J Tissue Viability 2016;25(2):157-163. [DOI] [PubMed] [Google Scholar]
- 80. Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev 2016;(5):CD006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cobos R, Aizpuru F, Parraza N, et al. Effectiveness and efficiency of platelet rich plasma in the treatment of diabetic ulcers. Curr Pharm Biotechnol 2015;16(7):630-634. [DOI] [PubMed] [Google Scholar]
- 82. Li L, Chen D, Wang C, et al. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen 2015;23(4):495-505. [DOI] [PubMed] [Google Scholar]
- 83. Kontopodis N, Tavlas E, Papadopoulos G, et al. Effectiveness of platelet-rich plasma to enhance healing of diabetic foot ulcers in patients with concomitant peripheral arterial disease and critical limb ischemia. Int J Low Extrem Wounds 2016;15(1):45-51. [DOI] [PubMed] [Google Scholar]
- 84. Kästenbauer T, Hörnlein B, Sokol G, Irsigler K. Evaluation of granulocyte-colony stimulating factor (Filgrastim) in infected diabetic foot ulcers. Diabetologia 2003;46(1):27-30. [DOI] [PubMed] [Google Scholar]
- 85. Yönem A, Cakir B, Güler S, et al. Effects of granulocyte-colony stimulating factor in the treatment of diabetic foot infection. Diabetes Obes Metab 2001;3(5):332-337. [DOI] [PubMed] [Google Scholar]
- 86. Reed KS, Pai MP. Adjunctive granulocyte colony-stimulating factor therapy for diabetic foot infections. Ann Pharmacother 2004;38(12):2150-2153. [DOI] [PubMed] [Google Scholar]
- 87. Cruciani M, Lipsky BA, Mengoli C, de Lalla F. Are granulocyte colony-stimulating factors beneficial in treating diabetic foot infections? A meta-analysis. Diabetes Care 2005;28(2):454-460. [DOI] [PubMed] [Google Scholar]
- 88. Papanas N, Demetzos C, Pippa N, et al. efficacy of a new heparan sulfate mimetic dressing in the healing of foot and lower extremity ulcerations in type 2 diabetes: a case series. Int J Low Extrem Wounds 2016;15(1):63-67. [DOI] [PubMed] [Google Scholar]
- 89. Chadwick P, McCardle J. Open, non-comparative, multi-centre post clinical study of the performance and safety of a gelling fibre wound dressing on diabetic foot ulcers. J Wound Care 2016;25(5):290-300. [DOI] [PubMed] [Google Scholar]
- 90. Crews RT, Shen BJ, Campbell L, et al. Role and determinants of adherence to off-loading in diabetic foot ulcer healing: a prospective investigation. Diabetes Care 2016;39(8):1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Elraiyah T, Prutsky G, Domecq JP, et al. A systematic review and meta-analysis of off-loading methods for diabetic foot ulcers. J Vasc Surg 2016;63(2 Suppl):59S-68S. [DOI] [PubMed] [Google Scholar]
- 92. Snyder RJ, Frykberg RG, Rogers LC, et al. The management of diabetic foot ulcers through optimal off-loading: building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc 2014;104(6):555-567. [DOI] [PubMed] [Google Scholar]
- 93. Morona JK, Buckley ES, Jones S, et al. Comparison of the clinical effectiveness of different off-loading devices for the treatment of neuropathic foot ulcers in patients with diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev 2013;29(3):183-193. [DOI] [PubMed] [Google Scholar]
- 94. Bus SA, Armstrong DG, van Deursen RW, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab Res Rev 2016;32(suppl 1):25-36. [DOI] [PubMed] [Google Scholar]
- 95. Ulbrecht JS, Hurley T, Mauger DT, Cavanagh PR. Prevention of recurrent foot ulcers with plantar pressure-based in-shoe orthoses: the CareFUL prevention multicenter randomized controlled trial. Diabetes Care 2014;37:1982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Banchellini E, Macchiarini S, Dini V, et al. Use of nanotechnology-designed footsock in the management of preulcerative conditions in the diabetic foot: results of a single, blind randomized study. Int J Low Extrem Wounds 2008;7:82-87. [DOI] [PubMed] [Google Scholar]
- 97. Abbruzzese L, Iacopi E, Coppelli A, et al. Safety and effectiveness of therapeutic magnetic resonance in the management of postsurgical lesion of the diabetic foot. Int J Low Extrem Wounds 2015;14:4-10. [DOI] [PubMed] [Google Scholar]
- 98. Malyar NM, Freisinger E, Meyborg M, et al. Amputations and mortality in in-hospital treated patients with peripheral artery disease and diabetic foot syndrome. J Diabetes Complications 2016;30(6):1117-1122. [DOI] [PubMed] [Google Scholar]
- 99. Weledjicor EP, Fokam P. Treatment of the diabetic foot – to amputate or not? BMC Surg 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Armstrong DG, Lavery LA. Diabetic foot ulcers: prevention, diagnosis and classification. Am Fam Physician 1998;57(6):1325-1332. [PubMed] [Google Scholar]
- 101. Dorresteijn JA, Kriegsman DM, Assendelft WJ, Valk GD. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev 2012;10:CD001488. [DOI] [PubMed] [Google Scholar]