Abstract
Objectives:
Immunotherapy that targets the programmed death-1 / programmed death-ligand 1 (PD-L1) axis has been approved for treatment of non-small cell lung cancer (NSCLC) patients in many countries. However, our current understanding of the role of immunotherapies on NSCLC patients with epidermal growth factor receptor (EGFR) mutation, following acquisition of resistance to EGFR tyrosine kinase inhibitors (TKIs), is so far unclear. Especially, there is little data on if each acquired resistance mechanism to EGFR-TKIs alters PD-L1 expression status which is employed as an important predictive biomarker for PD-1/PD-L1 targeting agents.
Materials and methods:
Lung cancer cell lines (HCC827, HCC4006, PC9, H1975, H358, SW900, and H647) and their daughter cells that acquired resistance to EGFR-TKIs or cytotoxic drugs (cisplatin or vinorelbine) were examined. PD-L1 expression was analyzed by immunohistochemistry, immunoblotting, and/or fluorescent imaging. Published microarray data were also employed to evaluate our findings.
Results and conclusion:
We found correlations between therapy-induced E-cadherin downregulation and decreased PD-L1 expression using our cell lines and published microarray data. ShRNA mediated E-cadherin knockdown decreased PD-L1 expression in parental cells, and dual immunofluorescent staining of E-cadherin and PD-L1 suggests co-localization of both molecules. We also observed marked downregulation of PD-L1 in cells with E-cadherin downregulation after chronic treatment with vinorelbine. These results indicate a correlation between therapy-induced E-cadherin downregulation and decreased PD-L1 expression, highlighting the importance of re-biopsy after acquisition of resistance to EGFR-TKIs, not only for the evaluation of resistance mechanisms but also for the determination of PD-L1 expression status.
Keywords: EGFR mutation, Immunotherapy, Acquired resistance, EGFR-TKIs, Epithelial to mesenchymal transition (EMT), Erlotinib
1. Introduction
Lung cancers with epidermal growth factor receptor (EGFR) mutations are one of the most common molecularly defined subtypes of lung cancers [1]. EGFR tyrosine kinase inhibitors (TKIs) demonstrate initial dramatic efficacy in these patients. However, acquisition of resistance to EGFR-TKIs is almost inevitable after a median of approximately 1 year [2]. Therefore, development and optimization of secondary or later treatments is essential to further improve outcomes for lung cancer patients with EGFR mutations. T790M-specific EGFR-TKIs, so called 3rd generation EGFR-TKIs, are a promising strategy to overcome the acquired resistance via EGFR T790M secondary mutation that accounts for ~50% of resistance mechanisms [3].
Another approach involves immune checkpoint inhibitors that target programmed death-ligand 1 (PD-L1) or programmed death-1 (PD-1) which have shown dramatic and / or durable responses in a subset of lung cancer patients in clinical trials [4–6]. Although high PD-L1 expression is a predictive biomarker for effective treatment with these drugs [7], immunotherapy proves less effective in lung cancers with EGFR mutation [8, 9] despite their higher PD-L1 expression status [10–13]. This demonstrates the need for a better understanding of immune marker expression in EGFR-mutant tumors, especially PD-L1 expression status after acquisition of resistance to EGFR-TKIs, including the effect of specific acquired resistance molecular mechanisms on PD-L1 expression.
Regulation of PD-L1 expression in tumor cells is complex and affected by several mechanisms including: PD-L1 genomic gains [14], structural variations of the 3’ region of the PD-L1 gene [15], oncogenic signaling activation such as AKT serine/threonine kinase (AKT) – mechanistic target of rapamycin (mTOR) pathway [16], Janus kinase (JAK) – signal transducer and activator of transcription (STAT) pathway [17], and mitogen-activated protein kinase 1 (MAPK1) – Jun proto-oncogene, AP-1 transcription factor subunit (JUN) pathway [18]. In addition, PD-L1 expression in tumor cells is influenced by a variety of factors such as release of IFN-gamma from T cells in the tumor microenvironment in vivo [19]. Therefore, the first step to elucidate the effect of acquired resistance mechanisms to EGFR-TKIs on the expression of PD-L1 would be the comparison of tumor cells between drug sensitive parental cells and drug resistant isogenic cells in the absence of the tumor microenvironment. To date, we have established several acquired resistance in vitro models from EGFR-mutated lung cancer cell lines via chronic exposure to EGFR-TKIs [20–23]. This study focuses on analyzing the effect of acquired resistance mechanisms to EGFR-TKIs on the expression of PD-L1 protein employing these in vitro models.
2. Materials and methods
2.1. Cell lines, reagents, and generation of in vitro resistant cell lines
All human lung cancer cell lines used in this study were obtained or established in our previous studies [20–25]. All cells were cultured in RPMI1640 medium supplemented with 10% fetal bovine serum (FBS) and 1× penicillin / streptomycin solution (Mediatech, Inc., Manassas, VA) at 37°C / 5% CO2. T790M-specific EGFR-TKI, AZD9291 and cytotoxic agents (vinorelbine and cisplatin) were purchased from Selleck Chemicals (Houston, TX). H1975-AZD cells, SW900-VNR cells, and H647-CDDP cells were developed via chronic, repeated exposure to AZD9291, vinorelbine and cisplatin, respectively, as described previously [20]. All experiments using acquired resistance cells, including the tissue microarray (TMA) preparation, were performed following removal of drug exposure to avoid the direct effects of drugs on PD-L1 expression.
2.2. TMA preparation, antibodies and immunohistochemistry (IHC) analysis
Formalin-fixed paraffin-embedded (FFPE) cell blocks were prepared to make a cell line TMA of drug sensitive parental cells and their acquired resistant descendants. Cultured cells were gently harvested using Accutase (Innovative Cell Technologies, Inc., San Diego, CA) and fixed with alcoholic formalin solution for 24 hours. Fixed cells were mixed with melted agarose solution, allowed to solidify, placed in the cassette, and submerged in 70% ethanol. Paraffin-embedding of the agarose cell pellet was performed at our pathology core lab.
The TMA was sectioned at a thickness of 4 μm, and mounted on charged glass slides. All staining was performed on the Benchmark XT automated stainer (Ventana Medical Systems, Inc., Tucson, AZ) or the Link 48 Autostainer (Dako – Agilent Technologies, Carpinteria, CA). Staining for PD-L1 (SP142, Ventana Medical Systems), E-cadherin (anti-E-cadherin (36) Mouse Monoclonal antibody, Ventana Medical Systems), and total-EGFR (2–18C9, Dako-Agilent Technologies) were performed using respective kit systems. Other antibodies were purchased from Cell Signaling Technology (Danvers, MA) and detailed antibody information was summarized in Table 1. The staining platform utilized the Ultraview development reagents (Ventana Medical Systems, Inc.) or the Envision FLEX visualization system (Dako – Agilent Technologies). PD-L1 staining was assessed by the percentage of positive cells. Other specimens were evaluated using the H-score assessment which combines staining intensity (0–3) and the percentage of positive cells (0–100%) as previously described [26].
Table 1.
H-scores for PD-L1, EGFR and downstream molecules
| Molecule | Antibody (dilution) | HCC4006 | HCC4006ER |
|---|---|---|---|
| PD-L1 | SP142 (kit system) | 120 | 0 |
| total EGFR | 2-18C9 (kit system) | 260 | 230 |
| pEGFR | 53A5 (1:250) | 100 | 100 |
| pMAPK | 20G11 (1:100) | 170 | 130 |
| pAkt | 736E11 (1:40) | 0 | <5 |
| pS6 | D57.2.2E (1:400) | 280 | 230 |
| pSTAT3 | D3A7 (1:200) | 8 | <5 |
| p4E-BP1 | 236B4 (1:1000) | 20 | 13 |
| 4E-BP1 | 53H11 (1:1000) | 110 | 110 |
2.3. Antibodies and western blot analysis
Antibodies against E-cadherin, PD-L1, and beta-actin were purchased from Cell Signaling Technology. Total cell lysates were prepared, and immunoblotting conducted as described elsewhere [22]. Briefly, cells were cultured until sub-confluent, rinsed with phosphate-buffered saline (PBS), lysed in sodium dodecyl sulfate (SDS) sample buffer and homogenized. The total cell lysate (10 μg) was subjected to SDS polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). PVDF membranes were cut to include the indicated sized protein, therefore full-length blots are not available. After blocking with 5% nonfat dry milk, membranes were incubated with primary antibodies, washed with PBS, reacted with secondary antibodies (Cell Signaling Technology), and signals visualized using ECL reagent (Clarity, Bio-Rad, Hercules, CA) and film.
2.4. Short hairpin-mediated knockdown
Lentiviral preparations with a short hairpin RNA (shRNA) that specifically target E-cadherin or a non-targeting control were purchased from UCD Genomics Core. Transfection was performed per established protocol using polybrene (Sigma-Aldrich Corporation, St. Louis, MO), and 10 μg/mL puromycin (Invitrogen, Carlsbad, CA) treatment was used to select for the transfected cells.
2.5. Confocal image analysis (immunofluorescence)
Cells were seeded in an 8-well Nunc Lab-Tek II Chamber Slide System (Thermo Scientific, Rochester, NY), and treated the day after with PBS or IFN-gamma (Cell Signaling Technology) for 24 hours. Immunofluorescence was performed according to the manufacture’s protocol. Briefly, cells were fixed with 4% formaldehyde, blocked with blocking buffer, and incubated with primary antibodies for E-cadherin (mouse monoclonal) or PD-L1 (rabbit monoclonal) at 4°C overnight. After the washing with PBS, cells were incubated with fluorochrome-conjugated secondary antibodies for anti-rabbit and anti-mouse (Cell Signaling Technology). Cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI). Microscopy was performed on an EVOS fluorescent microscope (Model FL, Life Technologies, Carlsbad, CA).
3. Results
3.1. Differential expression of PD-L1 in HCC4006 erlotinib resistant cells
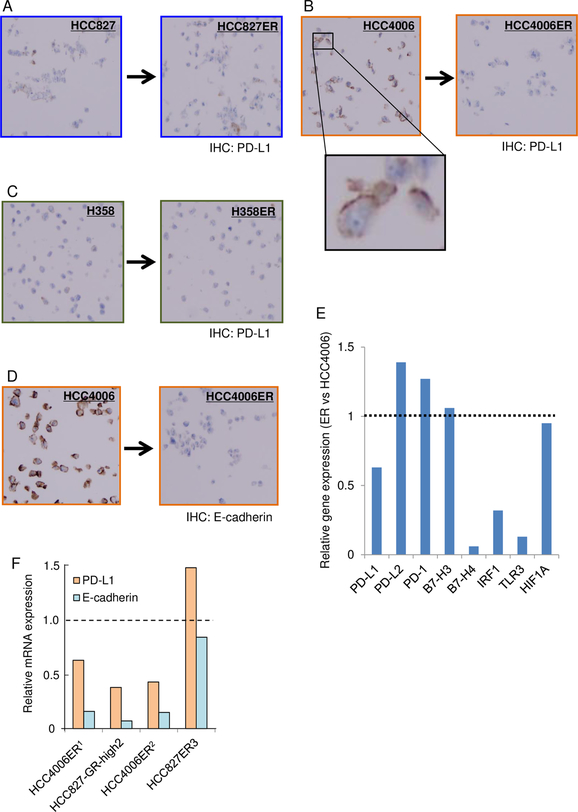
Initially, we screened PD-L1 expression in lung cancer cell lines by immunohistochemistry (IHC), the gold-standard for PD-L1 expression analysis in the clinic, comparing EGFR-TKI acquired resistant descendants to their parent cells in the absence of EGFR-TKIs (Fig 1A-C). HCC827 cells (del E746_A750) and HCC4006 cells (del L747_E749, A750P) harbor EGFR exon 19 deletion mutation, and H358 cells harbor KRAS G12C mutation but retain EGFR-TKI sensitivity via higher autocrine production of amphiregulin [27]. We found complete loss of PD-L1 expression in HCC4006 erlotinib resistant (ER) cells, while the parent cells showed high PD-L1 expression (IHC positive cells: 0% vs. 95%, respectively, Fig 1B). While HCC827ER cells, with acquired MET gene amplification as a resistance mechanism [20], showed slightly decreased PD-L1 expression compared with the parental HCC827 cells (IHC positive cells: 10% vs. 30%, respectively, Fig 1A). H358ER cells, with insulin-like growth factor 1 receptor activation [22], and the parental H358 cells both harbored < 5 % of PD-L1 positive cells (Fig 1C).
Figure 1.
As described in our previous report [21], HCC4006ER cells showed marked downregulation of E-cadherin (Fig 1D) and epithelial to mesenchymal transition (EMT) phenotype, and did not harbor any other candidate acquired resistance mechanisms including T790M secondary EGFR mutation, MET gene amplification, ERBB2 gene amplification, CRKL gene amplification or PTEN downregulation. Microarray analysis data comparing HCC4006ER cells with the parent HCC4006 cells, that was performed in our previous study [28], also identified decreased expression of PD-L1 mRNA. In addition, we observed decreased expression of B7-H4, while sparing the expression of PD-L2, PD-1, and B7-H3 (Fig 1E).
3.2. Role of EGFR and downstream signaling in the decreased expression of PD-L1 in HCC4006ER cells
As described above [16–18], the activation of oncogenic signaling pathways positively regulates the expression of PD-L1 [29]. Therefore, we compared the activation of EGFR and these downstream signaling molecules between HCC4006ER cells and HCC4006 parental cells using IHC. As shown in Table 1, H-scores for total EGFR, phosphorylated EGFR (Y1173), and phosphorylated downstream molecules (MAPK, AKT, S6, STAT3, 4E-BP1) are all similar between HCC4006ER cells and HCC4006 parental cells, confirming our previous results which used western blotting analysis [21, 30]. These results encouraged us to analyze the direct relationship between E-cadherin expression and PD-L1 expression in HCC4006 and HCC4006ER cell line models.
3.3. PD-L1 expression in the other “EMT-mediated” EGFR-TKI resistant cells
To generalize our findings in HCC4006ER cells, we searched for microarray data from the literature that reported EMT-mediated acquired resistance to EGFR-TKIs in lung cancers. We identified an additional three acquired resistance cell lines with EMT-features and available microarray data [31–33]. As shown in Fig 1F, three cell lines out of four showed decreased expression of PD-L1 mRNA compared with parental cells. HCC827-ER3 cells, the exception, had AXL overexpression together with EMT-features, and these cells harbor comparably higher E-cadherin expression than the other three cells that had decreased PD-L1 expression.
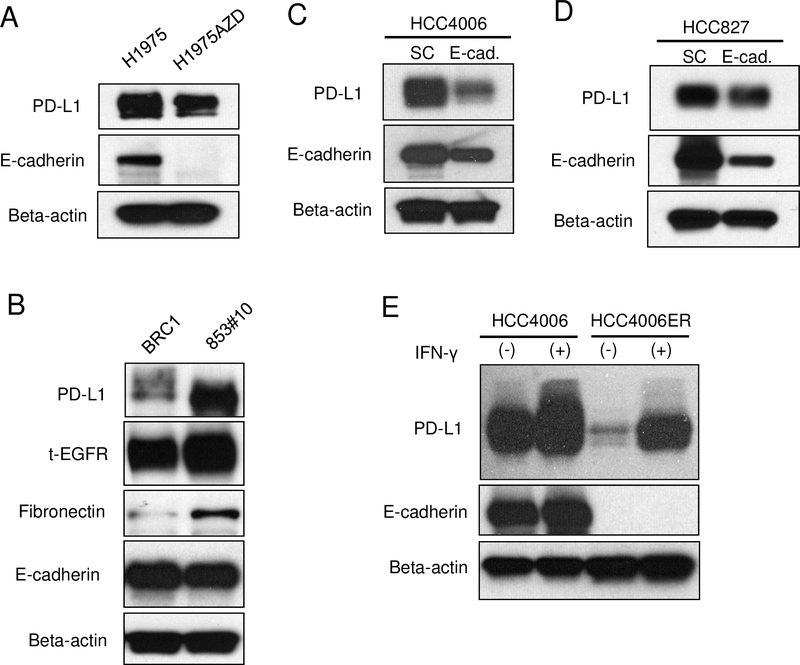
We also established H1975AZD cells (acquired resistant cells to AZD9291 also known as osimertinib) from H1975 lung cancer cells (EGFR L858R plus T790M mutation). We employed H1975 cells because they developed acquired resistance with decreased E-cadherin to irreversible EGFR-TKIs in previous studies [24, 34]. As expected, H1975AZD cells showed decreased expression of E-cadherin, in addition, H1975AZD cells showed decreased expression of PD-L1 by western blot analysis (Fig 2A). We also examined BRC1 cells (EGFR exon 19 deletion with T790M; derivative of PC9 cells) and 853#10 cells that were established from BRC1 cells via treatment with afatinib plus cetuximab in vivo [23]. Although 853#10 cells showed EMT-like features; with increased fibronectin and slightly decreased E-cadherin, 853#10 cells showed increased PD-L1 expression compared with their parental BRC1 cells (Fig 2B) similar to HCC827-ER3 cells (Fig. 1F). These data suggest that downregulation of PD-L1 is a general phenomenon in lung cancers with the EGFR mutation after acquisition of resistance to EGFR-TKIs if they concurrently acquire marked downregulation of E-cadherin.
Figure 2.
3.4. Effect of E-cadherin knockdown on PD-L1 expression in lung cancer cells with EGFR mutation
To address the relationship between E-cadherin downregulation and PD-L1 downregulation in lung cancer cells with EGFR mutations, we performed shRNA-mediated E-cadherin knockdown in HCC4006 parental cells. We observed decreased PD-L1 expression in HCC4006 cells transfected with E-cadherin shRNA compared with non-targeting shRNA transfected control cells (Fig 2C). A similar phenomenon was observed in HCC827 parental cells (Fig 2D), although the magnitude of PD-L1 decrease was not correlated with the magnitude of E-cadherin knockdown. These results indicate a direct relationship between E-cadherin and PD-L1 expression, but the magnitude of PD-L1 downregulation also depends on the other undetermined factor(s).
3.5. Effect of IFN-gamma treatment on parental and resistant cells with E-cadherin downregulation
It is also reported that cytokine signaling from the surrounding tumor microenvironment regulates PD-L1 expression in tumor cells [19]. To mimic an immune cell attack, we treated HCC4006 parental cells and HCC4006ER cells with IFN-gamma and measured PD-L1 expression. IFN-gamma treatment induced increased expression of PD-L1 in both HCC4006 and HCC4006ER cells. However, PD-L1 expression was still lower in HCC4006ER cells compared with HCC4006 parental cells after treatment with IFN-gamma (Figs 2E and 3).
Figure 3.
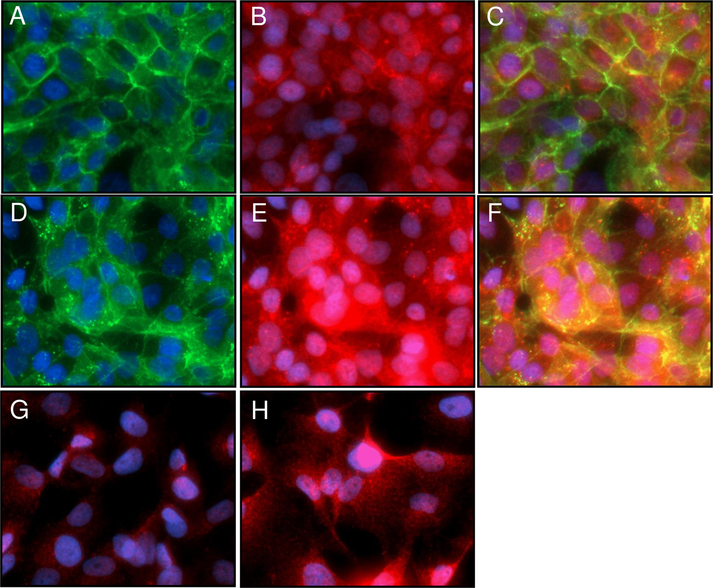
3.6. Co-localization assay for E-cadherin and PD-L1
To further evaluate the relationship between E-cadherin and PD-L1 in HCC4006 and HCC4006ER cells, we performed a co-localization assay for both proteins using the EVOS cell imaging system (ThemoFisher Scientific). PD-L1 was expressed at the cell membrane as well as in the cytoplasm in HCC4006 cells (Figs 3A-C), consistent with the IHC results (Fig 1B). Upon treatment with IFN-gamma (Figs 3D-F), E-cadherin was internalized as reported previously [35], and membranous PD-L1 expression was dramatically increased. Interestingly, PD-L1 was also internalized together with E-cadherin in HCC4006 parental cells treated with IFN-gamma (Fig 3F), although its biological implication is unclear.
In contrast, treatment with IFN-gamma did not alter expression of E-cadherin in HCC4006ER cells (Figs 3G and H). PD-L1 expression was increased in IFN-gamma treated HCC4006ER cells; however, overexpressed PD-L1 was mainly located in the cytoplasm (Fig 3H).
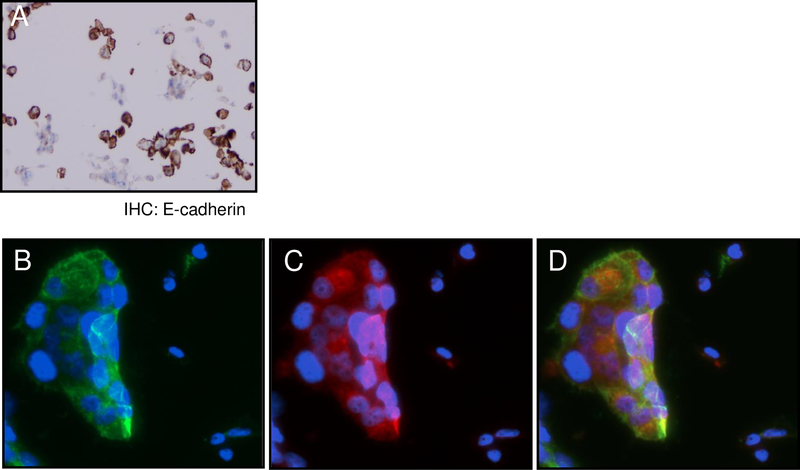
We have observed partial expression of PD-L1 in HCC827ER cells (Fig 1A). We also found E-cadherin expression in a part of HCC827ER cells (Fig 4A). The co-localization assay for E-cadherin and PD-L1 identified that a subset of cells expressed both proteins, while the other cells did not express either (Figs 4B-D), further supporting the positive relationship between E-cadherin and PD-L1 in lung cancer cells with acquired resistance to EGFR-TKIs.
Figure 4.
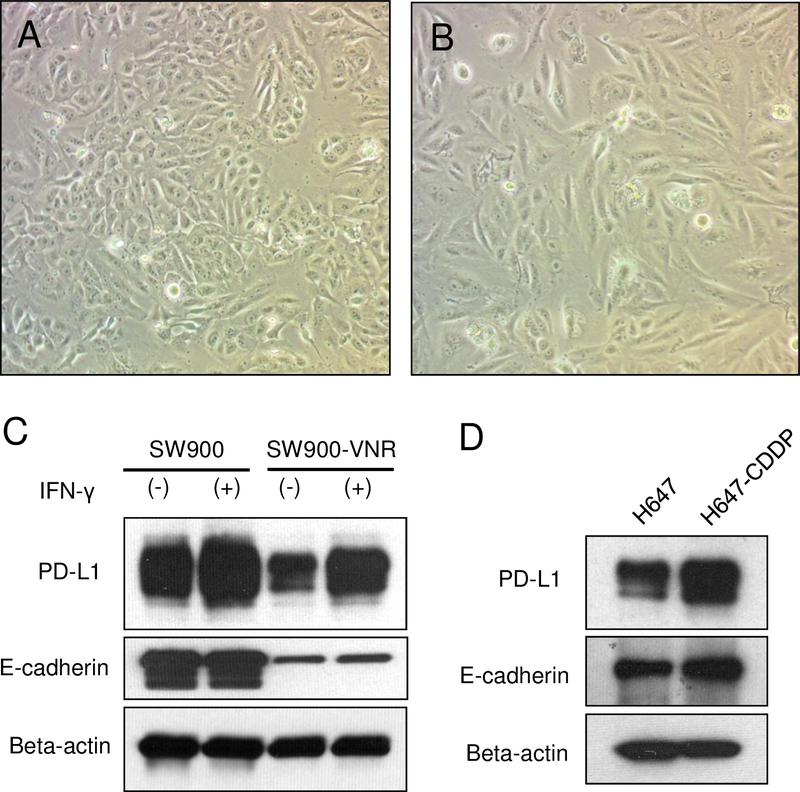
3.7. Chemotherapy induced EMT also decreased the expression of PD-L1
To expand our findings in chemotherapy induced EMT, we treated SW900 cells (lung squamous cell carcinoma) and H647 cells (lung adenosquamous carcinoma), which showed moderate expression of PD-L1 in our previous research, with cytotoxic agents including cisplatin (CDDP), gemcitabine, docetaxel, or vinorelbine (VNR). We successfully developed CDDP-resistant H647 cells (H647CDDP cells) and VNR-resistant SW900 cells (SW900VNR cells). As shown in Figs 5A and B, SW900VNR cells were spindle shaped with less cell-cell contact compared with SW900 parental cells. In western blot analysis, SW900VNR cells showed markedly decreased E-cadherin expression and decreased PD-L1 expression (Fig 5C), while H647CDDP cells showed increased expression of both proteins (Fig 5D). These results suggest that chemotherapy-induced E-cadherin downregulation has the same effect on PD-L1 expression, in addition to EGFR-TKI mediated E-cadherin downregulation.
Figure 5.
4. Discussion
EMT has long been recognized as playing a major role in cancer progression and metastasis via providing increased motility and invasive phenotypes to cancer cells. In addition, EMT is also reported as one of the acquired resistance mechanisms to anti-cancer drugs including cytotoxic agents and EGFR-TKIs [21, 28]. However, despite the wide use of the term “EMT”, the definition of EMT is still under debate.
Regarding the relationship between “EMT” and the expression of PD-L1, recent studies have reported increased PD-L1 expression in “de novo mesenchymal-type lung cancers” compared with epithelial-type ones [36–40]. However, these studies cannot elucidate the direct relationship between “EMT” and increased expression of PD-L1 due to many confounding factors. Briefly, it is possible that inherent mesenchymal-like lung cancers are distinct from epithelial-like lung cancers in their backgrounds such as patient smoking history or tumor stage that may also affect the expression status of PD-L1.
Therefore, it is not surprising that we found the opposite relationship between E-cadherin downregulation, the best-known hallmark of EMT, and PD-L1 expression status using isogenic cell line models. In this study, we found that acquired resistant cells with marked downregulation of E-cadherin showed decreased expression of PD-L1. We observed two exceptions; HCC827-ER3 cells (Fig 1F) from published microarray data and 853#10 cells (Fig 2B) that was established in our previous study [23] showed increased PD-L1 expression despite their “EMT” features. However, the magnitude of E-cadherin downregulation was quite small in these two cells compared with the other acquired resistant EMT cell lines with PD-L1 downregulation. Therefore, we consider that E-cadherin expression, but not the other EMT features, determines the expression of PD-L1. ShRNA mediated E-cadherin knockdown (Figs 2C and D) and co-localization assays (Fig 3) support this hypothesis. We also observed the same phenomenon in VNR resistant cells with marked downregulation of E-cadherin (Fig 5), suggesting that the downregulation of PD-L1 was not due to long-term inhibition of EGFR.
In this study, together with an extensive review for previous publication, we could not elucidate the molecular mechanism(s) how therapy-induced E-cadherin downregulation can affect the expression of PD-L1. However, the microarray data [28, 33] showed that toll-like receptor 3 (TLR3) and interferon regulatory factor-1 (IRF1), that positively regulate PD-L1 expression [41, 42], were markedly downregulated in erlotinib-resistant HCC4006 cells with E-cadherin downregulation. In addition, TLR3 and IRF1 were downregulated in HCC4006 cells transfected with E-cadherin shRNA compared with those transfected with non-targeting shRNA (data not shown), further suggesting a role of these molecules in the mechanism for PD-L1 downregulation together with E-cadherin downregulation.
Because PD-L1 expression status is the most significant predictive biomarker for candidates receiving anti-PD-1/PD-L1 antibody drugs, our results suggest reduced effectiveness of immunotherapy in patients who develop a marked downregulation of E-cadherin in response to EGFR-TKIs. Although the microarray data suggests slightly higher expression of PD-L2, another ligand for PD-1, in HCC4006ER cells, the expression level of PD-L2 is lower in lung cancers [43] and the role of PD-L2 as a predictive biomarker for anti-PD-1/PD-L1 therapy is currently unclear. We have shown that HCC4006ER cells were insensitive to anti-microtubule agents that are often used as cytotoxic agents in non-small cell lung cancers [28]. These results may indicate that EMT is one of the most intractable cancer status, and treatment strategies that may prevent EMT (or downregulation of E-cadherin), such as upfront polytherapy as recently reported [30, 44, 45], may be needed in future development of treatment strategies.
In this study, we had no data comparing clinical specimens before and after treatment due to the lack of adequate patients samples. We consider that the changes of immune checkpoint marker expression in patients are quite complicated as reported recently [46, 47]. They are affected by both the tumors themselves (including resistance mechanisms as shown in this study) and the microenvironment, which is itself affected by many factors such as tumor cell immunogenicity, patient characteristics, type of chemotherapeutic agents, and the duration of treatment holiday. We consider that detailed in vitro analyses provide important fundamental data regarding the role of resistance mechanisms on alteration of immune checkpoint markers.
In this study, we demonstrated that PD-L1 expression decreased in acquired resistant cells with E-cadherin downregulation. Our results support the importance of re-biopsy after acquisition of resistance to EGFR-TKIs, not only for the assessment of resistance mechanisms but also for the evaluation of PD-L1 expression status.
Acknowledgments
This work was supported by an International Association for the Study of Lung Cancer (IASLC) Young Investigator Award (2015 – 2017) to K. Suda. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest statement
T. Mitsudomi has received honoraria from AstraZeneca, Chugai, Boehlinger-ingelheim, Pfizer, and Roche; compensation from AstraZeneca, Chugai, Boehlinger-Ingelheim, Pfizer, Roche, and Clovis Oncology for participating in advisory boards; and research funding (through Kindai University Faculty of Medicine) from AstraZeneca and Chugai. F. R. Hirsch has received compensation from Genentech/Roche, Pfizer, BMS, Lilly, Merck, AstraZeneca, Boehringer-Ingelheim and Ventana/Roche for participating in advisory boards; and has received research funding (through the University of Colorado) from Genentech/Roche, BMS, Lilly, Bayer, Amgen and Ventana/Roche. All other authors declare that they have no conflict of interest related to this study.
References
- 1.Suda K, Mitsudomi T: Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch Toxicol 2015, 89(8):1227–1240. [DOI] [PubMed] [Google Scholar]
- 2.Suda K, Mizuuchi H, Maehara Y, Mitsudomi T: Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation-diversity, ductility, and destiny. Cancer Metast Rev 2012, 31(3–4):807–814. [DOI] [PubMed] [Google Scholar]
- 3.Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L et al. : AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015, 372(18):1689–1699. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E et al. : Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015, 373(17):1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E et al. : Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015, 373(2):123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016, 387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 7.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al. : Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadgeel S, Ciardiello F, Rittmeyer A, Barlesi F, Cortinovis D, Barrios C, et al. : PL04a.02: OAK, a randomized Ph III study of atezolizumab vs docetaxel in patients with advanced NSCLC: results from subgroup analyses. J Thorac Oncol 2017, 12, Suppl S9–10. [Google Scholar]
- 9.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC: Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer-A Meta-Analysis. J Thorac Oncol 2017, 12(2):403–407. [DOI] [PubMed] [Google Scholar]
- 10.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ et al. : Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013, 3(12):1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M et al. : Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014, 25(10):1935–1940. [DOI] [PubMed] [Google Scholar]
- 12.D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J, Coppi E et al. : PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015, 112(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, Chen N, Zhan J, He X, Qin T et al. : The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget 2015, 6(16):14209–14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue Y, Yoshimura K, Mori K, Kurabe N, Kahyo T, Mori H, Kawase A, Tanahashi M, Ogawa H, Inui N et al. : Clinical significance of PDL1 and PDL2 copy number gains in nonsmallcell lung cancer. Oncotarget 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataoka K, Shiraishi Y, Takeda Y, Sakata S, Matsumoto M, Nagano S, Maeda T, Nagata Y, Kitanaka A, Mizuno S et al. : Aberrant PD-L1 expression through 3’-UTR disruption in multiple cancers. Nature 2016, 534(7607):402–406. [DOI] [PubMed] [Google Scholar]
- 16.Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S et al. : Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res 2016, 76(2):227–238. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda S, Okamoto T, Okano S, Umemoto Y, Tagawa T, Morodomi Y, Kohno M, Shimamatsu S, Kitahara H, Suzuki Y et al. : PD-L1 Is Upregulated by Simultaneous Amplification of the PD-L1 and JAK2 Genes in Non-Small Cell Lung Cancer. J Thorac Oncol 2016, 11(1):62–71. [DOI] [PubMed] [Google Scholar]
- 18.Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, Zhang Y, He X, Zhou T, Qin T et al. : Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol 2015, 10(6):910–923. [DOI] [PubMed] [Google Scholar]
- 19.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH et al. : Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 2003, 33(11):3117–3126. [DOI] [PubMed] [Google Scholar]
- 20.Suda K, Murakami I, Katayama T, Tomizawa K, Osada H, Sekido Y, Maehara Y, Yatabe Y, Mitsudomi T: Reciprocal and complementary role of MET amplification and EGFR T790M mutation in acquired resistance to kinase inhibitors in lung cancer. Clin Cancer Res 2010, 16(22):5489–5498. [DOI] [PubMed] [Google Scholar]
- 21.Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T: Epithelial to Mesenchymal Transition in an Epidermal Growth Factor Receptor-Mutant Lung Cancer Cell Line with Acquired Resistance to Erlotinib. J Thorac Oncol 2011, 6(7):1152–1161. [DOI] [PubMed] [Google Scholar]
- 22.Suda K, Mizuuchi H, Sato K, Takemoto T, Iwasaki T, Mitsudomi T: The insulin-like growth factor 1 receptor causes acquired resistance to erlotinib in lung cancer cells with the wild-type epidermal growth factor receptor. Int J Cancer 2014. [DOI] [PubMed] [Google Scholar]
- 23.Pirazzoli V, Nebhan C, Song X, Wurtz A, Walther Z, Cai G, Zhao Z, Jia P, de Stanchina E, Shapiro EM et al. : Acquired resistance of EGFR-mutant lung adenocarcinomas to afatinib plus cetuximab is associated with activation of mTORC1. Cell Rep 2014, 7(4):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware KE, Hinz TK, Kleczko E, Singleton KR, Marek LA, Helfrich BA, Cummings CT, Graham DK, Astling D, Tan AC et al. : A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis 2013, 2:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizuuchi H, Suda K, Murakami I, Sakai K, Sato K, Kobayashi Y, Shimoji M, Chiba M, Sesumi Y, Tomizawa K et al. : Oncogene swap as a novel mechanism of acquired resistance to EGFR-tyrosine kinase inhibitor in lung cancer. Cancer Sci 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I et al. : Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst 2005, 97(9):643–655. [DOI] [PubMed] [Google Scholar]
- 27.Yonesaka K, Zejnullahu K, Lindeman N, Homes AJ, Jackman DM, Zhao F, Rogers AM, Johnson BE, Janne PA: Autocrine production of amphiregulin predicts sensitivity to both gefitinib and cetuximab in EGFR wild-type cancers. Clin Cancer Res 2008, 14(21):6963–6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mizuuchi H, Suda K, Sato K, Tomida S, Fujita Y, Koyabashi Y, Maehara Y, Sekido Y, Nishio K, Mitsudomi T: Collateral chemoresistance to anti-microtubule agents in a lung cancer cell line with acquired resistance to erlotinib. PloS one 2015, in press, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Jiang CC, Jin L, Zhang XD: Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol 2016, 27(3):409–416. [DOI] [PubMed] [Google Scholar]
- 30.Sesumi Y, Suda K, Mizuuchi H, Kobayashi Y, Sato K, Chiba M, Shimoji M, Tomizawa K, Takemoto T, Mitsudomi T: Effect of dasatinib on EMT-mediated-mechanism of resistance against EGFR inhibitors in lung cancer cells. Lung Cancer 2017, 104:85–90. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, Abdel-Rahman M, Wang X, Levine AD, Rho JK et al. : Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nature Genet 2012, 44(8):852–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shien K, Toyooka S, Yamamoto H, Soh J, Jida M, Thu KL, Hashida S, Maki Y, Ichihara E, Asano H et al. : Acquired Resistance to EGFR Inhibitors Is Associated with a Manifestation of Stem Cell-like Properties in Cancer Cells. Cancer Res 2013, 73(10):3051–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida T, Song L, Bai Y, Kinose F, Li J, Ohaegbulam KC, Munoz-Antonia T, Qu X, Eschrich S, Uramoto H et al. : ZEB1 Mediates Acquired Resistance to the Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. PloS one 2016, 11(1):e0147344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK et al. : Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011, 3(75):75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth D, Leung G, Fernando M, McKay DM: Reduced surface expression of epithelial E-cadherin evoked by interferon-gamma is Fyn kinase-dependent. PLoS one 2012, 7(6):e38441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W et al. : Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014, 5:5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, Skoulidis F, Parra ER, Rodriguez-Canales J, Wistuba II et al. : A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin Cancer Res 2016, 22(3):609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou Y, Diao L, Parra Cuentas ER, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C et al. : Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K, Takemoto T, Mitsudomi T: Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1). Lung Cancer 2016, 98:69–75. [DOI] [PubMed] [Google Scholar]
- 40.Kim S, Koh J, Kim MY, Kwon D, Go H, Kim YA, Jeon YK, Chung DH: PD-L1 expression is associated with epithelial-to-mesenchymal transition in adenocarcinoma of the lung. Human Pathol 2016, 58:7–14. [DOI] [PubMed] [Google Scholar]
- 41.Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, et al. : Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274). FEBS Lett 2006, 580(3):755–62. [DOI] [PubMed] [Google Scholar]
- 42.Boes M, Meyer-Wentrup F: TLR3 triggering regulates PD-L1 (CD274) expression in human neuroblastoma cells. Cancer Lett 2015, 361:49–56. [DOI] [PubMed] [Google Scholar]
- 43.Inoue Y, Yoshimura K, Nori K, Kurabe N, Kahyo T, Mori H, et al. : Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget 2016, 7:32113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soucheray M, Capelletti M, Pulido I, Kuang Y, Paweletz CP, Becker JH, Kikuchi E, Xu C, Patel TB, Al-Shahrour F et al. : Intratumoral heterogeneity in EGFR mutant NSCLC results in divergent resistance mechanisms in response to EGFR tyrosine kinase inhibition. Cancer Res 2015, 75:4372–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suda K, Rivard CJ, Mitsudomi T, Hirsch FR: Heterogeneity in Tumors and Resistance to EGFR TKI Therapy-Letter. Cancer Res 2016, 76(10):3109–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han JJ, Kim DW, Koh J, Keam B, Kim TM, Jeon YK, Lee SH, Chung DH, Heo DS: Change in PD-L1 Expression After Acquiring Resistance to Gefitinib in EGFR-Mutant Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016, 17:263–270. [DOI] [PubMed] [Google Scholar]
- 47.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR et al. : EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016, 22(18):4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]