Abstract
Objective
This study was to evaluate the effectiveness of ultrasound-guided percutaneous ozone injections around the cervical dorsal root ganglions of zoster-associated pain (ZAP) patients.
Study design
Retrospective comparative study.
Settings
The study was conducted at a pain center of a university hospital.
Patients and methods
From June 2016 to July 2017, a total number of 30 patients with ZAP were treated with ultrasound-guided percutaneous ozone injection around the cervical dorsal root ganglion (DRG) at the injured nerve level (C2-C8). A volume of 3 mL ozone-oxygen mixture at a concentration of 30 µg/mL was injected into the area around the DRG. Patients were divided into two groups according to their disease duration: group A (at or <3 months) and group B (>3 months). The pain severity was assessed according to a visual analog scale, and imaging changes were evaluated by ultrasound. Patient improvements in pain and neurologic function were evaluated during a follow-up period from 1 to 3 months.
Results
The data showed that ozone injections reduced pain in patients with ZAP. However, the success rate of group A was higher than group B. After the injection, the von Frey data demonstrated decreases in both groups, but, there were no significant differences between the groups. Moreover, univariate logistic regression analysis and multivariate regression analysis showed a history of diabetes mellitus had a significant effect on the treatment results.
Conclusions
Percutaneous ozone injection around the DRG might be a useful method for treatment-resistant cases of ZAP at the cervical level. Institutional Review Board (IRB) approval number: HK2017-1130.
Keywords: herpes zoster, neuropathic pain, post-herpetic neuralgia, ozone injection
Introduction
Herpes zoster (HZ) is caused by the reactivation of dormant varicella-zoster virus (VZV) in a sensory ganglion such as the dorsal root ganglion (DRG) or trigeminal ganglion, where the virus establishes lifelong latency.1,2 According to previous studies, the lifetime prevalence of HZ is about 30%, and the incidence of HZ increasing with age and declining immune status.3,4 The most common complication of HZ is post-herpetic neuralgia (PHN), described as refractory neuropathic pain which normally lasts for several days to weeks, and negatively affects the patient’s quality of life and ability to function.5 Aging, severe pain and rash severity, greater degree of sensory impairment, and psychological distress are risk factors for PHN.6 Neuralgia lasting more than 3 months is generally considered to be PHN.7
Multiple treatment options for the management of zoster-associated pain (ZAP) are available. Conventional analgesic drugs, antiviral drugs and tricyclic antidepressants are widely used, but they are associated with significant adverse events, especially in the elderly.8 In many countries, corticosteroids and local anesthetics are widely applied during the neural blockade administration; however, this treatment has a weak evidence base for preventing PHN.9 A randomized, double-blind, placebo-controlled trial showed that the use of HZ vaccine decreased morbidity from herpes zoster and PHN among older adults; however, the cost implications are high.10 Pulsed radiofrequency treatment (PRF) is also being used for conditions with refractory neuropathic pain such as trigeminal neuralgia, chronic cervical and lumbosacral pain.11–14 Nevertheless, the efficacy of PRF for the reduction of intractable pain is still controversial15 and PHN often remains intractable to existing treatments despite the many therapeutic options available.16
Ozone is a trivalent form of oxygen and it has been shown to have an effect on both chronic viral infections and circulatory disorders.17 One application method of ozone is percutaneous injection. In some previous studies, it has been indicated that percutaneous ozone injection can be an effective treatment for neuropathic symptoms without causing tissue damage within a certain concentration range (below 70 µg/mL).18 And this method is also one of several minimally invasive therapies that have been used for the treatment of chronic neuropathic pain such as herniated discs and trigemi-nal neuralgia11,19,20; although, the exact mechanism underlying ozone-induced analgesia is not well understood yet. At concentrations around 30 µg/mL, ozone not only could enhance oxygenation and reduce inflammation at the disease site due to the oxidizing effect on pain-producing mediators, but also lead to increased local oxygen supply and reduced nerve root ischemia and edema.19–21 We have a hypothesis that ozone therapy gradually decrease inflammation through decreasing concentrations of various pro-inflammatory cytokines and protect the nerve damage from further aggravation through the bidirectional regulation of inflammation.22
The DRGs lie at the entrance to the spinal cord receiving input from peripheral nerve terminals (PNS); they are important in the production of neurotransmitters involved in nociceptive signaling.23 Through appropriate treatment to inhibit the abnormal electrical activity to the spinal cord via the DRGs in HZ, they can decrease the incidence of hyperalgesia and allodynia by reducing central sensitization (characterized by reductions in threshold and increases in the responsiveness of dorsal horn neurons).24 Therefore, the DRGs are priority target for treatment of ZAP.12,24 However, the use of percutaneous ozone injections in ZAP has not yet been investigated.
In this article, we retrospectively evaluated the clinical effects of percutaneous ozone injection around cervical DRG in patients with HZ and patients with PHN. Therapeutic effectiveness and follow-up information was collected, and overall improvement in pain was observed up to the 3-month follow-up. In addition, we designed the study to analyze any influence of age, gender, pain duration, the number of treatments, hypertension history and diabetes mellitus history on pain relief after the ozone treatment.
Patients and methods
Study design
This research was a retrospective study. Permission to conduct this study was obtained from the Institutional Review Board of the Aviation General Hospital of China Medical University (approval number HK2017-1130). And the approval included a waiver of informed consent, because this study did not include direct contact with the study population. This retrospective analysis did not include patients’ identifiers.
Patients
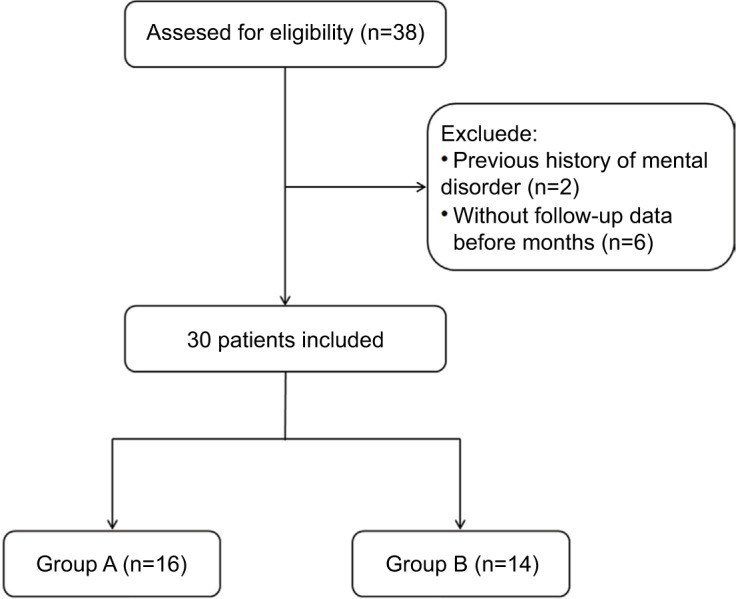
From June 2016 to July 2017, patients who underwent ozone injections around the cervical DRG due to ZAP at our pain center were included. Patients were excluded from the study if they fulfilled at least one of the following exclusion criteria: age less than 18 or older than 80 years; infection at the site of puncture; cardiac or pulmonary dysfunction; hyperthyroidism; previous radiofrequency treatment; history of mental disorders and drug abuse; lost to follow-up before 3 months after percutaneous ozone injection. The flow diagram of therapeutic process is shown in Figure 1.
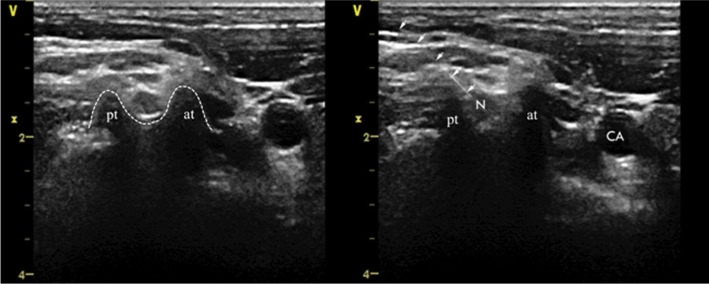
Figure 1.
Axial transverse ultrasound images of the anterior tubercle and the posterior tubercle of C5.
Abbreviations: V, the mark of the transducer probe; x, depth (cm); pt, posterior tubercle; at, anterior tubercle; N, nerve root; CA, carotid artery.
Procedural technique
During the treatment, heart rate, blood pressure and pulse oxygenation of patients were recorded. The ultrasound-guided technique and all injection procedures were carried out by a physician experienced in pain management.
We determined the injured nerve level according to the skin lesion area and nerve segments of the pain. All patients underwent percutaneous ozone injection around the cervical DRG using ultrasound guidance as previously described.25 With patients lying in the lateral position, an ultrasound device (Vivid-i; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) was used to perform the ultrasound examinations. The 11-L transducer probe covered with a sterile sheath was positioned transversely to the lateral aspect of the neck, so we could obtain a transverse axial view. Anatomically, the anterior tubercles of C7 are rudimentary and the posterior tubercles of the C7 are prominent, which are different from other cervical levels. The C6 level is visualized by sliding the transducer cranially. Determining the other cervical level by confirming the location of C7 is a convenient positioning method. Once the target cervical level is identified, the skin was disinfected and anesthetized with the injection of 1% lidocaine. Following this, a 22-gauge needle was inserted under ultrasound guidance at the foraminal opening between the anterior and posterior tubercles, which is most proximate to the DRG (Figure 2). After the needle tip was placed at the target point, the O2–O3 (3 mL) mixture was injected by an assistant at a concentration of 30 µg/mL, which was generated by an ozone therapy device (Ozomed® basic; Kastner-Praxisbedarf-GmbH, Rastatt, Germany). Ideally, the mixture should spread around the nerve root. The physicians who performed the procedure must determine the most appropriate pathway for needle insertion, in order to avoid important adjacent structures.
Figure 2.
The flow diagram of therapeutic process.
All patients were observed for 30 minutes after this procedure. During this time, we observed whether serious adverse reactions occurred such as: dizziness, nausea and vomiting, respiratory depression, low blood oxygen and blood pressure fluctuation.
Data collection
The following clinical characteristics of the patients were collected and analyzed from the medical records: age; gender; targeted DRG level; pain severity and duration; localization of the rash; tactile sensory; history of underlying diseases and follow-up records. The severity of burning pain and lancinating pain was evaluated with the use of a 10 cm visual analog scale (VAS), patients were shown a horizontal line (0 cm represented no pain and 10 cm the worst imaginable pain), a difference of at least of 3 cm of VAS score was considered clinically significant.26 Tactile sensation was evaluated through the use of a series of calibrated von Frey hairs (Stoelting Co., Wood Dale, IL, USA). This measurement was applied perpendicular to the affected skin area and was repeated several times. When the patients responded to the stimulus, the corresponding von Frey date was recorded. This test was performed before the first treatment and after the last treatment. Follow-up examinations were performed at 1 month and 3 months following the end of the treatment by physicians who were unaware of the patients’ treatment assignments. The severity of pain was evaluated before the first treatment, after the final treatment, at 1 month later, and then 3 months after the end of treatment.
Outcome measures
Thirty patients with ZAP at our pain center were treated with percutaneous ozone injection around the cervical DRG under ultrasound guidance. All patients who underwent ozone therapy were divided into two groups: one group included patients who underwent ozone therapy within 3 months after ZAP onset (Group A), while the second group included patients who underwent ozone therapy more than 3 months after onset of ZAP (Group B).
We assessed the analgesic efficacy of ozone therapy by VAS. A tactile sensory test, performed before the first treatment and immediately after the final treatment, was used as an evaluation of sensation. Clinically meaningful pain intensity is considered to be the presence of a VAS ≥ 3,26–28 so we used pain intensity of VAS <3 as a measure of success in relieving ZAP.
Statistical analysis
All data were analyzed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA). Numerical variables are presented as mean ± SD, such as age, disease duration and VAS. The Student’s t-test was used to compare the outcomes between the two groups for numerical variables, whereas the chi-squared test or Fisher’s exact test was used for categorical variables (gender, treatment location and history of underlying disease). Repeated measures analysis of variance was used to assess changes in pain intensity over time. We combined all patients from both of the groups for the logistic regression. By using univariate and multivariate regression, the factors associated with successful responses 3 months after ozone injection were analyzed. The most relevant factors associated with successful responses were included in the univariate logistic regression analysis. The inclusion of variables in the final multivariate logistic regression analysis to evaluate independent factors associated with successful responses was based on clinical importance, and statistical considerations. P-values of <0.05 were considered statistically significant.
Results
Initially, a total of 38 patients with ZAP who underwent percutaneous ozone injection around the cervical dorsal root ganglion were examined. Two patients provided a history of mental disorder and 6 patients were lost to follow-up 3 months after the ozone injection. As a result, a complete review of 30 patients’ medical records were available at 3 months following the percutaneous ozone injection, there were 16 patients in group A and 14 patients in group B. The patients’ general characteristics and pain intensity data details are summarized in Table 1.
Table 1.
Patient characteristics
| Patent no. | Age | Gender | Duration | Location | Cervical level | Pre VAS | Post VAS | 1 month follow-up VAS | 3 months follow-up VAS | Pre VonFrey | Post von Frey | Number of treatments | DM history | HTN history |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 58 | F | 2 | R | C5 | 10.00 | 4.00 | 4.00 | 5.00 | 0.008 | 0.008 | 5 | N | N |
| 2 | 64 | M | 2 | L | C5 | 7.00 | 3.00 | 4.00 | 2.00 | 0.008 | 0.008 | 10 | Y | Y |
| 3 | 73 | M | 1 | L | C2–C4 | 7.00 | 3.00 | 3.00 | 4.00 | 0.008 | 0.008 | 4 | N | Y |
| 4 | 77 | M | 2 | R | C3–C4 | 6.00 | 3.00 | 3.00 | 4.00 | 0.008 | 0.008 | 7 | N | Y |
| 5 | 65 | F | 1 | L | C2–C3 | 6.00 | 3.00 | 1.00 | 0.00 | 0.004 | 0.008 | 13 | N | N |
| 6 | 64 | F | 1 | L | C2–C5 | 6.00 | 2.00 | 2.00 | 0.00 | 0.04 | 0.008 | 3 | N | N |
| 7 | 60 | F | 2 | R | C5 | 9.00 | 7.00 | 7.00 | 6.00 | 0.07 | 0.008 | 13 | Y | Y |
| 8 | 61 | F | 2 | L | C4 | 9.00 | 5.00 | 5.00 | 5.00 | 0.02 | 0.008 | 17 | Y | Y |
| 9 | 67 | M | 1 | R | C5–C8 | 7.00 | 3.00 | 3.00 | 2.00 | 1 | 0.008 | 5 | N | Y |
| 10 | 40 | F | 1 | R | C2–C3 | 7.00 | 2.00 | 3.00 | 2.00 | 0.008 | 0.008 | 10 | N | Y |
| 11 | 72 | M | 1 | L | C3–C4 | 9.00 | 3.00 | 3.00 | 1.00 | 0.008 | 0.008 | 20 | N | N |
| 12 | 59 | F | 1 | R | C5–C6 | 10.00 | 3.00 | 4.00 | 2.00 | 0.008 | 0.008 | 14 | N | N |
| 13 | 64 | F | 1 | L | C5 | 6.00 | 2.00 | 2.00 | 0.00 | 0.008 | 0.008 | 4 | N | N |
| 14 | 79 | F | 1 | L | C5–C6 | 6.00 | 1.00 | 1.00 | 0.00 | 0.02 | 0.008 | 7 | N | N |
| 15 | 53 | M | 1 | R | C4–C6 | 7.00 | 4.00 | 5.00 | 6.00 | 0.008 | 0.008 | 12 | Y | N |
| 16 | 58 | F | 1 | L | C3 | 7.00 | 2.00 | 3.00 | 4.00 | 0.008 | 0.008 | 10 | Y | Y |
| 17 | 58 | F | 3 | R | C5 | 7.00 | 4.00 | 5.00 | 7.00 | 0.008 | 0.008 | 10 | Y | Y |
| 18 | 80 | F | 3 | L | C4–C5 | 7.00 | 5.00 | 5.00 | 6.00 | 0.008 | 0.008 | 15 | Y | N |
| 19 | 53 | F | 4 | R | C6 | 6.00 | 5.00 | 6.00 | 5.00 | 6 | 1 | 5 | Y | N |
| 20 | 79 | M | 4 | R | C2–C5 | 5.00 | 2.00 | 2.00 | 3.00 | 0.008 | 0.008 | 14 | N | Y |
| 21 | 59 | F | 36 | R | C2–C4 | 9.00 | 6.00 | 5.00 | 5.00 | 8 | 0.4 | 8 | Y | Y |
| 22 | 59 | F | 7 | L | C7–C8 | 7.00 | 5.00 | 5.00 | 5.00 | 0.008 | 0.008 | 10 | Y | Y |
| 23 | 77 | M | 48 | R | C6 | 7.00 | 3.00 | 4.00 | 5.00 | 0.008 | 0.008 | 11 | N | N |
| 24 | 66 | M | 18 | L | C7–C8 | 8.00 | 4.00 | 6.00 | 7.00 | 4 | 0.4 | 11 | Y | Y |
| 25 | 60 | M | 9 | R | C7 | 5.00 | 4.00 | 3.00 | 3.00 | 0.4 | 0.4 | 4 | N | Y |
| 26 | 70 | F | 24 | L | C5 | 6.00 | 3.00 | 4.00 | 2.00 | 1 | 1 | 10 | N | Y |
| 27 | 80 | F | 8 | R | C3–C4 | 7.00 | 5.00 | 4.00 | 4.00 | 0.2 | 0.2 | 3 | Y | N |
| 28 | 43 | F | 12 | R | C2–C3 | 5.00 | 1.00 | 2.00 | 2.00 | 0.008 | 0.008 | 5 | N | N |
| 29 | 70 | M | 14 | L | C5 | 5.00 | 4.00 | 2.00 | 4.00 | 0.002 | 0.008 | 15 | Y | N |
| 30 | 57 | F | 36 | R | C5 | 8.00 | 4.00 | 5.00 | 4.00 | 0.008 | 0.008 | 6 | N | N |
Abbreviations: VAS, visual analog scale; DM, diabetes mellitus; HTN, hypertension; M, male; F, female; L, left; R, right; Y, yes; N, no.
The two groups were not significantly different in terms of age, gender, treatment location, and the underlying disease. The duration of illness in group A and group B were 1.312±0.479 and 16.143±14.496 months respectively, which represented a significant difference between the two groups (P<0.001). The pretreatment VAS of group A and group B were 7.437±1.459 and 6.571±1.283, respectively which were not significantly different (Table 2).
Table 2.
Baseline patient characteristics
| Characteristics | Group A (n=16) |
Group B (n=14) |
P-value |
|---|---|---|---|
| Age (years, mean ±SD) | 63.375±9.528 | 65.071±11.364 | 0.660 |
| Total duration of pain (months, mean ±SD) | 1.312±0.479 | 16.143±14.496 | <0.001 |
| Gender (n) | 0.923 | ||
| Male | 6 | 5 | |
| Female | 10 | 9 | |
| VAS admission (mean ±SD) | 7.437±1.459 | 6.571±1.283 | 0.098 |
| von Frey admission (mean ±SD) | 0.077±0.247 | 1.404±2.625 | 0.082 |
| Treatment location (n) | 0.276 | ||
| Left | 9 | 5 | |
| Right | 7 | 10 | |
| History of underlying disease (n) | 0.331 | ||
| Diabetes | 1 | 4 | |
| Hypertension | 4 | 3 | |
| HTN and DM | 4 | 4 | |
| None | 7 | 3 |
Notes: Group A: pain duration <3 months; Group B: pain duration ≥3 months.
Abbreviations: HTN, hypertension; DM, diabetes mellitus.
The VAS of both groups decreased significantly over time following treatment but did not demonstrate significance between groups until the 3 month evaluation. At that 3 month post-procedure evaluation, the VAS of group A was significantly lower than that of group B (P <0.05) (Figure 3).
Figure 3.
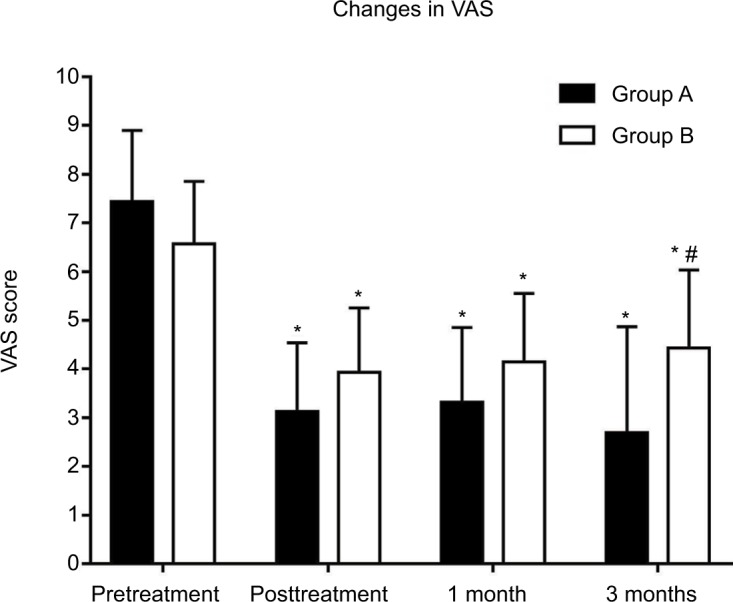
Pain level as measured by the visual analog scale in patients presented with ZAP. Both the two groups showed significant changes in the VAS over time, and showed no difference at any time point examined with the exception of the 3 month interval.
Notes: *P<0.05 compared with the pretreatment VAS level. #P<0.05 compared with the VAS level of group A.
Abbreviations: ZAP, zoster-associated pain; VAS, visual analog scale.
We assessed the pain degree at 3 months follow-up, the pain intensity of VAS <3 was used as a measure of success in relieving ZAP, and VAS ≥ 3 was considered poor pain relief. A total of 9 patients in group A (9/16, 56.3%) had excellent pain relief after the therapy (VAS <3), while 7 patients (7/16, 43.7%) showed poor improvement following ozone injection (VAS ≥ 3). Among these 7 patients, 1 patient demonstrated no change in pain degree (1/16, 6.2%); 5 patients (5/16, 31.2%) achieved satisfactory effect during and after the ozone injection, but still achieved poor pain relief (VAS ≥ 3). One patient (1/16, 6.2%) who initially achieved satisfactory analgesia during the ozone injection, noted a return of pain after the end of the ozone injection; however, this did not exceed the pretreatment level.
In the group B, at the 3 months follow-up, 2 patients (2/14, 14.3%) had satisfactory pain improvement (VAS <3); however, in the remaining 12 cases (12/14, 85.7%) satisfactory analgesia was unsuccessful (VAS ≥ 3). Three (3/14, 21.4%) of these12 patients noted no pain relief compared to their pretreatment level. Seven patients (7/14, 50.0%) did not reach a curative effect even though they got some initial pain relief (VAS ≥ 3). Two patients in group B (2/14, 14.3%), noticed that their pain returned but remained lower than their initial level (Figure 4).
Figure 4.
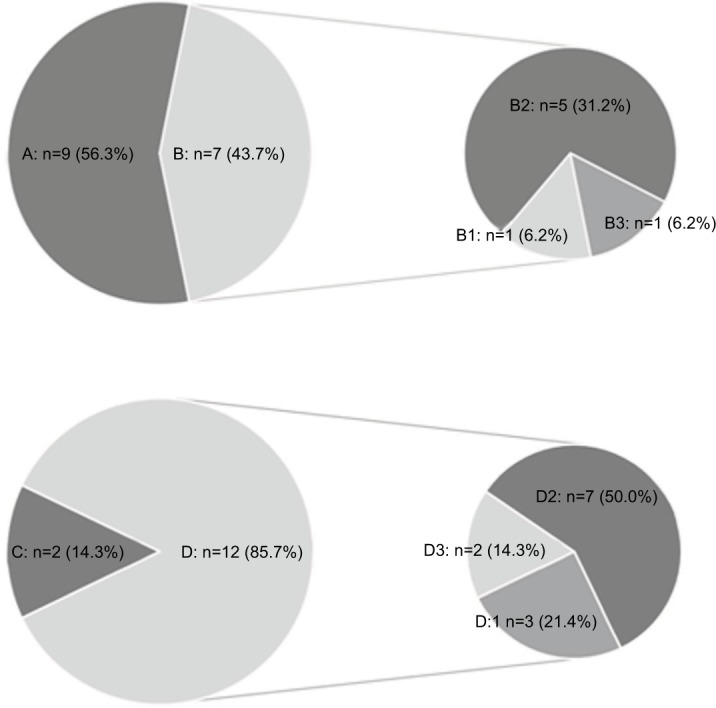
Pie charts depicting the response of the ZAP patients to ozone injection.
Notes: A, B, B1, B2 and B3: the response of the ZAP patients of group A to ozone injection. C, D, D1, D2 and D3: the response of the ZAP patients of group B to ozone injection. A and C: indicated patients satisfied with an obvious pain relief (VAS <3). B and D: indicated patients showed poor improvement in pain relief (VAS ≥ 3). B1 and D1: pain degree was no different. B2 and D2: patients got satisfactory effect during and after the ozone injection, but still achieved poor pain relief (VAS ≥ 3). B3 and D3: patients achieved satisfactory effect during the ozone injection, after the ozone injection ended the pain degree returned, but not exceeding the initial level.
Abbreviations: ZAP, zoster-associated pain; VAS, visual analog scale.
The von Frey measurements of both groups improved following treatment; however, this did not demonstrate a significant difference. Before the procedures, the von Frey of groups A and B were 0.077±0.247 and 1.404±2.625 g/mm2, respectively; which were not significantly different (Figure 5). Immediately following the treatments, we reassessed the tactile detection in all patients. In group A, the von Frey was 0.008±0.000 g/mm2, and in group B, the von Frey was 0.247±0.358 g/mm2. The posttreatment von Frey measurement of group B was significantly higher compared to group A (P <0.05).
Figure 5.
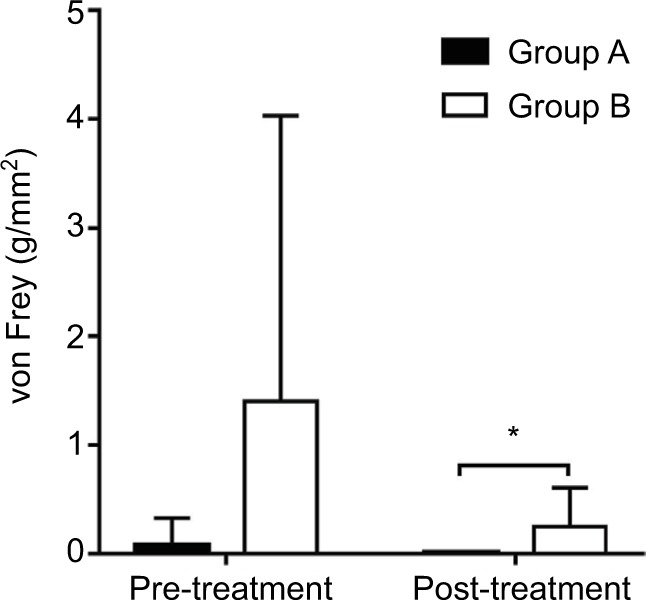
Changes in von Frey.
Notes: Evolution of tactile detections: before the procedure, the von Frey data of group A and group B have no significant difference. After the ozone treatment, no significant changes have been found in both groups, but the von Frey of group B was higher than group A and there was a significant difference. *P<0.05 compared with group A. Group A, pain duration <3 months; Group B, pain duration ≥3 months. Group A, pain duration <3 months; Group B, pain duration ≥3 months.
For all 30 patients, age was classified into two groups: <65 years and ≥ 65 years. Pain duration was treated as a potential predictive variable and classified as <3 months and ≥ 3 months. The number of treatments were classified as <10 and ≥ 10. We also classified the patients with regard to their diabetes mellitus (DM) status as with and without DM and with or without hypertension (HTN). Univariate logistic regression analysis showed that the disease duration (OR: 0.130, 95% CI: 0.022–0.779, P=0.026) and DM history (OR: 0.058, 95% CI: 0.006–0.557, P=0.014) were significantly associated with a successful response 3 months after ozone injection; however, age, gender and duration and number of treatments did not independently predict a clinically successful outcome (P>0.05). After adjusting for demographic differences for multivariate regression analysis, the association between disease duration and successful response was no longer significant. The history of DM was independently associated with a successful response 3 months after ozone injection (OR: 0.040; 95% CI: 0.002–0.937; P=0.045). Further details are provided in Tables 3 and 4.
Table 3.
Univariate analysis of possible outcome predictors for injection effectiveness after treatment
| Characteristics | Successful (n=11) | Unsuccessful (n=19) | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Age (years) | 1.146 | 0.257–5.115 | 0.858 | ||
| ≥65 | 5 | 8 | |||
| <65 | 6 | 11 | |||
| Gender | 1.939 | 0.388–9.696 | 0.420 | ||
| Male | 3 | 8 | |||
| Female | 8 | 11 | |||
| Duration | 0.130 | 0.022–0.779 | 0.026 | ||
| ≥3 months | 2 | 12 | |||
| <3 months | 9 | 7 | |||
| The number of treatments | 0.873 | 0.196–3.896 | 0.858 | ||
| ≥10 | 6 | 11 | |||
| <10 | 5 | 8 | |||
| DM history | 0.058 | 0.006–0.557 | 0.014 | ||
| With | 1 | 12 | |||
| Without | 10 | 7 | |||
| HTN history | 0.416 | 0.090–1.918 | 0.260 | ||
| With | 4 | 11 | |||
| Without | 7 | 8 |
Abbreviations: DM, diabetes mellitus; HTN, hypertension.
Table 4.
Multiple logistic regression analysis of possible outcome predictors for injection effectiveness after treatment
| Characteristics | OR | 95%CI | P-value |
|---|---|---|---|
| Age | 2.228 | 0.124–40.001 | 0.587 |
| Gender | 1.865 | 0.098–35.492 | 0.678 |
| Duration | 0.097 | 0.008–1.113 | 0.061 |
| The number of treatments | 5.165 | 0.321–83.169 | 0.247 |
| DM history | 0.040 | 0.002–0.937 | 0.045 |
| HTN history | 0.414 | 0.038–4.532 | 0.470 |
Abbreviations: DM, diabetes mellitus; HTN, hypertension.
Adverse event: In only 1 case was there a minor treatment problem. The patient underwent ozone injection around C4 DRG and experienced dizziness and an abrupt hemodynamic deterioration. This deterioration was exemplified as a drop in blood pressure (BP) from 120/82 to 84/60 mmHg and a decrease in his heart rate (HR) from 67 to 52 bpm. The patient was immediately returned to the supine position, intravenous access was quickly established and about 3 minutes later, the HR and BP of the patient recovered to initial levels and the dizziness disappeared.
Discussion
This retrospective study of patients with ZAP receiving per-cutaneous ozone injection around cervical DRGs suggests that this could provide long-term pain relief for this difficult to treat condition. Following treatment, the VAS of both groups significantly decreased over time (P<0.05). However, great pain reduction was achieved in the group A compared with the group B at all time points after ozone injection. Our study showed that an independent factor associated with successful responses to ozone injection was history of DM.
The DRG is an essential location for nociceptive signaling and suggests it is an essential site of generation of pain in HZ. This makes the DRG a priority target for intervention in managing ZAP.29 Although group B showed a significant decrease in VAS after the procedures, the success rate was much lower than group A. Some researches indicated that the reaction of VZV in sensory ganglia causes sustained neuronal damage,1,3 and the abnormal electrical activity to the spinal cord can lead to neuropathic processes and central sensitization with the development of disease,17 which could explain the superior clinical outcomes in group A in this study. Moreover, a natural healing process of acute HZ might be another reason for the better clinical outcomes of the group A in our research.12
After the statistics and analysis, we found that most of patients in this study achieved pain relief after treatment with ozone the results suggest that ozone injection procedure around cervical DRGs might be an effective therapy. However, a total of 30 ZAP patients were included in this analysis, the sample size was relative small, and this was one of our limitations. After infection with VZV, the virus becomes latent in sensory ganglion,2 once the dormant VZV is reactivated, newly synthesized viral particles are transported in both peripheral and central directions, resulting in neuropathic pain and sensory disturbance as result of intercellular disruption.1 There is an increase of chemical mediators from the injured tissue, including cytokines, substance P, histamine, etc.3,11 These changes also increase the ectopic discharge rate of C-fiber nociceptors. Repetitive discharge of C-fibers can lead to a prolonged response of neurons in the DRG, that may contribute to chronic pain states or PHN.30,31 We believe that appropriate and timely analgesic treatment decreases the incidence of hyperalgesia and allodynia by reducing central sensitization, and it is important for the prognosis.
Ozone therapy has been used to treat several diseases such as herniated discs, trigeminal neuralgia and refractory headache.11,19–21 An anti-inflammatory action induced by ozone has been reported and ozone notably increases the release of neutralizing pro-inflammatory cytokines such as interleukin IL-2, IL-8, IL-15, interferon-α and tumor necrosis factor-α.32,33 Steroids are also used to treat the ZAP patients; however, it may not only increase the risk of infection but also, is not an ideal option for the diabetic patient. It has been considered that chronic pain is mainly related to chronic inflammation,22 and the analgesia of ozone might be correlated to the anti-inflammation properties.11 Other probable mechanisms of ozone-induced pain relief could be activating the descending antinociceptive system and increasing the release of endorphins.22 The efficacy of standard treatments combined with the unique capacity of ozone therapy to reactivate the innate antioxidant system is the key to correcting the oxidative stress typical of chronic inflammatory diseases.18,22 Within the safe concentration range (below 70 µg/mL), ozone can be an efficient oxidative stress regulator: stimulating the antioxidant system of the cell and helping to normalize the redox balance altered by several pathological conditions such as, viral infection, aging or inflammatory-immune injuries.18 In addition, ozone can enhance oxygen delivery to ischemic tissues secondary to erythrocyte manipulation, as well as the local release of nitric oxide and carbon monoxide by the endothelium. Thus, ozone could induce vasodilation in ischemic areas and secondarily reduce hypoxia.20,34
Before ozone injection, the tactile discrimination at the lesion skin area of the ZAP patients became abnormal, especially in group B. Von Frey data following the ozone injection treatments, showed that both groups have an improvement in tactile discrimination, although there was no significant difference between groups before and after the treatments. We concluded that this treatment strategy has a protective effect on the recovery of the tactile sensitivity and it can be a safe and reliable method to relieve the pain intensity for ZAP patients. In future studies, it will be necessary to adopt more evaluation methods to analyze the effect of ozone injection therapy; these will include skin temperature and pain threshold of the lesion areas providing more objective evidence.
Additionally, in order to explore why differences exist in response to treatment within groups, we combined all the patients from both groups and designed the study to analyze any influence of age, gender, pain duration, the number of treatments, HTN history and DM history on the pain relief after the ozone treatment. As a result, we concluded that a history of DM independently predicts a clinically successful outcome and thus had a significant effect on the results. In a previous study, it was shown that patients with DM are at higher risk of HZ.35,36 DM as a chronic disease, can cause tissue damage throughout the body but especially in the nervous system. Neural changes such as axonal loss and sensory nerve fiber dysfunction, nerve demyelination, increased nerve water content and decreased nerve conduction velocity might lead to the development of diabetic peripheral neuropathy and have an influence on the ameliorative effect of ozone.37–39
In our study, we acquired anatomical images of cervical DRG through the ultrasound. In the future, ultrasound guidance would be the preferred method of guiding the needle tip to the proximity of the DRG. It would provide a real-time image and observation of gas diffusion process. An ultrasound device is portable, low cost and eliminates the exposure to X-ray radiation compared with the CT-guided methods.
Regarding the possible reason of the adverse event, one hypothesis is that, owing to the location of C4 DRG is nearer to the carotid sinus, the diffused O2–O3 mixture and the probe positioned might stimulate the carotid sinus and stimulate the vagus nerve, causing fluctuation of heart-rate and blood pressure. Hence, we suggest that pretreatment with a vagolytic should be considered prior to ozone therapy at the C4 level: especially in the elderly and patients with cardiovascular diseases.
Limitations
First, this study was a relatively small sample size and considered a short duration of action. Second, this was a retrospective study based on the analysis of medical records, additional prospective studies are necessary. Third, we need to supplement the measurement of anti-inflammatory action induced by ozone during the treatment in the future. Forth, in further research, we can set a control group, and more objective assessment methods should be used to evaluate the reliability and security of this treatment.
Conclusion
Ozone injection around cervical DRG might be an effective and safe, minimally invasive strategy for the treatment of ZAP condition.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Reichelt M, Zerboni L, Arvin AM. Mechanisms of varicella-zoster virus neuropathogenesis in human dorsal root ganglia. J Virol. 2008;82(8):3971–3983. doi: 10.1128/JVI.02592-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323(10):627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- 3.Volpi A, Gross G, Hercogova J, Johnson RW. Current management of herpes zoster: the European view. Am J Clin Dermatol. 2005;6(5):317–325. doi: 10.2165/00128071-200506050-00005. [DOI] [PubMed] [Google Scholar]
- 4.Brisson M, Edmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom—CORRIGENDUM. Epidemiol Infect. 2015;143(6):1332. doi: 10.1017/S0950268814002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzil KM, Griffin MR. Preventing shingles and its complications in older persons. N Engl J Med. 2016;375(11):1079–1080. doi: 10.1056/NEJMe1610652. [DOI] [PubMed] [Google Scholar]
- 6.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18(6):350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Liang L, Li X, Zhang G, Sun Y, Yu H, Jiao J. Pregabalin in the treatment of herpetic neuralgia: results of a multicenter Chinese study. Pain Med. 2015;16(1):160–167. doi: 10.1111/pme.12564. [DOI] [PubMed] [Google Scholar]
- 8.Meng FY, Zhang LC, Liu Y, et al. Efficacy and safety of gabapentin for treatment of postherpetic neuralgia: a meta-analysis of randomized controlled trials. Minerva Anestesiol. 2014;80(5):556–567. [PubMed] [Google Scholar]
- 9.van Wijck AJ, Opstelten W, Moons KG, et al. The PINE study of epidural steroids and local anaesthetics to prevent postherpetic neuralgia: a randomised controlled trial. Lancet. 2006;367(9506):219–224. doi: 10.1016/S0140-6736(06)68032-X. [DOI] [PubMed] [Google Scholar]
- 10.Harpaz R, Ortega-Sanchez IR, Seward JF, Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC) Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP. MMWR Recomm Rep. 2008;57(RR-5):1–30. [PubMed] [Google Scholar]
- 11.An JX, Liu H, Chen RW, et al. Computed tomography-guided percutaneous ozone injection of the Gasserian ganglion for the treatment of trigeminal neuralgia. J Pain Res. 2018;11:255–263. doi: 10.2147/JPR.S140369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim K, Jo D, Kim E. Pulsed radiofrequency to the dorsal root ganglion in acute herpes zoster and postherpetic neuralgia. Pain Physician. 2017;20(3):E411–E418. [PubMed] [Google Scholar]
- 13.Fang L, Ying S, Tao W, Lan M, Xiaotong Y, Nan J. 3D CT-guided pulsed radiofrequency treatment for trigeminal neuralgia. Pain Pract. 2014;14(1):16–21. doi: 10.1111/papr.12041. [DOI] [PubMed] [Google Scholar]
- 14.Thapa D, Ahuja V, Dass C, Verma P. Management of refractory tri-geminal neuralgia using extended duration pulsed radiofrequency application. Pain Physician. 2015;18(3):E433–E435. [PubMed] [Google Scholar]
- 15.Rohof OJ. Caudal epidural of pulsed radiofrequency in post herpetic neuralgia (PHN); report of three cases. Anesth Pain Med. 2014;4(3):e16369. doi: 10.5812/aapm.16369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacks GM. Unmet need in the treatment of postherpetic neuralgia. Am J Manag Care. 2013;19(1Suppl):207–213. [PubMed] [Google Scholar]
- 17.Ji RR, Kohno T, Moore KA, Woolf CJ, Rr J, Sensitization C. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Bocci V. Does ozone therapy normalize the cellular redox balance? Implications for therapy of human immunodeficiency virus infection and several other diseases. Med Hypotheses. 1996;46(2):150–154. doi: 10.1016/s0306-9877(96)90016-x. [DOI] [PubMed] [Google Scholar]
- 19.Gautam S, Rastogi V, Jain A, Singh AP. Comparative evaluation of oxygen-ozone therapy and combined use of oxygen-ozone therapy with percutaneous intradiscal radiofrequency thermocoagulation for the treatment of lumbar disc herniation. Pain Pract. 2011;11(2):160–166. doi: 10.1111/j.1533-2500.2010.00409.x. [DOI] [PubMed] [Google Scholar]
- 20.Clavo B, Santana-Rodriguez N, Gutierrez D, et al. Long-term improvement in refractory headache following ozone therapy. J Altern Complement Med. 2013;19(5):453–458. doi: 10.1089/acm.2012.0273. [DOI] [PubMed] [Google Scholar]
- 21.Murphy K, Elias G, Steppan J, et al. Percutaneous treatment of herniated lumbar discs with ozone: investigation of the mechanisms of action. J Vasc Interv Radiol. 2016;27(8):1242–1250. doi: 10.1016/j.jvir.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Bocci V, Borrelli E, Zanardi I, Travagli V. The usefulness of ozone treatment in spinal pain. Drug Des Devel Ther. 2015;9:2677–2685. doi: 10.2147/DDDT.S74518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The dorsal root ganglion as a therapeutic target for chronic pain. Reg Anesth Pain Med. 2016;41(4):511–519. doi: 10.1097/AAP.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 24.Erken G, Erken HA, Bilen C, Gencer N. The effects of ex vivo ozone treatment on human erythrocyte carbonic anhydrase enzyme. Arch Physiol Biochem. 2018;124(2):1–4. doi: 10.1080/13813455.2017.1371194. [DOI] [PubMed] [Google Scholar]
- 25.Narouze SN, Vydyanathan A, Kapural L, Sessler DI, Mekhail N. Ultrasound-guided cervical selective nerve root block: a fluoroscopy-controlled feasibility study. Reg Anesth Pain Med. 2009;34(4):343–348. doi: 10.1097/AAP.0b013e3181ac7e5c. [DOI] [PubMed] [Google Scholar]
- 26.Kanai A, Suzuki A, Kobayashi M, Hoka S. Intranasal lidocaine 8% spray for second-division trigeminal neuralgia. Br J Anaesth. 2006;97(4):559–563. doi: 10.1093/bja/ael180. [DOI] [PubMed] [Google Scholar]
- 27.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 28.Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5(6):344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Oaklander AL. Mechanisms of pain and itch caused by herpes zoster (shingles) J Pain. 2008;9(1):10–18. doi: 10.1016/j.jpain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Jeon YH, Zoster H. Herpes zoster and postherpetic neuralgia: practical consideration for prevention and treatment. Korean J Pain. 2015;28(3):177–184. doi: 10.3344/kjp.2015.28.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Treede RD. Gain control mechanisms in the nociceptive system. Pain. 2016;157(6):1199–1204. doi: 10.1097/j.pain.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 32.Bocci V. Ozone as Janus: this controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13(1):3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bocci V, Luzzi E, Corradeschi F, Paulesu L, di Stefano A. Studies on the biological effects of ozone: 3. An attempt to define conditions for optimal induction of cytokines. Lymphokine Cytokine Res. 1993;12(2):121–126. [PubMed] [Google Scholar]
- 34.Valacchi G, Bocci V. Studies on the biological effects of ozone: 11. Release of factors from human endothelial cells. Mediators Inflamm. 2000;9(6):271–276. doi: 10.1080/09629350020027573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen HH, Lin IC, Chen HJ, Yeh SY, Kao CH. Association of herpes zoster and type 1 diabetes mellitus. PLoS One. 2016;11(5):e0155175. doi: 10.1371/journal.pone.0155175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67(5):463–469. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Ahlawat A, Sharma S. A new promising simultaneous approach for attenuating type II diabetes mellitus induced neuropathic pain in rats: iNOS inhibition and neuroregeneration. Eur J Pharmacol. 2018;818:419–428. doi: 10.1016/j.ejphar.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Jin HY, Lee KA, Song SK, et al. Sulodexide prevents peripheral nerve damage in streptozotocin induced diabetic rats. Eur J Pharmacol. 2012;674(2-3):217–226. doi: 10.1016/j.ejphar.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 39.Vinik A, Emir B, Cheung R, Whalen E. Relationship between pain relief and improvements in patient function/quality of life in patients with painful diabetic peripheral neuropathy or postherpetic neuralgia treated with pregabalin. Clin Ther. 2013;35(5):612–623. doi: 10.1016/j.clinthera.2013.03.008. [DOI] [PubMed] [Google Scholar]