Abstract
Objective
The aim of the study was to assess the myostatin concentration and an improvement in the severity of urinary incontinence (UI) after pelvic floor muscle training (PFMT) in a group of elderly women with stress UI.
Methods
A total of 74 participants were included in the analysis: 40 participants in the experimental group (EG) and 34 participants in the control group (CG). The EG underwent PFMT, whereas no therapeutic intervention was applied to the CG. Myostatin concentration and UI severity (Revised Urinary Incontinence Scale [RUIS]) were assessed in all women before and after the treatment.
Results
By comparing the results before and after the treatment, we have been able to demonstrate a statistically significant decrease in myostatin concentration (P<0.0001) and an improvement in the severity of UI (RUIS) (P<0.0001) in the EG. No statistically significant differences in all measured variables were reported before and after the treatment in the CG. A lower myostatin concentration (P=0.0084) and an improvement in the severity of UI (RUIS) (P=0.0008) were observed after the treatment in the EG compared to that in the CG.
Conclusion
Effective PFMT causes downregulation of myostatin concentration and an improvement in the severity of UI in elderly women with stress UI. Further trials on a larger EG and an assessment of long-term treatment outcomes are required.
Keywords: urinary incontinence, pelvic floor muscle training, myostatin
Introduction
According to the epidemiological data, 4%–8% of the population suffer from urinary incontinence (UI). According to the data adopted during the sixth International Consultations on Incontinence (ICI), it is estimated that, in 2018, the number of people with UI around the world will be approximately 420–300 million women and 120 million men. Statistics clearly states that UI is a serious health, social, and economic problem.1,2 The percentage of women suffering from UI increases with age, and the condition often becomes more severe with time. Aging is associated with a progressive decline in muscle strength and mass, described as sarcopenia.3 Decrease in pelvic floor muscle mass may result in UI. Pelvic floor muscle mass reduction is caused by various mechanisms, such as type II myofiber atrophy as a result of motor neuron death, hormonal changes, reduced calorie and protein supply, inflammatory mediators, stress, reduced physical activity, and alteration in the synthesis of various proteins, including myostatin.4,5 Myostatin, known as Growth and Differentiation Factor-8 (GDF-8), is a member of the TGF-β superfamily.6 Myostatin is a protein produced by skeletal muscle cells that enters the bloodstream of living organisms and inhibits muscle growth.7 The myostatin gene acts as a mediator of gene expression, controls muscle fiber formation, and inhibits muscle growth by inhibiting myoblast proliferation.8 The level of myostatin increases in periods of skeletal muscle inactivity, and the inhibition of serum myostatin increases muscle strength and mass.9,10 Therapeutic interventions such as physical activity can suppress myostatin signaling for the purpose of ameliorating the effects of advancing age on skeletal muscle mass and function.11 There are some studies suggesting that myostatin inhibits human urethral rhabdosphincter satellite cell proliferation; therefore, inhibition of myostatin function might be a useful strategy for the treatment of stress UI.12
Physiotherapy is recommended as the first-line treatment for UI. According to the 2017 European Association of Urology guidelines on the assessment and nonsurgical management of UI, the available physical therapies include pelvic floor muscle training (PFMT), bladder training, electrical stimulation, posterior tibial nerve stimulation, and magnetic stimulation.13 The scientific basis for pelvic floor muscle rehabilitation was founded by an American gynecologist Arnold Kegel, who, in 1950, published results of a 15-year-old study, which covered the use of pelvic floor exercises in patients with UI. Kegel and Powell14 pointed out that systematic muscle activity causes muscles to lose four times less of their mass than compared with staying inactive, and hence, it is the optimal method for improving the anatomical and functional impairment of muscles. Two biological theories explain the mechanism through which PFMT may alleviate UI. According to one theory, the external sphincter muscle (musculus sphincter urethral externus) squeezes the urethra, and according to another theory, strong PFM may support the bladder neck.15,16
The aim of study
The aim of the study was to assess the myostatin concentration and the improvement in the severity of UI after PFMT in a group of elderly women with stress UI.
Methods
Study design
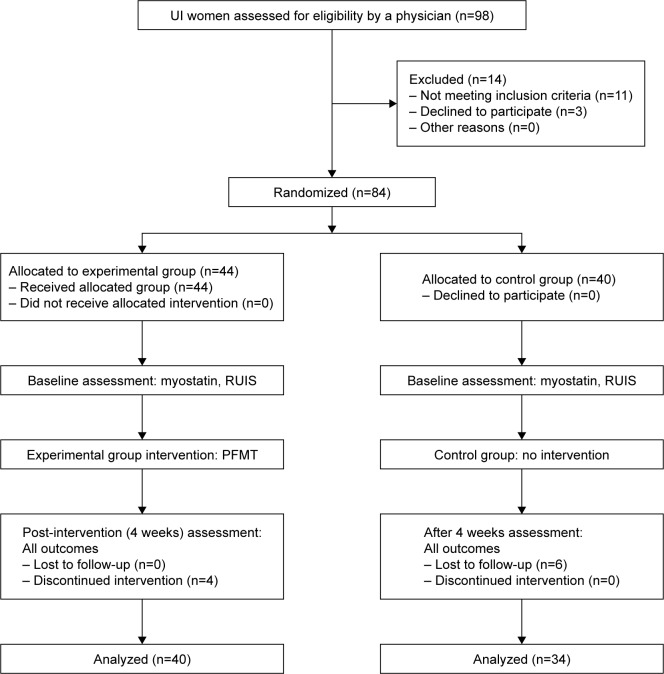
Between January 2017 and May 2017, 98 women with UI were enrolled into a randomized, double-blind, controlled study. Approval from the Bioethics Committee of the Collegium Medicum in Bydgoszcz, Nicolaus Copernicus University in Torun, was obtained for this study (768/2016 CM UMK). All patients provided written informed consent for the study. To ensure stratified randomization, subjects were allocated using a simple method of picking envelopes containing a group allocation number from a computer-generated random number table. The main investigator was blinded as to the intervention group allocation. In the first stage of the study, 14 women were excluded (11 women did not meet the inclusion criteria and three women refused to participate). A total of 84 women were then randomly assigned to the experimental group (EG) or the control group (CG). The total number of participants who did not complete the study was 10: four women withdrew from the EG during the 4-week intervention program and six women from the CG missed the final study visit. Hence, 74 women have completed the study (EG n=40; CG n=34). CONSORT statement was used to improve the RCT reporting quality (Figure 1).17
Figure 1.
The study flow diagram.
Abbreviations: PFMT, pelvic floor muscle training; RUIS, Revised Urinary Incontinence Scale; UI, urinary incontinence.
Before the treatment, all the women were asked about the circumstances of urine loss and the presence of comorbid conditions and contraindications to the treatment. Additionally, the Questionnaire for Urinary Incontinence Diagnosis (QUID) was used to diagnose UI type. The QUID is a 6-item UI symptom questionnaire, which was developed and validated to distinguish stress and urge UI. The QUID has acceptable psychometric characteristics and may be used as a UI outcome measure in clinical trials.18
Study inclusion criteria were as follows: age 60 years or older, diagnosed stress UI, and no contradictions to the treatment (active malignancy, recent surgeries, recent pelvic fractures, fever, acute inflammations, uterine tumors and myomas, urinary or genital tract infections, grade 3 or 4 hemorrhoids, stage 3 uterine prolapse [downward displacement of the uterus into the vagina]). Study exclusion criteria were as follows: age <60 years, diagnosed urge and mixed UI, recent therapeutic interventions in UI in the last 3 months (PFMT, magnetotherapy, electrostimulation, and biofeedback), and the presence of contraindications to the treatment.
Measurements
In order to objectify the results, myostatin concentration was measured for the EG and the CG before and after the treatment and the UI severity assessment (RUIS) was performed.
Myostatin level
The study design follows the procedure described in the Myostatin ELISA (Immundiagnostik AG, Bensheim, Germany) manual for the determination of myostatin in human serum and plasma (K 1012 catalog number). Six milliliters of blood was collected from each subject after at least 12 hours fasting into Vacuette tubes with EDTA anticoagulant. Participants had to fast over night with the last meal before 7 pm on the day before the investigation. The collected samples were centrifuged at 3,000 rpm for 15 minutes. The plasma obtained during centrifugation was then pipetted into smaller samples (about 500 µL) and was frozen at −80°C. The results were read using the BMG Labtech ELISA absorbance reader with a monochromator. The assay was used for the quantitative determination of myostatin in serum and EDTA plasma. In the first step, a biotinylated myostatin tracer was added to the samples, standards, and controls. Next, aliquots of the treated preparations were transferred and incubated in microtiter plate wells coated with polyclonal antimyostatin antibodies. During the incubation phase, the free target antigen present in the samples competes with the biotinylated myostatin tracer and, then, it binds with the polyclonal antimyostatin antibodies immobilized on the microtiter plate wells. Unbound components were removed at the washing step. During the second incubation step, a streptavidin-labeled peroxidase antibody, which binds to the biotinylated myostatin tracer, was added into each microtiter well. Following the washing step to remove the unbound components, the peroxidase substrate tetramethylbenzidine (TMB) was added. In the end, the enzymatic reaction was terminated by an acidic stop solution. Subsequently, the color changed from blue to yellow. The intensity of the yellow color is inversely proportional to the myostatin concentration in the sample. Consequently, high myostatin concentration in the sample reduces the concentration of the biotinylated myostatin tracer bound to the immobilized antimyostatin antibodies and weakens the photometric signal. The color intensity was measured at 450 nm.
The Revised Urinary Incontinence Scale (RUIS)
The RUIS is a valid 5-item scale that can be used to assess UI and to monitor patient outcomes following treatment. A score of 0–3 is considered non-UI, a score of 4–8 is considered mild UI, a score of 9–12 is considered moderate UI, and a score of 13 or above is considered severe UI.19
Intervention
At the beginning of the study, physiotherapists specializing in urogynecology introduced the EG participants to the topic of the anatomy of the pelvic floor muscles and the lower part of the urinary tract, as well as physiological mechanisms of UI. Women who participated in the therapy sessions learned how to contract their pelvic floor muscles correctly, as assessed by vaginal palpation. The PFMT program was launched after confirming the participants’ contraction technique. The intervention comprised a planned 4-week training program. The EG participated in 12 therapy sessions of supervised PFMT (45 minutes × three times a week, for 4 weeks). Training groups comprised 5–6 participants. Exercise type and difficulty were individually adjusted to the psychophysical fitness of the participants.
Statistical analyses
Statistical analysis of the collected data was performed using the Statistica 13.1 software. The normality distribution of measured variables was checked using the Shapiro–Wilk test. Since the variables did not show normal distribution, the median, lower quartile (Q1), and upper quartile (Q3) were measured. Differences between the two groups were estimated using the Mann–Whitney U-test. Differences within one group were estimated using the Wilcoxon test. The correlation between measured variables was evaluated using the Spearman correlation coefficient. The level of statistical significance was defined as P<0.05.
Results
Table 1 shows descriptive statistics and Mann–Whitney U-test results for all measured variables for the EG and CG at the initial assessment.
Table 1.
Comparative analysis of all measured variables for the EG and CG – the initial assessment
| Parameters | Statistics | EG (n=40) | CG (n=34) | P-value |
|---|---|---|---|---|
|
| ||||
| Age | IQR | 14.00 | 10.00 | 0.2452 |
| Med | 69.50 | 69.50 | ||
|
| ||||
| Myostatin concentration (ng/mL) | IQR | 38.69 | 79.58 | 0.6644 |
| Med | 142.74 | 145.32 | ||
|
| ||||
| RUIS (points) | IQR | 6.00 | 7.00 | 0.7408 |
| Med | 8.00 | 8.00 | ||
Note: P, significance level.
Abbreviations: EG, experimental group; CG, control group; Med, median; RUIS, Revised Urinary Incontinence Scale.
We compared the Mann–Whitney U-test P-value with the significance level of α=0.05 and found that there are no statistically significant differences between the EG and CG results at the initial assessment. This confirms the homogeneity of the study groups. In the next stage of the study, we correlated the RUIS scores with the myostatin concentration results in the EG and CG at the initial assessment. No correlation was found between the measured variables both in the EG (r=0.184) and the CG (r=0.051).
Table 2 shows descriptive statistics and Wilcoxon test results for all measured variables for the EG and CG at the initial and final assessments.
Table 2.
Comparative analysis of all measured variables for the EG and CG – the initial and final assessments
| Parameters | Statistics | EG (n=40)
|
P-value | CG (n=34)
|
P-value | ||
|---|---|---|---|---|---|---|---|
| Initial assessment | Final assessment | Initial assessment | Final assessment | ||||
|
| |||||||
| Myostatin concentration (ng/mL) | IQR | 38.69 | 48.57 | <0.0001* | 79.58 | 67.47 | 0.1852 |
| Med | 142.74 | 121.15 | 145.32 | 153.08 | |||
|
| |||||||
| RUIS (points) | IQR | 6.00 | 5.00 | <0.0001* | 7.00 | 5.00 | 0.1904 |
| Med | 8.00 | 6.00 | 8.00 | 9.00 | |||
Note:
Indicates statistical significance.
Abbreviations: EG, experimental group; CG, control group; Med, median; RUIS, Revised Urinary Incontinence Scale.
We compared the Wilcoxon test P-value with the significance level of α=0.05 and found that there is a statistically significant difference in all measured variables between the EG results at the initial and final assessments. A statistically significant decrease in myostatin concentration and reduction in the severity of UI were observed in the PFMT group at the final assessment. We compared the Wilcoxon test P-value with the significance level of α=0.05 and found that there are no statistically significant differences in all measured variables between the CG results at the initial and final assessments.
Table 3 shows the relationship between the assessment of myostatin concentration for the EG at the initial and final assessments and the severity of UI (RUIS).
Table 3.
The relationship between the assessment of myostatin concentration for the EG at the initial and final assessments and RUIS
| RUIS (points) | Statistics | Myostatin concentration (ng/mL)
|
P-value | |
|---|---|---|---|---|
| Initial assessment | Final assessment | |||
|
| ||||
| Mild | IQR | 33.59 | 31.79 | <0.0001* |
| Med | 138.89 | 120.10 | ||
|
| ||||
| Moderate | IQR | 61.60 | −82.43 | 0.0051* |
| Med | 146.46 | 125.37 | ||
|
| ||||
| Severe | IQR | 44.15 | 39.49 | 0.0277* |
| Med | 143.57 | 112.76 | ||
Note:
Indicates statistical significance.
Abbreviations: EG, experimental group; Med, median; RUIS, Revised Urinary Incontinence Scale.
We compared the Wilcoxon test P-value with the significance level of α=0.05 and found that there is a statistically significant difference in myostatin concentration at the final assessment in patients with mild, moderate, and severe UI.
Table 4 shows descriptive statistics and Mann–Whitney U-test results for all measured variables for the EG and CG at the final assessment.
Table 4.
Comparative analysis of all measured variables for the EG and CG – the final assessment
| Parameters | Statistics | EG (n=40) | CG (n=34) | P-value |
|---|---|---|---|---|
|
| ||||
| Myostatin concentration (ng/mL) | IQR | 48.57 | 67.47 | 0.0084* |
| Med | 121.15 | 153.08 | ||
|
| ||||
| RUIS (points) | IQR | 5.00 | 5.00 | 0.0008* |
| Med | 6.00 | 9.00 | ||
Note:
Indicates statistical significance.
Abbreviations: EG, experimental group; CG, control group; Med, median; RUIS, Revised Urinary Incontinence Scale.
We compared the Mann–Whitney U-test P-value with the significance level of α=0.05 and found that there is a statistically significant difference in all measured variables between the EG and CG at the final assessment.
In the last stage of the study, we correlated the RUIS scores with the myostatin concentration results in the EG and CG at the final assessment. No correlation was found between the measured variables both in the EG (r=0.184) and the CG (r=0.051).
Discussion
Pelvic muscle training is recommended as a first-line therapy for stress UI, and its effectiveness has been confirmed by numerous studies.20 In this study, we attempted to assess biochemical changes that occur after PFMT. We measured the myostatin level, since the inhibition of serum myostatin increases muscle strength and mass. Other authors have also attempted to assess myostatin levels in response to various forms of physical activity.21–25 However, none of these studies have looked at patients with UI. This is the first study, which assesses myostatin concentration after physical activity, namely PFMT. We observed a statistically significant, lower myostatin concentration (P<0.0001) in the EG after the treatment. We also decided to check whether the myostatin concentration had changed after treatment, regardless of the severity of UI. The results demonstrated a decrease in myostatin concentration in patients with mild, moderate, and severe UI. Furthermore, all the results were statistically significant. At the same time, no statistically significant differences in myostatin concentration were reported (P=0.1852) for the CG after therapy.
In addition to the biochemical parameters, we assessed the treatment effectiveness from subjects’ perspective. For this purpose, we used the RUIS, which is a short, reliable, and valid scale for evaluating UI and its response to treatment.15 The analysis of RUIS results in the EG at the initial assessment showed a mild incontinence in 65%, moderate incontinence in 28%, and severe UI in 7% of women. At the final assessment, the results showed nonincontinence in 33%, mild incontinence in 45%, and moderate incontinence in 22% of women, whereas the analysis of RUIS results in the CG at the initial assessment showed a mild incontinence in 55%, moderate incontinence in 43%, and severe incontinence in 2% of women. At the final assessment, the results showed mild incontinence in 33%, moderate incontinence in 55%, and severe incontinence in 12% of women. These results confirm the effectiveness of the therapeutic program and also demonstrate that the lack of therapeutic intervention can lead to worsening of UI symptoms.
The highest level of statistical significance was observed in patients with mild UI, whereas the lowest level of statistical significance was reported for patients with moderate UI. Further research is recommended to examine this, as this relationship can play a significant role in treatment planning.
Limitations
The authors acknowledge certain limitations of their analysis. These include a relatively small study group and the lack of assessment of long-term treatment outcomes. We therefore consider this research as a pilot study, and we plan to further study this topic.
Conclusion
Effective PFMT causes downregulation of myostatin concentration and an improvement in the severity of UI in elderly women with stress UI. Further trials on a larger EG and an assessment of long-term treatment outcomes are required.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Abrams P, Cardozo L, Wagg A, Wein A. Incontinence. 6th ed. Bristol UK: ICI-ICS. International Continence Society; 2017. [Google Scholar]
- 2.Serati M, Ghezzi F. The epidemiology of urinary incontinence: a case still open. Ann Transl Med. 2016;4(6):123. doi: 10.21037/atm.2016.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witham MD, Aihie Sayer A. Introduction to the Age and Ageing sarcopenia collection. Age Ageing. 2016;45(6):752–753. doi: 10.1093/ageing/afw145. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95(4):1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 6.Roberts SB, Goetz FW. Differential skeletal muscle expression of myostatin across teleost species, and the isolation of multiple myostatin isoforms. FEBS Lett. 2001;491(3):212–216. doi: 10.1016/s0014-5793(01)02196-2. [DOI] [PubMed] [Google Scholar]
- 7.Casas E, Shackelford SD, Keele JW, Stone RT, Kappes SM, Koohmaraie M. Quantitative trait loci affecting growth and carcass composition of cattle segregating alternate forms of myostatin. J Anim Sci. 2000;78(3):560–569. doi: 10.2527/2000.783560x. [DOI] [PubMed] [Google Scholar]
- 8.Shin S, Song Y, Ahn J, et al. A novel mechanism of myostatin regulation by its alternative splicing variant during myogenesis in avian species. Am J Physiol Cell Physiol. 2015;309(10):C650–C659. doi: 10.1152/ajpcell.00187.2015. [DOI] [PubMed] [Google Scholar]
- 9.Joulia-Ekaza D, Cabello G. Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res. 2006;312(13):2401–2414. doi: 10.1016/j.yexcr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Whittemore LA, Song K, Li X, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300(4):965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 11.Lynch GS. Emerging drugs for sarcopenia: age-related muscle wasting. Expert Opin Emerg Drugs. 2004;9(2):345–361. doi: 10.1517/14728214.9.2.345. [DOI] [PubMed] [Google Scholar]
- 12.Akita Y, Sumino Y, Mori K, Nomura T, Sato F, Mimata H. Myostatin inhibits proliferation of human urethral rhabdosphincter satellite cells. Int J Urol. 2013;20(5):522–529. doi: 10.1111/j.1442-2042.2012.03186.x. [DOI] [PubMed] [Google Scholar]
- 13.Burkhard FC, Bosch JLH, Cruz F, et al. EAU Guidelines on urinary incontinence; Presented at: EAU Annual Congress London; 2017; Arnhem, The Netherlands: EAU Guidelines Office. [Google Scholar]
- 14.Kegel AH, Powell TO. The physiologic treatment of urinary stress incontinence. J Urol. 1950;63(5):808–814. doi: 10.1016/S0022-5347(17)68832-3. [DOI] [PubMed] [Google Scholar]
- 15.Delancey JOL. Structural aspects of urethrovesical function in the female. Neurourol Urodyn. 1988;7(6):509–519. [Google Scholar]
- 16.Ptak M, Brodowska A, Ciećwież S, Rotter I. Quality of Life in Women with Stage 1 Stress Urinary Incontinence after Application of Conservative Treatment-A Randomized Trial. Int J Environ Res Public Health. 2017;14(6):pii:E577. doi: 10.3390/ijerph14060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diallo S, Cour F, Josephson A, et al. Evaluating single-incision slings in female stress urinary incontinence: the usefulness of the CONSORT statement criteria. Urology. 2012;80(3):535–541. doi: 10.1016/j.urology.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Bradley CS, Rahn DD, Nygaard IE, et al. The questionnaire for urinary incontinence diagnosis (QUID): Validity and responsiveness to change in women undergoing non-surgical therapies for treatment of stress predominant urinary incontinence. Neurourol Urodyn. 2010;29:727–734. doi: 10.1002/nau.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansoni J, Hawthorne G, Fleming G, Owen E, Marosszeky N. Technical manual and instructions: revised incontinence and patient satisfaction tools; Centre for Health Service Development, University of Wollon-gong; 2011. [Accessed September 17, 2018]. Available from: http://www.bladderbowel.gov.au/assets/doc/ncms/Phase3InformationAndEvidence/TechnicalManualForTh-eRevisedIncontinenceAndPatientSatisfactionTools.pdf. [Google Scholar]
- 20.Radzimińska A, Strączyńska A, Weber-Rajek M, Styczyńska H, Strojek K, Piekorz Z. The impact of pelvic floor muscle training on the quality of life of women with urinary incontinence: a systematic literature review. Clin Interv Aging. 2018;13:957–965. doi: 10.2147/CIA.S160057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lenk K, Erbs S, Höllriegel R, et al. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Prev Cardiol. 2012;19(3):404–411. doi: 10.1177/1741826711402735. [DOI] [PubMed] [Google Scholar]
- 22.Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA. Myostatin gene expression is reduced in humans with heavy-resistance strength training: a brief communication. Exp Biol Med (Maywood) 2003;228(6):706–709. doi: 10.1177/153537020322800609. [DOI] [PubMed] [Google Scholar]
- 23.Walker KS, Kambadur R, Sharma M, Smith HK. Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc. 2004;36(5):787–793. doi: 10.1249/01.mss.0000126384.04778.29. [DOI] [PubMed] [Google Scholar]
- 24.Willoughby DS. Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc. 2004;36(4):574–582. doi: 10.1249/01.mss.0000121952.71533.ea. [DOI] [PubMed] [Google Scholar]
- 25.Hosseini SRA, Moeinnia N, Rad MM. The effect of two intensities resistance training on muscle growth regulatory myokines in sedentary young women. Obesity Medicine. 2017;2(5):25–28. [Google Scholar]