Although the precise mechanisms are uncertain, essential tremor (ET) is thought to be caused by tremulous activity within a central tremor neural network, which involves the ventral intermediate nucleus (VIM) of the thalamus.1, 2 Clinical evidence supports targeting the VIM to treat tremor symptoms in ET with various methods.2 Previous studies have shown that electrical median nerve stimulation evokes activity within the VIM and other regions of the central tremor network.3 Based on these reports, we hypothesized that median and radial nerve stimulation at the wrist could reduce hand tremor. The objective of this study was to evaluate the efficacy of median and radial nerve stimulation as a noninvasive, nonpharmacological treatment to aid in the symptomatic relief of hand tremor in individuals with ET.
Twenty‐three blinded subjects were examined at a single site under an institutional review board‐approved protocol (Fig. S1, Table S1). Subjects were randomized to treatment or sham groups. For stimulation, hydrogel electrodes were positioned on the wrist over the median and radial nerves (Fig. 1A; see Supporting Information). Efficacy was measured as the change in the Tremor Research Group's Essential Tremor Rating Assessment Scale (TETRAS) Archimedes spiral drawing task following stimulation compared with prestimulation (Fig. 1B,C).4 The response in the treatment group was significant compared with both baseline and sham. In the treatment group, blinded rater scores significantly improved following stimulation (1.77 ± 0.21) compared with prestimulation (2.77 ± 0.22; P = 0.01; Fig. 1D). This response was achieved without the risks of surgical or pharmacological intervention, such as the risk of hemorrhage or infection with DBS implantation,5 or side effects of ET medications, including the first‐line therapies propranolol and primidone.6 In the sham group, scores did not change significantly following stimulation (2.37 ± 0.22) compared with prestimulation (2.62 ± 0.14; P = 0.37; Fig. 1E). The response to treatment corresponded to an estimated hand tremor amplitude reduction of 60% ± 8.4% and was significantly greater in the treatment than in the sham group (P = 0.02; Fig. 1F). Three subjects experienced transient redness and/or itchiness under the hydrogel electrodes that resolved without intervention. No unanticipated device effects occurred during the study.
Figure 1.
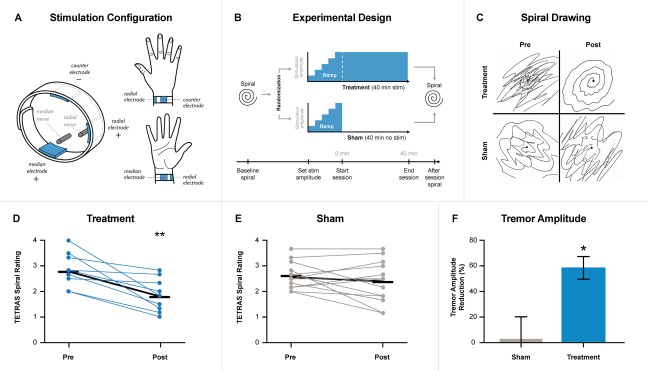
(A) Electrode placement on subject's wrist to target median and radial nerves, with counterelectrode positioned on posterior surface of the wrist. (B) Spiral drawing assessments were performed before and after treatment or sham stimulation. Both groups underwent the same frequency calibration and stimulation amplitude setting. Treatment consisted of an average of a 1‐minute ramp‐up of stimulation followed by a 40‐minute stimulation, whereas sham included an average of a 1‐minute ramp‐up followed by a rapid ramp‐down of the stimulation. (C) Representative spirals pre‐ and posttreatment and sham stimulation. (D) Treatment group (n = 10) TETRAS Spiral rating scores with average rating marked with a black line for prestimulation (2.77) and poststimulation (1.77). Two subjects had the same change in rating and had overlapping points. (E) Sham group (n = 13) TETRAS Spiral rating scores with average rating marked with a black line for prestimulation (2.62) and poststimulation (2.37). (F) Tremor amplitude reduction comparison between sham and treatment following stimulation. *P ≤ 0.05; **P ≤ 0.01.
This was a pilot study with too few subjects for subanalyses of the effects of age, medication status, or medical history. Future studies should expand the subject count, investigate the response rate, repeatability, durability, and effects of chronic use, and add assessments of quality of life. This therapeutic approach was inspired by the idea that peripheral stimulation evokes central activity in brain regions such as the VIM, a thalamic target widely accepted to improve tremor with DBS.5 Although our data support this idea, other potential mechanisms are possible, including circuitry modulated in previous studies demonstrating tremor reduction by manipulation of peripheral sensory input.7 Future studies that are able to better characterize the precise mechanism may facilitate improvements to therapy. Nonetheless, this randomized, sham‐controlled pilot study suggests that noninvasive neuroperipheral therapy may offer clinically meaningful symptomatic relief from hand tremor in ET with a favorable side effect profile compared with other available therapies.
Authors Roles
Peter Lin: principal investigator on the research; research project execution, manuscript and statistical analysis review and critique.
Erika Ross: manuscript writing, review, statistical analysis design, execution, review and critique.
Kathryn Rosenbluth: research project conception, organization, manuscript writing, statistical analysis, review and critique.
Samuel Hamner: research project execution, manuscript writing, review and critique.
Paula Chidester: research project execution, manuscript review and critique.
Serena Wong: research project execution.
Terence Sanger: research project conception, manuscript and statistical analysis review and critique.
Mark Hallett: research project conception, manuscript and statistical analysis review and critique.
Scott Delp: research project conception, organization, manuscript and statistical analysis review and critique.
Financial Disclosures of all authors (for the preceding 12 months)
Dr. Lin was a consultant for Cala Health, Inc. during the period of this research. Dr. Lin is also a principal investigator for clinical trials sponsored by Acadia Pharmaceuticals, Inc. and US WorldMeds, and acts as a consultant to Apple Inc. and Cofactor Genomics.
Dr. Ross is employed by Cala Health, Inc. She is a Co‐I on DARPA contracts N66001‐17‐2‐4010 and N66001‐17‐2‐4018.
Dr. Rosenbluth, Ms. Chidester, and Dr. Hamner are employed by Cala Health, Inc. Dr. Sanger is a consultant and scientific adviser to Cala Health, Inc. Dr. Wong was an employee of Cala Health, Inc. during this research.
Dr. Hallett serves as chair of the Medical Advisory Board for and may receive honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US patent 6,780,413 B2 (Issued: August 24, 2004): Immunotoxin (MAB‐Ricin) for the treatment of focal movement disorders, and US patent 7,407,478 (Issued: August 5, 2008): Coil for Magnetic Stimulation and methods for using the same [H‐coil]); in relation to the latter, he has received license fee payments from the NIH (from Brainsway) for licensing of this patent. He is on the editorial boards of approximately 20 journals and received royalties and/or honoraria for publishing from Cambridge University Press, Oxford University Press, and Elsevier. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by UniQure for a clinical trial of AAV2‐GDNF for Parkinson Disease, Merz for treatment studies of focal hand dystonia, Allergan for studies of methods to inject botulinum toxins, and Medtronic, Inc. for a study of DBS for dystonia.
Dr. Delp has received research support from NIH grants U54 EB020405, P2C HD065690, R01 GM107340, and R01 NS080954 and DARPA contract W911QX12C0018, on which he is the principal investigator. He also has received support from NIH grant R01 EB009351 as a coinvestigator. Dr. Delp is a consultant, scientific adviser, and board member of Cala Health, Inc., Circuit Therapeutics Inc., and Zebra Medical Technologies, Inc. and receives compensation for this service.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Table S1. Patient Demographics & Therapy Information
Figure S1
Relevant conflicts of interest/financial disclosures: Erika Ross, Paula Chidester, Kathryn Rosenbluth, Samuel Hamner, and Serena Wong are all current or former employees of Cala Health, Inc. Terence Sanger and Scott Delp are consultants and scientific advisers to Cala Health, Inc. Mark Hallett is a scientific adviser to Cala Health, Inc. Peter Lin was a consultant of Cala Health, Inc. while conducting this research.
Funding agencies: Funding for this study was provided by Cala Health, Inc.
References
- 1. Brittain JS, Cagnan H, Mehta AR, Saifee TA, Edwards MJ, Brown P. Distinguishing the central drive to tremor in Parkinson's disease and essential tremor. J Neurosci 2015;35(2):795‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis ED. Essential tremor. Lancet Neurol 2005;4(2):100‐110. [DOI] [PubMed] [Google Scholar]
- 3. Hanajima R, Chen R, Ashby P, et al. Very fast oscillations evoked by median nerve stimulation in the human thalamus and subthalamic nucleus. J Neurophysiol 2004;92(6):3171‐3182. [DOI] [PubMed] [Google Scholar]
- 4. Elble R, Comella C, Fahn S, et al. Reliability of a new scale for essential tremor. Mov Disord 2012;27(12):1567‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Putzke JD, Wharen RE Jr, Obwegeser AA, et al. Thalamic deep brain stimulation for essential tremor: recommendations for long‐term outcome analysis. Can J Neurol Sci 2004;31(3):333‐342. [DOI] [PubMed] [Google Scholar]
- 6. Koller WC, Vetere‐Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology 1989;39(12):1587‐1588. [DOI] [PubMed] [Google Scholar]
- 7. Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. Modulation of postural tremors at the wrist by supramaximal electrical median nerve shocks in essential tremor, Parkinson's disease and normal subjects mimicking tremor. J Neurol Neurosurg Psychiatry 1993;56(10):1085‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Table S1. Patient Demographics & Therapy Information
Figure S1


