Abstract
Objective
To better understand the utility of using pain freedom and most bothersome headache‐associated symptom (MBS) freedom as co‐primary endpoints in clinical trials of acute migraine interventions.
Background
Adhesive dermally applied microarray (ADAM) is an investigational system for intracutaneous drug administration. The recently completed pivotal Phase 2b/3 study (ZOTRIP), evaluating ADAM zolmitriptan for the treatment of acute moderate to severe migraine, was one of the first large studies to incorporate MBS freedom and pain freedom as co‐primary endpoints per recently issued guidance by the US Food and Drug Administration. In this trial, the proportion of patients treated with ADAM zolmitriptan 3.8 mg, who were pain‐free and MBS‐free at 2 hours post‐dose, was significantly higher than for placebo.
Methods
We undertook a post‐hoc analysis of data from the ZOTRIP trial to examine how the outcomes from this trial compare to what might have been achieved using the conventional co‐primary endpoints of pain relief, nausea, photophobia, and phonophobia.
Results
Of the 159 patients treated with ADAM zolmitriptan 3.8 mg or placebo, prospectively designated MBS were photophobia (n = 79), phonophobia (n = 43), and nausea (n = 37). Two‐hour pain free rates in those with photophobia as the MBS were 36% for ADAM zolmitriptan 3.8 mg and 14% for placebo (P = .02). Corresponding rates for those with phonophobia as the MBS were 14% and 41% (P = .05). For those whose MBS was nausea, corresponding values were 56% and 16%, respectively (P = .01). Two‐hour freedom from the MBS for active drug vs placebo were 67% vs 35% (P < .01) for photophobia, 55% vs 43% (P = .45) for phonophobia, and 89% vs 58% for nausea (P = .04). MBS freedom but not pain freedom was achieved in 28%. Only 1 patient (1%) achieved pain freedom, but not MBS freedom. The proportion with both pain and MBS freedom was highest (56%) among those whose MBS was nausea.
Conclusion
In this study, the use of MBS was feasible and seemed to compare favorably to the previously required 4 co‐primary endpoints.
Keywords: migraine, headache, triptan, zolmitriptan, drug delivery, intracutaneous, adhesive dermally applied microarray
Abbreviations
- ADAM
adhesive dermally appliedmicroarray
- CMH
Cochran–Mantel–Haenszel test
- FDA
Food and Drug Administration
- MBS
most bothersome symptom
Primary efficacy endpoints in clinical trials of acute migraine medications have evolved over time and have included headache response/relief (improvement from moderate to severe pain at baseline to mild or no pain 2‐hour post‐dose); pain freedom at 2 hours post‐dose; and the co‐primary endpoints of pain relief, nausea, photophobia, and phonophobia.1, 2 In February 2018, the US Food and Drug Administration (FDA) issued a final guidance document for developing drugs for the acute treatment of migraine.1 The guidance suggested an alternative, preferred approach: having patients specify their most bothersome migraine‐associated symptom (MBS) other than pain, either at a baseline visit or at the time of the attack, and using MBS freedom and pain freedom as co‐primary endpoints. Per the guidance, the use of this endpoint “aims to better align the study outcome with the symptom(s) of primary importance to patients.” This approach may also substantially reduce the burden of conducting trials with 4 co‐primary endpoints, while still addressing the need to alleviate migraine‐associated symptoms in addition to pain, and may better harmonize migraine randomized controlled trial design with “real world” treatment experience, as has been suggested in the case of cluster headache trials.3
Adhesive dermally applied microarray (ADAM) is an investigational system for intracutaneous drug administration. In a Phase 1 study evaluating the pharmacokinetics of zolmitriptan delivered with ADAM, absorption was considerably faster than for oral zolmitriptan, with higher exposure in the first 2 hours.4 The recently completed pivotal Phase 2b/3 study (ZOTRIP, NCT02745392), evaluating ADAM zolmitriptan for the treatment of acute moderate to severe migraine, was one of the first large studies to incorporate MBS freedom and pain freedom as co‐primary endpoints. In this trial, the proportion of patients treated with ADAM zolmitriptan 3.8 mg, who were pain‐free and MBS‐free at 2 hours post‐dose (co‐primary endpoints), was significantly higher than for placebo.5
As this is the only published pivotal acute migraine trial using pain freedom and MBS freedom as co‐primary endpoints,5 we undertook a post‐hoc analysis of the ZOTRIP trial to better understand the utility of this approach and the relationships among achieving each of the endpoints. Specifically, our aims were to provide summary statistics for each MBS group, to compare response rates and time course of response among each of these groups, and to examine the correlation between MBS freedom and pain freedom. We were further interested in how the results might theoretically differ if the trial had been designed using the traditional 4 co‐primary endpoints.
METHODS
This was a multicenter, randomized, double‐blind, placebo‐controlled, parallel group, Phase 2b/3 study conducted at 36 sites in the US. Detailed methods and primary study results were previously reported.5 Eligible patients experienced 2‐8 migraine headaches (with or without aura) during a 28‐day run‐in period. On the first day of the run‐in period, patients declared the MBS other than pain occurring most of the time with their migraine headaches, choosing prospectively among nausea (with or without vomiting), photophobia, or phonophobia. Patients were randomly assigned in a 1:1:1:1 ratio, stratified by MBS, to receive ADAM zolmitriptan 1 mg, 1.9 mg, 3.8 mg, or placebo to treat 1 migraine at moderate or severe levels of pain. Patients were required to have their prospectively chosen MBS as one of their symptoms for the headache to qualify for treatment. Symptoms were recorded using an electronic diary (e‐Diary).
The co‐primary endpoints of the study were the proportion of patients reporting pain freedom at 2 hours post‐dose and the proportion reporting MBS freedom at 2 hours post‐dose. Prespecified secondary endpoints included (but were not limited to) freedom at 2 hours post‐dose from photophobia, phonophobia, or nausea (irrespective of MBS).
All authors had full access to all study data.
Data were analyzed via Cochran–Mantel–Haenszel (CMH) test stratified by MBS. A fixed sequential testing methodology was applied to control the overall type 1 error. A test was considered statistically significant only if the corresponding CMH test had a P value <.05 and all previous tests had a P value <.05. Last observation carried forward was used to impute missing data.
For the post‐hoc analyses presented here (Table 1), CMH testing was also employed. Data are only reported for the 3.8 mg dose and placebo, as this was the only dose that clearly showed a significant treatment effect for the co‐primary endpoints of pain freedom and MBS freedom at 2 hours post‐dose.
Table 1.
Percentage of Patients Who Had 2‐Hour Pain Freedom or 2‐Hour MBS Freedom by Prespecified MBS (mITT Population)
| Photophobia | Phonophobia | Nausea | ||||
|---|---|---|---|---|---|---|
| Placebo, n = 37 | ADAM Zolmitriptan 3.8 mg, n = 42 | Placebo, n = 21 | ADAM Zolmitriptan 3.8 mg, n = 22 | Placebo, n = 19 | ADAM Zolmitriptan = 3.8 mg, n = 18 | |
| 2‐hour pain freedom, n (%) | 5 (14) | 15 (36) | 3 (14) | 9 (41) | 3 (16) | 10 (56) |
| Treatment difference † | 22% | 27% | 40% | |||
| P value ‡ | .02 | .05 | .01 | |||
| 2‐hour MBS freedom, n (%) | 13 (35) | 28 (67) | 9 (43) | 12 (55) | 11 (58) | 16 (89) |
| Treatment difference † | 32% | 12% | 31% | |||
| P value ‡ | <.01 | .45 | .04 | |||
ADAM zolmitriptan 3.8 mg minus placebo.
ADAM zolmitriptan 3.8 mg vs placebo.
RESULTS
A total of 365 patients were randomized; 321 were treated and had at least 1 post‐treatment symptom assessment (mITT population). Patient demographics and baseline characteristics as well as primary study results were reported previously.5 Of the 321 treated, 77 received placebo and 82 were treated with ADAM zolmitriptan 3.8 mg.
For the primary outcome measures, 11 (14%) patients receiving placebo and 34 (42%) of patients receiving ADAM zolmitriptan 3.8 mg were pain‐free at 2 hours post‐dose (P < .01), and 33 (43%) patients receiving placebo and 56 (68%) patients receiving ADAM zolmitriptan 3.8 mg were free from MBS (P < .01).5
The most frequently prespecified MBS was photophobia, chosen by 79 patients (50%), 37 of whom received placebo and 42 ADAM zolmitriptan 3.8 mg. Phonophobia was the next most commonly chosen, by a total of 43 patients (27%), 21 of whom were assigned to placebo and 22 to ADAM zolmitriptan 3.8 mg. Nausea was selected as the MBS for 37 (23%) patients, 19 in the placebo group and 18 in the ADAM zolmitriptan 3.8 mg group.
Among patients with photophobia or nausea as their MBS, significantly more patients in the ADAM zolmitriptan 3.8 mg group were pain‐free and/or MBS‐free at 2 hours post‐dose compared with those who received placebo (Table 1). In patients whose MBS was phonophobia, the difference between ADAM zolmitriptan 3.8 mg and placebo was not significant for either pain freedom or MBS freedom at 2 hours post‐dose.
To evaluate how frequently 2‐hour pain freedom and 2‐hour MBS freedom coincided, we determined the percentages of patients who achieved both 2‐hour pain freedom and 2‐hour MBS‐freedom, freedom from either pain or MBS, and those who did not achieve either pain or MBS freedom (Figure 1). Overall, 33 (40%) patients in the ADAM zolmitriptan 3.8 mg group and 11 (14%) in the placebo group were both pain‐free and MBS‐free at 2 hours post dose. Freedom from MBS, but not from pain, occurred in 23 (28%) patients in the ADAM zolmitriptan 3.8 mg group and 22 (29%) in the placebo group. Only 1 patient (1%) achieved freedom from pain, but not from their MBS; this patient reported photophobia as their MBS. In the ADAM zolmitriptan 3.8 mg group, the proportion who reported both 2‐hour pain freedom and MBS‐freedom was highest (n = 10, 56%) among those whose MBS was nausea. This proportion was smallest (n = 14, 33%) among those whose MBS was photophobia.
Figure 1.
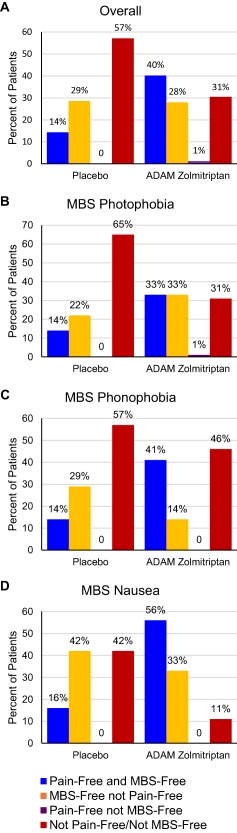
Concordance and discordance percentages between MBS and pain freedom [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 2 shows the time course of pain freedom and MBS freedom. Overall, the percentage of patients who achieved pain freedom in the ADAM zolmitriptan 3.8 mg group increased over the course of 4 hours post‐dose. Freedom from MBS appeared to occur more rapidly than pain freedom with only a slight increase in the number who achieved MBS freedom between 2 and 4 hours.
Figure 2.
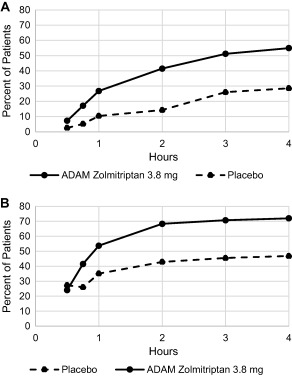
Pain freedom (A) and MBS freedom (B) rates over time
All patients in this trial were required to have a qualifying migraine that included the presence of their prespecified MBS, even if that symptom was not the most bothersome at the time of that particular headache. As shown in Table 2, the percentage of patients who reported the presence of one or more of photophobia, phonophobia, or nausea at the time of treatment was considerably higher than the percentage who chose each of these symptoms as prespecified MBS.
Table 2.
Characteristics of Qualifying Migraine at the Time of Treatment and Symptom Relief/Freedom at 2 Hours Post‐Dose (mITT Population)
| Placebo, n = 77, n (%) | ADAM Zolmitriptan 3.8 mg, n = 82, n (%) | P value † | |
|---|---|---|---|
| Characteristics of qualifying migraine | |||
| Photophobia present | 75 (97) | 78 (95) | — |
| Phonophobia present | 72 (94) | 71 (87) | — |
| Nausea present | 51 (66) | 59 (72) | — |
| Outcomes 2 hours post‐dose | |||
| Pain relief ‡ | 40 (59) | 55 (80) | <.01 |
| Photophobia‐free | 32 (42) | 57 (70) | <.01 |
| Phonophobia‐free | 43 (56) | 57 (70) | .06 |
| Nausea‐free | 49 (64) | 67 (82) | .01 |
Nominal P value ADAM zolmitriptan 3.8 mg vs placebo due fixed sequential testing.
Defined as improvement to a rating of none or mild without the use of rescue medications.
Table 2 also presents the results corresponding to the previously often‐used 4 co‐primary endpoints: pain relief, and absence of nausea, photophobia, and phonophobia at 2 hours post‐dose. The latter 3 endpoints were secondary endpoints in this pivotal trial and although nominal statistical superiority was achieved for absence of photophobia and phonophobia, due to the fixed sequential testing methodology employed, formal statistical significance was not established for any of these endpoints.
DISCUSSION
In the last 25 years, new therapies for the acute treatment of migraine have been evaluated in studies with 4 primary endpoints at 2 hours post‐treatment: pain relief, photophobia freedom, phonophobia freedom, and nausea freedom. Pain freedom is now recommended in place of pain relief as a primary efficacy endpoint, as it is considered more clinically meaningful. It is also the recommended primary endpoint for acute migraine treatment trials by the International Headache Society.5 The major associated symptoms of migraine—photophobia, phonophobia, and nausea—are important for assessing a therapy's effect on the migraine, but all symptoms are not always present. For example, nausea rates at the time of treatment range anywhere from 40% to 70%.6 Therefore, requiring the elimination of a symptom that was never present and requiring a therapy to have a higher response rate than placebo in nausea freedom at 2 hours may require a large sample size per treatment group. The designation of MBS prerandomization, and the requirement that the symptom be present at the time of treatment, solves the problem of any given symptom being absent at the time of treatment and allows for a proper comparison of the differential effect of active vs placebo.
The ZOTRIP trial is the only published pivotal trial to use prespecified usual MBS freedom as a co‐primary endpoint along with pain freedom at 2 hours.5 In this multicenter, randomized, double‐blind, placebo‐controlled, parallel group Phase 2b/3 study, patients treated with ADAM zolmitriptan 3.8 mg were significantly more likely to be pain‐free and/or MBS‐free at 2 hours post‐treatment than those receiving placebo.5 Approximately 80 patients per treatment group were sufficient to show a clear difference between an effective therapy and placebo. Using the previous 4 co‐primary endpoints, ADAM‐Zolmitriptan 3.8 mg would have also been found to be effective for pain relief, nausea, and photophobia (Table 2). The study was not powered for the 4 endpoints, however, and the P value for phonophobia was .06 for ADAM‐Zolmitriptan 3.8 mg, suggesting a larger sample size is needed to reach significance on this endpoint. Using a 2‐sided Mantel‐Haenszel test in 3 strata and assuming a significance level of 0.05 and 80% power, ∼170 subjects per group are needed. Considering this, our experience with MBS in this trial suggests that its use may be an improvement over requiring statistically significant improvement for each of the endpoints of photophobia freedom, phonophobia freedom, and nausea freedom to demonstrate efficacy. The MBS endpoint may allow for smaller, faster, and less expensive trials to be conducted without any loss in sensitivity.
In this trial, patients' treatment assignment was stratified based on prespecified (on the first day of the run‐in period) MBS to avoid imbalance across treatment groups. We chose this approach as it would have been logistically cumbersome and burdensome to subjects to withhold drug assignment until the time of a migraine in an outpatient study, especially in this trial in which there were 4 possible treatments. Given that an individual's migraine‐associated symptoms often vary from attack to attack, requiring patients to pre‐select MBS has the inherent risk that the selected MBS may not be present or is not their most bothersome symptom when they treat the migraine headache. This study controlled for this by not allowing patients to treat unless their prespecified MBS was present. Future studies might also add a question to the e‐Diary to assess how many times there is discordance between the preselected most bothersome symptom and which symptom is the most bothersome symptom at the time of treatment.
An important finding in this trial was that only 1 patient (<1%) achieved pain freedom without achieving MBS freedom. In addition, the placebo response rate for 2‐hour MBS freedom (42%) was substantially higher than that for 2‐hour pain freedom (14%) in this trial,5 as has been observed in other trials,6, 7 which may increase the number of trial participants necessary to show freedom for both endpoints. Further work is required to elucidate if this concordance rate is maintained across trials. If so, it may argue for the feasibility of a single pain freedom endpoint in the future as a means to decrease trial cost and duration.
The limitation of this analysis is its post‐hoc nature. Results should therefore be considered preliminary and the usefulness of including MBS as a co‐primary endpoint will require confirmation in additional and larger studies.
CONCLUSIONS
Experience in the ZOTRIP trial indicates that MBS as a co‐primary endpoint is a feasible and desirable alternative to using 4 co‐primary endpoints in acute migraine treatment trials. Furthermore, the trial demonstrated a very high concordance rate between MBS freedom and pain freedom.
STATEMENT OF AUTHORSHIP
Category 1
(a) Conception and Design
David W. Dodick, Amy A. Gelfand, Deborah I. Friedman, Donald J. Kellerman, Stewart J. Tepper, Peter C. Schmidt
(b) Acquisition of Data
Donald J. Kellerman, Peter C. Schmidt
(c) Analysis and Interpretation of Data
David W. Dodick, Amy A. Gelfand, Deborah I. Friedman, Donald J. Kellerman, Stewart J. Tepper, Peter C. Schmidt
Category 2
(a) Drafting the Manuscript
David W. Dodick, Amy A. Gelfand, Deborah I. Friedman, Donald J. Kellerman, Stewart J. Tepper, Peter C. Schmidt
(b) Revising It for Intellectual Content
David W. Dodick, Amy A. Gelfand, Deborah I. Friedman, Donald J. Kellerman, Stewart J. Tepper, Peter C. Schmidt
Category 3
(a) Final Approval of the Completed Manuscript
David W. Dodick, Amy A. Gelfand, Deborah I. Friedman, Donald J. Kellerman, Stewart J. Tepper, Peter C. Schmidt
Acknowledgment
Statistical analysis was performed by Jean Engels and funded by Zosano Pharma. Medical writing support was provided by Pamela Foreman and funded by Zosano Pharma.
Conflict of Interest: DD has received compensation from serving on advisory boards and/or consulting within the past 5 years for: Allergan, Amgen, Alder, Arteaus, Pfizer, Colucid, Merck, NuPathe, Eli Lilly and Company, Autonomic Technologies, Ethicon J&J, Zogenix, Supernus, Labrys, Boston Scientific, Medtronic, St. Jude, Bristol‐Myers Squibb, Lundbeck, Impax, MAP, Electrocore, Tonix, Novartis, Teva, Alcobra, Zosano, Insys, GBS/Nocira, Acorda eNeura, Charleston Laboratories, Gore, Biohaven, Bioventric, Magellan, Theranica, Xenon, Dr. Reddy's/Promius Pharma. Dr. Dodick owns equity in Epien, GBS/Nocira, Second Opinion, Healint, and Theranica. Dr. Dodick has received funding for travel, speaking, editorial activities, or royalty payments from IntraMed, SAGE Publishing, Sun Pharma, Allergan, Oxford University Press, American Academy of Neurology, American Headache Society, West Virginia University Foundation, Canadian Headache Society, Healthlogix, Universal Meeting Management, WebMD, UptoDate, Medscape, Oregon Health Science Center, Albert Einstein University, University of Toronto, Starr Clinical, Decision Resources, Synergy, MedNet LLC, Peer View Institute for Medical Education, Medicom, Chameleon Communications, Academy for Continued Healthcare Learning, Haymarket Medical Education, Global Scientific Communications, HealthLogix, Miller Medical, MeetingLogiX, and Wiley Blackwell. Dr. Dodick, through his employer, has consulting use agreements with NeuroAssessment Systems and Myndshft. He holds Board of Director positions with King‐Devick Technologies and Epien Inc. He holds the following Patent 17189376.1‐1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis (no compensation).
DF served on advisory boards for Supernus, Alder BioPharmaceuticals, Amgen, Avanir, Biohaven, Eli Lilly and Company, Teva, electoCore, and Zosano Pharma. She received grant support from Merck, Eli Lilly and Company, and Autonomic Technologies. She serves as a consultant for Eli Lilly and Company and Trigemina. She is a speaker for Allergan and Supernus. She is on the editorial board of Neurology Reviews and Headache, a contributing author to MedLink Neurology, and serves on the Board of Directors of the American Headache Society.
ST serves as a consultant for Acorda Therapeutics, Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, Biovision, electroCore, Eli Lilly and Company, eNeura, Gerson Lehman Group, Guidepoint Global, Kimberly‐Clark, Pernix Therapeutics, Pfizer, Slingshot Insights, Supernus, Teva Pharmaceutical Industries, and Zosano Pharma. He serves on the advisor's board for Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc, Avanir Pharmaceuticals, Charleston Laboratories, Dr. Reddy's, Kimberly‐Clark, Pfizer, Scion Neurostim, Teva Pharmaceutical Industries, and Zosano Pharma. He performs research (without personal compensation) for Alder Biopharmaceuticals, Allergan, Amgen, Autonomic Technologies, Inc., Avanir Pharmaceuticals, Dr. Reddy's, electroCore, eNeura, Scion Neurostim, Teva Pharmaceutical Industries, Theranica, and Zosano Pharma. He has received stock options from Autonomic Technologies, Inc. and receives royalties from University of Mississippi Press and Springer. He receives salary compensation from Dartmouth‐Hitchcock Medical Center and the American Headache Society.
PS and DK are employees of Zosano Pharma.
AG is a consultant for Zosano Pharma, Eli Lilly and Company, and Biohaven. She receives royalty payments from UpToDate and has received research funding from eNeura. She has received payment from JAMA Neurology for work as an associate editor. Her spouse consults for Genentech and receives research support from Genentech, Quest Diagnostics, and MedDay.
REFERENCES
- 1.Migraine: Developing Drugs for Acute Treatment Guidance for Industry. https://www.fda.gov/downloads/drugs/guidances/ucm419465.pdf
- 2. Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: Detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia. 2002;22:633‐658. [DOI] [PubMed] [Google Scholar]
- 3. Martelletti P, Curto M. Headache: Cluster headache treatment ‐ RCTs versus real‐world evidence. Nat Rev Neurol. 2016;12:557‐558. [DOI] [PubMed] [Google Scholar]
- 4. Kellerman DJ, Ameri M, Tepper SJ. Rapid systemic delivery of zolmitriptan using an adhesive dermally applied microarray. Pain Manage. 2017;7:559. [DOI] [PubMed] [Google Scholar]
- 5. Spierings EL, Brandes JL, Kudrow DB, et al. Randomized, double‐blind, placebo‐controlled, parallel‐group, multi‐center study of the safety and efficacy of ADAM zolmitriptan for the acute treatment of migraine. Cephalalgia. 2018;38:215‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geraud G, Olesen J, Pfaffenrath V, et al. Comparison of the efficacy of zolmitriptan and sumatriptan: Issues in migraine trial design. Cephalalgia. 2000;20:30‐38. [DOI] [PubMed] [Google Scholar]
- 7. Wietecha LA, Kuca B, Case MG, Seltzler KJ, Aurora SK. Phase 3 study (SPARTAN) of lasmiditan compared to placebo for acute treatment of migraine. Cephalalgia. 2017;37:319‐374. 29067828 [Google Scholar]


