Abstract
Fluctuations in food availability are a major challenge faced by primates living in seasonal climates. Variation in food availability can be especially challenging for females, because of the high energetic costs of reproduction. Therefore, females must adapt the particular demands of the different reproductive stages to the seasonal availability of resources. Madagascar has a highly seasonal climate, where food availability can be extremely variable. We investigated the seasonal changes in diet composition, nutrient and energy intake of female and male sifakas (Propithecus verreauxi) in a dry deciduous forest in western Madagascar. We examined how females adjust their diet to different reproductive stages. Seasonality affected the diet of both sexes; particularly in the dry season (Apr–Oct) with low availability of food items, especially fruits, males and females had a reduced nutrient and energy intake compared to the wet season (Nov–Mar) with higher food and fruit availability. The comparison of the diet between sexes in different reproductive stages showed that during the late stage of lactation (Nov–Jan) females had higher food intake, and as a result they had a higher intake of macronutrients (crude protein, fat and non‐structured carbohydrates (TNC)) and energy than males. These differences were not present during the pregnancy of females, with both sexes having similar intake of macronutrients and energy during that stage. The increase in the intake of macronutrients observed for females during late lactation could be related to the higher energetic demands of this stage of reproduction. Thus, the observed pattern in the diet indicates that sifaka females are following a capital breeding strategy, whereby females potentially store enough nutrients to cope with the reproduction costs in periods of low food availability.
Keywords: capital breeder, food quality, primates, reproduction, seasonality
1. INTRODUCTION
Fluctuations in food availability are a major challenge faced by primates living in seasonal climates. The variance in availability of food can have different impact for males and females, due to differences in energy requirements between sexes (reviewed in Key & Ross, 1999). Reproduction is highly demanding for females (National Research Council, 2003). Therefore, it is expected that reproductive females are more affected by changes in food availability than males and non‐reproductive females (Hemingway, 1999; McCabe & Fedigan, 2007; Oftedal, 1985). For instance, to assure adequate nutrition, pregnant females can spend more time feeding than non‐pregnant ones (Boinski, 1988; Hemingway, 1999; Lee, 1984), select higher quality diets (McCabe & Fedigan, 2007) or ingest larger amounts of food (Hemingway, 1999; Rothman, Dierenfeld, Hintz, & Pell, 2008). Moreover, in green monkeys (Chlorocebus sabaeus), lactating females adopted an energy conservation strategy during periods of food scarcity, in which they increased the time resting and avoided excessive activity in competition with others over food (Gittleman & Thompson, 1988; Harrison, 1983).
In many mammalian species, including primates, males are typically larger than females and have to cope with high costs of body maintenance (Ralls, 1976; reviewed in Key & Ross, 1999). However, in some species there is little sexual dimorphism in size, including the majority of Lemuriformes (Jolly, 1984; Kappeler, 1990, 1991). Interestingly, the absence of sexual dimorphism in size may be energetically advantageous to males, since they are exempt from costly maintenance of a larger body size (Richard, 1992), while females still cope with the costs of reproduction (Jolly, 1984). Additionally, in lemurs, females are typically dominant to males and have priority of access to food (Dunham, 2008; Jolly, 1984; Kappeler, 1990; Richard & Nicoll, 1987). Since lemurs occur in Madagascar with a highly seasonal climate (Fleagle, 1999; Wright, 1999), females can ensure through social dominance to access enough resources for their survival and reproduction (Jolly, 1984; Richard & Nicoll, 1987; Wright, 1999).
With respect to the timing of reproductive stages with the availability of resources, animals can adopt different reproductive strategies to adapt the energy requirements of reproduction to the environmental conditions. In that sense animals can be classified as income or capital breeders (Drent & Daan, 1980; Stearns, 1989, 1992). Income breeders synchronize the most demanding period of their reproduction with the period when availability of food is high, while capital breeders rely on nutrients stored previously to pay for the costs of their reproduction.
Sifakas are among the largest diurnal lemurs in Madagascar. They include large amounts of leaves in their diets (Richard, 1978), and rely on anatomical adaptations such as high molar crests, enlarged stomach, and elongated cecum and colon (Hill, 1953) to digest a fibrous diet. The highly seasonal climate of Madagascar, particularly in the dry deciduous forests where the majority of trees lose their leaves during the dry season (Sorg & Rohner, 1996), raises important questions on how animals adapt their diet to the fluctuation of food availability among seasons.
Despite the fact that sifakas are seasonal breeders with a short mating season (Brockman, Whitten, Richard, & Schneider, 1998; Mass, Heistermann, & Kappeler, 2009), adult females cope with the demands of reproduction year round, spending 6 months being pregnant and another 6 months lactating (Jolly, 1984; Kappeler & Fichtel, 2012). The lack of male parental care and the fact that infants are born during the peak of the dry season, when food availability is low (Lewis & Kappeler, 2005a; Richard & Nicoll, 1987) contributes to the high reproductive costs for sifaka females (Jolly, 1984).
The reproductive strategy adopted by sifaka females, being classified as capital or income breeders, however, is still debated. According to Richard, Dewar, Schwartz, and Ratsirarson (2000) sifakas are capital breeders, with females storing energy during the wet season to cope with the scarcity of food and the expenses of reproduction over the dry season. In contrast, Lewis and Kappeler (2005a) classified sifakas as “classic breeders.” This classification is very similar to the definition of income breeding, where females synchronize the most demanding portion of their reproduction (mid/late lactation) with the period of highest availability of food.
Several studies investigated the impact of seasonality on the diet and nutrient intake in sifakas (Hemingway, 1999; Irwin, Raharison, Raubenheimeir, Chapman, & Rothman, 2014; Lewis & Kappeler, 2005a, 2005b; Norscia, Carrai, & Borgognini‐Tarli, 2006; Richard et al., 2000), showing that they had lower intake of food and macronutrients during the dry season. However, detailed information on sex differences in the diet and nutrient intake across reproductive seasons is still missing.
To build on this past research, we investigated the effect of seasonality, sex, and female reproductive stages in diet patterns of a population of Verreaux's sifakas (Propithecus verreauxi) in a dry deciduous forest in western Madagascar. We examined the following aspects of diet of sifakas between seasons, sexes and reproductive stages: (1) the diet composition in terms of the importance of food items in the diet (fruits, leaves, and flowers); (2) the intake of macronutrients and energy. We first investigated the general impact of seasonality to the diet of both sexes. Second, we compared the diet of males and females across different reproductive stages. And finally, we explored the concepts of “capital” and “income” breeders, discussing them according to the characteristics of diet adopted by females in different reproductive stages. This comparison will contribute to our comprehension on important traits of Lemuriformes, such as female dominance and lack of sexual dimorphism, that have been related to the high costs of reproduction of females in a seasonal environment.
2. METHODS
2.1. Study site and behavioral observations
The study was conducted in the forest concession of Kirindy/CNFEREF, a dry‐deciduous forest in western Madagascar (44°39′E, 20°03′S) (Kappeler & Fichtel, 2012). The climate in this region is characterized by a long dry season from April to early November, and a short wet season between mid‐November and the end of March (Sorg & Rohner, 1996).
We observed the behavior of 23 habituated adults (9 females and 14 males), of eight neighboring groups of Verreaux's sifakas from March 2012 to April 2013. However, given that not all 23 individuals were present in all seasons (due to dispersal or death), in the present study we used data only from focal individuals that were present in all seasons (N = 18, nine females and nine males) for a better comparison of diet between sexes and seasons.
Two observers conducted simultaneous observations of the adults in two different groups. All groups were followed every month, and each focal individual was observed continuously for 1 hr using the focal animal sampling (Altmann, 1974). We recorded the beginning and end of each defined behavioral state such as resting, locomotion, feeding, and social. Information on feeding behavior was collected in more detail as presented next. Each observer followed one group for 3 hr (three focals) in the morning from 7:30 (±30 min) to 10:30 (±30 min) and a different group for 3 hr (three focals) in the afternoon from 14:00 (±30 min) to 17:00 (±30 min), thereby focal individuals from four different groups were observed per day. We followed this particular protocol including hourly observations instead of full day focal observations because the present study was part of a project that aimed to investigate other questions apart of feeding ecology, and for those questions hourly observations were more suitable. Observations of focal individuals and groups followed a rotation system that alternates the orders of observation to achieve full statistical days for all individuals. Sifakas were habituated and individually marked with combinations of colored nylon collars and pendants or color‐coded radio collars, respectively (Kappeler & Fichtel, 2012). Group size ranged from three to eight individuals, with one adult female and one to three adult males per group, with the exception of one group where two adult females were present during the study. We recorded 1,046 hr of observation, with an average of 59 hr (±3) for each focal.
In order to investigate sex differences in the diet, we compared the diet of males and females across different reproductive stages of females. We categorized the reproductive stages of females into pregnancy and lactation according to earlier studies (Kappeler & Fichtel 2012). Because the different stages of lactation and pregnancy have different energy requirements, we split the categories of gestation and lactation in a more detailed classification, as follows: early pregnancy (February to April), late pregnancy (May to July), early lactation (August to October), and late lactation (November to January). The period of pregnancy was determined retrospectively after each infant was born. Because gestation is known to be about 6 months in length (Jolly, 1984), the total gestation period can be determined in retrospect once the infant is born. Since the estrus in sifaka females is synchronized (Mass et al., 2009), all females give birth around the same time (within a month). We used nursing behavior as the criterion to classify a female in the lactation category. Because all groups are monitored on a daily basis as part of the long term data collection, the exact birthday of each infant was known. During the period of this study the first infant was born on 20 June 2012 and the last infant was born on 21 July 2012. In the subsequent year (based on information from the long term data collection) the first infant was born on 20 June 2013 and the last one on 23 July 2013. The sample size used for the comparison between reproductive stages among males and females was nine adult males and seven females. One female did not give birth in 2012 and in 2013, and another female lost the infant shortly after giving birth in 2012.
2.2. Feeding behavior
Feeding bouts started when a focal individual inserted food in its mouth and ended when it stopped feeding for at least 30 s. For each feeding bout, we recorded the food type (young or mature leaves, unripe or ripe fruits, open flowers or flower bud, barks, and seeds), the tree species, and the location of the tree with a GPS (Garmin® GPS 60CSx, Garmin, Schaffhausen, Switzerland). Given the high diversity in the diet of sifakas we were not able to collect samples of all foods they fed on. For that reason, we only collected samples from “important food resources” (IFR), defined as food items of the same species consumed by a focal individual consecutively for more than 5 min. For feeding bouts on IFR we also estimated the feeding rates (intake of food per minute) specific for each food (combination of item and species). In the case of small food items one bite often meant one item ingested. For larger items, we counted the bites necessary to ingest the whole item, and then we converted this number to the number of items ingested in a certain period of time. Intake rates were calculated as follows: whenever we processed a sample we weighed a single food item from that sample, for example, one single young leaf from species X. Afterwards, we multiplied the weight of this one food item by the total number of items ingested per minute, obtaining the intake in grams per minute. Therefore, if the focal animal had an intake of 10 leaves per minute, fed for 5 min on this particular food, and supposing that each leaf weight 0.05 g, then this animal had a total intake of 2.5 g. All calculations were based on dry weight.
The intake rates (bites per minute) were observed by FK, and we used the averages for each item‐species to complete the dataset of the field assistant. FK recorded 651 feeding rates and the intake of all possible combinations of item‐species included in the diet of sifakas was rated at least once. For foods consumed more often, few intakes were recorded and averaged afterwards. We used the same averages for males and females because there was no sex difference in the feeding rates (GLMM χ2 = 0.74, df = 1, P = 0.38).
The collection of samples was performed on the same day or within a maximum of 3 days after the feeding was recorded, and whenever possible, from the same tree from which sifakas were feeding. All IFR were sampled regardless if the same species had been already sampled (since foods can be intra‐specifically variable in nutritional content (Chapman, Chapman, Rode, Hauck, & Mcdowell, 2003)), resulting in 1,143 plant samples that were used for nutritional analyses (see below). When nutritional and intake information for certain foods were not available, we followed the method used by Irwin, Raharison, Raubenheimeir, Chapman, and Rothman (2014), using the average of all samples from the same species and food item to replace missing values.
The availability of food was based on monitoring monthly phenology of 690 trees from 166 species distributed in 47 families. Five trees were randomly selected (whenever possible) based on a list of species available in Kirindy Forest. This list was produced on a pilot study conducted by FK when two methods of diversity were implemented and more than 25,000 trees distributed within the home range of the eight groups of sikakas were identified on the species level by a local field assistant. We used a semi‐quantitative method (Fournier, 1974) in which the availability for each food item was scored ranging from 0 to 4, where 0 was the complete absence of the item and 4 represented its maximum abundance (100%). We calculated the average of the scores from all trees for each item per month to infer its availability. To investigate if sifakas selected their diet based on the availability of items, we performed a Spearman correlation between the importance of items in the diet (based on time spent feeding) per month and its availability (based on the scores of abundance of food items).
2.3. Processing samples and chemical analyses
The samples were processed, weighed, and prepared for drying by placing them in paper bags and storing them in containers filled with dried ECO silica (non‐toxic, 1.3 mm pearls with color indicator Roth®, Karlsruhe, Germany) where they stayed until they were completely dried (i.e., water content did not change). The containers were inspected at least twice a day to control for possible mold. The silica gel was oven dried and replaced on a daily basis. Before drying, fruit pulp was sliced into small pieces, and seeds (in the case sifakas ate the seeds) were dried separately from the pulp of the fruits to optimize drying.
After samples were dried, they were ground in an analytical mill (IKA, A11) through a 1 mm screen, and stored in plastic tubes. The samples were analyzed for neutral detergent fiber (hereafter, NDF) (NDF‐ANKOM fiber analyzer), nitrogen (Kjeldahl), ash (combustion), and fat (ether extract) (Donati, Baldi, Morelli, Ganzhorn, & Borgognini‐Tarli, 2009; Naumann & Bassler, 1976; Van Soest, Robertson, & Lewis, 1991; Voigt et al., 2004) following standard chemical procedures. A comparison of methods is provided by Ortmann, Bradley, Stolter, and Ganzhorn, (2006) and Rothman, Chapman, and Soest (2011).
Due to the large number of samples for analysis, we applied near infrared spectroscopy (NIRS) (Foley et al., 1998; Rothman, Chapman, Hansen, Cherney, & Pell, 2009; Stolter, Julkunen‐Tiitto, & Ganzhorn, 2006) in the Department of Zoology of the University of Hamburg (Germany). NIRS models were developed with the plant samples collected for the present study and with the Quant two‐method using partial least squares (PLS) regression with the software Opus NT Version 2.02 (Bruker GmbH, Germany). We used cross‐validation (jack‐knifing, internal validation) and test‐set‐validation (external validation) to test the accuracy of the developed NIRS models (Table 1). Standard NIRS procedures use the same data for generating the NIRS models and to test them. Though these procedures are used routinely, they can result in erroneous estimates when applied to samples that had not been used in model development (Stolter et al., 2006). We therefore applied the NIRS models to another test set of samples that had not been used for model development (independent test set validation; following Stolter et al. (2006)). The original NIRS model was only used when the concentrations predicted by the NIRS model deviated less than 10% from the results obtained by wet chemical analyses of the independent test set (Stolter et al., 2006). Since the NIRS models were unreliable for fat, all 255 fruit samples were analyzed for fat using ether extraction. Though nitrogen can be predicted reliably with NIRS models, 346 samples of fruits and flowers were analyzed for total nitrogen with the Kjeldahl method as the data were needed for other purposes. NIRS model performance is listed in Table 1.
Table 1.
Model performance of NIRS‐models used to estimate concentrations of nitrogen, NDF and ash; sample size in brackets
| Plant part | Component | Validation a | R 2 | RMSEP/RMSECV b |
|---|---|---|---|---|
| Fruits | NDF (19) | Cross | 99.35 | 1.140 |
| Flowers | NDF (13) | Cross | 95.41 | 1.600 |
| Mature leaves | Nitrogen (57) | Test‐set | 96.15 | 0.144 |
| NDF (34) | Cross | 80.29 | 3.440 | |
| Young leaves | Nitrogen (57) | Test‐set | 96.15 | 0.144 |
| NDF (34) | Cross | 90.32 | 2.800 | |
| All parts | Ash (89) | Test‐set | 89.79 | 0.941 |
Cross, cross validation; test‐set, test‐set validation.
RMSEP: root of the mean square error of the prediction based on the test‐set validation; RMSECV: root of the mean square error of the prediction of cross validation [Stolter et al., 2006].
We estimated crude protein as nitrogen *6.25. Total non‐structural carbohydrates (TNC) were calculated following the formula:
This measure of TNC has flaws as the errors of each analysis accumulate in the calculation (Rothman, Chapman, & Soest, 2011), but is nevertheless an estimation of the nonstructural and most digestible carbohydrates in a food item. The calculations of energy from TNC, fiber, protein, and fat were based on the conventional conversion values of 4 kcal per gram protein, 4 kcal per gram of TNC, and 9 kcal per gram of fat (National Research Council, 2003). A specific value for digestibility of fiber is currently not available for Verreaux's sifakas. Therefore, the digestibility coefficient for fiber used in this study was 40% of 3 kcal, and it was based on a study that investigated digestibility in two species of Propithecus (P. coquereli and P. tattersalli) in captivity (Campbell, Eisemann, Glander, & Crissey, 1999). In the case of fiber, we used a conversion factor of 1.2 kcal per gram, instead of 4 kcal, since we subtracted 1 kcal which is lost to the anaerobic microbes processing the fermentable fractions, plus the coefficient of digestibility for fiber (40%) (Campbell et al., 1999; Conklin & Wrangham, 1994). Leaves were not analyzed for “fat” because ether extracts from leaves are very low. Therefore, only fruits were analyzed for fat contents. In the case of leaves, we set the concentrations for “fat” = 0 in energy calculations. We calculated the nutrient concentration per food sifakas fed on based on the percentage of dry matter. All analyses of nutrient intake were based on grams per hour of time spent feeding.
2.4. Statistical analysis
For the statistical analyses, we calculated the weighted averages for the nutritional content of food seen eaten by each focal in each hour of observation. Despite the fact that we counted on a balanced data set, we divided the total nutritional content consumed from each focal in each season and reproductive stage by the total hours of observation of each focal individual in each period. We included in the analyses only focal individuals that were present in all seasons (N = 18 individuals, nine males and nine females). Linear Mixed Models [LMM, Baayen, 2008] in R (R, version 3.1.2; R Core Team, 2014), from the package lmer4 (Bates, Maechler, & Bolker, 2012) were applied to investigate the influence of season on energy and macronutrient intake. Seasons were the predictors, and food intake, intake of macronutrients (TNC, crude protein, NDF, fat, measured in grams) and the intake of energy (measured in calories) were our response factors. Since the availability of food in Kirindy Forest drops gradually (Lewis & Kappeler, 2005a; Norscia et al., 2006) we divided the wet and dry seasons into: early dry season (April to July), late dry season (August to October), early wet season (November to December), and late wet season (January to March) to investigate seasonal patterns in more detail. The analyses were done separately for each macronutrient; therefore each LMM had the intake from a particular macronutrient, for instance crude protein, as a response variable, and season as the explanatory factor. Squared root and log transformations were applied to variables that were not normally distributed in order to achieve normality. Figures are representing the original data without transformation, which were used only for the statistical models. All models were controlled for focal and group identity by integrating them as random factors (individual ID nested in group ID). For the LMMs, P values were obtained with the R‐package lmerTest (Kuznetsova, Brockhoff, & Bojesen Christensen, 2013). We checked all the relevant assumptions (multicollinearity, and existence of influential cases) for each linear mixed model, and we verified the significance of the full model (including the predictors and controlled factors) to the null model (only with the controlled factors) using ANOVA.
Non‐parametric tests (Friedman and Wilcoxon Signed Rank Test) were applied for the comparison between sexes and seasons on time spent feeding on different food items: flowers (FL), fruits (FR), young leaves (YL), and mature leaves (ML). In order to correct for multiple testing, we reduced the value of P from 0.05 to 0.008 (0.05/6 different tests) using the method “Bonferroni” in the “P‐adjust” function from the package “Stats” (version 3.1.0) in R.
With respect to sex differences in diet across reproductive stages (N = 7 females) we used LMM to compare food intake, energy and macronutrient intake between males and females in different reproductive stages of females. This comparison was relevant due to the lack of sexual size dimorphism in sifakas, and was done to confirm that differences in diet between the sexes were due to the high demands of reproduction for females, rather than to the increase of food availability in the forest. All statistical analyses were performed in R.
3. RESULTS
3.1. Seasonal differences
Sifakas in Kirindy spent 47% of their time feeding. Their diet was composed of 118 species from 44 plant families. During the wet season, sifakas spent 42% of their time feeding from 88 species, while during the dry season they spent 48% of their time feeding and included 99 species.
The time spent feeding on different food items differed between seasons for all food categories (Friedman tests: fruits: χ2 = 92.25, df = 4, P < 0.001; flowers: χ2 = 71, df = 4, P < 0.001; mature leaves: χ2 = 154.84, df = 4, P < 0.001; young leaves: χ2 = 67.73, df = 4, P < 0.001). Sifakas spent more time feeding on fruits during the late wet season; on flowers in the transition between late dry and early wet seasons; on mature leaves during the early dry season, and more time feeding on young leaves during the early wet season (Supplementary Table S1A). The proportion of fruits included in the diet of sifakas was correlated with the availability of this item (R 2 = 0.52, df = 10, F = 13.06, P = 0.005, Figure 1). However, they did not select their diet based on the availability of young and mature leaves, and flowers.
Figure 1.
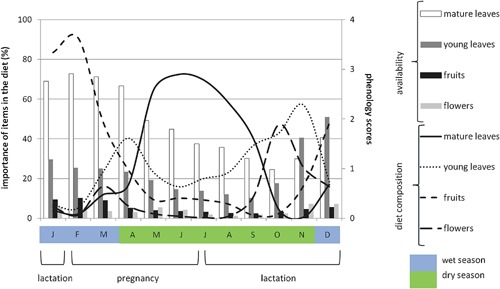
Diet composition and food availability across seasons. Lines indicate the percentage of items in the diet. The bars indicate the monthly availability of food items in the forest, based on the phenology
There was no difference in food intake (amount of food eaten) between the dry and the wet season (Supplementary Table S1B). However, sifakas had a higher intake of macronutrients during the wet season, in particular during the late wet season (Jan–Mar). They had a higher intake of TNC (χ2 = 13.87, df = 3, P < 0.001), crude protein (χ2 = 28.08, df = 3, P < 0.001), NDF (χ2 = 24.58, df = 3, P < 0.001), fat (χ2 = 152.3, df = 3, P < 0.001), and energy (χ2 = 62.99, df = 3, P < 0.001) compared to the other season stages (Figure 2). Results of Linear Mixed Models are available in supplementary material (TNC: Supplementary Table S1C; crude protein: Table S1D; NDF: Table S1E; fat: Table S1F; and energy: Table S1G).
Figure 2.
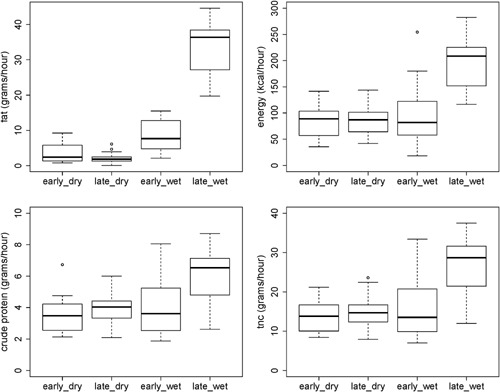
Intake of macronutrients (grams/hour) and energy (cal/hour) during season stages. The intake of all macronutrients and energy was high during the late wet season in comparison to the other stages (LMM, P < 0.001, tables with model parameters are available in SM)
3.2. Sex differences
In terms of food items, females spent more time feeding on young leaves (V = 53, P = 0.002), mature leaves (V = 48, P = 0.04), and fruits (V = 54, P = 0.003) than males. There was no sex difference in the time spent feeding on flowers (V = 40, P = 0.23) (Figure 3).
Figure 3.
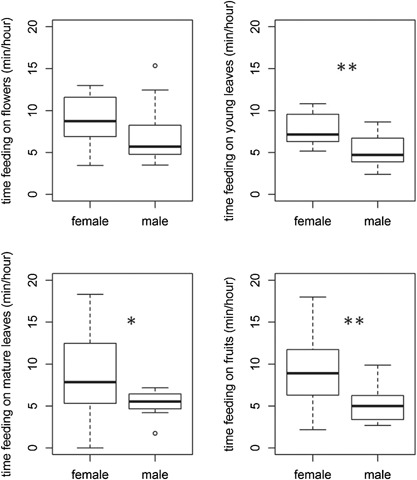
Time spent feeding (min/hour of feeding time) on fruits, flowers, young leaves, and mature leaves between females and males (Wilcoxon‐signed paired test, significance levels *P < 0.05, **P < 0.01)
Across reproductive stages, females had a higher intake of food than males during the late stage of lactation (Nov–Jan) (χ2 = 11.68, df = 1, P < 0.001, Supplementary Table S1H). Females also had a higher intake of macronutrients during the late stage of lactation than males with a higher intake of TNC (χ2 = 8.38, df = 1, P = 0.003, Supplementary Table S1J), crude protein (χ2 = 8.19, df = 1, P = 0.004, Supplementary Table S1K), NDF (χ2 = 6.47, df = 1, P = 0.01, Supplementary Table S1L), fat (χ2 = 8.35, df = 1, P = 0.004, Supplementary Table S1M), and energy (χ2 = 4.73, df = 1, P = 0.03, Supplementary Table S1N) (Figure 4). There was no sex difference in intake of macronutrients and energy within the other reproductive stages.
Figure 4.
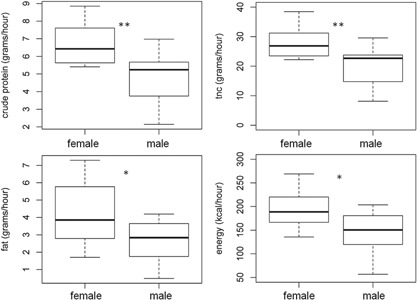
Sex differences in the intake of macronutrients (grams/hour) during the late stage of lactation (LMM, Significance levels *P < 0.05, **P < 0.01, tables with model parameters are available in SM)
4. DISCUSSION
Our study shows that sex and seasonality influenced feeding patterns of Verreaux's sifakas. The dry season was indeed a period in which the availability of food dropped drastically, and both males and females had lower intake of nutrients in comparison to the wet season. Sifakas consumed fruits in relation to their availability, but did not do so for other plant parts. The comparison of dietary patterns between sexes across reproductive stages showed that females had higher intake of macronutrients and energy than males, but this pattern was present only during the late stage of lactation and not during pregnancy. Our results on seasonal patterns of diet agree with Norscia et al. (2006) who worked on the same population of Verreaux's sifakas in Kirindy Forest. The authors reported the negative impact of the dry season on the intake of macronutrients. Sifakas were also highly selective in their diet, giving priority to high quality foods (here defined as high protein/low fiber content) regardless of the general availability of food in the forest.
One possible explanation for the sex differences in nutrient intake can be related to different requirements for body maintenance (Key & Ross, 1999). However, that is not the case for sifakas since there is no sex dimorphism in size (Kappeler, 1991), or any other physical or physiological difference between males and females, other than reproduction that could justify differences in energy and nutrient intake. Therefore, we suggest that the sex differences in diet observed in our study are due to the high costs of reproduction for females.
Reproduction is indeed a highly demanding period for mammals, particularly the lactation period for females (Coelho, 1974; reviewed in Gittleman & Thompson, 1988). For example, in white‐faced capuchins (Cebus capucinus), lactating females consumed more food than cycling or pregnant females, suggesting that lactation is the most costly period of reproduction (McCabe & Fedigan, 2007). The same pattern has been observed in howler monkeys (Alouatta palliata), in which reproductive females had higher intake of energy than non‐reproductive ones (Serio‐Silva, Hernández‐Salazar, & Rico‐Gray, 1999). In the case of lemurs, reproduction has been suggested to be even more costly for females than in other primates because of the highly seasonal climate of Madagascar (reviewed in Wright, 1999), altricial infants and low basal metabolic rates (Jolly, 1984).
Reproductive stages require different amounts of energy from females. It has been suggested that mid/late lactation is the most demanding stage for female mammals (Coelho, 1974; Payne & Wheeler, 1968). Since the availability of foods with easily digestible carbohydrates such as fruits is concentrated in a short period of the year in Madagascar (Janson & Verdolin, 2005; Wright, 1999), sifakas have to strategically adjust the reproductive stages, in particular the most demanding ones, across fluctuations in food availability (Richard et al., 2000; Wright, 1999).
In that regard females may synchronize the most demanding period of their reproduction, mid/late lactation, to the period of highest availability of food, following the income breeding strategy. Alternatively, females can store energy from the periods of high food availability in order to pay for the costs of reproduction in periods of low food availability, following then the capital breeding strategy. It has been debated if sifakas are following a capital or an income breeding strategy, and there is no consensus on this aspect up to this date (Janson & Verdolin, 2005; Lewis & Kappeler, 2005a, 2005b; Richard et al., 2000; Van Schaik & Brockman, 2005; Wright, 1999). Reproductive females in our study indeed increased their food intake during the most demanding period of reproduction (late lactation). However, the period of late lactation was not synchronized with the period of highest availability of food. In addition, by the time of the peak of abundance of food (late wet season), infants were already weaned (total length of lactation period was around 5 months, based on our observations, and unpublished data from C. Fichtel).
Although we did not include measurements of body mass in our study as an indicator of storing energy, previous studies already showed that both male and female sifakas lose weight during the dry season and gain weight during the wet season (Lewis & Kappeler, 2005a; Richard et al., 2000). This pattern of seasonal oscillation in body mass is in fact more accentuated in females (Meyers & Wright, 1993; Richard et al., 2000, 2002). Richard, Dewar, Schwartz, and Ratsirarson (2002) suggest that the higher oscillation of body mass in females is due to the necessity of storing nutrients from the wet season to pay for the costs of reproduction. Females that have a better body condition (higher body mass) around the mating season had higher chances of giving birth, and were more successful in caring for their infant (Lewis & Kappeler, 2005a; Richard et al., 2000). Our findings indeed show that females had a higher intake of TNC than males during the late lactation. Carbohydrates can be stored as glycogen or fat for later use (National Research Council, 2003).
In our study, females synchronized the timing of weaning their infants with the period of high availability of food. This seems to be an adaptive strategy for two reasons: first from the perspective of the infants that will have plenty of high quality food to explore and to get prepared for the harsh dry season; second from the perspective of females that can concentrate all their energy in recovering the body condition and storing enough nutrients for their next reproduction (Janson & Verdolin, 2005; Richard et al., 2000). Additionally, females in our study gave birth during the peak of the dry season and were dealing with at least half of the lactation period while the availability of food in the forest was still low, probably relying on reserves. The increase in food intake, which resulted in higher intake of energy and macronutirents, observed during the late lactation probably indicates that the amount of nutrients stored by female sifakas during the abundant season is probably not enough to pay for all the costs of reproduction, as seen in classical capital breeders. Therefore, our results are in accordance with Richard et al. (2000) and indicate that sifakas are capital breeders.
In conclusion, our study showed that despite the fact that seasonality affected the diet of both sexes, reproductive females managed to have a higher intake of food than males. Since we can exclude different costs of body maintenance between sexes, we suggest that the high costs of reproduction drive the patterns of the diet of females in this species. As suggested, it is likely that one of the mechanisms used by female sifakas to ensure their access to a better diet is their priority to access food resources through social dominance over males (Jolly, 1966). In addition, the capacity for storing nutrients and the synchronization of reproductive stages, including the period weaning infants, to the seasonal fluctuations of food, also contribute to the improvement of their diet after scarce periods and thereby to their reproductive success. The storage capacity has been also described for red‐tailed sportive lemurs (Lepilemur ruficaudatus), another folivorous lemur species inhabiting the same forest (Ganzhorn, 2002; but see also Dröscher, Rothman, Ganzhorn, & Kappeler, 2016 for Lepilemur leucopus). Likewise, storage capacity is one prerequisite for hibernation in gray mouse lemurs (Microcebus murinus) and fat‐tailed dwarf lemurs (Cheirogaleus medius, Dausmann, 2014; Schmid, 2000). Thus, this might be a basic trait of lemur biology that requires reconsideration of the importance of lean and rich seasons for lemur evolution. Hence, factors such as social organization, reproductive strategy, and storage capacity are supporting the successful persistence of sifakas in extreme seasonal environments.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information.
ACKNOWLEDGMENTS
We are thankful to Nicoletta Righini for organizing this special issue on nutritional ecology in primates, and for giving us the opportunity to participate in it. We thank the Commission Tripartite de Direction des Eaux et Forêts, and the CNFEREF Morondava for their authorization and support for this study. We would like to thank the team of field assistants in Kirindy Forest, in particular to Mamy Razafindrasamba for his help with behavioral observations, and Patrick Solondrainy for the collection of plant samples. We thank Irene Tomaschewski and Kain Irretier for their help with the chemical and NIRS analyses. We are grateful to Nicoletta Righini, Paul Garber, and three anonymous reviewers for the comments on the manuscript. This study was funded by the German Research Foundation (DFG, FI 929/5‐1). This research complied with the laws of Madagscar and the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
Koch F, Ganzhorn JU, Rothman JM, Chapman CA, Fichtel C. Sex and seasonal differences in diet and nutrient intake in Verreaux's sifakas (Propithecus verreauxi). Am J Primatol. 2017;79:e22595. 10.1002/ajp.22595
REFERENCES
- Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–267. [DOI] [PubMed] [Google Scholar]
- Baayen, R. H. (2008). Analyzing linguistic data. Cambridge: Cambridge University Press. [Google Scholar]
- Bates, D. , Maechler, M. , Bolker, B. 2012. lme4—Linear mixed effects models using S4 classes. Available at: http://CRAN.R-project.org/package=lme4 R package
- Boinski, S. (1988). Sex differences in the foraging behavior of squirrel monkeys in a seasonal habitat. Behavioral Ecology and Sociobiology, 23, 177–186. [Google Scholar]
- Brockman, D. K. , Whitten, P. L. , Richard, A. F. , & Schneider, A. (1998). Reproduction in free‐ranging male Propithecus verreauxi: The hormonal correlates of mating and aggression. American Journal of Physical Anthropology, 105, 137–151. [DOI] [PubMed] [Google Scholar]
- Campbell, J. L. , Eisemann, J. H. , Glander, K. E. , & Crissey, S. D. (1999). Intake, digestibility, and passage of a commercially designed diet by two Propithecus species. American Journal of Primatology, 48, 237–246. [DOI] [PubMed] [Google Scholar]
- Chapman, C. A. , Chapman, L. J. , Rode, K. D. , Hauck, E. M. , & Mcdowell, L. R. (2003). Variation in the nutritional value of primate foods: Among trees, time periods, and areas. International Journal of Primatology, 24, 317–333. [Google Scholar]
- Coelho, A. M. (1974). Socio‐bioenergetics and sexual dimorphism in primates. Primates, 15, 262–269. [Google Scholar]
- Conklin, N. L. O. U. , & Wrangham, R. W. (1994). The value of figs to a hind‐gut fermenting frugivore: A nutritional analysis. Biochemical Systematics and Ecology, 22, 137–151. [Google Scholar]
- Dausmann, K. H. (2014). Flexible patterns in energy savings: Heterothermy in primates. Journal of Zoology, 292, 101–111. [Google Scholar]
- Donati, G. , Baldi, N. , Morelli, V. , Ganzhorn, J. U. , & Borgognini‐Tarli, S. M. (2009). Proximate and ultimate determinants of cathemeral activity in brown lemurs. Animal Behaviour, 77, 317–325. [Google Scholar]
- Drent, R. , & Daan, S. (1980). The prudent parent: Energetic adjustments in avian breeding. Ardea, 68, 225–252. [Google Scholar]
- Dröscher, I. , Rothman, J. M. , Ganzhorn, J. U. , & Kappeler, P. M. (2016). Nutritional consequences of folivory in a small‐bodied lemur (Lepilemur leucopus): Effects of season and reproduction on nutrient balancing. American Journal of Physical Anthropology, 207, 197–207. [DOI] [PubMed] [Google Scholar]
- Dunham, A. E. (2008). Battle of the sexes: Cost asymmetry explains female dominance in lemurs. Animal Behaviour, 76, 1435–1439. [Google Scholar]
- Fleagle J. G. (1999). Primate evolution and adaptation. New York, NY: Academic Press. [Google Scholar]
- Foley, W. J. , McIlwee, A. , Lawler, I. , Aragones, L., Woolnough, A. P. , & Berding, N. (1998). Ecological applications of near infrared reflectance spectroscopy: A tool for rapid, cost‐effective prediction of the composition of plant and animal tissues and aspects of animal performance. Oecologia, 116, 293–305. [DOI] [PubMed] [Google Scholar]
- Fournier, L. A. (1974). Un método cuantitativo para la medición de caracteristicas fenológicas en árboles. Turrialba, 24, 422–423. [Google Scholar]
- Ganzhorn, J. U. (2002). Distribution of a folivorous lemur in relation to seasonally varying food resources: Integrating quantitative and qualitative aspects of food characteristics. Oecologia (Berlin), 131, 427–435. [DOI] [PubMed] [Google Scholar]
- Gittleman, J. L. , & Thompson, S. D. (1988). Energy allocation in mammalian reproduction. Integrative and Comparative Biology, 28, 863–875. [Google Scholar]
- Harrison, M. J. S. (1983). Age and sex differences in the diet and feeding strategies of the green monkey, Cercopithecus sabaeus . Animal Behaviour, 31, 969–977. [Google Scholar]
- Hemingway, C. A. (1999). Time budgets and foraging in a Malagasy primate: Do sex differences reflect reproductive condition and female dominance? Behavioral Ecology and Sociobiology, 45, 311–322. [Google Scholar]
- Hill, W. C. O. (1953). Primates: Comparative anatomy and taxonomy: I Strepsirhini. Edinburgh: Edinburgh University Press. [Google Scholar]
- Irwin, M. T. , Raharison, J. , Raubenheimeir, D. , Chapman, C. A. , & Rothman, J. M. (2014). Nutritional correlates of the “lean season”: Effects of seasonality and frugivory on the nutritional ecology of diademed sifakas. American Journal of Physical Anthropology, 153, 78–91. [DOI] [PubMed] [Google Scholar]
- Janson, C. , & Verdolin, J. (2005). Seasonality of primate births in relation to climate In Brockman D. K., & van Schaik C. P. (Eds.), Seasonality in primates: Studies of living and extinct human and non‐human primates (pp. 307–350). Cambridge: Cambridge University Press. [Google Scholar]
- Jolly, A. (1966). Lemur behavior. Chicago: University of Chicago Press. [Google Scholar]
- Jolly, A . (1984). The puzzle of female feeding priority In Small M. (Ed.), Female primates: Studies by women primatologists (pp. 197–215). New York, NY: A.R. Liss. [Google Scholar]
- Kappeler, P. M. , & Fichtel, C. (2012). A 15‐year pespective on the social organization and life history of sifaka in kirindy forest In Kappeler P., & Watts D. P. (Eds.), Long‐term field studies of primates (pp 21–45). Berlin: Springer. [Google Scholar]
- Kappeler, P. M. (1990). The evolution of sexual size dimorphism in prosimian primates. American Journal of Primatology, 21, 201–214. [DOI] [PubMed] [Google Scholar]
- Kappeler, P. M. (1991). Patterns of sexual dimorphism in body weight among prosimian primates. Folia Primatologica, 57, 132–146. [DOI] [PubMed] [Google Scholar]
- Key, C. , & Ross, C. (1999). Sex differences in energy expenditure in non‐human primates. Proceedings of the Royal Society of Biological Sciences, 266, 2479–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova, A. , Brockhoff, P. B. , & Bojesen Christensen, R. H. (2013). lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). Available at: http://CRAN.R-project.org/package=lmerTest.Rpackage
- Lee, P. C. (1984). Early infant development and maternal care in free‐ranging vervet monkeys. Primates, 25, 36–47. [Google Scholar]
- Lewis, R. J. , & Kappeler, P. M. (2005a). Seasonality, body condition, and timing of reproduction in Propithecus verreauxi verreauxi in the Kirindy Forest. American Journal of Primatology, 67, 347–364. [DOI] [PubMed] [Google Scholar]
- Lewis, R. J. , & Kappeler, P. M. (2005b). Are Kirindy sifaka capital or income breeders? It depends. American Journal of Primatology, 67, 365–369. [DOI] [PubMed] [Google Scholar]
- Mass, V. , Heistermann, M. , & Kappeler, P. M. (2009). Mate‐guarding as a male reproductive tactic in Propithecus verreauxi . International Journal of Primatology, 30, 389–409. [Google Scholar]
- McCabe, G. M. , & Fedigan, L. M. (2007). Effects of reproductive status on energy intake, ingestion rates, and dietary composition of female Cebus capucinus at Santa Rosa, Costa Rica. International Journal of Primatology, 28, 837–851. [Google Scholar]
- Meyers, D. M. , & Wright, P. C. (1993). Resource tracking: Food availability and Propithecus seasonal variation In Kappeler P. M., & Ganzhorn J. U. (Eds.), Lemur social systems and their ecological basis (pp 179–192). New York, NY: Plenum Press. [Google Scholar]
- Naumann, C. , & Bassler, R. (1976). Die chemische untersuchung von futtermitteln. Darmstadt, Germany: VDLUFA‐Verlag. [Google Scholar]
- Norscia, I. , Carrai, V. , & Borgognini‐Tarli, S. M. (2006). Influence of dry Season and food quality and quantity on behavior and feeding strategy of Propithecus verreauxi in Kirindy, Madagascar. International Journal of Primatology, 27, 1001–1022. [Google Scholar]
- National Research Council (NRC). (2003). Nutrient requirements of nonhuman primates. Washington, DC: The National Academies Press. [Google Scholar]
- Oftedal, O. T . (1985). Pregnancy and lactation In Hudson R. J., & White R. G. (Eds.), Bioenergetics of wild herbivores (pp. 215–238). Florida: CRC Press. [Google Scholar]
- Ortmann, S. , Bradley, B. , Stolter, C. , & Ganzhorn, J. U. (2006). Estimating the quality and composition of wild animal diets: A critical survey of methods In Hohmann G., Robbins M. M., Boesch C. (Eds.), Feeding ecology in apes and other primates: ecological, physical and behavioral aspects (pp. 396–420). Cambridge: Cambridge University Press. [Google Scholar]
- Payne, P. R. , & Wheeler, E. F. (1968). Comparative nutrition in pregnancy and lactation. Proceedings of the Nutrition Society, 27, 129–138. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Ralls, K. (1976). Mammals in which females are larger than males. Quarterly Review of Biology, 51, 245–276. [DOI] [PubMed] [Google Scholar]
- Richard, A. F. (1978). Variability in the feeding behavior of a Malagasy prosimian, Propithecus verreauxi: Lemuriformes In Montgomery G. G. (Ed.), The ecology of arboreal folivores (pp. 519–533). Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Richard, A. F. , Dewar, R. E. , Schwartz, M. , & Ratsirarson, J. (2000). Mass change, environmental variability and female fertility in wild Propithecus verreauxi . Journal of Human Evolution, 39, 381–391. [DOI] [PubMed] [Google Scholar]
- Richard, A. F. , Dewar, R. E. , Schwartz, M. , & Ratsirarson, J. (2002). Life in the slow lane? Demography and life histories of male and female sifaka (Propithecus verreauxi verreauxi). Journal of Zoology, 256, 421–436. [Google Scholar]
- Richard, A. F. , & Nicoll, M. E. (1987). Female social dominance and basal metabolism in a Malagasy primate, Propithecus verreauxi . American Journal of Primatology, 12, 309–314. [DOI] [PubMed] [Google Scholar]
- Richard, A. F. (1992). Aggressive competition between males, female‐controlled polygyny and sexual monomorphism in a Malagasy primate, Propithecus verreauxi . Journal of Human Evolution, 22, 395–406. [Google Scholar]
- Rothman, J. M. , Chapman, C. A. , Hansen, J. L. , Cherney, D. J. R. , & Pell, A. N. (2009). Rapid assessment of the nutritional value of foods eaten by mountain gorillas: Applying near‐infrared reflectance spectroscopy to primatology. International Journal of Primatology, 30, 729–742. [Google Scholar]
- Rothman, J. M. , Chapman, C. A. , & Soest, P. J. (2011). Methods in primate nutritional ecology: A user's guide. International Journal of Primatology, 33, 542–566. [Google Scholar]
- Rothman, J. M. , Dierenfeld, E. S. , Hintz, H. F. , & Pell, A. N. (2008). Nutritional quality of gorilla diets: Consequences of age, sex, and season. Oecologia, 155, 111–122. [DOI] [PubMed] [Google Scholar]
- Schmid, J. (2000). Daily torpor in the gray mouse lemur (Microcebus murinus) in Madagascar: Energetic consequences and biological significance. Oecologia, 123, 175–183. [DOI] [PubMed] [Google Scholar]
- Serio‐Silva, J. C. , Hernández‐Salazar, L. T. , & Rico‐Gray, V. (1999). Nutritional composition of the diet of Alouatta palliata mexicana females in different reproductive states. Zoo Biology, 513, 507–513. [Google Scholar]
- Sorg, J. , & Rohner, U. (1996). Climate and tree phenology of the dry deciduous forest of Kirindy forest. Primate Report, 46, 57–81. [Google Scholar]
- Stearns, S. C. (1989). Trade‐offs in life‐history evolution. Funtional Ecology, 3, 259–268. [Google Scholar]
- Stearns, S. C. (1992). The evolution of life histories. New York, NY: Oxford University Press. [Google Scholar]
- Stolter, C. , Julkunen‐Tiitto, R. , & Ganzhorn, J. U. (2006). Application of near infrared reflectance spectroscopy (NIRS) to assess some properties of a sub‐arctic ecosystem. Basic and Applied Ecology, 7, 167–187. [Google Scholar]
- Van Schaik, C. P. , & Brockman, D. K. (2005). Seasonality in primate ecology, reproduction, and life history: An overview In Brockman D. K., & van Schaik C. P. (Eds.), Seasonality in primates: Studies of living and extinct human and non‐human primates (pp. 3–20). Cambridge: Cambridge University Press. [Google Scholar]
- Van Soest, P. V. , Robertson, J. B. , & Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science, 74, 3583–3597. [DOI] [PubMed] [Google Scholar]
- Voigt, F. A. , Bleher, B. , Fietz, J. , Ganzhorn, J. U. , Schwab, D. , & Böhning‐Gaese, K . (2004). A comparison of morphological and chemical fruit traits between two sites with different frugivore assemblages. Oecologia, 141, 94–104. [DOI] [PubMed] [Google Scholar]
- Wright, P. C. (1999). Lemur traits and Madagascar ecology: Coping with an island environment. American Journal of Physical Anthropology, 29, 31–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information.


