Abstract
Objective
Anti–tumor necrosis factor (anti‐TNF) medications are effective in controlling chronic inflammatory diseases, but information about their use and safety in pregnancy is limited. Consequently, anti‐TNF agents are often discontinued early in gestation. Certolizumab pegol (CZP), a PEGylated, Fc‐free anti‐TNF agent approved for the treatment of rheumatic diseases and/or Crohn's disease, has minimal to no active placental transfer. This analysis was undertaken to evaluate pregnancy outcomes in women receiving CZP, especially those exposed during early pregnancy.
Methods
Prospective and retrospective data on maternal CZP exposure were extracted from the UCB Pharma safety database through March 6, 2017. Analysis was limited to prospective reports to avoid potential bias associated with retrospective submissions. The numbers of live births, miscarriages, elective abortions, stillbirths, and major congenital malformations were ascertained.
Results
Of 1,137 prospectively reported pregnancies with maternal exposure to CZP, 528 (including 10 twin pregnancies) had 538 known outcomes: 459 live births (85.3%), 47 miscarriages (8.7%), 27 elective abortions (5.0%), and 5 stillbirths (0.9%). There were 8 major congenital malformations (1.7%) among the 459 infants. First trimester exposure occurred in 367 (81.2%) of 452 pregnancies resulting in 459 live births. Exposure during all 3 trimesters occurred in 201 (44.5%) of 452 pregnancies.
Conclusion
This analysis represents the largest cohort of pregnant women exposed to an anti‐TNF agent for management of chronic inflammatory diseases. Analysis of pregnancy outcomes does not indicate a teratogenic effect of CZP, compared to the general population, nor an increased risk of fetal death. The data are reassuring for women of childbearing age considering treatment with CZP.
For women affected by chronic inflammatory diseases, such as rheumatoid arthritis (RA), axial spondyloarthritis (SpA), psoriatic arthritis (PsA), and Crohn's disease (CD), disease onset, diagnosis, and initiation of treatment increasingly overlap with peak reproductive age 1, 2. For many of these patients, disease activity during pregnancy requires effective management to maintain maternal health and ensure optimal fetal outcomes. Up to half of pregnant patients with rheumatic diseases and the majority of those with inflammatory bowel disease (IBD) experience active disease and need treatment 3, 4. High disease activity is associated with an increased risk of pregnancy complications and adverse outcomes, including preeclampsia, preterm delivery, low birth weight, small for gestational age, and fetal loss 5, 6, 7, 8, 9, 10, 11, 12, 13. Adequate disease control during pregnancy is therefore paramount for fetal and maternal health, as acknowledged by recent publications 6, 14, 15, 16, and the risks of untreated disease during pregnancy should be evaluated against the risks related to drug exposure of mother and fetus. However, some treatments are not used in pregnant patients, due to proven teratogenicity (for example, methotrexate) or because of concerns due to the absence of a well‐established safety profile in pregnancy 16.
Clinical trial data on the effects of drugs on pregnancy outcomes are sparse, since pregnant women are generally excluded from participation in clinical studies, and women who become pregnant during a trial have treatment stopped immediately 14. Therefore, evidence generated through pharmacovigilance reporting and pregnancy registries is extremely important to help physicians and patients make informed decisions regarding anti–tumor necrosis factor (anti‐TNF) treatment for rheumatic diseases and CD during pregnancy 15, 17.
The UCB Pharma safety database contained reports of 625 pregnancies exposed to certolizumab pegol (CZP) up to 2014 18, while the 2017 data cutoff presented here includes 1,600 CZP‐exposed pregnancies. With a substantial number of pregnant patients now being exposed to CZP across the different indications, more data are available to gain a better understanding of the timing of gestational exposure and its impact on maternal and infant health. In particular, exposure during the first trimester, when most organogenesis takes place, is of interest. The objective of our investigation is to provide information on pregnancy outcomes, including major congenital malformations and other adverse maternal and fetal outcomes, with a specific focus on the timing of CZP exposure.
Patients and methods
The methodology of pregnancy data collection and analysis has been described previously 18. Reports of CZP‐exposed pregnancies were obtained from the UCB Pharma safety database from the start of CZP clinical development through the cutoff date of March 6, 2017. We report the totality of the data up to the cutoff date, including those given in the previous data cut 18. Information on pregnancy dating, CZP exposure, congenital malformations, and other pregnancy outcomes, maternal comorbidities, and concomitant medications was extracted.
Pregnancies were classified as prospective or retrospective 19. Prospective pregnancies were those that were reported to UCB Pharma while ongoing and for which no fetal abnormalities were known. In contrast, pregnancies whose outcomes (including abnormal ultrasound results) were already known at initial notification were categorized as retrospective. The focus of this analysis was on prospectively reported pregnancies to avoid potential bias in outcome reporting rates.
Pregnancy outcomes were categorized as follows: live birth, miscarriage (non‐induced embryonic or fetal death or passage of products of conception before 20 weeks of gestation), induced abortion (medically indicated or elective), and stillbirth (a fetal death occurring at or after 20 weeks of gestation). Ongoing pregnancies included any pregnancy with an estimated (or calculated) due date less than 3 months before the cutoff date of March 6, 2017. If no information on pregnancy outcome was available after 3 months following the estimated pregnancy due date, it was considered lost to follow‐up and reported as having an unknown outcome.
Trimesters of pregnancy were defined by time since the first day of the last menstrual period (LMP) and distinguished as first (up to 12 weeks and 6 days’ gestation), second (13–28 weeks and 6 days’ gestation), and third (any time at or after 29 weeks’ gestation) 20. Timing of CZP exposure during pregnancy was categorized into first, second, and third trimester by entering the exposure start, stop, and restart dates (where applicable) into an algorithm when sufficient data regarding the LMP were available.
Live birth characteristics of infants analyzed included the gestational age at delivery (i.e., the number of completed weeks starting from LMP), low birth weight (≤2,500 gm), small for gestational age (a birth weight less than the tenth percentile for infants of the same gestational age and sex), and prematurity (infants born before 37 weeks’ gestation). Mothers’ indications, comorbidities, adverse events, and concomitant medications were also analyzed.
Information on mother and infant was recorded in Council for International Organization of Medical Sciences forms for cases of major congenital malformations, which were reviewed by a clinical teratologist. The clinical review assessed the presence and characterization of malformations among live‐born infants and the possible temporal association between malformation, organogenesis, and medication exposure.
Results
Overview of pregnancies. A total of 1,600 reports of CZP‐exposed pregnancies were entered in the UCB Pharma safety database from the start of CZP clinical development (earliest report July 12, 2001) through March 6, 2017, with 1,541 maternal and 59 paternal CZP exposures (Figure 1). The majority (n = 1,137) of pregnancies with maternal exposure were prospective. Outcomes were known for 528 prospective pregnancies with maternal CZP exposure, which included 10 twin pregnancies; 198 prospective pregnancies were ongoing at the time of data cutoff (Figure 1). The outcome was not known for 411 cases.
Figure 1.
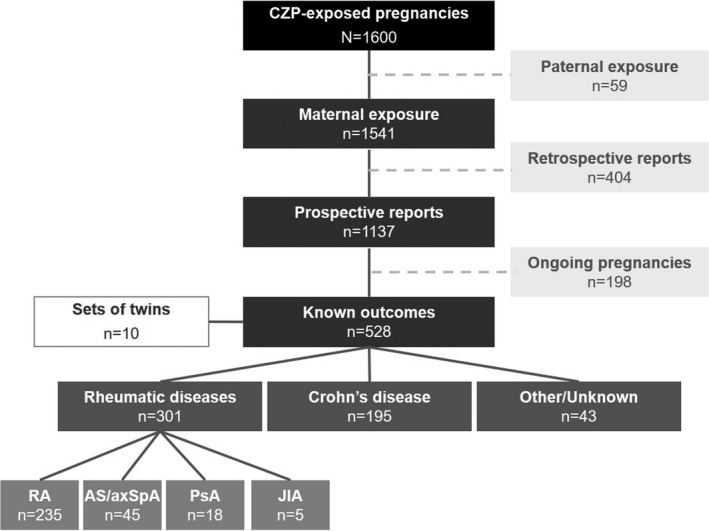
Overview of pregnancies exposed to certolizumab pegol (CZP). Eleven patients were included in more than 1 treatment indication category (rheumatic diseases, Crohn's disease, or other) due to multiple reported indications. RA = rheumatoid arthritis; AS = ankylosing spondylitis; axSpA = axial spondyloarthritis; PsA = psoriatic arthritis; JIA = juvenile idiopathic arthritis.
The main indications of women with CZP exposure and known prospective pregnancy outcomes were rheumatic diseases (n = 301) and CD (n = 195). Rheumatic diseases included RA (n = 235), ankylosing spondylitis/axial SpA (n = 45), PsA (n = 18), and juvenile idiopathic arthritis (n = 5) (Figure 1). Of note, with regard to indications for treatment, 11 patients were counted more than once due to multiple reported indications.
Prospective pregnancies. Maternal demographics. Almost half (46.4%) of prospective pregnancies with maternal CZP exposure and known outcomes were spontaneous reports (including those found in the literature); the remaining 53.2% of reports came from other sources, with interventional clinical trials accounting for 12.3% of pregnancy reports (Table 1). The majority of pregnancy reports originated from North America (60.0%) and Europe (22.5%). The median age at the estimated due date (EDD) was 31 years (range 17–44 years), with 26.8% of women age 35 years or older (Table 1). Of the women with CD, 87.7% were in North America, compared to 42.9% of those with rheumatic diseases (Table 1). Of note, more women with rheumatic diseases (33.1%) than with CD (18.0%) were older than 35 years at EDD (Table 1). The maternal demographics of prospective pregnancies with unknown outcome were also reviewed for comparison. These reports originated almost exclusively from spontaneous reporting (Table 1). Of note, the mother's age was reported for only 50% of these patients (Table 1).
Table 1.
Maternal demographics by indication for CZP use in prospectively reported pregnancies with maternal CZP exposurea
| Known outcomes | Unknown outcomes (n = 411)d | |||
|---|---|---|---|---|
| Total (n = 528)b | Rheumatic diseases (n = 301)c | CD (n = 195) | ||
| Report source | ||||
| Spontaneous reportse | 245 (46.4) | 125 (41.5) | 97 (49.7) | 378 (92.0) |
| Clinical trials | 65 (12.3) | 37 (12.3) | 21 (10.8) | 7 (1.7) |
| NIS/OLEs/compassionate usef | 218 (41.3) | 139 (46.2) | 77 (39.5) | 17 (4.1) |
| Region | ||||
| North America | 317 (60.0) | 129 (42.9) | 171 (87.7) | 289 (70.3) |
| Europe | 119 (22.5) | 85 (28.2) | 22 (11.3) | 84 (20.4) |
| Asia | 62 (11.7) | 61 (20.3) | 0 (0.0) | 14 (3.4) |
| Latin America | 9 (1.7) | 8 (2.7) | 0 (0.0) | 6 (1.5) |
| Middle East | 11 (2.1) | 10 (3.3) | 0 (0.0) | 7 (1.7) |
| Australia/New Zealand | 9 (1.7) | 8 (2.7) | 1 (0.5) | 8 (2.0) |
| Africa | 1 (0.2) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Maternal age at EDD | ||||
| Number with available data | 455 | 254 | 178 | 200 |
| 18–34 years | 332 (73.0) | 169 (66.5) | 146 (82.0) | 154 (77.0) |
| <18 years | 1 (0.2) | 1 (0.4) | 0 (0.0) | 2 (1.0) |
| ≥35 years | 122 (26.8) | 84 (33.1) | 32 (18.0) | 44 (22.0) |
| Median (range) years | 31 (17–44) | 32 (17–44) | 30 (18–43) | 30 (16–55) |
Except where indicated otherwise, values are the number (%). CD = Crohn's disease; NIS = non‐interventional studies; OLEs = open‐label extension studies; EDD = estimated due date.
Includes pregnancy reports where the indication for treatment of the mother with certolizumab pegol (CZP) was unknown (n = 43) and excludes 11 pregnancies where more than 1 indication was reported.
Includes rheumatoid arthritis, ankylosing spondylitis, axial spondyloarthritis, psoriatic arthritis, and juvenile idiopathic arthritis.
Includes pregnancy reports where the indication for treatment (n = 50), report source (n = 9), and region (n = 3) were unknown.
Including registries (but excluding Organization of Teratology Information Services), social media reports, and reports from the literature.
Includes healthy volunteers.
Pregnancy outcomes and CZP exposure. A total of 528 pregnancies (including 10 twin pregnancies) had 538 known outcomes: 452 pregnancies resulted in 459 live births (85.3% of 538), and there were 47 miscarriages (8.7% of 538), 27 elective abortions (5.0% of 538), and 5 stillbirths (0.9% of 538) (Figure 2). Analysis of pregnancy outcomes demonstrated similar trends for women with rheumatic diseases and those with CD: live births were the most common pregnancy outcome in both indications (Figure 2). Of note, 2 cases of neonatal death were reported: 1 member of a female twin pair (born at 25 weeks), from brain damage and pneumoperitoneum; and 1 member of a female twin pair (born at 27 weeks) with a heart defect who developed an unspecified infection of the intestines ~8 weeks after birth and underwent surgery. The infant did not survive the surgery.
Figure 2.
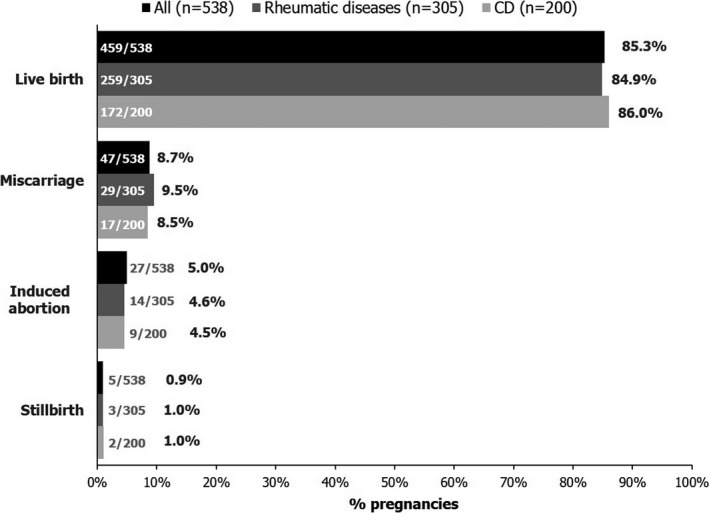
Known outcomes for prospectively reported pregnancies with maternal exposure to certolizumab pegol. Ten twin pregnancies are included in the total number of pregnancy outcomes (4 in mothers with rheumatic diseases, 5 in mothers with Crohn's disease [CD], and 1 in a mother with unknown indication). Miscarriage was defined as embryonic or fetal death before 20 weeks of gestation. Induced abortion includes both elective and medically indicated abortions.
Serious maternal infections occurred in 22 (4.2%) of 528 prospective pregnancies. For the 452 CZP‐exposed women whose prospective pregnancies resulted in a live birth, maternal comorbidities included preeclampsia (in 5 patients [1.1%]) and gestational diabetes (in 6 patients [1.3%]).
Maternal exposure to CZP occurred during at least the first trimester for 367 (81.2%) of the 452 prospective pregnancies resulting in a live birth, including 132 (29.2%) exposed during the first trimester only (Figure 3A). CZP exposure during all 3 trimesters of pregnancy was reported by 201 pregnant women with a live birth outcome (44.5%) (Figure 3A). Over half of the pregnant women with CD who had live births (55.6%) were exposed to CZP during all 3 trimesters, compared to 37.1% of those with rheumatic diseases (Figure 3B). More women with rheumatic diseases than women with CD were exposed to CZP during the first trimester only or during the first and second trimesters (Figure 3B), reflecting the differing clinical practice between the treatment of CD and the treatment of rheumatic diseases.
Figure 3.
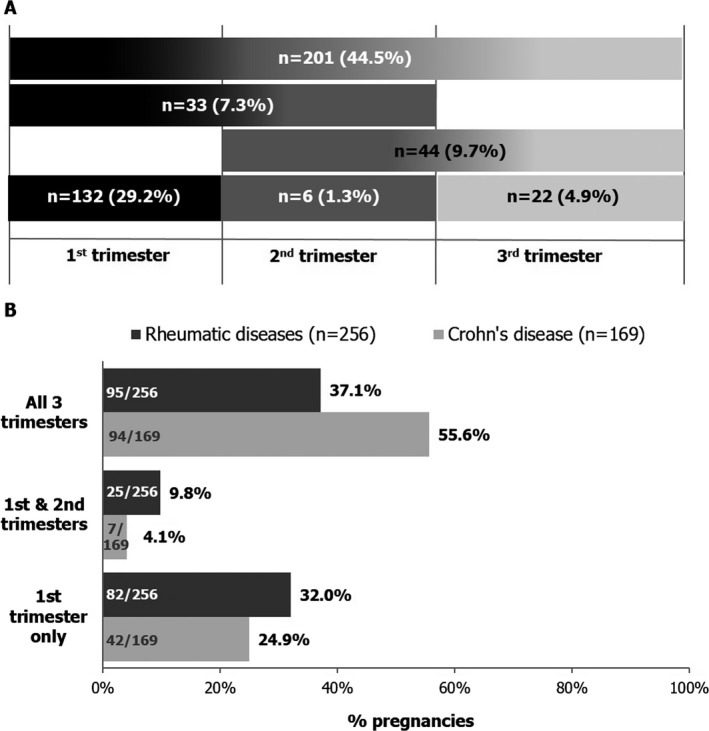
Trimesters of maternal certolizumab pegol (CZP) exposure for prospectively reported pregnancies resulting in a live birth. A, Timing of CZP exposure for all pregnancies. A total of 452 pregnancies resulted in a live birth, including those in which the mother was exposed preconception (n = 5; CZP exposure stopped >14 days and ≤70 days prior to the first trimester) and pregnancies for which the timing of exposure could not be determined (n = 8), as well as 1 patient exposed in the first and third trimesters. Black, dark gray, and light gray shading represent CZP exposure during the first, second, and third trimesters, respectively; shading gradients reflect exposure during multiple trimesters. B, Exposure by indication for CZP use. There were 8 live births for which the timing of exposure could not be determined (n = 2 for patients with rheumatic disease and n = 6 for patients with Crohn's disease).
Concomitant medications were reported for only 382 pregnancies, and the type of medication was unspecified for 3 of those pregnancies. Nonsteroidal antiinflammatory drugs and steroids were the most frequent medications used; 7 miscarriages were reported in patients who received concomitant methotrexate.
Live birth characteristics. Among women with CZP exposure, 452 prospective pregnancies resulted in 459 live births; 17 infants from 10 twin pregnancies were reported. Four twin pregnancies occurred in women with rheumatic diseases and 5 in women with CD. The indication of 1 mother of twins was unknown. Among the singleton live births, 31.4% of women had a cesarean section (24.2% of women with rheumatic diseases and 42.7% of women with CD) (Table 2). Of the live births, 11.9% were preterm, with similar proportions in the different indications; the median gestational age at birth was 39 weeks (minimum 27 weeks, maximum 43 weeks) (Table 2). Twenty‐four (11.7%) of 205 infants had low birth weight (Table 2); no differences were observed between infants of mothers with rheumatic diseases and those with CD. A low number of infants (4 [0.9%] of 442) were reported as small for their gestational age.
Table 2.
Characteristics of live‐born singletons from prospectively reported pregnancies with maternal exposure to CZPa
| Characteristic | All (n = 442)b | Rheumatic diseases (n = 252)c | CD (n = 164)c |
|---|---|---|---|
| Cesarean section | 139 (31.4) | 61 (24.2) | 70 (42.7) |
| Gestational age at birth | |||
| Number with available data | 326 | 195 | 120 |
| 20 to <32 weeks | 4 (1.2) | 1 (0.5) | 2 (1.7) |
| 32 to <37 weeks | 35 (10.7) | 22 (11.3) | 12 (10.0) |
| ≥37 weeks | 287 (88.0) | 172 (88.2) | 106 (88.3) |
| Median (range) weeks | 39 (27–43) | 39 (27–43) | 39 (31–42) |
| Birth weight | |||
| Number with available data | 205 | 145 | 55 |
| Low birth weightd | 24 (11.7) | 17 (11.7) | 7 (12.7) |
Except where indicated otherwise, values are the number (%). Of 442 singleton infants, 4 (0.9%) were small for gestational age (2 [0.8%] of 252 born to mothers with rheumatic diseases and 2 [1.2%] of 164 born to mothers with Crohn's disease [CD]). CZP = certolizumab pegol.
Includes patients with multiple and unknown indications.
Includes patients who had both rheumatic diseases and CD.
≤2,500 gm.
Major congenital malformations. Adverse events reported for the infants did not show any patterns or clusters of events suggesting a specific safety signal in newborns. Major congenital malformations were reported for 8 live‐born infants (1.7%): 4 infants of mothers with RA, 1 infant of a mother with axial SpA, and 3 infants of mothers with CD (Table 3). Except for the mothers of the infants with cerebral ventricle dilatation, congenital heart disease, and hydronephrosis, all mothers of the infants with major congenital malformations were exposed to CZP at least during the first trimester. The women who had infants with accessory auricle and polydactyly were exposed during all 3 trimesters of their pregnancies (Table 3).
Table 3.
Major congenital malformations in live‐born infants from prospectively reported pregnancies and timing of maternal CZP exposurea
| Infant abnormality | Mother's indication for CZP use | CZP exposure | ||
|---|---|---|---|---|
| First trimester | Second trimester | Third trimester | ||
| Accessory auricle | RA | X | X | X |
| Anal fistula | RA | X | – | – |
| Congenital heart diseaseb | RA | – | X | X |
| Polydactyly | RA | X | X | X |
| Cerebral ventricle dilatationb | Axial SpA | – | – | X |
| Talipes | CD | X | – | – |
| Vesicoureteric reflux | CD | X | X | – |
| Hydronephrosis | CD | – | – | X |
A total of 452 pregnancies resulted in live births. CZP = certolizumab pegol; RA = rheumatoid arthritis; SpA = spondyloarthritis; CD = Crohn's disease.
Premature birth.
No discernible patterns of birth defects among the CZP‐exposed infants were observed. The teratologist review concluded no temporal association with CZP exposure for the case of hydronephrosis, owing to evidence of anomalies on ultrasound prior to the initiation of medication (during the third trimester). The possibility of an association could not be ruled out for the cases of anal fistula, accessory auricle, vesicoureteric reflux, talipes, and congenital heart disease. It was not possible to assess temporality for the cerebral ventricle dilatation. It should be noted that a familial predisposition was present for the infant born with polydactyly: the father, the father's sister, and the paternal grandmother all had the same malformation at birth.
Discussion
The large majority (85.3%) of CZP‐exposed pregnancies evaluated in this analysis resulted in live births, and the rate of major congenital malformations for CZP‐exposed infants was similar to those reported for the general population in the US and Europe 21, 22. Most pregnancies were exposed to CZP at least during the first trimester, when organogenesis primarily occurs; 201 of 452 pregnancies resulting in a live birth were exposed during all 3 trimesters. No discernible trends linking major congenital malformations to the timing of CZP exposure were observed, similar to data from other prospective studies 23, 24, 25. The frequencies of the pregnancy‐associated comorbidities preeclampsia and gestational diabetes were similar to those in the general population 26, 27, and no cases of eclampsia were reported. The frequency of serious infections during pregnancy was consistent with that reported in CZP‐exposed patients 28, 29, 30, 31.
Our review showed that CZP treatment during all 3 trimesters of pregnancy was more common in women with CD (55.6%) than in women with rheumatic diseases (37.1%). Spontaneous disease improvement during pregnancy is less likely in women with IBD than in women with RA and other rheumatic diseases, and many patients with IBD may require treatment throughout pregnancy, thus potentially reducing the risk of adverse outcomes, such as spontaneous miscarriage, premature birth, low birth weight, and small for gestational age 13, 32, 33. Compared to the incidence of low birth weight in the US general population (8.1%) 34, the incidence of low birth weight among singletons in our review (11.7%) was slightly elevated; however, this is consistent with previous reports for anti‐TNF agents 35. Pregnancy outcomes, gestational age, low birth weight and small size at birth, and the rate of congenital malformations were similar for mothers with CD and their infants, compared to mothers with rheumatic diseases and their infants.
Despite the increasing evidence regarding the safety of anti‐TNF therapies, patients and physicians still have concerns regarding their use during pregnancy. The potential risks to the fetus associated with the use of drugs during pregnancy are strongly related to the timing of exposure. During the first trimester, drug exposure can affect organogenesis and increase rates of miscarriage or congenital malformations. Preconception and birth control assessment is therefore important to provide information regarding the safety of anti‐TNF agents before and during the time to conception, as well as during early pregnancy 36. Until December 2017, the European Union (EU) label advised women to take birth control measures for at least 5 months after the last CZP administration 37. As of January 2018, the EU label has been revised to mention that for women planning pregnancy, contraception may be considered, while taking into account their need for treatment 38. There are no contraception recommendations in place in the US label regarding CZP use 39, which likely contributes to the majority of the pregnancy reports in this review originating in North America.
This analysis of prospective pregnancy reports from the UCB Pharma safety database represents the largest published cohort of pregnant women exposed to an anti‐TNF agent for the management of chronic inflammatory diseases. It addresses the need for new and detailed studies of the impact of drug exposure during pregnancy and supports the conclusion that exposure to CZP in utero does not appear to increase the risk of major congenital malformations or fetal loss. The results are also consistent with the growing body of evidence becoming available on anti‐TNF exposure during pregnancy, which indicates no association with an increased risk of adverse neonatal outcomes or congenital malformations 40.
Recent systematic reviews and meta‐analyses have found no link to adverse outcomes for pregnancies with anti‐TNF exposure during the first trimester 17, 40, 41, and more encouraging data are becoming available that support the use of these therapeutics during pregnancy 17, helping to alleviate women's and physicians’ concerns regarding the impact of exposure. Exposure in late pregnancy and the resulting potential risk of postnatal infections 17 are associated with active placental drug transfer from mother to infant, mediated by binding of the Fc region to the neonatal Fc receptor (FcRn) 42. In contrast to other anti‐TNF agents, the CZP molecule lacks the Fc moiety, preventing binding to placental FcRn, thus limiting placental transport 43.
Limitations to our analysis include the fact that, while the UCB safety database is an appropriate tool for standard safety evaluations, the data collection process is designed for pharmacovigilance purposes, rather than being an analysis tool for scientific pregnancy research. In addition, no untreated control group was available for comparison; therefore, a formal assessment of data by statistical analysis was not possible. However, it is acknowledged that for studies with a focus on pregnancy, inclusion of a control group is not always possible 44. Uncontrolled large data sets are still of value, in particular when assessing the frequency of rare events like congenital malformations, since this information is readily available for the general population for comparison.
Another limitation of this analysis is the fact that pregnancy outcome was unknown for a third of the cases. Missing outcomes data is an inherent issue with passive reporting of pregnancy outcomes, despite adequate follow‐up attempts. In addition, the small number of available data for certain birth characteristics, such as low birth weight, impedes drawing any definite conclusions based on this information. Accurate and extrapolatable rates of miscarriage are also hard to estimate from spontaneously reported data, since reports are received at various times throughout gestation, with a report received later on being at lower risk of spontaneous abortion than a report received earlier. Furthermore, data collected via spontaneous reporting systems are affected by reporting bias and often associated with inherent limitations, due to the passive and voluntary nature of the reporting systems. To reduce bias toward the reporting of adverse outcomes associated with retrospective data, we focused our analysis on prospective pregnancy reports to allow a quantitative assessment of outcomes and evaluation of the temporal association between CZP exposure and outcomes.
The results of our analysis represent the most comprehensive published data set for pregnancies exposed to a single anti‐TNF agent to date, with a particular focus on assessment of the temporal association. The fact that 528 CZP‐exposed pregnancies resulted in 459 live births, with 367 women being exposed to CZP during the first trimester, and that only 8 cases of major congenital malformations were reported, confirms and expands the known CZP safety profile. Data from other sources, such as the ongoing MotherToBaby study from the Organization of Teratology Information Specialists 45, will provide further important information to confirm the findings described here. However, these data are reassuring for women of childbearing age affected by chronic inflammatory diseases who need an anti‐TNF agent to control their condition and wish to become pregnant or are pregnant during CZP treatment.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Clowse had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Cooney, Shaughnessy, Vanderkelen.
Acquisition of data
Cooney, Shaughnessy, Vanderkelen.
Analysis and interpretation of data
Clowse, Scheuerle, Chambers, Afzali, Kimball, Cush, Cooney, Shaughnessy, Vanderkelen, Förger.
Role of the study sponsor
UCB Pharma sponsored the analysis and the development of this manuscript and reviewed the text to ensure that from UCB Pharma perspective, the data presented in the publication are scientifically, technically, and medically supportable, that they do not contain any information that has the potential to damage the intellectual property of UCB Pharma, and that the publication complies with applicable laws, regulations, guidelines, and good industry practice. The authors approved the final version to be published after critically revising the manuscript/publication for important intellectual content. Publication of this article was contingent upon approval by UCB Pharma.
Acknowledgments
The authors thank the patients and health care providers who reported data included in this analysis. The authors acknowledge Marie Teil, Cécile Ecoffet, and Simone E. Auteri (UCB Pharma, Brussels, Belgium) for publication coordination, and Julia Bárdos, PhD (Costello Medical, Cambridge, UK) for writing and editorial assistance in preparing the manuscript for publication, based on the authors’ input and direction.
Supported by UCB Pharma.
Dr. Clowse has received consulting fees, speaking fees, and/or honoraria from UCB Pharma (more than $10,000) and research grants from Pfizer and Janssen. Dr. Scheuerle is a contractor with UCB Pharma, INC Research, and Genentech. Dr. Chambers has received research grants from AbbVie, Amgen, Bristol‐Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Pfizer, Hoffmann‐La Roche/Genentech, Genzyme Sanofi‐Aventis, Seqirus, Takeda, and UCB Pharma. Dr. Afzali has received consulting fees, speaking fees, and/or honoraria from AbbVie, IBD Horizons (less than $10,000 each), UCB Pharma, and Takeda (more than $10,000 each) and research grants from UCB Pharma. Dr. Kimball has received consulting fees, speaking fees, and/or honoraria from UCB Pharma, Dermira, Janssen (less than $10,000 each), and AbbVie (more than $10,000). Dr. Cush has received consulting fees, speaking fees, and/or honoraria from AbbVie, UCB Pharma, Bristol‐Myers Squibb, Celgene (less than $10,000 each), Novartis, Amgen, Genentech, Eli Lilly and Company, and Horizon (more than $10,000 each) and research support from Pfizer, Janssen, AbbVie, Celgene, Novartis, AstraZeneca, and Genentech. Dr. Förger has received speaking fees from Mepha, Roche, and UCB Pharma (less than $10,000 each) and research grants from UCB Pharma.
References
- 1. Kavanaugh A, Cush JJ, Ahmed MS, Bermas BL, Chakravarty E, Chambers C, et al. Proceedings from the American College of Rheumatology Reproductive Health Summit: the management of fertility, pregnancy, and lactation in women with autoimmune and systemic inflammatory diseases. Arthritis Care Res (Hoboken) 2015;67:313–25. [DOI] [PubMed] [Google Scholar]
- 2. Chakravarty E, Clowse ME, Pushparajah DS, Mertens S, Gordon C. Family planning and pregnancy issues for women with systemic inflammatory diseases: patient and physician perspectives. BMJ Open 2014;4:e004081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van den Brandt S, Zbinden A, Baeten D, Villiger PM, Ostensen M, Forger F. Risk factors for flare and treatment of disease flares during pregnancy in rheumatoid arthritis and axial spondyloarthritis patients. Arthritis Res Ther 2017;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedersen N, Bortoli A, Duricova D, D Inca R, Panelli MR, Gisbert JP, et al. The course of inflammatory bowel disease during pregnancy and postpartum: a prospective European ECCO‐EpiCom study of 209 pregnant women. Aliment Pharmacol Ther 2013;38:501–12. [DOI] [PubMed] [Google Scholar]
- 5. Boyd HA, Basit S, Harpsoe MC, Wohlfahrt J, Jess T. Inflammatory bowel disease and risk of adverse pregnancy outcomes. PLoS One 2015;10:e0129567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McConnell RA, Mahadevan U. Pregnancy and the patient with inflammatory bowel disease: fertility, treatment, delivery, and complications. Gastroenterol Clin North Am 2016;45:285–301. [DOI] [PubMed] [Google Scholar]
- 7. Jakobsson GL, Stephansson O, Askling J, Jacobsson LT. Pregnancy outcomes in patients with ankylosing spondylitis: a nationwide register study. Ann Rheum Dis 2016;75:1838–42. [DOI] [PubMed] [Google Scholar]
- 8. Ma KK, Nelson JL, Guthrie KA, Dugowson CE, Gammill HS. Adverse pregnancy outcomes and risk of subsequent rheumatoid arthritis. Arthritis Rheumatol 2014;66:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Norgaard M, Larsson H, Pedersen L, Granath F, Askling J, Kieler H, et al. Rheumatoid arthritis and birth outcomes: a Danish and Swedish nationwide prevalence study. J Intern Med 2010;268:329–37. [DOI] [PubMed] [Google Scholar]
- 10. De Man YA, Dolhain RJ, van de Geijn FE, Willemsen SP, Hazes JM. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum 2008;59:1241–8. [DOI] [PubMed] [Google Scholar]
- 11. Mouyis MA, Thornton CC, Williams D, Giles IP. Pregnancy outcomes in patients with psoriatic arthritis. J Rheumatol 2017;44:128–9. [DOI] [PubMed] [Google Scholar]
- 12. Polachek A, Li S, Polachek IS, Chandran V, Gladman D. Psoriatic arthritis disease activity during pregnancy and the first‐year postpartum. Semin Arthritis Rheum 2017;46:740–5. [DOI] [PubMed] [Google Scholar]
- 13. Mahadevan U, McConnell RA, Chambers CD. Drug safety and risk of adverse outcomes for pregnant patients with inflammatory bowel disease. Gastroenterology 2017;152:451–62.e2. [DOI] [PubMed] [Google Scholar]
- 14. Blehar MC, Spong C, Grady C, Goldkind SF, Sahin L, Clayton JA. Enrolling pregnant women: issues in clinical research. Womens Health Issues 2013;23:e39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flint J, Panchal S, Hurrell A, van de Venne M, Gayed M, Schreiber K, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding‐Part I: standard and biologic disease modifying anti‐rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2016;55:1693–7. [DOI] [PubMed] [Google Scholar]
- 16. Ostensen M, Forger F. Management of RA medications in pregnant patients. Nat Rev Rheumatol 2009;5:382–90. [DOI] [PubMed] [Google Scholar]
- 17. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, Fischer‐Betz R, Elefant E, Chambers C, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795–810. [DOI] [PubMed] [Google Scholar]
- 18. Clowse ME, Wolf DC, Forger F, Cush JJ, Golembesky A, Shaughnessy L, et al. Pregnancy outcomes in subjects exposed to certolizumab pegol. J Rheumatol 2015;42:2270–8. [DOI] [PubMed] [Google Scholar]
- 19. Medicines and Healthcare products Regulatory Agency . Best practice in reporting of individual case safety reports (ICSRs). London: Medicines and Healthcare products Regulatory Agency; 2011. [Google Scholar]
- 20. National Institutes of Health Office of Communications . About pregnancy. January 2017. URL: https://www.nichd.nih.gov/health/topics/pregnancy/conditioninfo.
- 21. Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep 2012;60:1–21. [PubMed] [Google Scholar]
- 22. European surveillance of congenital anomalies (EUROCAT) web site. URL: http://www.eurocat-network.eu/homepage.
- 23. Hoxha A, Calligaro A, Di Poi E, Peccatori S, Favaro M, Del Ross T, et al. Pregnancy and foetal outcomes following anti‐tumor necrosis factor α therapy: a prospective multicentre study. Joint Bone Spine 2017;84:169–73. [DOI] [PubMed] [Google Scholar]
- 24. Mahadevan U, Martin CF, Sandler RS, Kane SV, Dubinsky M, Lewis JD, et al. 865 PIANO: a 1000 patient prospective registry of pregnancy outcomes in women with IBD exposed to immunomodulators and biologic therapy. Gastroenterology 2012;142:S–149. [Google Scholar]
- 25. Weber‐Schoendorfer C, Oppermann M, Wacker E, Bernard N, Beghin D, Cuppers‐Maarschalkerweerd B, et al. Pregnancy outcome after TNF‐α inhibitor therapy during the first trimester: a prospective multicentre cohort study. Br J Clin Pharmacol 2015;80:727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- 27. DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev Chronic Dis 2014;11:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bykerk VP, Cush J, Winthrop K, Calabrese L, Lortholary O, de Longueville M, et al. Update on the safety profile of certolizumab pegol in rheumatoid arthritis: an integrated analysis from clinical trials. Ann Rheum Dis 2015;74:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loftus EV Jr, Colombel JF, Schreiber S, Randall CW, Regueiro M, Ali T, et al. Safety of long‐term treatment with certolizumab pegol in patients with Crohn's disease, based on a pooled analysis of data from clinical trials. Clin Gastroenterol Hepatol 2016;14:1753–62. [DOI] [PubMed] [Google Scholar]
- 30. Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, et al. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24‐week results of a Phase 3 double‐blind randomised placebo‐controlled study (RAPID‐PsA). Ann Rheum Dis 2014;73:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van der Heijde D, Dougados M, Landewe R, Sieper J, Maksymowych WP, Rudwaleit M, et al. Sustained efficacy, safety and patient‐reported outcomes of certolizumab pegol in axial spondyloarthritis: 4‐year outcomes from RAPID‐axSpA. Rheumatology (Oxford) 2017;56:1498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nguyen GC, Seow CH, Maxwell C, Huang V, Leung Y, Jones J, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016;150:734–57.e1. [DOI] [PubMed] [Google Scholar]
- 33. Mahadevan U, Wolf DC, Dubinsky M, Cortot A, Lee SD, Siegel CA, et al. Placental transfer of anti‐tumor necrosis factor agents in pregnant patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11:286–92; quiz e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep 2017;66:1. [PubMed] [Google Scholar]
- 35. Komaki F, Komaki Y, Micic D, Ido A, Sakuraba A. Outcome of pregnancy and neonatal complications with anti‐tumor necrosis factor‐α use in females with immune mediated diseases; a systematic review and meta‐analysis. J Autoimmun 2017;76:38–52. [DOI] [PubMed] [Google Scholar]
- 36. Ostensen M. Contraception and pregnancy counselling in rheumatoid arthritis. Curr Opin Rheumatol 2014;26:302–7. [DOI] [PubMed] [Google Scholar]
- 37. Cimzia summary of product characteristics. URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001037/WC500069763.pdf.
- 38. Electronic Medicines Compendium . Cimzia. URL: http://www.medicines.org.uk/emc/medicine/32367.
- 39. Cimzia prescribing information. Smyrna (GA): UCB; 2016. [Google Scholar]
- 40. Calligaro A, Hoxha A, Ruffatti A, Punzi L. Are biological drugs safe in pregnancy? Reumatismo 2015;66:304–17. [DOI] [PubMed] [Google Scholar]
- 41. Komaki Y, Yamada A, Komaki F, Kudaravalli P, Micic D, Ido A, et al. Efficacy, safety and pharmacokinetics of biosimilars of anti‐tumor necrosis factor‐α agents in rheumatic diseases: a systematic review and meta‐analysis. J Autoimmun 2017;79:4–16. [DOI] [PubMed] [Google Scholar]
- 42. Saji F, Samejima Y, Kamiura S, Koyama M. Dynamics of immunoglobulins at the feto‐maternal interface. Rev Reprod 1999;4:81–9. [DOI] [PubMed] [Google Scholar]
- 43. Porter C, Armstrong‐Fisher S, Kopotsha T, Smith B, Baker T, Kevorkian L, et al. Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): consequences for FcRn‐mediated in vitro transcytosis and ex vivo human placental transfer. J Reprod Immunol 2016;116:7–12. [DOI] [PubMed] [Google Scholar]
- 44. European Medicines Agency/Committee for Medicinal Products for Human Use . Guidelines on risk assessment of medicinal products on human reproduction and lactation: from data to labelling. URL: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003307.pdf.
- 45. MotherToBaby: medications & more during pregnancy & breastfeeding web site. URL: https://mothertobaby.org/.


