Abstract
Patients with peritoneal carcinomatosis (PC) from colorectal origin may undergo cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) as a curative approach. One major prognostic factor that affects survival is completeness of cytoreduction. Molecular Fluorescence Guided Surgery (MFGS) is a novel intraoperative imaging technique that may improve tumor identification in the future, potentially preventing over‐ and under‐treatment in these patients. This narrative review outlines a chronological overview of MFGS development in patients with PC of colorectal origin.
Keywords: colorectal cancer, molecular fluorescence‐guided surgery, peritoneal carcinomatosis, review
1. INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers worldwide, with an incidence of 40 patients per 100 000 population and a mortality rate of 15 per 100 000 persons.1, 2 Of these patients, 8‐25% develop peritoneal carcinomatosis (PC).3, 4, 5, 6 Over the past decades, the treatment of PC of colorectal origin has evolved considerably, from palliative care toward a more successful treatment approach with curative intent.7, 8 In particular, the introduction of cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has contributed significantly to this change.9, 10 After surgical cytoreduction of all macroscopic tumor tissue, the abdominal cavity is perfused with heated chemotherapy in order to eliminate remaining microscopic disease. Up to date, only one randomized clinical trial has been performed studying patients with PC of colorectal origin. A median overall survival of 22 months was seen for patients after undergoing CRS in combination with HIPEC, compared to 13 months for patients receiving only systemic chemotherapy with or without palliative surgery.11, 12 The authors report a 5‐years survival of 43% for patients in whom all macroscopic tumor was removed, compared to 0% for patients in whom residual lesions of more than 2.5 mm were left behind.11 These findings emphasize the importance of patient selection and a macroscopically complete cytoreduction, mainly because incomplete cytoreduction followed by HIPEC does not contribute to a prolonged survival, but potentially does introduce a high risk of postoperative complications, an extensive rehabilitation period and subsequently decreased quality of life.12, 13, 14, 15
Although the technical quality of the complete CRS/HIPEC procedure has improved, still up to 88% of the patients undergoing CRS and HIPEC for PC of CRC develop recurrent disease within 2 years.16 Currently, many imaging modalities are available for preoperative staging, such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET) scans. Unfortunately, all of these imaging modalities are insufficient for the preoperative assessment of tumor load, or determination of a preoperative Peritoneal Cancer Index (PCI), the most important staging system in PC. CT, MRI, and fluorodeoxyglucose (FDG‐) PET scans have a poor sensitivity and specificity to estimate PCI by detection of individual tumor deposits, due to the limited spatial resolution.17 For example, the detection of individual peritoneal deposits using a CT‐scan varies from 9.1 to 24.3% for tumor sizes <1 cm, to up to 59.3‐66.7% for tumor size of over 5 cm.18 These results are in accordance with other previous studies.19, 20, 21 Current hybrid PET/CT scanners have a limited spatial resolution of 5‐8 mm,22 whereas MRI seems to be more promising in detecting peritoneal lesions.23
For intraoperative differentiation between benign and malignant lesions, surgeons currently depend on visual and tactile inspection only. Unfortunately, the human eye and palpation are not competent enough to detect molecular changes in intra‐abdominal lesions that have the same color and physical properties, or to distinguish tumor lesions from benign scar tissue originating from previous surgery. Today, to the best of our knowledge, no intraoperative imaging modalities provided by the more classical modalities like PET, are available to assist in the real‐time identification of peritoneal cancer deposits, loco‐regional metastases, and tumor‐positive resection margins.
Considering the high tumor recurrence rates after the CRS and HIPEC procedure, there is a clear need for an imaging modality that can aid the oncological surgeon in the differentiation between tumor and benign tissue intraoperatively. In recent years, optical molecular imaging using tumor‐targeted fluorescence tracers has emerged as a promising imaging technique for real‐time guidance in oncological surgery.24, 25, 26 This technique can be applied intraoperatively to serve as a “red‐flag” imaging technique to assist in optimal tumor identification. Improved detection of tumor tissue could not only help attain a more complete cytoreduction, but might also facilitate tailored surgery, avoiding unnecessary resections of benign lesions and organs.
This narrative review explains the principles of intraoperative optical molecular imaging and provides a chronological overview of the development of Molecular Fluorescence Guided Surgery (MFGS) in patients with PC of colorectal origin.
2. PRINCIPLES OF INTRAOPERATIVE OPTICAL MOLECULAR IMAGING
In colorectal surgery, as in surgical oncology in general, radical surgery and tumor‐free resection margins are essential for optimizing patient prognosis. Optical molecular imaging using fluorescence imaging agents can provide real‐time intraoperative feedback with high resolution, that is in concordance with the natural surgical field of view of a surgeon and based on the molecular characteristics of the tissue (Figure 1). The technique makes use of non‐ionizing imaging agents and can be implemented relatively easily in the current surgical workflow.
Figure 1.
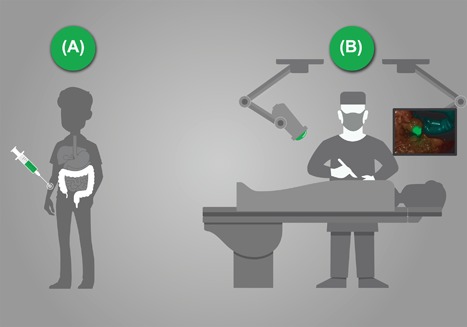
Concept of molecular fluorescence guided surgery (MFGS). Prior to surgery a fluorescent target tracer is injected intravenously (A). During the operation the surgeon will receive real‐time feedback by a molecular fluorescence camera in the detection tumor tissue (B). Unpublished figure from previously published study Harlaar et al107
Over the past decades, there has been an increased interest in the clinical application of optical molecular imaging using fluorescence imaging agents. Fluorescence occurs when a photon or fluorescent dye absorbs light at a certain wavelength, subsequently triggering the release of a photon with a longer wavelength.27 The quality of fluorescence imaging is influenced by different factors such as changes in photon directions (ie, scattering) and absorption of photons by the tissue. Multiple tissue components play an important role in fluorophore absorption, with the most relevant being hemoglobin, water, and lipids. As the scattering and absorption properties of tissue are lower in light with longer wavelengths, the near‐infrared (NIR) light spectrum (700‐900 nm) is considered the optimal clinical diagnostic window for fluorescence imaging.28 These characteristics result in deeper penetration depths of up to one to three centimeters that can be obtained in the NIR light spectrum, leading to higher signal‐to‐background (SBR) ratios compared to the visible light spectrum (ie, red‐green‐blue white‐light, 380‐700 nm).29, 30
NIR fluorescence light is invisible to the human eye and therefore special imaging devices are required to visualize fluorescence during surgery. In general, these camera systems are equipped with two different light sources: a white‐light source and a NIR fluorescence light source. Due to the use of a dichroic mirror and specific filters installed in the camera system itself, the visible light derived from the tissue can be separated from NIR fluorescence light, which enables simultaneous imaging of both visible and NIR fluorescence light. Next to that, an overlay of fluorescence signals can be projected on the “normal” white‐light images by use of computer software.27 In the operating theatre, all three images can be displayed on monitors at the same time, providing real‐time imaging related to the natural surgical field of view (Figure 2). Currently, there are several different intraoperative NIR fluorescence imaging devices available for research and clinical use.31, 32, 33, 34, 35, 36, 37, 38
Figure 2.
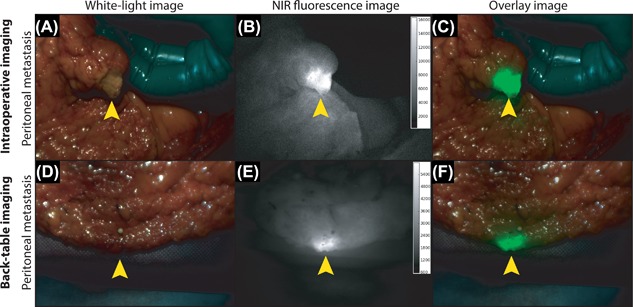
Intraoperative imaging with white‐light, NIR fluorescence and the overlay of both. Intraoperative imaging of a patient with PC of colorectal origin following intravenous administration of 4.5 mg of the fluorescent tracer bevacizumab‐800CW targeting VEGF‐A. A white‐light image (A), NIR fluorescence image (B), and overlay of both (C) clearly show fluorescent signals at the location of a clinically suspect peritoneal lesion. Back‐table imaging directly after surgery of a different peritoneal lesion of the same patient is depicted (D‐F). Both peritoneal lesions proved to be tumor metastasis upon final histopathology. Unpublished figures from previously published study Harlaar et al.107
Fluorescence signals in tissue arise by either an endogenous tissue component (ie, autofluorescence), or an intravenously administered exogenous optical contrast agent. At present, various types of optical contrast agents are available enabling intraoperative imaging, which can roughly be divided into non‐targeted and targeted imaging agents.
The effect of non‐targeted imaging agents is mainly based on vascularization and perfusion (ie, also the so‐called Enhanced Permeability and Retention (EPR) effect), whereas targeted imaging agents specifically bind to a receptor or protein that is present in a tumor cell. Due to genetic alterations that occur in cancer development, various receptors and proteins become upregulated, which can potentially be used as targets for imaging purposes.39 Prior to developing such targeted imaging agents, it is essential to identify which genes or proteins become upregulated for each specific tumor type.40, 41
3. UPREGULATED GENES AND PROTEINS RELATED TO CRC
The potential application of targeted imaging agents for intraoperative tumor visualization is dependent on the expression levels of biomarkers. A biomarker is a specific component present on or secreted by the tumor cell itself.
Most colorectal cancers are thought to develop via the “adenoma‐to‐carcinoma sequence,” arising from normal cells through the stepwise asset of different genetic alterations.42, 43 In these expressed genes different functional categories can be identified: genes related to proliferation and metabolic rates, to cell adhesion and communication, to transcription and mitosis regulation, or to apoptosis.44, 45 Knowing which biomarkers are encoded by which genes is important when searching for which target to develop a fluorescence imaging agent for.
Cardoso et al44 presented a list of 128 different genes that were found to be upregulated in CRC compared to normal colorectal tissue. Since protein expression is not always synchronously upregulated, not all of these genes result in overexpression of the related proteins or receptors. Previously, an extensive literature search has been performed on this specific list of genes, in order to identify which genes gave an upregulation of the related proteins or receptors as confirmed by immunohistochemical analysis. 46 As a result, 29 targets were identified, that could be used for imaging purposes during CRC surgery.
4. TARGET SELECTION CRITERIA (TASC)
To select the most optimal target for imaging purposes from this large set of upregulated biomarkers, the TArget Selection Criteria (TASC) scoring system was developed.46 The aim of the TASC was to improve the selection of suitable biomarkers for tumor‐targeted imaging of all types of cancer. Seven of the most relevant target characteristics were identified based on literature, that each could be scored with 0‐6 points. The following characteristics were identified by which a biomarker is validated: i) extracellular biomarker localization—either on the cell membrane or in close proximity of the tumor cell; ii) expression pattern; iii) tumor‐to‐healthy tissue ratio (T/N); iv) percentage of positive tumors; v) reported successful use of the biomarker in in vivo imaging studies; vi) enzymatic activity; and vii) internalization.46 Based on extensive testing of the TASC on a variety of biomarkers, cut‐off values were determined for target selection. A total score of 18 or more indicates that a biomarker can be considered a potential candidate for tumor‐targeted imaging.
As mentioned before, 29 targets were identified that may be used as potential targets for intraoperative imaging of CRC.46 Using the TASC‐scoring system, six biomarkers were considered the most promising: Epithelial Cell Adhesion Molecule (EpCAM), CXC Chemokine Receptor 4 (CXCR4), Mucin 1 (Muc1), Matrix MetalloProteinases (MMPs), Epidermal Growth Factor Receptor (EGFR), and Carcino‐Embryonic Antigen (CEA). Although the Vascular Endothelial Growth Factor‐A (VEGF‐A) scored a total of 17 points, it was still considered a suitable potential target as well, given the extensive experience there already is in VEGF‐A targeted imaging. For the clinical translation of these seven suitable biomarkers, specific fluorescence imaging agents need to be available to facilitate MFGS of CRC and PC of colorectal origin.
5. FLUORESCENCE IMAGING AGENTS
As stated before, fluorescence imaging agents or probes that can be used for MFGS can roughly be divided into two categories: non‐targeted fluorescent probes and targeted fluorescent probes. The main difference between these two categories is based on their mechanism of action (MOA).
5.1. Non‐targeted fluorescent probes
Non‐targeted fluorescent probes accumulate “passively” in solid tumors due to physiological properties such as increased angiogenesis, pressure differences, and high metabolic activity (Figure 3A). It is commonly known that the majority of solid tumor cells stimulate angiogenesis and therefore are highly vascularized. This feature combined with the lack of efficient lymphatic drainage results in more accumulation in tumor tissue compared to normal surrounding tissue, thereby enhancing contrast and enabling a differentiation between the tumor and surrounding tissue. This phenomenon is also known as the enhanced permeability and retention (EPR) effect.
Figure 3.
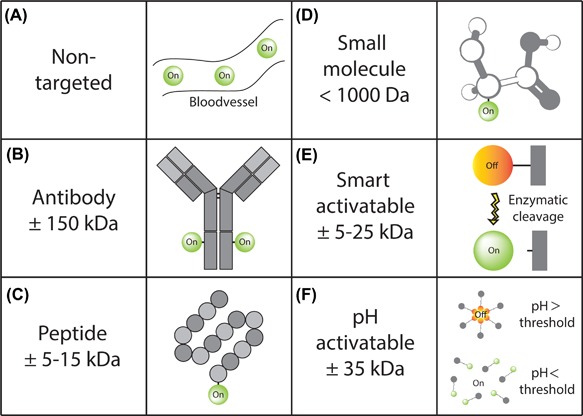
Fluorescence imaging probes. Overview of fluorescent imaging probes with different mechanisms of action. The effect of non‐targeted fluorescent probes is based on tissue distribution by perfusion (A), whereas antibody‐based (B), peptide‐based (C), and small molecule‐ based (D) imaging enables targeted fluorescence imaging through binding to specific receptors or proteins overexpressed by the tumor. Smart activatable fluorescent probes are activated upon cleavage by specific enzymes or proteases secreted by the tumor (E), whereas pH activated probes becomes fluorescent through a change in molecular structure due to the characteristic acidotic environment of a tumor (F)
Many non‐targeted fluorescent probes have already been used in humans to enhance contrast during surgery in a variety of different indications, such as for example fluorescein for retinal fluorescein angiography, indocyanine green (ICG) for liver perfusion47, 48, 49 and lymph node detection,50, 51 or methylene blue for sentinel lymph node detection in breast cancer patients.52, 53
ICG is the most commonly known fluorescent probe, which was already approved by the Food and Drug Administration (FDA) back in 1959. ICG has several advantages over to fluorescein as it only fluoresces in the NIR light spectrum (instead of the visible light spectrum) and therefore is less influenced by tissue optical properties such as scattering and absorption. As ICG binds to plasma proteins, it has a negligible toxicity and is excreted rapidly by the liver into the bile, with a plasma half‐life of only 3‐4 min.54, 55 These features make ICG a very attractive contrast agent for assessment of macro‐ and micro‐circulatory status of different organs based on its intravascular distribution.56
Ever since its first clinical application in hepatology for liver condition monitoring in 1957,57 it has been widely applied and studied to visualize perfusion in ophthalmology for identification of retinal blood vessels, in cardiac bypass surgery for evaluation of anastomoses and for monitoring cardiac output.58, 59, 60, 61, 62 More recent studies have reported the potential application of ICG for intraoperative fluorescence angiography in a broad range of other indications such as neuro‐, coronary‐, reconstructive‐, liver‐, and vascular surgery.59, 63, 64, 65, 66, 67, 68, 69, 70
The first potential application of ICG‐based fluorescence imaging in patients with peritoneal metastases of colorectal origin was demonstrated in 2016.71 In this study, peritoneal metastases from non‐mucinous adenocarcinoma were accurately identified following the intravenous administration of free ICG during surgery, leading to an adjustment in clinical decision making in 29% of patients. However, the benefit was minimal in patients with mucinous adenocarcinoma. Despite the positive results demonstrated in this study, the main disadvantage still lies in the fact it is not tumor‐specific and therefore leads to a low sensitivity and specificity.
5.2. Targeted fluorescent probes
Due to the low sensitivity and specificity of non‐targeted fluorescence probes, its application in surgical oncology is still limited. Therefore, to increase contrast, this resulted in a shift toward the development and clinical translation of targeted optical imaging agents enhancing surgical vision based on the molecular characteristics of cancer cells.
The MOA of targeted fluorescent probes is based on the concept of a carrier molecule that is conjugated to a fluorescent dye, specifically binding to a certain tumor target. Carrier molecules can either be monoclonal antibodies, (small) peptides, small molecules, or other molecules that specifically target certain cell surface markers that become overexpressed due to genetic variances that occur in every tumor (Figure 3B).72 Moreover, the increased metabolic activity that characterizes certain tumor types can be used as a target.40
Besides a suitable carrier molecule, the fluorescent dye itself also plays an important role. The development of new fluorescence probes is challenging since each agent needs separate regulatory approval, which is an expensive and time‐consuming process.73 As mentioned before, fluorescent dyes that emit light in the NIR light spectrum provide several advantages over dyes that emit light in the visible light spectrum. Although there is a wide variety of fluorescent dyes available for conjugation to carrier molecules, the most preclinical and clinical experience has been obtained with the NIR fluorescent dye IRDye800CW, developed by LI‐COR Biosciences Inc. (Lincoln, NE). The IRDye800CW has a peak emission wavelength at 794 nm and is ideal for protein and antibody labeling, as conjugation to a carrier molecule is relatively easy and extensive toxicity studies have been performed.74
The increasing clinical application of therapeutic monoclonal antibodies specifically targeting certain biomarkers of cancer is an interesting development in the perspective of optical molecular imaging. Targeting certain tumor‐specific receptors with fluorescently labeled antibodies seems to have great potential for visualization of cancer during interventions, also in CRC.72, 75, 76, 77, 78 Multiple targeted probes have already been testes successfully in several preclinical studies.75, 77, 78, 79, 80, 81, 82, 83, 84
The first in‐human proof‐or‐principle of targeted optical molecular imaging using a fluorescent probe was provided by van Dam et al24 in 2011, demonstrating the potential of MFGS in patients with PC originating from ovarian cancer using the fluorescent tracer folate‐FITC, targeting the folate receptor α. Ever since, targeted optical imaging has been applied for many different indications.
6. POTENTIAL FLUORESCENCE IMAGING AGENTS FOR DETECTION OF COLORECTAL CANCER
As mentioned before, using the TASC scoring system, seven potential targets for optical molecular imaging of PC of colorectal origin have been identified: CXCR4, EpCAM, EGFR, CEA, Muc1, MMPs, and VEGF‐A.46 The specifics of these proteins and receptors are summarized in Table 1.85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109 Several fluorescent imaging probes targeting these biomarkers have already been investigated in humans in a broad variety of indications.
Table 1.
Potential targets for optical molecular imaging in PC of colorectal origin using the TASC scoring system
| Target | Name | Location | Function | Over‐expression in CRC | Carrier molecule clinically available | GMP‐labeled fluorescent tracer | Clinical trials in humans |
|---|---|---|---|---|---|---|---|
| CXCR4 | Chemokine Receptor 4 | Cell surface | Homing of hematopoietic stem cells to the bone‐marrow | ±70%85 | AMD3100 (molecule)86 SDF‐1a (peptide)86 12G5 (mAb)86 | ‐ | ‐ |
| EpCAM | Epithelial Cell Adhesion Molecule | Cell surface | Cell adhesion | >80%87, 88 | Edrecolomab Catumaxomab | 323/A3‐800CW89 | ‐ |
| EGFR | Epidermal Growth Factor Receptor | Cell surface | Cell proliferation, differentiation, adhesion and migration | ±80%90, 91 | Cetuximab Panitumumab | Cetuximab‐800CW Panitumumab‐800CW | NCT0313484692 |
| CEA | Carcino‐embryogenic antigen | Cell surface | Cell adhesion | >90%93, 94, 95 | Arcitumomab | SGM‐10196 | ‐ |
| Muc1 | Mucin‐1 | Cell surface | Forming protective mucous barriers on epithelial surfaces, intracellular signaling (cell adhesion and anti‐adhesion) | ±50%97, 98 | Muc1‐targeting peptide C595 (mAb) Bispecific anti‐Muc1 antibody | ‐ | ‐ |
| MMP | Matrix Metalloproteinases | Tumor micro‐environment | Degrading proteins in extracellular matrix | 30‐95% depending on the type97, 99, 100, 101, 102 | ‐ | ‐ | ‐ |
| VEGF‐A | Vascular Endothelial Growth Factor‐A | Tumor micro‐environment | Angiogenesis | Up to 96%103, 104 | Bevacizumab | Bevacizumab‐800CW105 | NCT02113202 NCT01972373 NCT02583568 NCT02975219 NCT02743975 NCT01691391 106, 107, 108, 109 |
For example, the NIR fluorescent tracer cetuximab‐800CW targeting EGFR has been applied in humans for surgical navigation in head‐and‐neck squamous cell carcinoma.92 Moreover, cetuximab‐800CW is being used in a phase‐I clinical trial in the University Medical Center Groningen in patients with head and neck squamous cell carcinoma (NCT03134846).
Besides, the NIR fluorescent tracer bevacizumab‐800CW targeting VEGF‐A has been applied for detection of a variety of different tumor types, among which locally advanced rectal cancer (NCT01972373), pancreatic cancer (NCT02743975), breast cancer109 (NCT02583568), and esophageal cancer.108 The feasibility of MFGS using bevacizumab‐800CW is also being investigated for intraoperative guidance in benign diseases such as endometriosis (NCT02975219) or for endoscopic detection of familial adenomatous polyposis (NCT02113202).
In CRC and specifically peritoneal metastases of colorectal origin, so far two phase‐I feasibility studies have been performed in humans.
7. FEASABILITY STUDIES IN PC OF COLORECTAL ORIGIN
In 2016, Harlaar et al107 used the NIR fluorescent tracer bevacizumab‐RDye800CW targeting VEGF‐A for MFGS in seven patients with PC from CRC origin, that were scheduled to undergo CRS and HIPEC. Intravenous administration of bevacizumab‐800CW 3 days prior to surgery proved to be safe, as no (serious) adverse events that were related to tracer administration occurred in any of the patients. Fluorescence signals were observed in all patients during surgery. Additional tumor tissue that had not been identified by the surgeons using only visual and tactile inspection was detected in two patients using fluorescence imaging. The fresh surgical specimens were imaged back‐table at the operating theatre. A total of 80 peritoneal areas were imaged using the intraoperative camera system and analyzed by a pathologist. All 29 resected, but non‐fluorescent areas proved to be benign on final histopathology, thus potentially indicating a sensitivity of 100%. In 27 out of 57 fluorescent areas in the fresh surgical specimen, tumor tissue was identified. Although the authors state that their study was not powered to investigate the sensitivity and specificity, the results are very promising. In conclusion, in this study MFGS using bevacizumab‐800CW was safe and feasible and could potentially improve CRS and patient selection.
The second feasibility study was performed in 2018 by Boogerd et al,96 in which SGM‐101, a fluorescent anti‐CEA monoclonal antibody, was administered intravenously 2–4 days before surgery, to investigate the feasibility of MFGS in CRC and PC of colorectal origin. Patients with PC of colorectal origin that were scheduled for open surgical removal were included. First, a dose‐finding study was performed in the first nine patients. Subsequently, the most optimal dose of SGM‐101 was investigated in another 17 patients. SGM‐101 showed no treatment‐related (serious) adverse events. However, a total of eight possibly related mild adverse events occurred throughout the study. Using MFGS, in six patients a total of 19 additional peritoneal lesions were identified as potentially tumor‐positive, and therefore treatment strategies were changed. The authors report a sensitivity of 98% and a specificity of 62%.
Interestingly, although both studies used different fluorescent tracers, more or less the same conclusions were drawn. Most importantly, both bevacizumab‐800CW and SGM‐101 were deemed safe in combination with MFGS. Moreover, it appeared that with both fluorescent tracers, a very high sensitivity could be obtained. If these results are validated in a larger patient cohort and indeed clinically suspect, but non‐fluorescent lesions turn out to be benign, non‐fluorescent lesions may be left in situ in the future and subsequently decrease morbidity. Interestingly, this might also imply that currently visual and tactile inspection‐based surgery leads to unnecessary resections when compared to MFGS. The majority of complications and revalidation time is probably related to the extent of the cytoreduction itself. This might also improve the current morbidity of 22‐34% and mortality of 0.8‐4.1%.110, 111, 112, 113, 114, 115
The specificity in these two feasibility studies appears to be relatively low, with a substantial amount of false positive lesions when applying intraoperative fluorescence imaging. This might be due to technical limitations of the fluorescence camera system that still need to be improved, such as the multispectral substration techniques. Currently, quantification of fluorescence with most of the present generation of clinically approved fluorescence camera systems is still limited, making the interpretation of fluorescence signals subjective. If a threshold could be set to give the surgeon a “yes” or “no” answer to the question whether a peritoneal lesion is tumor‐positive with a certain sensitivity and specificity, this could potentially improve interpretation of fluorescence signals. Last, for some tracers, the optimal dose might still need further optimization.107
Additional research and studies need to be performed to investigate novel fluorescence imaging agents in humans for MFGS of PC of colorectal origin. Theoretically, multiple fluorescence imaging agents can be intravenously administered to the same patients simultaneously, a so‐called “tracer cocktail,” in order to improve sensitivity and specificity. Therefore, novel fluorescence imaging agents need to be validated in a standardized way, with the emphasis on the determination of the safety, feasibility, optimal agent dose, and optimal timing for surgical intervention in phase‐I feasibility studies.
8. FUTURE PERSPECTIVES
8.1. Target selection for MFGS
Currently, there are many carrier molecules that seem promising for potential validation in phase‐I feasibility studies according to the TASC scoring system.46 Additionally, new strategies have been developed recently to identity biomarkers that are upregulated in cancer development, such as functional genomic mRNA (FGM) profiling.116 This method corrects expression data of numerous genes for relevant non‐genetic variables. It is likely that in the near future new promising targets will be identified by this gene expression analysis, that may be used as targets, providing new possibilities for imaging of PC of colorectal origin.
8.2. Novel fluorescence imaging probes
Next to the validation of potential targets for imaging, novel fluorescent probes are being developed.72, 117 Different types of carrier molecules have different pharmacokinetics. The substantial molecular weight of monoclonal antibodies (generally ± 150 kDa, Figure 3B) results in a relatively long blood circulation time of several days up to weeks. Although there is extensive experience with the use of monoclonal antibodies, even smaller molecules may provide favorable pharmacokinetic properties, such as nanobodies.118 A faster clearance from background tissue results in sufficient signal‐to‐background ratios that occur within a much shorter period of time. Therefore, peptides or small molecules might be logistically favorable compared to antibodies for MFGS (Figures 3C and D).118 On the other hand, smaller molecules are in general more difficult to conjugate to a fluorescence dye, as even small structural changes can influence pharmacokinetics and binding efficacy significantly.118, 119
Another subgroup of imaging probes has come forward in recent years: targeted smart‐activatable probes (Figure 3E).119 The working mechanism of these probes is based on the principle of photochemical quenching or ligand‐targeted activation. Smart activatable probes only fluoresce when bound to the tumor or cleaved by specific proteases or peptidases excreted by the tumor, which improves signal‐to‐background ratios due to limited background fluorescence.118, 120 The first clinical studies to investigate smart activatable probes have been performed already.117 However, to the best of our knowledge, this has not yet been done for intraoperative imaging of CRC or PC of colorectal origin.
A similar “on‐or‐off” concept has been applied in the development of a pH‐activatable fluorescent probe. This probe becomes activated upon contact with a certain threshold pH (pH ≤ 6.9), as the majority of solid tumors are acidotic. Although this probe does not target a tumor biomarker, it is still highly specific due to the pH transistor concept.121 The benefit of such a probe is that it can be applied in a broad range of oncological indications. However, the first proof‐of‐concept in human study using a pH‐activated probe still needs to be conducted.
8.3. Phase II/III clinical studies
Although different fluorescent probes are being developed, so far only two phase‐I feasibility studies have been finalized in relatively small numbers of patients with PC of colorectal origin.96, 107 The ability of fluorescence imaging to detect peritoneal metastases that are missed by visual and tactile inspection and to aid in the differentiation between malignant and benign tissue, may have the potential to change clinical decision making. Although these results seem promising, further validation in phase‐II clinical studies is required, with larger patient cohorts that are sufficiently powered to estimate the diagnostic accuracy. Eventually, in phase‐III studies, the impact of MFGS in CRS and HIPEC surgery on clinical endpoints such as progression‐free and overall survival need to be evaluated, hoping to improve the current median progression‐free survival of only 12.6 months.11
8.4. Photodynamic therapy
Although current clinical studies are mainly aimed to investigate the feasibility of optical imaging for cancer detection, in the future intraoperative imaging may also be used as a therapeutic modality. Carrier molecules that specifically target the tumor can also be labeled to a photoactive dye (ie, photosensitizer), to allow targeted photodynamic therapy (tPDT). When excited with light of a specific wavelength, photosensitizers not only fluoresce, but also form reactive oxygen species that oxidize the cells they target, thereby killing them.122 Potentially, tPDT may be applied after CRS, to assist in the elimination of microscopic peritoneal lesions. As there is only superficial activation of the targeted photosensitizers, side‐effects are estimated to be limited. The first clinical trials have already been performed to investigate the safety and feasibility of tPDT using a variety of different photosensitizers. Phase I and II clinical trials have been conducted for treatment of colorectal cancer,123 pelvic recurrence of CRC,124 colorectal liver metastases (NCT00068068),125 and locally advanced rectal cancer.126 Moreover, tPDT has also been applied for the treatment of peritoneal metastases originating from ovarian cancer and sarcomas,127 and different gastrointestinal tumors128 with promising results. These studies demonstrate that tPDT could potentially be used as an effective treatment for both CRC and PC. However, future studies are required to determine the effect on PC of colorectal origin, when combined with MFGS.
9. CONCLUSION
In conclusion, treatment of PC of colorectal origin with curative intent consists of CRS followed by HIPEC. Up to date, surgeons still rely on visual and tactile inspection for intraoperative differentiation between tumor and benign tissue. The ultimate goal during cytoreduction is to obtain a macroscopically complete cytoreduction by resecting malignant tissue only. Therefore, there is a clear need for an intraoperative imaging technique improving tumor detection. The first phase‐I clinical trials have been performed showing the potential benefit of MFGS for patients with PC of colorectal origin. Even though no conclusions can be drawn with regard to the impact of these studies on clinical decision making, it appears MFGS has the potential to improve both cytoreduction and patient selection, facilitating patient‐tailored surgery. However, to reliably determine the sensitivity and specificity of MFGS during CRS and HIPEC, subsequent phase‐II studies are required.
CONFLICTS OF INTEREST
None.
SYNOPSIS
This paper gives a chronological overview in the development of Molecular Fluorescence Guided Surgery during Cytoreductive Surgery and HIPEC treatment in patients with Peritoneal Carcinomatosis of colorectal origin.
Hentzen JE, de Jongh SJ, Hemmer PH, van der Plas WY, van Dam GM, Kruijff S. Molecular fluorescence‐guided surgery of peritoneal carcinomatosis of colorectal origin: A narrative review. J Surg Oncol. 2018;118:332–343. 10.1002/jso.25106
Judith E. K. R. Hentzen and Steven J. de Jongh contributed equally to this work and therefore share first author position.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. Ca Cancer J Clin. 2017; 67:177–193. [DOI] [PubMed] [Google Scholar]
- 3. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108. [DOI] [PubMed] [Google Scholar]
- 4. Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann surg. 2006; 243:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 6. Segelman J, Granath F, Holm T, et al. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012; 99:699–705. [DOI] [PubMed] [Google Scholar]
- 7. Yan TD, Chu F, Links M, et al. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma: non‐mucinous tumour associated with an improved survival. Eur J Surg Oncol. 2006; 32:1119–1124. [DOI] [PubMed] [Google Scholar]
- 8. Sugarbaker PH. A curative approach to peritoneal carcinomatosis from colorectal cancer. Semin Oncol. 2005; 32:S68–S73. [DOI] [PubMed] [Google Scholar]
- 9. Sugarbaker PH. Peritonectomy procedures. Cancer Treat Res. 2007; 134:247–264. [DOI] [PubMed] [Google Scholar]
- 10. Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995; 221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verwaal VJ, Bruin S, Boot H, et al. 8‐year follow up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systematic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann surg Oncol. 2008; 15:2426–2432. [DOI] [PubMed] [Google Scholar]
- 12. Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003; 21:3737–3743. [DOI] [PubMed] [Google Scholar]
- 13. Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French Study. J Clin Oncol. 2010; 28:63–68. [DOI] [PubMed] [Google Scholar]
- 14. Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012; 13:e362–e369. [DOI] [PubMed] [Google Scholar]
- 15. Shan LL, Saxena A, Shan BL, Morris DLL. Quality of life after cytoreductive surgery and hyperthermic intra‐peritoneal chemotherapy for peritoneal carcinomatosis: a systematic review and meta‐analysis. Surg Oncol. 2014; 23:199–210. [DOI] [PubMed] [Google Scholar]
- 16. Braam HJ, van Oudheusden TR, de Hingh IH, et al. Patterns of recurrence following complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. J Surg Oncol. 2014; 109:841–847. [DOI] [PubMed] [Google Scholar]
- 17. Klumpp BD, Schwenzer N, Aschoff P, et al. Preoperative assessment of peritoneal carcinomatosis: intraindividual comparison of 18F‐FDG PET/CT and MRI. Abdom Imaging. 2013; 38:64–71. [DOI] [PubMed] [Google Scholar]
- 18. De Bree E, Koops W, Kröger R, et al. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol. 2004; 86:64–73. [DOI] [PubMed] [Google Scholar]
- 19. Dromain C, Leboulleux S, Auperin A, et al. Staging of peritoneal carcinomatosis: enhanced CT vs PET/CT. Abdom Imaging. 2008; 33:87–89. [DOI] [PubMed] [Google Scholar]
- 20. Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993; 72:1631–1636. [DOI] [PubMed] [Google Scholar]
- 21. Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002; 223:495–499. [DOI] [PubMed] [Google Scholar]
- 22. González‐Moreno S, González‐Bayón L, Ortega‐Pérez G‐H. Imaging of peritoneal carcinomatosis. Cancer J. 2009; 15:184–189. [DOI] [PubMed] [Google Scholar]
- 23. Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2015; 22:1708–1715. [DOI] [PubMed] [Google Scholar]
- 24. van Dam GM, Themelis G, Crane LM, et al. Intraoperative tumor‐specific fluorescence imaging in ovarian cancer by folate receptor‐α targeting: first in‐human results. Nat Med. 2011; 17:1315–1319. [DOI] [PubMed] [Google Scholar]
- 25. Tummers QR, Hoogstins CE, Gaarenstroom KN, et al. Intraoperative imaging of folate receptor alpha positive ovarian and breast cancer using the tumor specific agent EC17. Oncotarget. 2016; 7:32144–32155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Boer E, Warram JM, Tucker MD, et al. In vivo fluorescence immunohistochemistry: localization of fluorescently labeled cetuximab in squamous cell carcinomas. Sci Rep. 2015; 5:10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Boer E, Harlaar NJ, Taruttis A, et al. Optical innovations in surgery. Br J Surg. 2015; 102:e56–e72. [DOI] [PubMed] [Google Scholar]
- 28. Weissleder R, Ntziachristos V. Shedding light into live molecular targets. Nat Med. 2003; 9:123–128. [DOI] [PubMed] [Google Scholar]
- 29. Frangioni JV. In vivo near‐infrared fluorescence imaging. Curr Opin Chem Biol. 2003; 7:626–634. [DOI] [PubMed] [Google Scholar]
- 30. Hilderbrand SA, Weissleder R. Near‐infrared fluorescence: application to in vivo molecular imaging. Curr Opin in Chem Biol. 2010; 14:71–79. [DOI] [PubMed] [Google Scholar]
- 31. de Grand AM, Frangioni JV. An operational near‐infrared fluorescence imaging system prototype for large animal surgery. Technol Cancer Res Treat. 2003; 2:553–562. [DOI] [PubMed] [Google Scholar]
- 32. Nakayama A, del Monte F, Hajjar RJ, Frangioni JV. Functional near‐infrared fluorescence imaging for cardiac surgery and targeted gene therapy. Mol Imaging. 2002; 1:365–377. [DOI] [PubMed] [Google Scholar]
- 33. Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near‐infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003; 13:195–208. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka E, Choi HS, Fuji H, et al. Image‐guided oncologic surgery using invisible light: completed pre‐clinical development for sentinel lymph node mapping. Ann Surg Oncol. 2006; 13:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Themelis G, Yoo JS, Soh KS, et al. Real‐time intraoperative fluorescence imaging system using light‐absorption correction. J Biomed Opt. 2009; 14:064012. [DOI] [PubMed] [Google Scholar]
- 36. Troyan SL, Kianzad V, Gibbs‐Strauss SL, et al. The FLARE intraoperative near‐infrared fluorescence imaging system: a first‐in‐human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009; 16:2943–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glatz J, Varga J, Garcia‐Allende PB, et al. Concurrent video‐rate color and near‐infrared fluorescence laparoscopy. J Biomed Opt. 2013; 18:10132. [DOI] [PubMed] [Google Scholar]
- 38. Garcia‐Allende PB, Glatz J, Koch M, et al. Towards clinically translatable NIR fluorescence molecular guidance for colonoscopy. Biomed Opt Express. 2013; 5:78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100:57–70. [DOI] [PubMed] [Google Scholar]
- 40. Keereweer S, Kerrebijn JD, van Driel PB, et al. Optical image‐guided surgery − where do we stand?. Mol Imaging Biol. 2011; 13:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles’ heel. Cancer Cell. 2008; 13:472–482. [DOI] [PubMed] [Google Scholar]
- 42. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–674. [DOI] [PubMed] [Google Scholar]
- 43. Day DW, Morson BC. The adenoma‐carcinoma sequence. Major Probl Pathol. 1978; 10:58–71. [PubMed] [Google Scholar]
- 44. Cardoso J, Boer J, Morreau H, Fodde R. Expression and genomic profiling of colorectal cancer. Biochim Biophys Acta. 2007; 1775:103–137. [DOI] [PubMed] [Google Scholar]
- 45. Lin YM, Furukawa Y, Tsunoda T, et al. Molecular diagnosis of colorectal tumors by expression profiles of 50 genes expressed differentially in adenomas and carcinomas. Oncogene. 2002; 21:4120–4128. [DOI] [PubMed] [Google Scholar]
- 46. van Oosten M, Crane LM, Bart J, et al. Selecting potential targetable biomarkers for imaging purposes in colorectal cancer using target selection criteria (TASC): a novel target identification tool. Transl Oncol. 2011; 4:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paumgartner G, Probst P, Kraines R, Leevy CM. Kinetics of indocyanine green removal from the blood. Ann N Y Acad Sci. 1970; 170:134–147. [Google Scholar]
- 48. Cherrik GR, Stein SW, Leevy CM, Davidson CS. Indocyanine green: observation on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960; 39:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007; 13:207–214. [DOI] [PubMed] [Google Scholar]
- 50. Wada H, Hyuan H, Vargas C, et al. Sentinel lymph node mapping of liver. Ann Surg Oncol. 2015; 22:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toyota T, Fujito H, Suganami AM, et al. Near‐infrared‐fluorescence imaging of lymph nodes by using liposomally formulated indocyanine green derivatives. Bioorg Med Chem. 2014; 22:721–727. [DOI] [PubMed] [Google Scholar]
- 52. Paulinelli RR, Freitas‐Junior R, Rahal RM, et al. A prospective randomized trial comparing patent blue and methylene blue for the detection of the sentinel lymph node in breast cancer patients. Rev Assoc Med Bras. 1992; 63:118–123. [DOI] [PubMed] [Google Scholar]
- 53. Fattahi AS, Tavassoli A, Rohbakhshfar O, et al. Can methylene blue dye be used as an alternative to patent blue dye to find the sentinel lymph node in breast cancer surgery?. J Res Med Sci. 2014; 19:918–922. [PMC free article] [PubMed] [Google Scholar]
- 54. Landsman ML, Kwant G, Mook GA, Zijlstra WG. Light‐absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol. 1976; 40:575–583. [DOI] [PubMed] [Google Scholar]
- 55. Muckle TJ. Plasma proteins binding of indocyanine green. Biochem Med. 1976; 15:17–21. [DOI] [PubMed] [Google Scholar]
- 56. Mothes H, Dönicke T, Friedel R, et al. Indocyanine‐green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma. 2004; 57:1018–1024. [DOI] [PubMed] [Google Scholar]
- 57. Leevy CM, Smith F, Longueville J, et al. Indocyanine green clearance as a test for hepatic function. Evaluation by dichromatic ear densinometry. JAMA. 1967; 200:236–240. [PubMed] [Google Scholar]
- 58. Reuthebuch O, Häussler A, Genoni M, et al. Novadaq SPY: intraoperative quality assessment in off‐pump coronary artery bypass grafting. Chest. 2004; 125:418–424. [DOI] [PubMed] [Google Scholar]
- 59. Desai N, Fremes SE. Intraoperative indocyanine green angiography: ready for prime time?. J Thorac Cardiovasc Surg. 2007; 133:1396–1397. [DOI] [PubMed] [Google Scholar]
- 60. Desai ND, Miwa S, Kodama D, et al. A randomized comparison of intraoperative indocyanine green angiography and transit‐time flow measurement to detect technical errors in coronary bypass grafts. J Thorac Cardiovasc Surg. 2006; 132:585–594. [DOI] [PubMed] [Google Scholar]
- 61. Rubens FD, Ruel M, Fremes SE. A new and simplified method for coronary and graft imaging during CABG. Heart Surg Form. 2002; 5:141–144. [PubMed] [Google Scholar]
- 62. Balacumaraswami L, Abu‐Omar Y, Choudhary B, et al. A comparison of transit‐time flowmetry and intraoperative fluorescence imaging for assessing coronary artery bypass graft patency. J thorac Cardiovasc Surg. 2005; 130:315–320. [DOI] [PubMed] [Google Scholar]
- 63. Schaafsma BE, Mieog SJ, Hutteman M, et al. The clinical use of indocyanine green as a near‐infrared fluorescent contrast agent for image‐guided oncologic surgery. J Surg Oncol. 2011; 104:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ishizawa T, Fukushima N, Shibahara J, et al. Real‐time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009; 115:2491–2504. [DOI] [PubMed] [Google Scholar]
- 65. Raabe A, Beck J, Gerlach R, et al. Near‐infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery. 2003; 52:132–139. [DOI] [PubMed] [Google Scholar]
- 66. Raabe A, Nakaji P, Beck J, et al. Prospective evaluation of surgical microscope‐integrated intraoperative near‐infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg. 2005; 103:982–989. [DOI] [PubMed] [Google Scholar]
- 67. Singh SK, Desai ND, Chikazawa G, et al. The graft imaging to improve patency (GRIIP) clinical trial results. J Thorac Cardiovasc Surg. 2010; 139:294–301. [DOI] [PubMed] [Google Scholar]
- 68. Zimmermann A, Roenneberg C, Wendorff H, et al. Early postoperative detection of tissue necrosis in amputation stumps with indocyanine green fluorescence angiography. Vasc Endovascular Surg. 2010; 44:269–273. [DOI] [PubMed] [Google Scholar]
- 69. Holm C, Mayr M, Höfter E, et al. Assessment of the patency of microvascular anastomoses using microscope‐integrated near‐infrared angiography: a preliminary study. Microsurgery. 2009; 29:509–514. [DOI] [PubMed] [Google Scholar]
- 70. Holm C, Dornseifer U, Sturtz G, Ninkovic M. Sensitivity and specificity of ICG angiography in free flap reexploration. J Reconstr Microsurg. 2010; 26:311–316. [DOI] [PubMed] [Google Scholar]
- 71. Liberale G, Vankerckhove S, Caldon MG, et al. Fluorescence imaging after indocyanine green injection for detection of peritoneal metastases in patients undergoing cytoreductive surgery for peritoneal carcinomatosis from colorectal cancer: a pilot study. Ann Surg. 2016; 264:1110–1115. [DOI] [PubMed] [Google Scholar]
- 72. Zhang RR, Schroeder AB, Grudzinski JJ, et al. Beyond the margins: real‐time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol. 2017; 14:347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Verbeek FPR, van der Vorst JR, Schaafsma BE, et al. Image‐guided hepatopancreatobiliary surgery using near‐infrared fluorescent light. J Hepatobiliary Pancreat Sci. 2012; 19:626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Marshall MV, Draney D, Sevick‐Muraca EM, Olive DM. Singe‐dose intravenous toxicity study of IRDye 800CW in Sprague‐Dawley rats. Mol Imaging Biol. 2010; 12:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Day KE, Sweeny L, Kulbersh B, et al. Preclinical comparison of near‐infrared‐labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Mol Imaging Biol. 2013; 15:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stummer W, Reulen HJ, Novotny A, et al. Fluorescence‐guided resections of malignant gliomas − an overview. Acta Neurochir Suppl. 2003; 88:9–12. [DOI] [PubMed] [Google Scholar]
- 77. Heath CH, Deep NL, Sweety L, et al. Use of panitumumab‐IRDye800 to image microscopic head and neck cancer in an orthotopic surgical model. Ann Surg Oncol. 2012; 19:3879–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Terwisscha van Scheltinga AG, van Dam GM, Nagengast WB, et al. Intraoperative near‐infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J Nucl Med. 2011; 52:1778–1785. [DOI] [PubMed] [Google Scholar]
- 79. Mieog JS, Vahrmeijer AL, Hutteman M, et al. Novel intraoperative near‐infrared fluorescence camera system for optical image‐guided cancer surgery. Mol Imaging. 2010; 9:223–231. [PubMed] [Google Scholar]
- 80. Mieog JSD, Hutteman M, Vorst JR, et al. Image‐guided tumor resection using real‐time near‐infrared fluorescence in a syngeneic rat model of primary breast cancer. Breast Cancer Res Treat. 2011; 128:679–689. [DOI] [PubMed] [Google Scholar]
- 81. Hutteman M, Mieog JS, van der Vorst JR, et al. Intraoperative near‐infrared fluorescence imaging of colorectal metastases targeting integrin a(v)β(3) expression in a syngeneic rat model. Eur J Surg Oncol. 2011; 37:252–257. [DOI] [PubMed] [Google Scholar]
- 82. Adams KE, Ke S, Kwon S, et al. Comparison of visible and near‐infrared wavelength‐excitable fluorescent dyes for molecular imaging of cancer. J Biomed Opt. 2007; 12:024017. [DOI] [PubMed] [Google Scholar]
- 83. Weissleder R, Tung CH, Mahmood U. Bogdanov A Jr. In vivo imaging of tumors with protease‐activated near‐infrared fluorescent probes. Nat Biotechnol. 1999; 17:375–378. [DOI] [PubMed] [Google Scholar]
- 84. Korb ML, Hartman YE, Kovar J, et al. Use of monoclonal antibody‐IRDye800CW bioconjugates in the resection of breast cancer. J Surg Res. 2014; 188:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rubie C, Kollmar O, Frick VO, et al. Differential CXC receptor expression in colorectal carcinomas. Scand J Immunol. 2008; 68:635–644. [DOI] [PubMed] [Google Scholar]
- 86. Nayak TR, Hong H, Zhang Y, Cai W. Multimodality imaging of CXCR4 in cancer: current status towards clinical translation. Curr Mol Med. 2013; 13:1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Paret C, Hildebrand D, Weitz J, et al. C4.4A as a candidate marker in the diagnosis of colorectal cancer. Br J Cancer. 2007; 97:1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xie X, Wang CY, Cao YX, et al. Expression pattern of epithelial cell adhesion molecule on normal and malignant colon tissues. World J Gastroenterol. 2005; 11:344–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Van Driel PBAA, Boonstra MC, Prevoo HAJM, et al. EpCAM as multi‐tumour target for near‐infrared fluorescence guided surgery. BMC Cancer. 2016; 12:884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cunningham MP, Essapen S, Thomas H, et al. Coexpression of the IGF‐IR, EGFR and HER‐2 is common in colorectal cancer patients. Int J Oncol. 2006; 16:329–335. [PubMed] [Google Scholar]
- 91. Goldstein NS, Armin M. Epidermal growth factor receptor immune‐histochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma: implications for a standardized scoring system. Cancer. 2001; 92:1331–1346. [DOI] [PubMed] [Google Scholar]
- 92. Rosenthal EL, Warram JM, de Boer E, et al. Safety and tumor specificity of cetuximab‐IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res. 2015; 21:3658–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li M, Li JY, Zhao AL, et al. Comparison of carcinoembryonic antigen prognostic value in serum and tumour tissue of patients with colorectal cancer. Colorectal Dis. 2009; 11:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kim JC, Gong G, Roh SA, Park KC. Carcinoembryonic antigen gene and carcinoembryonic antigen expression in the liver metastasis of colorectal carcinoma. Mol Cells. 1999; 9:133–137. [PubMed] [Google Scholar]
- 95. Suwanagool P, Fujimori T, Maeda S. Value of tissue carcinoembryonic antigen in patients with colorectal carcinoma. Asian Pac J Allergy Immunol. 1990; 8:33–37. [PubMed] [Google Scholar]
- 96. Boogerd LSF, Hoogstins CES, Schaap DP, et al. Safety and effectiveness of SGM‐101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose‐escalation pilot study. Lancet Gastroenterol Hepatol. 2018; 3:181–191. [DOI] [PubMed] [Google Scholar]
- 97. Kaneko I, Tanaka S, Oka S, et al. Immunohistochemical molecular markers as predictors of curability of endoscopically resected submucosal colorectal cancer. World J Gastro‐Enterol. 2007; 13:3829–3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Suzuki H, Shoda J, Kawamoto T, et al. Expression of MUC1 recognized by monoclonal antibody MY.1E12 is a useful biomarker for tumor aggressiveness of advanced colon carcinoma. Clin Exp Metastasis. 2004; 21:321–329. [DOI] [PubMed] [Google Scholar]
- 99. McKerrow JH, Bhargava V, Hansell E, et al. A functional proteomics screen of proteases in colorectal carcinoma. Mol Med. 2000; 6:450–460. [PMC free article] [PubMed] [Google Scholar]
- 100. Jeffery N, McLean MH, El Omar EM, Murray GI. The matrix metalloproteinase/tissue inhibitor of matrix metalloproteinase profile in colorectal polyp cancers. Histopathology. 2009; 54:820–828. [DOI] [PubMed] [Google Scholar]
- 101. Madoz‐Gúrpide J, López‐Serra P, Martinez‐Torrecuadrada JL, et al. Proteomics‐based validation of genomic data: applications in colorectal cancer diagnosis. Mol Cell Proteomics. 2006; 5:1471–1483. [DOI] [PubMed] [Google Scholar]
- 102. Emmert‐Buck MR, Roth MJ, Zhuang Z, et al. Increased gelatinase A (MMP‐2) and cathepsin B activity in invasive tumor regions of human colon cancer samples. Am J Pathol. 1994; 145:1285–1290. [PMC free article] [PubMed] [Google Scholar]
- 103. Cao D, Hou M, Guan YS, et al. Expression of HIF‐1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009; 9:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Abdou AG, Aiad H, Asaad N, et al. Immunohistochemical evaluation of vascular endothelial growth factor (VEGF) in colorectal carcinoma. J Egypt Natl Canc Inst. 2006; 18:311–322. [PubMed] [Google Scholar]
- 105. Ter Weele EJ, Terwisscha van Scheltinga AG, Linssen MD, et al. Development, preclinical safety, formulation, and stability of clinical grade bevacizumab‐800CW, a new near infrared fluorescent imaging agent for first in human use. Eur J Pharm Biopharm. 2016; 104:226–234. [DOI] [PubMed] [Google Scholar]
- 106. Tjalma JJ, Garcia‐Allende PB, Hartmans E, et al. Molecular fluorescence endoscopy targeting vascular endothelial growth factor A for improved colorectal polyp detection. J Nucl Med. 2016; 57:480–485. [DOI] [PubMed] [Google Scholar]
- 107. Harlaar NJ, Koller M, de Jongh SJ, et al. Molecular fluorescence‐guided surgery of peritoneal carcinomatosis of colorectal origin: a single‐centre feasibility study. Lancet Gastroenterol Hepatol. 2016; 1:283–290. [DOI] [PubMed] [Google Scholar]
- 108. Nagengast WB, Hartmans E, Garcia‐Allende PB, et al. Near‐infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut. 2017; 0:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lamberts LE, Koch M, de Jong JS, et al. Tumor‐specific uptake of fluorescent bevacizumab‐IRDye800CW microdosing in patients with primary breast cancer: a phase I feasibility study. Clin Cancer Res. 2017; 23:2730–2741. [DOI] [PubMed] [Google Scholar]
- 110. Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi‐institutional experience. J Clin Oncol. 2009; 27:6237–6242. [DOI] [PubMed] [Google Scholar]
- 111. Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi‐institutional study of 1,290 patients. Cancer. 2010; 116:5608–5618. [DOI] [PubMed] [Google Scholar]
- 112. Chua TC, Moran BJ, Sugarbaker PH, et al. Early‐ and long‐term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012; 30:2449–2456. [DOI] [PubMed] [Google Scholar]
- 113. Bakrin N, Bereder JM, Decullier E, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013; 39:1435–1443. [DOI] [PubMed] [Google Scholar]
- 114. Kuijpers AM, Mirck B, Aalbers AG, et al. Cytoreduction and HIPEC in the Netherlands: nationwide long‐term outcome following the Dutch protocol. Ann Surg Oncol. 2013; 20:4224–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Levine EA, Stewart JH, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014; 218:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Fehrmann RS, Karjalainen JM, Krajewska M, et al. Gene expression analysis identifies global gene dosage sensitivity in cancer. Nat Genet. 2015; 47:115–125. [DOI] [PubMed] [Google Scholar]
- 117. Garland M, Yim JJ, Bogyo M. A bright future for precision medicine: advances in fluorescent chemical probe design and their clinical application. Cell Chem Biol. 2016; 23:122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Srinivasarao M, Galliford CV, Low PS. Principles in the design of ligand‐targeted cancer therapeutics and imaging agents. Nat Rev Drug Discov. 2015; 14:203–219. [DOI] [PubMed] [Google Scholar]
- 119. Kobayashi H, Choyke PL. Target‐cancer‐cell‐specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res. 2011; 44:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kobayashi H, Ogawa M, Alford R, et al. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev. 2010; 110:2620–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhao T, Huang G, Li Y, et al. A transistor‐like pH nanoprobe for tumour detection and image‐guided surgery. Nat Biomed Eng. 2016; 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Plaetzer K, Krammer B, Berlanda J, et al. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med Sci. 2009; 24:259–268. [DOI] [PubMed] [Google Scholar]
- 123. Kawczyk‐Krupka A, Bugaj AM, Latos W, et al. Photodynamic therapy in colorectal cancer treatment: the state of the art in clinical trials. Photodiagnosis and Photodyn Ther. 2015; 12:545–553. [DOI] [PubMed] [Google Scholar]
- 124. Herrera‐Ornelas L, Petrelli NJ, Mittelman A, et al. Photodynamic therapy in patients with colorectal cancer. Cancer. 1986; 57:677–684. [DOI] [PubMed] [Google Scholar]
- 125. van Duijnhoven FH, Rovers JP, Engelmann K, et al. Photodynamic therapy with 5,10,15,20‐tetrakis(m‐hydroxyphenyl) bacteriochlorin for colorectal liver metastases is safe and feasible: results from a Phase I study. Ann Surg Oncol. 2005; 12:808–816. [DOI] [PubMed] [Google Scholar]
- 126. Kashtan H, Papa MZ, Wilson BC, et al. Use of photodynamic therapy in the palliation of massive advanced rectal cancer. Phase I/II study. Dis Colon Rectum. 1991; 34:600–605. [DOI] [PubMed] [Google Scholar]
- 127. Sindelar WF, DeLaney TF, Tochner Z, et al. Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms. Phase I study. Arch Surg. 1991; 126:318–324. [DOI] [PubMed] [Google Scholar]
- 128. Webber J, Kessel D, Fromm D. Side effects and photosensitization of human tissues after aminolevulinic acid. J Surg Res. 1997; 68:31–37. [DOI] [PubMed] [Google Scholar]


