Abstract
Aim
This study evaluated the usefulness of daily walking for gestational diabetes mellitus (GDM) management by analyzing the relationship between daily walking and glucose tolerance in pregnant women with GDM who were in the second trimester.
Methods
This longitudinal study was conducted at TOYOTA Memorial Hospital in Toyota, Japan, from January 2015 to June 2016. Pregnant women with GDM wore accelerometers on the waist for 7–12 weeks.
Results
Seventy‐three women with GDM were included in the present study; data collected from 24 women were analyzed. The estimated number of steps walked daily showed a significant positive correlation (r = 0.798, P = 0.000) with energy expenditure related to physical activity. There was a significant negative correlation (r = −0.603, P = 0.014) between the post‐ to pre‐research casual glucose level (CGL) ratio and the number of steps walked daily. No significant correlation (r = −0.004, P = 0.986) was detected between the ratio of hemoglobin A1c and the number of steps taken. When the study was completed, the 11 participants who walked ≥6000 steps/day showed significantly lower CGL (95 + 10 mg/dL [mean + SD]) than the 13 participants in the <6000 steps/day group (111 + 18 mg/dL) (P = 0.013).
Conclusion
Simple walking for light intensity physical activity is effective for controlling the CGL in pregnant women with GDM. We recommend that pregnant women with GDM should walk a minimum of 6000 steps/day.
Keywords: casual glucose level, daily walking, gestational diabetes mellitus, physical activity
Introduction
Gestational diabetes mellitus (GDM) is a major perinatal complication.1 On the basis of the universal definitions and diagnostic criteria for GDM proposed by the hyperglycemia and adverse pregnancy outcome study in 2008,2 the prevalence of GDM was reported to be about 8% in Japan,3 indicating that GDM occurs commonly in pregnant women.
It is known that GDM is associated with other disorders, such as high fetal body weight for gestational age and congenital malformations.4, 5 Fetal macrosomia causes shoulder dystocia and increases the possibility of a cesarean delivery. Moreover, hypertensive disorders in pregnancy shows relatively higher complication rates when occurring with concurrent GDM.6 Glucose intolerance during the perinatal period affects women even after delivery. Previous studies have reported that women who experienced GDM developed type 2 diabetes at a seven times higher rate than women without GDM.7, 8 Because diabetes is a major cause of death worldwide,9 primary prevention of GDM is essential. Although the recognition of GDM as clinical diabetes seems to be important for early detection and treatment, there is still no consensus on appropriate measures for glucose control during pregnancy to prevent the development of type 2 diabetes.
Diet, exercise and drug therapies are used to treat glucose intolerance in patients with type 2 diabetes. Diet and exercise therapy should be attempted first, with drug therapy added if diet and exercise alone cannot correct glucose tolerance sufficiently. A recent study has shown that physical activity (PA) is more effective than diet for improving insulin resistance in patients with type 2 diabetes.10 Appropriate PA can effectively improve glucose tolerance in the general population.11 The American College of Obstetricians and Gynecologists (ACOG) recommends at least 30 min of moderate‐intensity PA on most, if not all, days of the week.12
Regarding pregnant women, studies have consistently found that increasing the pace of walking is significantly and inversely associated with the risk of GDM.12 The pregnant abdomen in the second trimester causes changes in physical function. The body's center of gravity is inclined forward, and the feet become difficult to see. Therefore, pregnant women lean backward while walking for safety.13 Moderate‐intensity PA for non‐pregnant women is equivalent to high‐intensity PA for pregnant women. Daily walking is popular during pregnancy because of its lower intensity and higher accessibility.14, 15
Daily walking does not require special equipment and may be the most suitable light‐intensity PA for pregnant women. However, there is insufficient scientific evidence on the effects of daily walking during the gestation period. Therefore, this study aimed to evaluate the usefulness of daily walking for GDM management by analyzing the relationship between walking and glucose tolerance in pregnant women with GDM who were in the second trimester.
Methods
Subjects
This longitudinal study was conducted at TOYOTA Memorial Hospital in Toyota, Japan, from January 2015 to June 2016. Pregnant women who visited the hospital and who were diagnosed with GDM in the second trimester were included. Pregnant women who were less than 20 years old, who could not complete a questionnaire, who had type 1 or type 2 diabetes, who were using steroids, who required hospitalization, who had a pregnancy complicated by fetal disorder, or who performed habitual moderate and vigorous PA were excluded.
This hospital had 776 first trimester outpatients for perinatal care at the obstetrical department for the recruiting period of 18 months. Among the 716 women (92.3%) who underwent biochemical testing, 245 women (34.2%) had a casual glucose level (CGL) greater than 100 mg/dL in the first trimester (less than 16 weeks of gestation) or a level greater than 140 mg/dL in a 50‐g glucose challenge test at the second trimester (24–28 weeks of gestation). The 75‐g oral glucose tolerance test (OGTT) was performed in 184 women (75.1%) to diagnose GDM according to the following standard criteria: blood glucose ≥92 mg/dL at fasting, ≥180 mg/dL at 60 min after loading or ≥153 mg/dL at 120 min after loading. A total of 63 patients were found to have GDM. The calculated occurrence rate for GDM was 8.8%. An additional 10 cases that had already been diagnosed with GDM with 75‐g OGTT were referred to the hospital by nearby private clinics. Among 73 pregnant women with GDM, 32 (43.8%) agreed to participate in the study. Two women voluntarily withdrew, one relocated and three received medication, including insulin, during the research; the remaining 26 women completed the study. However, final data were analyzed for only 24 pregnant women with GDM because sufficient daily walking data were not obtained from two women (Fig. 1).
Figure 1.
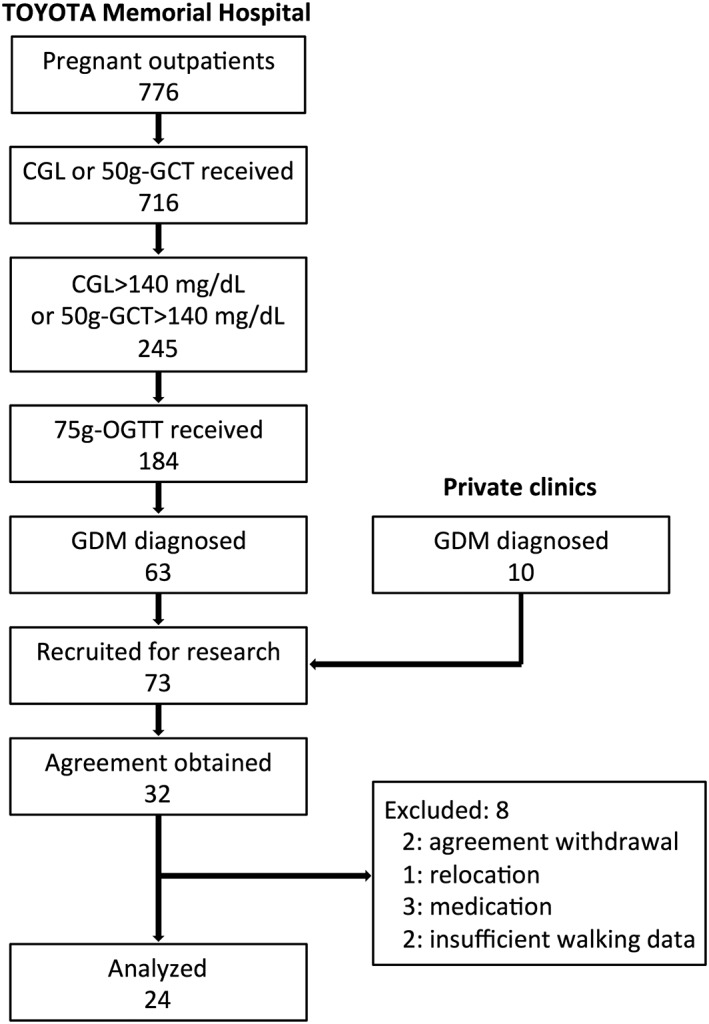
The recruitment and entry process for women with gestational diabetes mellitus (GDM) for the present study.
Research protocol
Women with GDM were recruited in the second trimester while awaiting routine examination in an outpatient hospital room; questionnaires provided to them assessed dietary intake. Background information, including maternal age, gestational week, pre‐pregnancy body mass index (BMI), pregnancy history, fetus number, pregnancy complications and laboratory biochemical data were obtained from patient medical charts. Body weight was measured except the women who were followed as antenatal care at other clinics.
The total amount of daily walking was estimated from the number of steps taken and the amount of exercise performed daily, as measured with an accelerometer (Lifecorder EX; Suzuken Co Ltd).16 Participants attached the accelerometer to the waistbands of their skirts or pants, as instructed at the time of recruitment by investigators. The accelerometers assessed daily walking for a total of 7–12 weeks because periodic pregnancy examinations were performed every 4 weeks on the basis of the number of steps taken and the amount of exercise performed every day from the second trimester to the third trimester. The accelerometers were removed during sleeping and bathing. In the third trimester, the participants removed the accelerometers permanently and completed questionnaires that assessed dietary intake.
Each participant was given detailed information on the study protocol, and all provided written informed consent. This study procedure was reviewed and approved by the Ethics Committees of Kyoto University School of Medicine (No. E2279) and TOYOTA Memorial Hospital. It was registered in the University Hospital Medical Information Network (UMIN) Center (ID: R000017881).
Measurements
Daily walking was assessed using the accelerometer, which measured steps, PA‐related intensity and PA‐related energy expenditure (PAEE). PA‐related intensity was judged from the strength and frequency of impact as transmitted to an acceleration sensor. PAEE was calculated every 4 s, using PA‐related intensity and body weight. The accelerometer frequency was 32 Hz, and its time base range was 0.06–1.94 g. As accelerometers only have two axes, their detection range is limited. No reports have indicated adverse events related to accelerometer use in pregnant women.
CGL, hemoglobin (Hb) and hemoglobin A1c (HbA1c) were measured at the initiation and completion of the research to estimate pre‐ and post‐research carbohydrate metabolism.
Dietary intake during the most recent month was assessed with a brief self‐administered diet history questionnaire (BDHQ), and the amount of daily intake for 50 foods and selected nutrients17, 18 was calculated from the BDHQ.
Statistical analyses
The Mann–Whitney U‐test and Spearman's correlation coefficient were performed using BellCurve for Excel Version 2.03 (Social Survey Research Information Co. Ltd). Two‐tailed P‐values less than 0.05 were considered statistically significant.
Clinical trial registration
UMIN (https://upload.umin.ac.jp/cgi-bin/icdr/ctr_reg_list.cgi)
UMIN ID:UMIN000015663. Official scientific title of the study: Verification of the effect of exercise on pregnant women with glucose intolerance.
Results
Participant characteristics
The mean values (range) for age (years), pre‐pregnant BMI (kg/m2), gestational weeks at the initiation and completion of the study, and the duration of walking measurements (weeks) for the 24 GDM women were 35.9 (29–42), 24.1 (16.7–36.8), 21.9 (14–27), 30.5 (25–35) and 8.6 (7–12), respectively (Table 1). Also body weight at the initiation and completion of the study and overall body weight gain of the women were 64.7 (41.4–99.2), 68.7 (47.8–110) and 8.2 (−0.2–14.4), respectively. One woman had a pre‐pregnancy BMI <18 kg/m2, and two had a BMI >30 kg/m2. Twelve women were primiparas. Two of the multiparas had GDM in prior pregnancies. The mean values of Hb (g/dL) at the initiation and completion of the study were 12.0 (10.1–14.0) and 11.3 (8.7–13.1). The research was initiated at 21.9 (14–28) gestational weeks and completed at 30 (25–35) weeks. The research duration was 8.6 (7–12) weeks.
Table 1.
Participant characteristics and parameters
| No. | Age (years) | P/M | History of GDM | Pre‐pregnant BMI (kg/m2) | No. of steps walked/day | PAEE | At initiation of research | At completion of research | Research duration (weeks) | BW gain during study period (kg) | Overall BW gain (kg) | Ratio of CGL change (initiation/completion) | Dietary intake (kcal/day) | Child birth | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational weeks | BW (kg) | CGL (mg/dL) | HbA1c (%) | Hb (g/dL) | Gestational weeks | BW (kg) | CGL (mg/dL) | HbA1c (%) | Hb (g/dL) | Gestational weeks at delivery | Delivery mode | Birth weight (g) | ||||||||||||
| 1 | 30 | P | 36.8 | 2947 | 112 | 24 | 99.2 | 97 | 5.6 | 11.5 | 32 | 110.0 | 111 | 6.0 | 10.9 | 8 | 11 | 15 | 1.14 | 1319 | 40 | NVD | 3746 | |
| 2 | 29 | M | + | 19.9 | 3804 | 69 | 23 | 51.2 | 69 | 5.2 | 11.5 | 31 | ND | 115 | 5.3 | ND | 8 | ND | ND | 1.67 | 1938 | 38 | NVD | 3012 |
| 3 | 37 | M | − | 24.1 | 3836 | 100 | 19 | 69.0 | 74 | 5.5 | 12.9 | 28 | 71.0 | 99 | 5.3 | 11.0 | 9 | 2 | 5.6 | 1.34 | 1814 | 40 | VD (forceps) | 3562 |
| 4 | 42 | M | − | 25.6 | 4013 | 122 | 27 | 77.0 | 87 | 5.4 | 10.2 | 35 | 80.0 | 97 | 5.3 | 9.0 | 8 | 3 | 12 | 1.11 | 2761 | 40 | NVD | 3460 |
| 5 | 31 | M | − | 18.3 | 4059 | 125 | 25 | 56.0 | 81 | 5.6 | 10.3 | 33 | 60.0 | 117 | 5.7 | 9.1 | 8 | 4 | 14.4 | 1.44 | 1677 | 40 | NVD | 4124 |
| 6 | 35 | M | − | 22.5 | 4092 | 99 | 19 | ND | 92 | 5.4 | ND | 27 | ND | 95 | 5.3 | ND | 8 | ND | ND | 1.03 | 1605 | 41 | NVD | 3416 |
| 7 | 30 | P | 26.7 | 4167 | 111 | 25 | 66.0 | 87 | 4.4 | 13.8 | 33 | 73.0 | 124 | 4.4 | ND | 8 | 7 | 8 | 1.43 | 1712 | 38 | CS (elective) | 2950 | |
| 8 | 35 | P | 21.0 | 4602 | 105 | 27 | 63.2 | 127 | 5.3 | 11.2 | 35 | 65.0 | 104 | 5.3 | 11.5 | 8 | 1.8 | 7 | 0.82 | 1503 | 40 | NVD | 3144 | |
| 9 | 42 | P | 24.6 | 4602 | 108 | 22 | 65.0 | 113 | 5.1 | 13.2 | 30 | 66.4 | 95 | 5.2 | 12.8 | 8 | 1.4 | −0.2 | 0.84 | 1210 | 35 | NVD | 1764 | |
| 10 | 40 | M | − | 25.3 | 4796 | 132 | 25 | 79.0 | 90 | 5.1 | 11.9 | 33 | 80.0 | 128 | 5.3 | 11.0 | 8 | 1 | ND | 1.42 | 1358 | 40 | NVD | 3066 |
| 11 | 35 | P | 21.0 | 4813 | 100 | 19 | 56.0 | 77 | 4.8 | 11.1 | 31 | 64.5 | 86 | 5.2 | ND | 12 | 8.5 | 15 | 1.12 | 1355 | 40 | CS (emergency) | 2990 | |
| 12 | 39 | P | 23.8 | 4855 | 116 | 14 | 65.0 | 103 | 6.0 | 12.0 | 26 | 63.0 | 122 | 5.3 | 12.0 | 12 | −2 | 7.2 | 1.18 | 1515 | 41 | NVD | 2946 | |
| 13 | 38 | P | 29.6 | 5391 | 125 | 26 | 77.5 | 173 | 5.2 | 13.0 | 34 | 77.8 | 154 | 5.3 | 12.5 | 8 | 0.3 | 6.8 | 0.89 | 1204 | 34 | CS (emergency) | 1554 | |
| 14 | 42 | M | − | 18.7 | 6159 | 119 | 24 | 53.4 | 102 | 5.3 | 11.2 | 31 | 54.0 | 94 | 5.9 | 11.2 | 7 | 0.6 | 6.8 | 0.92 | 1810 | 38 | NVD | 3074 |
| 15 | 35 | M | − | 20.8 | 6567 | 168 | 24 | 63.0 | 154 | 5.1 | 10.9 | 35 | 63.0 | 83 | 5.2 | 11.1 | 11 | 0 | 11.8 | 0.54 | 1868 | 38 | NVD | 2966 |
| 16 | 37 | P | 24.1 | 7004 | 185 | 14 | 62.8 | 123 | 5.8 | 10.9 | 25 | 69.0 | 99 | 6.0 | 8.7 | 11 | 6 | 13 | 0.80 | 2235 | 41 | NVD | 3658 | |
| 17 | 35 | M | − | 25.8 | 7094 | 184 | 28 | 67.2 | 108 | 5.4 | 12.9 | 35 | 66.5 | 106 | 5.7 | ND | 7 | −0.7 | 6 | 0.98 | 2403 | 39 | NVD | 3102 |
| 18 | 41 | P | 25.1 | 7305 | 169 | 27 | 59.2 | 97 | 5.8 | 12.5 | 35 | 73.2 | 119 | 5.9 | 11.6 | 8 | 14 | 3.2 | 1.23 | 1682 | 37 | CS (elective) | 2218 | |
| 19 | 32 | P | 26.1 | 7896 | 152 | 16 | 60.0 | 156 | 5.4 | 11.6 | 25 | 62.0 | 88 | 4.9 | 11.4 | 9 | 2 | 8.6 | 0.56 | 1125 | 41 | NVD | 3432 | |
| 20 | 29 | M | − | 30.9 | 8270 | 266 | 24 | 80.5 | 149 | 5.4 | 13.2 | 32 | 78.6 | 96 | 5.6 | 12.9 | 8 | −1.9 | 0.6 | 0.64 | 1601 | 38 | NVD | 2988 |
| 21 | 31 | M | + | 25.2 | 8632 | 196 | 17 | 66.4 | 98 | 5.4 | 13.1 | 25 | 67.8 | 96 | 5.3 | 13.1 | 8 | 1.4 | 8.8 | 0.98 | 1667 | 40 | VD (vacuum) | 3646 |
| 22 | 35 | P | 16.7 | 8977 | 147 | 19 | 41.4 | 102 | 4.7 | 10.1 | 28 | 47.8 | 87 | 4.7 | 10.9 | 9 | 6.4 | ND | 0.85 | 2063 | 38 | VD (vacuum) | 2752 | |
| 23 | 42 | M | − | 25.3 | 9037 | 238 | 18 | 61.0 | 83 | 5.4 | 12.0 | 25 | 65.8 | 82 | 5.4 | ND | 7 | 4.8 | 5 | 0.99 | 2057 | 38 | CS (elective) | 3030 |
| 24 | 40 | P | 20.5 | 9205 | 169 | 19 | 50.0 | 128 | 5.3 | 14.0 | 27 | 53.0 | 93 | 5.3 | 12.5 | 8 | 3 | 9.8 | 0.73 | 1552 | 41 | NVD | 3200 | |
| Mean | 35.9 | 24.1 | 5921.8 | 142.4 | 21.9 | 64.7 | 107.1 | 5.3 | 12.0 | 30.5 | 68.7 | 103.8 | 5.4 | 11.3 | 8.6 | 3.3 | 8.2 | 1.0 | 1709.8 | 39.0 | 3075 | |||
| SD | 4.4 | 4.3 | 1937.9 | 46.0 | 4.2 | 11.9 | 27.5 | 0.3 | 1.1 | 3.6 | 12.3 | 16.8 | 0.4 | 1.3 | 1.4 | 4.0 | 4.2 | 0.3 | 386.6 | 1.8 | 570 | |||
BMI, body mass index; BW, body weight; CGL, casual glucose level; CS, cesarean section; GDM, gestational diabetes mellitus; Hb, hemoglobin; HbA1c, hemoglobin A1c; M, multipara; ND, not determined; NVD, normal vaginal delivery; P, primipara; PAEE, physical activity‐related energy expenditure; VD, vaginal delivery.
The CGL values on the first and last day of walking measurements were 107 (69–156) and 104 (82–154), respectively. Thirteen women showed a CGL decrease during the research period.
Gestational complications and deliveries
Two women (nos. 13 and 19) showed hypertension in pregnancy and one (no. 9) indicated preeclampsia after the research had completed (Table 1). Mean gestational weeks at delivery was 39.0; two women (nos. 9 and 13) had preterm childbirths. Delivery treatment of forceps (no. 3) or vacuum extraction (nos. 21 and 22) was applied in three cases, and five women received elective (nos. 7, 18 and 23) or emergency (nos. 11 and 13) cesarean section. Following delivery, disseminated intravascular coagulation occurred in patient no. 3 and was treated. Mean birth weight was 3075 g; macrosomia (more than 4000 g) was observed in infant no. 5, and three infants (nos. 9, 13 and 18) demonstrated low birth weight (less than 2500 g).
Number of steps walked and PAEE
The number of mean steps taken daily by the 24 participants was 5922 steps, ranging from 2947 to 9205. A significant positive correlation (r = 0.798, P = 0.000) was observed between the number of steps walked and the PAEE, which indicated that daily walking as PA does consume sufficient energy, even in pregnant women.
The relationship between walking and glucose tolerance
The ratios of CGL and HbA1c changes (completed/initiated) ranged from 0.54 to 1.67 and 0.83 to 1.10, respectively. As shown in Fig. 2a, there was a significant (r = −0.603, P = 0.013) negative correlation between the number of steps walked daily and the CGL ratios. In contrast, the number of steps walked daily showed no clear correlation (r = −0.071, P = 0.755) with the ratios of HbA1c (Fig. 2b). On the other hand, no clear correlation was observed between the CGL ratios and body weight gain during the study period (r = 0.338, P = 0.124).
Figure 2.
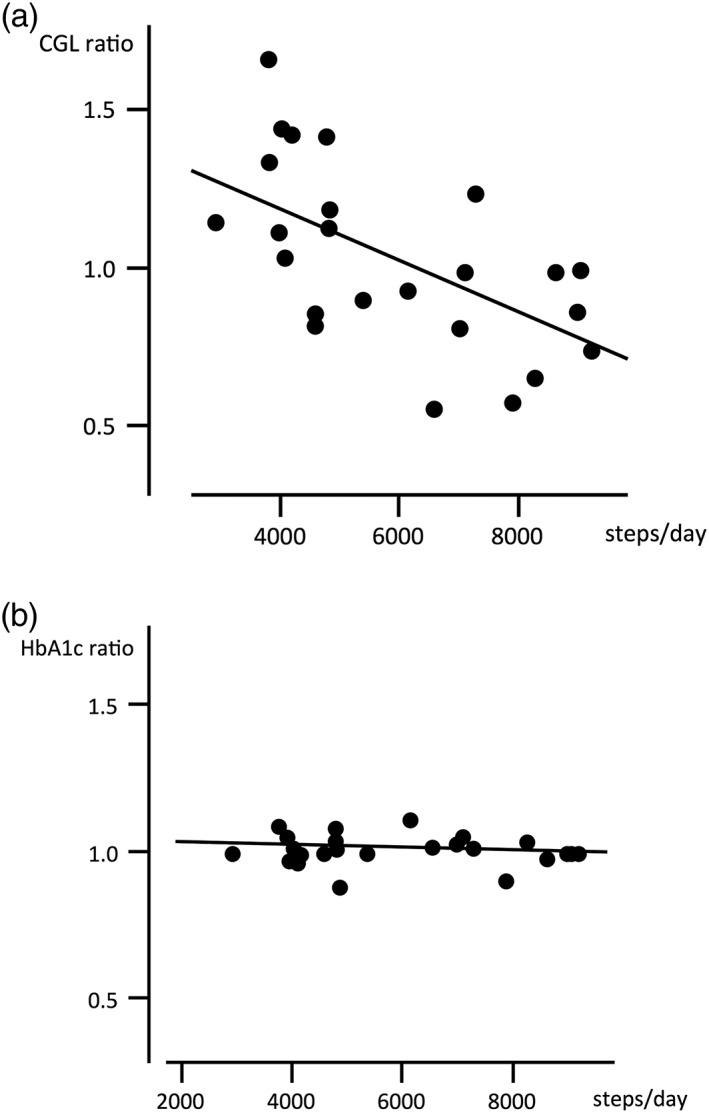
The correlation between the number of steps walked daily and the ratio of post‐ to pre‐research casual glucose level (CGL) (a) and hemoglobin A1c (HbA1c) (b). The number of steps walked correlated significantly with CGL ratio (r = −0.603, P = 0.013) but not with the HbA1c ratio (r = −0.071, P = 0.755).
PA and dietary intake
The dietary intakes evaluated by BDHQ ranged between 1125 and 2761 kcal/day (Table 1). No statistically significant correlation was observed between the number of steps walked and the dietary energy intake or the each intake of carbohydrate, protein and fat (data not shown).
Discussion
Since women who experience GDM often subsequently develop type 2 diabetes, it is very important to prevent GDM or to modify lifestyle habits such as diet and exercise after GDM develops. Our present study demonstrates that simple walking as light intensity PA is effective for controlling CGL independent of dietary intake in pregnant women with GDM. Our results suggest that pregnant women with GDM can benefit from walking, which can be measured as the number of steps per day.
CGL measurement is more convenient and less burdensome for pregnant women than fasting blood sugar measurement and/or a 75‐g OGTT. It is known that high‐intensity exercise can easily decrease CGL.19 Barakat et al.20 demonstrated that an exercise program for healthy pregnant women consisting of three sessions of aerobics and swimming per week resulted in a decrease in CGL in the intervention group. However, such an exercise program is not likely to be feasible. Prospective epidemiological studies have shown that PA is associated with a reduction in the risk of developing type 2 diabetes.21, 22 Low PA leads to glucose intolerance, and pregnant women with GDM may not exercise adequately. Thus, pregnant women with GDM require a lifestyle change, such as PA after delivery, to prevent type 2 diabetes. Despite the difficulty of obtaining adequate exercise in many pregnant women with GDM, it can be important for them to develop habits to improve non‐exercise activity thermogenesis.23 The finding of a significant negative correlation between the changes of CGL and the number of steps walked daily should be of interest in that it suggests that walking would be very beneficial for pregnant women with GDM.
In another report, improvement in HbA1c level was found to be directly proportional to exercise intensity, but there was no significant HbA1c improvement on the basis of the amount of exercise.24 This finding suggests that CGL improved in proportion to exercise intensity, rather than the amount of exercise. In our research, the exercise intensity of the walking was low, which may be why no significant negative correlation between HbA1c level and the number of steps walked daily was observed.
One question that remains is: What is the minimum number of steps that is suitable for GDM management in pregnant women? Although our present results suggest that the higher the number of steps walked, the better the results may be in terms of glucose tolerance improvement, it was clear from our findings that middle‐aged women can prevent metabolic syndrome by walking 6000 steps or more per day.25 Our previous study also demonstrated that walking ≥6000 steps per day could improve CGL in healthy pregnant women in the second trimester.26 Thus, the participants in this study were divided into two groups according to the cutoff of 6000 walking steps, and CGL recorded at the point at which the research was completed were analyzed. The CGL in the group of ≥6000 steps walked (n = 11) was 95 ±10 mg/dL (mean ±SD), which was significantly lower than that (111 ±18) in the <6000 steps walked group (n = 13) (P = 0.013). No significant differences in CGL were detected when the patients were instead separated according to the cutoffs of walking 5000 (P = 0.264) or 7000 (P = 0.062) steps, respectively.
There are several limitations of this study. First, it is known that there are ethnic variations in glucose tolerance, but only Asians participated in the study. As they show the least insulin secretion on the basis of genetic factors among all ethnic groups,27, 28, 29 it is possible for Asians to develop glucose intolerance without concomitant obesity. Thus, it is necessary for pregnant Asian women to be cognizant not only of their diet, but also of PA. Second, as the study was conducted in one hospital, our results may not be generalizable. Because the hospital is located in Toyota city, where the population of car users is highest in Japan, people may generally walk less than people in other regions. Third, although GDM occurs in all populations, the participant number was small in the present study. This may have resulted in insufficient walking data collected. Wearing of the accelerometer constantly for more than 8 weeks might also bother pregnant women during the second and third trimesters. For critical analyses, we restricted the participants to those who could complete the research protocol. Fourth, participants could not be blinded to the data from the accelerometers. Proper use was individually instructed, but number of steps walked may have been underestimated by incorrect use. Fifth, the influence of meals could not be ruled out, because CGL, rather than fasting blood sugar level, was evaluated.
In conclusion, we can recommend walking as a light‐intensity PA for pregnant women for the management of GDM. However, it is necessary to follow‐up sequentially with women with GDM after delivery for the long‐term evaluation of the effects of this exercise regimen. Further studies should be performed to confirm that not only CGL, but also insulin resistance, is improved by walking in pregnant women with GDM.
Disclosure
None declared.
Author contributions
A. H. participated in designing the research plan, coordinated the data analyses and contributing to the writing of the manuscript. Y. K. and Y. B. collected the data. N. S. coordinated the field work, designed the research plan and considered the composition of the paper. H. O. and J. S. collaborated and performed the study.
Acknowledgment
This work was supported by KAKENHI (Grant Number 25670970) from Japan Society for the Promotion of Science to N. S. We would like to thank all of the participants for their cooperation and the staff members at TOYOTA Memorial Hospital for their great help. We acknowledge Dr Kunihide Ino (President, ACCPREC Inc., Takatsuki, Japan) for teaching and helping us with the necessary statistical analyses. We would like to thank Editage (http://www.editage.jp) for English language editing.
References
- 1. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32 (Suppl 1): S62–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. HAPO Study Cooperative Research Group , Metzger BE, Lowe LP, Dyer AR et al Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991–2002. [DOI] [PubMed] [Google Scholar]
- 3. Morikawa M, Yamada T, Yamada T et al Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract 2010; 90: 339–342. [DOI] [PubMed] [Google Scholar]
- 4. Steel JM, Johnstone FD, Hepburn DA, Smith AF. Can pre‐pregnancy care of diabetic women reduce the risk of abnormal babies? BMJ 1990; 301: 1070–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fuhrmann K, Reiher H, Semmler K, Fischer F, Fischer M, Glöckner E. Prevention of congenital malformations in infants of insulin‐dependent diabetic mothers. Diabetes Care 1983; 6: 219–223. [DOI] [PubMed] [Google Scholar]
- 6. Landon MB, Spong CY, Thom E et al A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009; 361: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta‐analysis. Lancet 2009; 373: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 8. O'Sullivan JB. Diabetes mellitus after GDM. Diabetes 1991; 40 (Suppl 2): 131–135. [DOI] [PubMed] [Google Scholar]
- 9. WHO . Global health risks: Mortality and burden of disease attributable to selected major risks. 2009. [Cited 1 Jun 2018.] Available from URL: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf
- 10. Tamura Y, Tanaka Y, Sato F et al Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191–3196. [DOI] [PubMed] [Google Scholar]
- 11. Nelson JD, Poussier P, Marliss EB, Albisser AM, Zinman B. Metabolic response of normal man and insulin‐infused diabetics to postprandial exercise. Am J Physiol 1982; 242: E309–E316. [DOI] [PubMed] [Google Scholar]
- 12. American College of Obstetricians and Gynecologists (ACOG) . Physical activity and exercise during pregnancy and the postpartum period. Committee opinion 650. Obstet Gynecol 2015; 126: e135–e142. [DOI] [PubMed] [Google Scholar]
- 13. Sunaga Y, Kanemura N, Anan M, Takahashi M, Shinkoda K. Estimation of inertial parameters of the lower trunk in pregnant Japanese women: A longitudinal comparative study and application to motion analysis. Appl Ergon 2016; 55: 173–182. [DOI] [PubMed] [Google Scholar]
- 14. Mottola MF, Campbell MK. Activity patterns during pregnancy. Can J Appl Physiol 2003; 28: 642–653. [DOI] [PubMed] [Google Scholar]
- 15. Pereira MA, Rifas‐Shiman SL, Kleinman KP, Rich‐Edwards JW, Peterson KE, Gillman MW. Predictors of change in physical activity during and after pregnancy: Project viva. Am J Prev Med 2007; 32: 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kumahara H, Schutz Y, Ayabe M et al The use of uniaxial accelerometry for the assessment of physical‐activity‐related energy expenditure: A validation study against whole‐body indirect calorimetry. Brit J Nutr 2004; 91: 235–243. [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi S, Murakami K, Sasaki S et al Comparison of relative validity of food group intakes estimated by comprehensive and brief‐type self‐administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr 2011; 14: 1200–1211. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi S, Honda S, Murakami K et al Both comprehensive and brief self‐administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol 2012; 22: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi A, Suganuma N. Physical activity for gestational diabetes mellitus. Clinics Mother Child Health 2016; 13: 238, 10.4172/2090-7214.1000238. [DOI] [Google Scholar]
- 20. Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: A randomised controlled trial. Br J Sports Med 2012; 46: 656–661. [DOI] [PubMed] [Google Scholar]
- 21. Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS Jr. Physical activity and reduced occurrence of non‐insulin‐dependent diabetes mellitus. N Engl J Med 1991; 325: 147–152. [DOI] [PubMed] [Google Scholar]
- 22. Manson JE, Stampfer MJ, Colditz GA et al Physical activity and incidence of non‐insulin‐dependent diabetes mellitus in women. Lancet 1991; 338: 774–778. [DOI] [PubMed] [Google Scholar]
- 23. Levine JA, Lanningham‐Foster LM, McCrady SK et al Interindividual variation in posture allocation: Possible role in human obesity. Science 2005; 28: 584–586. [DOI] [PubMed] [Google Scholar]
- 24. Tamura Y, Tanaka Y, Sato F et al Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity type 2 diabetic patients. J Clin Endocrinol Metab 2005; 90: 3191–3196. [DOI] [PubMed] [Google Scholar]
- 25. Colpani V, Oppermann K, Spritzer PM. Association between habitual physical activity and lower cardiovascular risk in premenopausal, perimenopausal, and postmenopausal women: A population‐based study. Menopause 2013; 20: 525–531. [DOI] [PubMed] [Google Scholar]
- 26. Hayashi A, Matsuzaki M, Kusaka M, Shiraishi M, Haruna M. Daily walking decreases casual glucose level among pregnant women in the second trimester. Drug Discov Ther 2016; 10: 218–222. [DOI] [PubMed] [Google Scholar]
- 27. Unoki H, Takahashi A, Kawaguchi T et al SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in east Asian and European populations. Nature Genet 2008; 40: 1098–1102. [DOI] [PubMed] [Google Scholar]
- 28. Okamoto K, Iwasaki N, Nishimura C et al Identification of KCNJ15 as a susceptibility gene in Asian patients with type 2 diabetes mellitus. Am J Hum Genet 2010; 86: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamauchi T, Hara K, Maeda S et al A genome‐wide association study in the Japanese population identifies susceptibility loci for type 2 diabetes at UBE2E2 and C2CD4A‐C2CD4B. Nature Genet 2010; 42: 864–868. [DOI] [PubMed] [Google Scholar]


