Abstract
Background
How patients experience the symptoms/signs of psoriasis is highly relevant for assessing treatment response.
Objectives
Compare outcomes with guselkumab, placebo and adalimumab utilizing the novel, validated Psoriasis Symptoms and Signs Diary (PSSD).
Methods
VOYAGE 1 is an ongoing, phase III, double‐blinded, controlled trial of patients with moderate‐to‐severe psoriasis. Patients were randomized to guselkumab 100 mg every 8 weeks; placebo‐to‐guselkumab 100 mg every 8 weeks; or adalimumab 40 mg every 2 weeks. The PSSD was self‐administered to assess symptoms (i.e. itch, skin tightness, burning, stinging and pain) and signs (i.e. dryness, cracking, scaling, shedding/flaking, redness and bleeding) of psoriasis (0–10 [absent‐to‐worst‐imaginable]) every 24 h. Symptom and sign summary scores were derived (0–100) based on average scores of the individual symptoms and signs. Proportions of patients with clinically meaningful improvements and symptom‐ and sign‐free scores of 0 were evaluated across treatment groups at weeks 16, 24 and 48.
Results
At baseline, 652 of 837 randomized patients had PSSD scores. The proportion of patients achieving clinically meaningful improvements in PSSD summary scores was significantly higher in the guselkumab group compared with the placebo group at week 16 (P < 0.001) and compared with the adalimumab group at weeks 24 (P = 0.002) and 48 (P < 0.001). The proportions of patients achieving PSSD symptom and sign summary scores of 0 (i.e. symptom‐ and sign‐free) were significantly higher for guselkumab vs. placebo at week 16 and vs. adalimumab at weeks 24 and 48 (all P < 0.001).
Conclusions
Based on PSSD scores, greater improvements in symptoms and signs of psoriasis were reported by patients treated with guselkumab compared with placebo at week 16 or adalimumab through 48 weeks.
Introduction
Psoriasis is a chronic immune‐mediated disease that has a significant impact on health‐related quality of life (HRQoL).1, 2 While numerous clinical studies have led to the approval of new agents for moderate‐to‐severe psoriasis and subsequent advancements in treatment, evaluations in these trials have focused primarily on physician‐evaluated measures of skin lesion severity,3, 4 which are required for approval. More recently, self‐evaluation of improvement, particularly of symptoms, by patients has gained prominence and has been acknowledged by regulatory agencies for labelling.5
Fully validated instruments that measure classic symptoms and signs of disease are critical for evaluating patients’ perspectives. Patient‐reported outcome (PRO) instruments have been developed to meet this need, but they did not satisfy product‐labelling regulatory requirements and claims and were not designed to be used exclusively by individuals with psoriasis.6, 7 More recent instruments have been proprietary and/or limited in scope of evaluation.8, 9 Therefore, a new PRO measure, the Psoriasis Symptoms and Signs Diary (PSSD), was developed to assess symptoms and patient‐observable signs in individuals with moderate‐to‐severe plaque psoriasis.10, 11, 12 The PSSD was used for the first time in the development programme for guselkumab in psoriasis and has been validated for use in clinical trials as a self‐administered tool for capturing the individual patient's perspective.
Guselkumab (CNTO 1959; Janssen Research & Development, LLC, Spring House, PA) is a fully human IgG1 lambda monoclonal antibody that binds to the p19 subunit of interleukin (IL)‐23 and inhibits intracellular downstream signalling of IL‐23, which is required for terminal differentiation and survival of T helper (Th)17 cells.13 The efficacy and safety of guselkumab were confirmed in three phase III trials in psoriasis: VOYAGE 1, VOYAGE 2 and NAVIGATE.14, 15, 16 Limited PSSD results from these studies have been reported previously. We report here the full results of the PSSD from VOYAGE 1 through the first year of the study.
Methods
Study design
VOYAGE 1 is a phase III, randomized, double‐blinded, placebo‐ and active comparator‐controlled trial. The first year of the study included a placebo‐controlled period (weeks 0–16) and an active‐comparator period, during which guselkumab was compared with adalimumab (week 0–48). At baseline, patients were randomized (2:1:2) to receive either (i) guselkumab 100 mg at weeks 0, 4, 12 and every 8 weeks thereafter; (ii) placebo at weeks 0, 4, 12 with crossover to guselkumab 100 mg at weeks 16, 20 and every 8 weeks thereafter; or (iii) adalimumab 80 mg at week 0, 40 mg at week 1 and 40 mg every 2 weeks thereafter (Fig. 1). Adult patients (aged ≥18 years) were eligible for enrolment if they had moderate‐to‐severe plaque psoriasis for at least 6 months and were candidates for systemic therapy or phototherapy. Moderate‐to‐severe psoriasis was defined as an Investigator's Global Assessment [IGA] score of ≥3, Psoriasis Area and Severity Index [PASI] score of ≥12 and per cent body surface area involvement of >10%. Exclusion criteria and other details of the study have been reported previously.14
Figure 1.
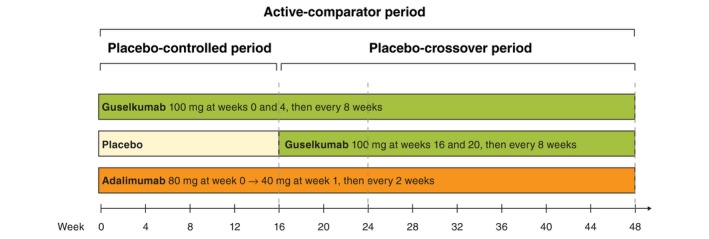
Study Schema.
Patient‐reported outcomes
The PSSD is a PRO questionnaire designed and validated by Janssen Research & Development, LLC, and has been translated and linguistically validated into 13 languages. The severity of psoriasis is evaluated to assess treatment benefit based on 11 individual items covering symptoms (i.e. itch, pain, stinging, burning and skin tightness) and patient‐observable signs (i.e. skin dryness, cracking, scaling, shedding/flaking, redness and bleeding) using a 0‐10 numerical rating scale for severity (Table S1).10, 11 The average score of the five symptoms and the average score of the six signs are used to derive a symptom summary score and a sign summary score. Summary scores range from 0 to 100, and a higher score indicates more severe disease.
Thresholds for clinically meaningful improvement have been established for the symptom and sign summary scores as well as each individual item scale score as follows: (i) an improvement of ≥40 points from baseline for the PSSD symptom and sign summary scores; (ii) an improvement of ≥3 points from baseline for the bleeding and stinging scores; (iii) an improvement of ≥4 points from baseline for the itch, dryness, cracking, skin tightness, burning and pain scores; and (iv) an improvement of ≥5 points from baseline for the scaling, shedding/flaking and redness scores.12
Patient‐reported outcome data were entered by patients directly into electronic tablet devices. The 24‐h PSSD was completed at home daily, and the Dermatology Life Quality Index (DLQI) was completed at the study site during selected study visits. The impact of skin disease on HRQoL was self‐reported by patients across six domains (i.e. symptoms and feelings, daily activities, leisure, work or school performance, personal relationships and treatment), for an overall DLQI score of 0 (no effect) to 30 (extremely large effect) (6). Disease severity was evaluated by clinicians using two instruments: the IGA (range, 0 [cleared] to 4 [severe]) and PASI (range, 0–72; higher score indicates more severe disease).3
Statistical analyses
All analyses of PSSD scores included only patients with PSSD scores at baseline. Continuous response parameters were compared based on an analysis of variance model stratified by investigator site. The Cochran–Mantel–Haenszel test stratified by investigator site (pooled) was used to compare the proportions of patients responding to treatment. Spearman's correlations were calculated between PSSD scores and DLQI (including the six domain scores), PASI and IGA scores. All statistical testing was performed at the two‐sided 0.05 significance level, and nominal P‐values are presented.
Baseline PSSD scores were defined as the average score of at least 4 days during the 7 days prior to the week 0 visit. To validate the assumption that having a missing PSSD score at baseline was a random event, the differences in baseline disease and demographic characteristics and selected efficacy endpoints between patients with and without baseline PSSD data were evaluated.
Patients who discontinued study agent due to lack of efficacy or an adverse event of worsening of psoriasis, or who started a protocol‐prohibited medication/therapy that could improve psoriasis, were considered treatment failures. After weekly data were derived and treatment failure rules were applied, data handling rules (last observation carried forward) were utilized for both binary and continuous endpoints for missing PSSD data.
Results
Patient characteristics
Baseline demographic and disease characteristics among patients with PSSD scores at baseline were comparable across treatment groups (Table 1). Baseline PSSD scores were missing in 185 of the 837 randomized patients (22%) due to technical difficulties associated with the electronic device used to collect data in the early stages of the study. The proportions of patients with missing PSSD scores at baseline were 25.9% (45/174), 24.3% (80/329) and 18.0% (60/334) in the placebo, guselkumab and adalimumab groups, respectively. Overall, compared with patients with baseline PSSD scores, key baseline disease characteristics were generally similar for those with missing baseline PSSD scores (Table S2). Additionally, there were no meaningful differences in clinical and HRQoL improvements as assessed by PASI, IGA and DLQI over time between those with and without baseline PSSD scores (Table S3).
Table 1.
Baseline demographic and disease characteristics of randomized patients with PSSD scores at baseline
| Placebo | Guselkumab | Adalimumab | Total | |
|---|---|---|---|---|
| Number of patients | 129 | 249 | 274 | 652 |
| Age, years | 45.3 ± 12.89 | 44.0 ± 12.78 | 43.3 ± 12.52 | 44.0 ± 12.70 |
| Men | 89 (69.0) | 176 (70.7) | 203 (74.1) | 468 (71.8) |
| White race | 107 (82.9) | 194 (77.9) | 223 (81.4) | 524 (80.4) |
| BMI (kg/m 2 ) | 29.4 ± 7.15 | 30.2 ± 6.24 | 29.6 ± 6.35 | 29.8 ± 6.47 |
| Duration of psoriasis, years | 17.1 ± 11.73 | 18.5 ± 12.68 | 17.3 ± 11.24 | 17.7 ± 11.90 |
| Body surface area involvement | 24.7 ± 15.68 | 27.8 ± 16.95 | 28.0 ± 16.80 | 27.3 ± 16.67 |
| IGA score, moderate/severe (3/4) | 129 (100.0) | 249 (100.0) | 272 (99.3) | 650 (99.7) |
| PASI score (0–72) | 20.0 ± 8.69 | 21.7 ± 9.24 | 22.2 ± 8.88 | 21.6 ± 9.00 |
| DLQI score (0–30) a | 13.7 ± 7.23 | 14.5 ± 7.58 | 14.8 ± 7.15 | 14.5 ± 7.33 |
| PSSD scores | ||||
| Symptom score (0–100) | 48.3 ± 23.77 | 54.4 ± 24.63 | 53.9 ± 25.79 | 53.0 ± 25.03 |
| Itch score (0–10) | 5.7 ± 2.29 | 6.4 ± 2.38 | 6.4 ± 2.48 | 6.3 ± 2.41 |
| Skin score tightness (0–10) | 5.4 ± 2.49 | 5.9 ± 2.50 | 5.9 ± 2.56 | 5.8 ± 2.52 |
| Burning score (0–10) | 4.2 ± 2.71 | 4.9 ± 2.88 | 4.8 ± 3.08 | 4.7 ± 2.94 |
| Stinging score (0–10) | 4.1 ± 2.85 | 4.7 ± 2.91 | 4.5 ± 3.18 | 4.5 ± 3.02 |
| Pain score (0–10) | 4.7 ± 2.74 | 5.3 ± 3.00 | 5.3 ± 3.12 | 5.2 ± 3.01 |
| Sign score (0–100) | 53.6 ± 20.34 | 56.9 ± 21.30 | 58.5 ± 21.73 | 56.9 ± 21.34 |
| Dryness score (0–10) | 6.1 ± 2.17 | 6.4 ± 2.34 | 6.6 ± 2.25 | 6.4 ± 2.27 |
| Cracking score (0–10) | 5.1 ± 2.65 | 5.4 ± 2.69 | 5.6 ± 2.74 | 5.4 ± 2.70 |
| Scaling score (0–10) | 6.1 ± 1.99 | 6.3 ± 2.31 | 6.5 ± 2.21 | 6.3 ± 2.21 |
| Shedding/flaking score (0–10) | 6.0 ± 2.14 | 6.5 ± 2.25 | 6.6 ± 2.36 | 6.4 ± 2.28 |
| Redness score (0–10) | 5.9 ± 2.38 | 6.1 ± 2.33 | 6.3 ± 2.36 | 6.2 ± 2.35 |
| Bleeding score (0–10) | 3.0 ± 2.64 | 3.3 ± 2.80 | 3.5 ± 2.96 | 3.4 ± 2.84 |
Values are reported as n (%) or mean ± standard deviation.
BMI, body mass index; DLQI, Dermatology Life Quality Index; IGA, Investigator's Global Assessment; PASI, Psoriasis Area and Severity Index; PSSD, Psoriasis Symptoms and Signs Diary.
DLQI scores were missing for one patient each in the placebo and guselkumab groups.
Clinically meaningful improvement in PSSD
Based on definitions described in the Methods, greater proportions of patients in the guselkumab group achieved clinically meaningful improvements in their PSSD symptom (73.6% vs. 7.7%, respectively) and sign (73.1% vs. 4.2%, respectively) summary scores at week 16 when compared with the placebo group (both P < 0.001) (Table 2). Greater proportions of patients in the guselkumab group also achieved clinically meaningful improvements in all individual item scales for both symptom and sign summary scores at week 16 when compared with the placebo group (all P < 0.001).
Table 2.
Patients who achieved clinically meaningful improvement in PSSD summary and individual scale scores at weeks 16, 24 and 48 among patients with at least minimal severity at baseline
| Week 16 | Week 24 | Week 48 | |||||
|---|---|---|---|---|---|---|---|
| Placebo | Guselkumaba | Adalimumab | Guselkumabb | Adalimumab | Guselkumabb | Adalimumab | |
| Symptom summary score ≥40 at baseline, n | 78 | 174 | 188 | 174 | 188 | 174 | 188 |
| ≥40‐point improvement | 6 (7.7) | 128 (73.6) | 124 (66.0) | 139 (79.9) | 120 (63.8) | 141 (81.0) | 113 (60.1) |
| Itch score ≥4 at baseline, n | 105 | 217 | 231 | 217 | 231 | 217 | 231 |
| ≥4‐point improvement | 6 (5.7) | 163 (75.1) | 147 (63.6) | 170 (78.3)c | 151 (65.4) | 177 (81.6) | 144 (62.3) |
| Skin tightness score ≥4 at baseline, n | 98 | 205 | 217 | 205 | 217 | 205 | 217 |
| ≥4‐point improvement | 8 (8.2) | 163 (79.5) | 154 (71.0) | 172 (83.9)c | 157 (72.4) | 172 (83.9) | 145 (66.8) |
| Burning score ≥4 at baseline, n | 75 | 158 | 173 | 158 | 173 | 158 | 173 |
| ≥4‐point improvement | 8 (10.7) | 127 (80.4) | 124 (71.7) | 135 (85.4) | 123 (71.1) | 134 (84.8) | 111 (64.2) |
| Stinging score ≥3 at baseline, n | 85 | 184 | 187 | 184 | 187 | 184 | 187 |
| ≥3‐point improvement | 15 (17.6) | 152 (82.6) | 139 (74.3) | 162 (88.0) | 137 (73.3) | 164 (89.1) | 125 (66.8) |
| Pain score ≥4 at baseline, n | 82 | 174 | 192 | 174 | 192 | 174 | 192 |
| ≥4‐point improvement | 10 (12.2) | 132 (75.9) | 131 (68.2) | 141 (81.0) | 129 (67.2) | 146 (83.9) | 125 (65.1) |
| Sign summary score ≥40 at baseline, n | 95 | 197 | 221 | 197 | 221 | 197 | 221 |
| ≥40‐point improvement | 4 (4.2) | 144 (73.1) | 149 (67.4) | 155 (78.7)c | 144 (65.2) | 162 (82.2) | 140 (63.3) |
| Dryness score ≥4 at baseline, n | 112 | 218 | 240 | 218 | 240 | 218 | 240 |
| ≥4‐point improvement | 12 (10.7) | 169 (77.5) | 167 (69.6) | 184 (84.4) | 171 (71.3) | 184 (84.4) | 153 (63.8) |
| Cracking score ≥4 at baseline, n | 91 | 189 | 210 | 189 | 210 | 189 | 210 |
| ≥4‐point improvement | 10 (11.0) | 149 (78.8) | 155 (73.8) | 164 (86.8)c | 161 (76.7) | 164 (86.8) | 145 (69.0) |
| Scaling score ≥5 at baseline, n | 100 | 194 | 223 | 194 | 223 | 194 | 223 |
| ≥5‐point improvement | 3 (3.0) | 139 (71.6) | 141 (63.2) | 150 (77.3)c | 140 (62.8) | 159 (82.0) | 132 (59.2) |
| Shedding score ≥5 at baseline, n | 97 | 197 | 221 | 197 | 221 | 197 | 221 |
| ≥5‐point improvement | 4 (4.1) | 149 (75.6) | 146 (66.1) | 160 (81.2) | 147 (66.5) | 162 (82.2) | 135 (61.1) |
| Redness score ≥5 at baseline, n | 85 | 190 | 218 | 190 | 218 | 190 | 218 |
| ≥5‐point improvement | 7 (8.2) | 126 (66.3) | 126 (57.8) | 145 (76.3) | 136 (62.4) | 145 (76.3) | 124 (56.9) |
| Bleeding score ≥3 at baseline, n | 63 | 124 | 150 | 124 | 150 | 124 | 150 |
| ≥3‐point improvement | 15 (23.8) | 112 (90.3) | 115 (76.7) | 114 (91.9)c | 121 (80.7) | 115 (92.7) | 111 (74.0) |
Data are presented as number of patients (%). PSSD, Psoriasis Symptoms and Signs Diary.
P < 0.001 for comparisons between guselkumab and placebo, unless otherwise indicated.
P < 0.001 for comparisons between guselkumab and adalimumab, unless otherwise indicated.
P < 0.01 for comparison between guselkumab and adalimumab at week 24.
When compared with adalimumab at weeks 24 and 48, greater proportions of patients in the guselkumab group achieved clinically meaningful improvements (≥40 points) in symptom and sign summary scores (all P < 0.001, except for P = 0.002 for signs summary score at week 24) (Table 2, Fig. 2). Similarly, the proportions of patients achieving clinically meaningful improvements in all individual symptom and sign item scale scores were greater in the guselkumab group at week 24 and 48 when compared with the adalimumab group (all P < 0.01) (Table 2).
Figure 2.
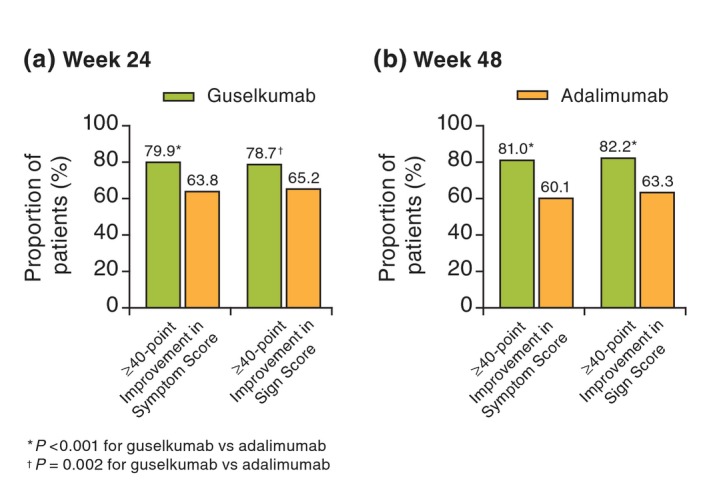
Proportion of patients achieving a clinically meaningful improvement (≥40 points) in PSSD symptom and sign summary scores at (a) week 24 and (b) week 48. PSSD, Psoriasis Symptoms and Signs Diary.
Symptom‐ and sign‐free PSSD scores
The proportions of patients achieving PSSD symptom and sign summary scores of 0 (i.e. symptom‐ and sign‐free) were significantly higher in the guselkumab group compared with the placebo group at week 16 and with the adalimumab group at weeks 24 and 48 (all P < 0.001; Table 3; Fig. 3).14 Greater proportions of patients in the guselkumab group achieved a score of 0 on each of the individual item scales for both the symptom and sign summary scores compared with placebo at week 16 (all P < 0.001) (Table 3). Similarly, greater proportions of patients in the guselkumab group achieved a score of 0 on each individual item scale for both symptoms and signs compared with adalimumab at weeks 24 and 48 (all P < 0.01) (Table 3).
Table 3.
Patients who achieved symptom‐ and sign‐free scores on the PSSD summary and individual scale scores at weeks 16, 24 and 48 among patients with PSSD scores >0 at baseline
| Week 16 | Week 24 | Week 48 | |||||
|---|---|---|---|---|---|---|---|
| Placebo | Guselkumaba | Adalimumab | Guselkumabb | Adalimumab | Guselkumabb | Adalimumab | |
| Symptom summary score >0 at baseline, n | 129 | 248 | 273 | 248 | 273 | 248 | 273 |
| Symptom summary score = 0 | 1 (0.8) | 67 (27.0) | 45 (16.5) | 90 (36.3) | 59 (21.6) | 104 (41.9) | 63 (23.1) |
| Itch score >0 at baseline, n | 129 | 247 | 268 | 247 | 268 | 247 | 268 |
| Itch score = 0 | 3 (2.3) | 83 (33.6) | 54 (20.1) | 101 (40.9) | 66 (24.6) | 112 (45.3) | 72 (26.9) |
| Skin tightness score >0 at baseline, n | 126 | 244 | 270 | 244 | 270 | 244 | 270 |
| Skin tightness score = 0 | 4 (3.2) | 109 (44.7) | 90 (33.3) | 127 (52.0) | 99 (36.7) | 136 (55.7) | 97 (35.9) |
| Burning score >0 at baseline, n | 118 | 232 | 238 | 232 | 238 | 232 | 238 |
| Burning score = 0 | 9 (7.6) | 139 (59.9) | 104 (43.7) | 147 (63.4) | 113 (47.5) | 157 (67.7) | 102 (42.9) |
| Stinging score >0 at baseline, n | 112 | 225 | 232 | 225 | 232 | 225 | 232 |
| Stinging score = 0 | 10 (8.9) | 131 (58.2) | 112 (48.3) | 139 (61.8)c | 117 (50.4) | 152 (67.6) | 108 (46.6) |
| Pain score >0 at baseline, n | 121 | 232 | 249 | 232 | 249 | 232 | 249 |
| Pain score = 0 | 8 (6.6) | 118 (50.9) | 98 (39.4) | 132 (56.9)c | 110 (44.2) | 145 (62.5) | 107 (43.0) |
| Sign summary score >0 at baseline, n | 129 | 248 | 274 | 248 | 274 | 248 | 274 |
| Sign summary score = 0 | 0 | 50 (20.2) | 32 (11.7) | 73 (29.4) | 40 (14.6) | 89 (35.9) | 51 (18.6) |
| Dryness score >0 at baseline, n | 129 | 248 | 274 | 248 | 274 | 248 | 274 |
| Dryness score = 0 | 0 | 81 (32.7) | 64 (23.4) | 112 (45.2) | 69 (25.2) | 115 (46.4) | 70 (25.5) |
| Cracking score >0 at baseline, n | 122 | 239 | 261 | 239 | 261 | 239 | 261 |
| Cracking score = 0 | 5 (4.1) | 136 (56.9) | 114 (43.7) | 154 (64.4) | 121 (46.4) | 158 (66.1) | 124 (47.5) |
| Scaling score ≥0 at baseline, n | 129 | 245 | 274 | 245 | 274 | 245 | 274 |
| Scaling score = 0 | 2 (1.6) | 92 (37.6) | 70 (25.5) | 116 (47.3) | 77 (28.1) | 124 (50.6) | 82 (29.9) |
| Shedding score ≥0 at baseline, n | 129 | 247 | 272 | 247 | 272 | 247 | 272 |
| Shedding score = 0 | 1 (0.8) | 99 (40.1) | 79 (29.0) | 126 (51.0) | 85 (31.3) | 132 (53.4) | 91 (33.5) |
| Redness score ≥0 at baseline, n | 128 | 247 | 272 | 247 | 272 | 247 | 272 |
| Redness score = 0 | 2 (1.6) | 83 (33.6) | 65 (23.9) | 110 (44.5) | 78 (28.7) | 118 (47.8) | 89 (32.7) |
| Bleeding score ≥0 at baseline, n | 100 | 202 | 220 | 202 | 220 | 202 | 220 |
| Bleeding score = 0 | 16 (16.0) | 157 (77.7) | 129 (58.6) | 162 (80.2) | 143 (65.0) | 164 (81.2) | 132 (60.0) |
Data are presented as number of patients (%). PSSD, Psoriasis Symptoms and Signs Diary
P < 0.001 for comparisons between guselkumab and placebo, unless otherwise indicated.
P < 0.001 for comparisons between guselkumab and adalimumab, unless otherwise indicated.
P < 0.01 for comparison between guselkumab and adalimumab at week 24.
Figure 3.
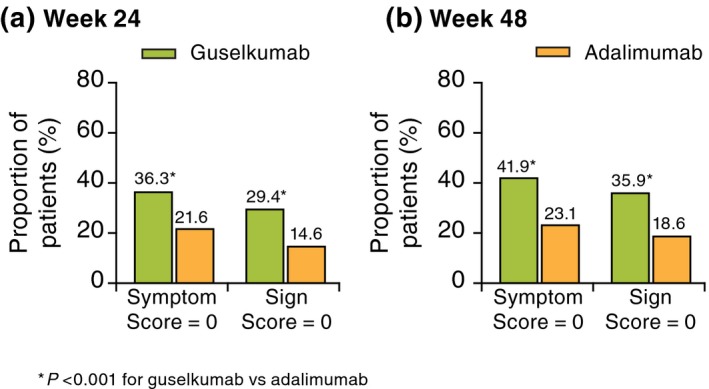
Proportion of patients achieving PSSD symptom and sign summary scores of 0 at (a) week 24 and (b) week 48. PSSD, Psoriasis Symptoms and Signs Diary.
Mean improvements from baseline in PSSD scores
At week 16, the improvements from baseline in the PSSD summary score were significantly greater for guselkumab compared with placebo (mean change ± standard deviation, −41.9 ± 24.61 vs. −3.0 ± 19.56 [symptom] and −44.6 ± 22.00 vs. −4.1 ± 17.87 [sign]; both P < 0.001 for guselkumab vs. placebo) (Fig. 4). At week 24, mean changes (improvements) in PSSD summary scores were significantly greater for guselkumab than for adalimumab for both the symptom (−44.0 ± 24.57 vs. −36.0 ± 28.36) and sign (−47.2 ± 22.19) vs. −40.1 ± 26.49) summary scores (both P < 0.001) (Fig. 4). Likewise, at week 48, mean changes for guselkumab were significantly greater than those for adalimumab: symptom (−45.3 ± 25.51 vs. −32.5 ± 31.14) and sign (−47.9 ± 23.08 vs. −36.6 ± 29.28) summary scores (both P < 0.001) (Fig. 4).14
Figure 4.

Mean improvements from baseline in PSSD (a) symptom and (b) sign summary scores in the placebo, guselkumab, placebo → guselkumab and adalimumab groups through week 48. PSSD, Psoriasis Symptoms and Signs Diary.
At week 16, mean improvement in scores for each individual PSSD symptom and sign item scale was also greater in the guselkumab group compared with the placebo group (P < 0.001). At week 24, improvements in individual symptom scores were also greater for guselkumab compared with adalimumab (P = 0.005 for burning; P < 0.0001 for all others). Greater mean improvements were observed for the guselkumab vs. the adalimumab group for most individual sign scores (P = 0.011 for cracking; P = 0.001 for scaling; P = 0.002 for redness; P < 0.001 for all others); improvement in the bleeding score was better for guselkumab, but the difference was not statistically significant (−3.0 vs. −2.6, P = 0.214). By week 48, mean improvement in all individual scores was significantly different between the active treatment groups (P = 0.014 for bleeding; P < 0.001 for all others [guselkumab vs. adalimumab]).
Correlations between changes in PSSD and PASI, IGA and DLQI
Spearman correlation analyses showed a moderate correlation between improvements in PSSD summary scores and PASI (r = 0.622 [PSSD symptom] and r = 0.686 [PSSD sign]) and IGA (r = 0.605 [PSSD symptom] and r = 0.664 [PSSD sign]) scores and a slightly stronger correlation with improvements in the total DLQI score (r = 0.782 [PSSD symptom] and r = 0.788 [PSSD sign]) at week 16. Among the individual DLQI domains, the correlation with improvements in PSSD symptom score was strongest for the symptoms and feelings domain (r = 0.816) and weakest for the work or school domain (r = 0.509); results were similar for correlations with the sign summary score (DLQI symptoms and feelings [r = 0.815] and DLQI work or school [r = 0.499]). Note that all correlations were significant (P < 0.0001).
Discussion
The PSSD was developed and validated as a self‐administered PRO instrument to measure the severity of symptoms (itch, burning, stinging, skin tightness and pain) and signs (skin dryness, cracking, scaling, shedding/flaking, redness and bleeding) in psoriasis patients.10, 11, 12 Our results from the VOYAGE 1 trial demonstrate that, based on self‐reported 24‐h recall using the PSSD, patient‐reported perceptions of broad improvements in symptoms and signs over time were high and correlate with the high efficacy of guselkumab. Compared with placebo at week 16 and adalimumab at weeks 24 and 48, guselkumab demonstrated better responses in mean summary scores as well as higher proportions of patients with either clinically meaningful improvements or symptom‐ and sign‐free status (scores of 0). Results were consistent for both symptom and sign summary scores as well as for individual item scores and were maintained through week 48 when compared with adalimumab. Furthermore, improvements in the PSSD correlated with changes in physician‐reported evaluations of disease severity (PASI and IGA) and patient‐reported assessments of HRQoL (DLQI). These findings are consistent with those from the PSSD validation study, which was based on data from the phase III NAVIGATE study of patients with moderate‐to‐severe psoriasis.12
The PSSD has unique properties compared with other PRO instruments used in psoriasis trials. Unlike the DLQI, the PSSD was developed with guidance from the United States Food and Drug Administration,5 is specific for psoriasis and measures individual signs and symptoms; in addition, the version used in the study has a short recall period of 24 h. Furthermore, the individual items of the PSSD instrument were developed based on input collected directly from patients. Most of these items, which have been commonly reported in the literature, are included in other recently published PRO instruments (such as the Psoriasis Symptom Diary and the Psoriasis Symptom Inventory) for the evaluation of symptoms and signs in clinical trials of psoriasis.8, 9 The more comprehensive PRO data from the PSSD allow for separating summary scores for psoriasis symptoms and psoriasis signs. Additionally, the symptoms summary score can be used as a primary or secondary endpoint for assessing clinical efficacy (e.g. becoming symptom‐free) and the signs summary score, as a secondary endpoint to supplement physician assessments such as PASI score.
One limitation of the study is that baseline PSSD scores were missing in 22% of randomized patients due to technical issues related to the use of the electronic tablet device at the start of the study. Because baseline data were required for the PSSD analyses, patients without baseline PSSD scores were excluded. However, baseline demographic and disease characteristics and trends in clinical improvement over time were similar between those with and without baseline PSSD scores, suggesting that the absence of data at baseline was random and no systematic bias was introduced. Another limitation is that patient‐reported assessments of improvements reported in this manuscript are limited to the first 48 weeks of the study. Durability of PSSD responses will be assessed over the remainder of this long‐term study, as patient‐reported symptoms and signs will continue to be evaluated using the 7‐day version of the PSSD.
In conclusion, patients treated with guselkumab reported clinically meaningful improvements from baseline in both symptoms and signs based on the PSSD, a new validated psoriasis‐specific PRO measure. Levels of improvement with guselkumab were superior to those reported by placebo‐treated patients at week 16 and by adalimumab‐treated patients at weeks 24 and 48. In addition, higher proportions of patients achieving clinically meaningful improvements from baseline and symptom‐ and sign‐free scores over time further substantiated the better response with guselkumab treatment. Given the totality of these data and the consistency across the endpoints at all timepoints, these results may assist clinicians in making treatment decisions for patients with psoriasis. The PSSD is a promising instrument for evaluating future psoriasis treatments, particularly because input from individual patients is assuming an increasingly prominent role in more fully assessing disease status.
Supporting information
Table S1. Psoriasis Symptoms and Signs Diary.
Table S2. Patient characteristics among randomized patients with missing PSSD scores at baseline.
Table S3. Summary of PASI, IGA, and DLQI for randomized patients with and without baseline PSSD scores
Acknowledgements
The authors thank Cynthia Arnold, BSc, CMPP, Janssen Scientific Affairs, LLC, Spring House, PA, USA, and Cynthia Guzzo, MD, HireGenics, Duluth, GA, USA, for their editorial assistance and writing support. Christopher Griffiths is a National Institute for Health Research Senior Investigator.
Conflict of Interests
Dr. Papp has been a consultant, scientific adviser, investigator, scientific officer, and/or speaker for AbbVie, Akros, Allergan, Amgen, Anacor, Astellas, AstraZeneca, Baxalta, Baxter, Boehringer Ingelheim, Bristol‐Myers Squibb, CanFite, Celgene, Dermira, Devonian, Dow Pharma, Eli Lilly, Galderma, Genentech, GlaxoSmithKline, Janssen, Kyowa Hakko Kirin, Leo, Medimmune, Meiji Seika Pharma, Merck Sharp & Dohme, Merck‐Serono, Mitsubishi Pharma, Mylan, Novartis, Pfizer, Regeneron, Roche, Sanofi/Genzyme, Stiefel, Takeda, UCB and Valeant. Dr. Blauvelt has served as a scientific adviser and clinical study investigator for AbbVie, Aclaris, Allergan, Almirall, Amgen, Boehringer Ingelheim, Celgene, Dermavent, Dermira, Eli Lilly, Genentech/Roche, GlaxoSmithKline, Janssen, Leo, Merck Sharp & Dohme, Meiji, Novartis, Pfizer, Purdue Pharma, Regeneron, Sandoz, Sanofi‐Genzyme, Sienna Pharmaceuticals, Sun Pharma, UCB, Valeant and Vidac and as a paid speaker for Eli Lilly, Janssen, Regeneron and Sanofi‐Genzyme. Dr. Kimball has received grants/research funding and honoraria as a consultant and investigator for AbbVie, Dermira, Janssen, Novartis and Regeneron; received honoraria as a consultant for Eli Lilly and UCB and received fellowship programme funding as an investigator from Abbvie and Janssen. Drs. Han, Randazzo, Wasfi, Shen and Li are employees of Johnson and Johnson and have received stock or stock options. Dr. Griffiths has received honoraria as an advisory board member and/or speaker from AbbVie, Almirall, Eli Lilly, Janssen, Leo, Novartis, Pfizer, Sun Pharma and/or UCB Pharma and research grants from AbbVie, Eli Lilly, Janssen, Leo, Novartis, Pfizer and Sandoz.
Funding
This study (clinicaltrials.gov: NCT02207231) was sponsored by Janssen Research & Development, LLC, Spring House, PA, USA.
References
- 1. Armstrong AW, Schupp C, Wu J et al Quality of life and work productivity impairment among psoriasis patients: findings from the National Psoriasis Foundation survey data 2003–2011. PLoS ONE 2012; 7: e52935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finlay AY, Coles EC. The effect of severe psoriasis on the quality of life of 369 patients. Br J Dermatol 1995; 132: 236–244. [DOI] [PubMed] [Google Scholar]
- 3. Fredriksson T, Pettersson U. Severe psoriasis ‐ oral therapy with a new retinoid. Dermatologica 1978; 157: 238–244. [DOI] [PubMed] [Google Scholar]
- 4. Berth‐Jones J, Grotzinger K, Rainville C et al A study examining inter‐ and intrarater reliability of three scales for measuring severity of psoriasis: Psoriasis Area and Severity Index, Physician's Global Assessment and Lattice System Physician's Global Assessment. Br J Dermatol 2006; 155: 707–713. [DOI] [PubMed] [Google Scholar]
- 5. United States Food and Drug Administration . Guidance for Industry: Patient‐reported outcome measures: use in medical product development to support labelling claims. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf (last accessed on October 24, 2017).
- 6. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–216. [DOI] [PubMed] [Google Scholar]
- 7. Chren MM, Lasek RJ, Quinn LM et al Skindex, a quality‐of‐life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol 1996; 107: 707–713. [DOI] [PubMed] [Google Scholar]
- 8. Lebwohl M, Swensen AR, Nyirady J et al The Psoriasis Symptom Diary: development and content validity of a novel patient‐reported outcome instrument. Int J Dermatol 2014; 53: 714–722. [DOI] [PubMed] [Google Scholar]
- 9. Mease PJ. The Psoriasis Symptom Inventory: An effective patient‐reported outcome measure of psoriasis severity. J Rheumatol 2015; 42: 1034–1036. [Google Scholar]
- 10. Mathias SD, Feldman SR, Crosby RD et al Measurement properties of a patient reported outcome measure assessing psoriasis severity: the Psoriasis Symptoms and Signs Diary. J Dermatol Treat 2016; 27: 322–327. [DOI] [PubMed] [Google Scholar]
- 11. Feldman SR, Mathias SD, Schenkel B et al Development of a patient‐reported outcome questionnaire for use in adults with moderate‐to‐severe plaque psoriasis: The Psoriasis Symptoms and Signs Diary. J Dermatol Dermatol Surg 2016; 20: 19–26. [Google Scholar]
- 12. Armstrong A, Puig L, Langley R et al Validation of psychometric properties and development of response criteria for the Psoriasis Symptoms and Signs Diary (PSSD): results from a phase III clinical trial. J Dermatol Treat 2017. [DOI] [PubMed] [Google Scholar]
- 13. Teng MWL, Bowman EP, McElwee JJ et al IL‐12 and IL‐23 cytokines: from discovery to targeted therapies or immune‐mediated inflammatory diseases. Nat Med 2015; 21: 719–729. [DOI] [PubMed] [Google Scholar]
- 14. Blauvelt A, Papp KA, Griffiths CEM et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: Results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76: 405–417. [DOI] [PubMed] [Google Scholar]
- 15. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: Results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431. [DOI] [PubMed] [Google Scholar]
- 16. Langley RG, Tsai T‐F, Flavin S et al Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results from a phase III clinical trial. Br J Dermatol 2018; 178: 114–123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Psoriasis Symptoms and Signs Diary.
Table S2. Patient characteristics among randomized patients with missing PSSD scores at baseline.
Table S3. Summary of PASI, IGA, and DLQI for randomized patients with and without baseline PSSD scores


