Abstract
Objectives
A prospective study was performed to examine the relationship of childhood socioeconomic status (SES) with cognition and the rate of change in a nationally representative sample of community‐dwelling middle‐aged and older Chinese population.
Methods
This study mainly focused on 3 composite measures of cognitive function, including Telephone Interview of Cognitive Status, word recall, and drawing a figure successfully. Childhood SES was evaluated by parental occupation and education, childhood residence, and self‐evaluated financial status. We designed an analysis strategy adding predictors incrementally in different models to examine the changes of effects of childhood SES on cognition by latent growth curve models.
Results
Finally, a total of 10 533 respondents were prospectively studied, including 5980 respondents aged 45–59 and 4553 aged 60–90. Cognition in younger cohort showed a curvilinear change, while cognition in older cohort showed a linear decline. After controlling for covariates, middle‐aged respondents with higher self‐evaluated financial status (β: −0.22, P < .001), better health status (β: −0.13, P < .001), higher parental education (β: 0.17 and 0.10, P < .001), who had lived in city/town before 16 years (β: 0.69, P < .001), and whose fathers engaged in nonfarming work (β: 0.43, P < .001) were associated with the better baseline cognition. Similar results were found in older cohort. Additionally, early‐life SES was not associated with cognitive decline in both cohorts.
Conclusions
This study indicates that childhood SES is associated with mid‐life and late‐life baseline cognition, but it is not contributed to cognition decline. Interventions in early‐life focused on improving childhood SES might have positive impacts on baseline cognition in later‐life.
Keywords: cognition, cognitive decline, life‐course, socioeconomic status
Key points.
Cognition in younger cohort showed a curvilinear change, with slight increase in 2013 and subsequent decline in 2015, while cognition in older cohort showed a linear decrease.
After adult SES and demographic factors adjusted, higher childhood SES, such as higher paternal education, higher self‐evaluated financial status, and better health status, indicated higher cognitive performance at baseline in both middle‐aged and older group. Childhood SES was not a contributor to the later‐life cognitive decline in younger or older cohorts.
Childhood SES played different roles in cognition among middle‐aged and older adults. For example, paternal occupation, maternal education, and the residence in childhood were the predictors of the baseline cognition in younger cohort, while played no role in cognition in older cohorts.
Our results implicated that adult SES is the main contributor to the baseline cognition, and the adult education is main driver to the decline in 2 age groups.
1. INTRODUCTION
With the rapid aging progression, the prevalence of cognition dysfunction is increasing dramatically.1, 2 Cognition dysfunction, such as Alzheimer's disease and other dementias, is associated with lower quality of life, increased the medical care, and causes more than 10 millions of disability adjusted of life years, leading enormous social and economic burden to societies.2, 3, 4 Reportedly, there will be over 70% of global dementia cases expected to occur in developing countries by 2050.5 Accumulating evidence has examined in developed countries that life‐course drivers played important roles in cognition. However, little research has investigated these associations in elder population in developing countries,6 such as China, which is one of the most rapidly aging societies in Asia, facing a great threat of dementia.7, 8 Thus, an additional examination of this issue targeting Chinese appeared to be warranted.
A growing number of studies regarding late‐life cognition have concentrated on the effects of early‐life and late‐life factors, such as childhood deprivation and adversity, adult socioeconomic status (SES), and lifestyles.9, 10, 11, 12 Childhood SES is a powerful predictor of health outcomes in later life, always measured by the parental education and occupation or household income. Recently, emerging data have indicated that both higher childhood and adult SES can prevent poor cognitive function in later‐life.13, 14, 15, 16 Adverse adult SES is also considered to not only associate with the risk of the cognition impairment but also with the rate of cognition decline in late‐life.17 However, previous studies have yielded conflicting findings in whether childhood SES is contributed to the rate of cognition decline, with several,13, 18, 19, 20 but not all,15, 21, 22, 23 suggesting that childhood SES may not be associated with late‐life cognition decline once adult SES is taken into account. Gonzalez et al18 used the American Health and Retirement Study data and found that the rate of cognition change in older adults was not predicted by the childhood SES. In contrast, Marden and their colleagues22 also used the same data and suggested that both the early and adult SES were related to the memory function or slowing cognition decline. Moreover, several gaps in the evidence base challenge the relationship of childhood SES with late‐life cognition.
From a life course perspective, more and more researchers have argued that it is necessary to consider the relationship between early‐life and late‐life SES. However, empirical studies regarding on the relative contributions did not take the adult SES into account18; therefore, it could not interpret how accumulations and interactions of these conditions affect cognitive function. Furthermore, different birth cohorts experienced different historical events and lifestyles, which may have carried different impacts on later life. However, research on age group differences in the linkages between childhood SES and late/mid life cognition is scarce and inconclusive, most of studies exploring these associations based on mixed sample, ignoring that cognition is age‐related.6 Additionally, studies conducted in older population may be obscured by aging‐related pathophysiological changes. Therefore, detection of cognitive decline in at‐risk middle‐aged and older groups has become a research priority. However, most commonly used neuropsychological tests have norms for older populations aged 60 years or above. The norms of cognitive function are relatively under‐researched among Chinese middle‐aged and older adults owing to the lack of large‐scale community‐based studies.
The aim of this study is to examine the relative effects of childhood SES on mid‐life and late‐life cognitive function and rate of change varied in 2 different age groups (45–59 years old and 60+ years old), based a nationally representative cohort study of middle‐aged and older Chinese adults. Four main questions are needed to address: Is there an association between childhood SES and mid‐life or late‐life cognitive function? Whether the early‐life conditions contribute to the cognition decline? If so, are these associations modified when adult SES and demographic covariates added in the model? Whether childhood SES play different roles in different life stages.
2. METHODS
2.1. Data and sample
This study was based on a nationally representative longitudinal survey of community‐dwelling middle‐aged and older Chinese population from the China Health and Retirement Longitudinal Study (CHARLS), which is intended to provide a high‐quality public microdatabase with a wide range of information on ageing‐related issues. A multistage probability sampling design and a proportional sampling method were used in the baseline survey to ensure the representative of the sample. Detailed description of the sampling procedure is available from the previous study.24 The baseline survey was performed in 2011, and the follow‐up surveys were conducted every 2 years. Finally, a total of 17 708 respondents from 150 counties/districts in China were included in baseline survey. Out of them, 13 978 individuals (78.9%) provided anthropometric and physical performance measures. The second wave successfully reinterviewed 15 788 individuals, and the third wave reinterviewed 15 333 participants. The sample included in final analysis was restricted to respondents who were aged 45 or older at baseline, took part in the cognition tests, provided anthropometric and physical performance measures, and completed the 3 time follow up (n = 10 533).
2.2. Cognitive function
In line with the American Health and Retirement Study, this study mainly focused on 3 composite measures of cognitive functioning. We used the sum of 3 measures to represent the respondent's cognitive status as a whole, with total scores ranging from 0 to 21. The first measurement is based on Telephone Interview of Cognitive Status. Telephone Interview of Cognitive Status reflects the mental status of cognition and involves 10 questions, including recalling today's date (month, day, year), the day of the week and season of the year, and serial 7 subtraction from 100 (up to 5 times). This dimension score is calculated on the number of correct answers, ranging from 0 to 10. The second measurement of cognition relies on word recall. It mainly tests episodic memory of cognition. After the interviewer reading a list of 10 Chinese words, the participant is asked to repeat the words in any order immediately. About 4 minutes later, the respondent is asked to recall the list of words again. The word recall score is based on the average of the number of correct answers, ranging from 0 to 10. The third cognitive measure is a test of the ability to draw a picture of 2 overlapping pentagons successfully. Respondents who draw the picture successfully can receive a score of 1, and those who fail to do so receive a score of 0. This is an overall measure of the respondent's cognitive function.
2.3. Childhood SES and health
Childhood SES was assessed by parents' occupation, parents' educational attainments, childhood residence, and self‐evaluated financial status. Parents' occupation dichotomized in 2 groups, including farming and not farming groups. Parents' educational attainments were divided into 11 categories: “illiterate,” “did not finish primary school but capable of reading and/or writing,” “home school,” “elementary school,” “middle school,” “high school,” “vocational school,” and 4 higher levels in CHARLS design. However, as most of the respondents parents had no‐schooling at all, we divided only 2 categories in our analyses, including “no schooling” and “capable of read or writing.” Childhood residence was divided into village and city/town, because of a deep rural‐urban disparity in China, such as the economic level, accessibility of health care, and the living conditions.25 Childhood self‐evaluated financial status was assessed by the respondents' rating of their financial status when compared with their neighbors before age 16 years on a 5‐point scale ranging from 1 to 5.
Childhood health status was assessed by self‐reported health status before 16 years old on a 5‐point scale, which ranged from excellent to poor. This measurement of childhood health status was proved reasonably well reliability and validity by previous studies.26
2.4. Adult SES
Adult SES was assessed by the proxies of adult educational attainment, family food expenditure of last week, and the total expenditure (except for food) last month. Adult educational attainment was coded into 5 categories: “no schooling,” “primary or less than,” “middle school,” “High school,” and “college, university, or high.” We used the household expenditure as the alternative of self‐reported household income or wealth, because there were a large number of missing values in these variables. Previous evidence indicated that compared with household income and assets, household expenditure can provide a more reliable and valid assessment for living standards in developing counties.27 Food expenditure and other consumption expenditure were considered as the measurements of the household expenditure in this study.
2.5. Other covariates
Based on the analysis of previous studies, following potential covariates at baseline requiring adjustment were identified and assessed: (1) socio‐demographic characteristics, such as age, gender (male = 1 and female = 2), marital status (current married = 1, divorced/separated = 2, widowed = 3, and never married = 4), and hukou status (agricultural hukou = 1 and nonagricultural hukou = 2). Hukou is the registration system in China created in 1955 to restrict internal population movement, especially rural‐to‐urban migration, divided Chinese into 2 categories: agricultural hukou and non‐agricultural hukou.28 (2) Lifestyles and health behaviors, including smoking status (current smoker = 1 and not current smoker = 2) and alcohol consumption (drinking more than once a month = 1, drinking less than once a month = 2, and none of these = 3). (3) Adult health status was assessed by a count of diseases, activities of the daily living (ADL), and depressive symptoms. The number of self‐reported health chronic diseases was coded into 3 groups (0 = 1, 1–2 = 2, and ≥ 3 = 3) at baseline suvery. Activities of the daily living and the depressive symptoms were assessed at every 2‐year follow‐ups. Activities of the daily living were assessed by using 5 types of instrumental ADL and 6 types of ADL, including bathing, dressing, eating, indoor transferring, toileting, and continence, with answers varying from “no difficulty” to “much difficulty” and scores ranging from 0 to 3. Depressive symptoms were evaluated by using the 10‐item Center for Epidemiologic Studies Depression Scale short form, which has been viewed as a valid and reliable instrument to assess the depression in China.29
2.6. Data analysis
Previous evidence has revealed that the influence of childhood environment on cognition differed by birth cohort in China. Therefore, we performed the data analysis separately in middle‐aged group and older group. The descriptive statistics were conducted by using the SPSS software version 18.0. The latent growth curve model (LGCM) analyses were performed by using MPLUS, version 7.0 (Muthén & Muthén, Los Angeles, CA, USA). A missing value analysis was conducted to identify the missingness mechanism. Based on the results, we created a hypothesis in this research that data were missing at random. Statistical analyses for LGCM were conducted by using a full information maximum likelihood estimation method, which estimated the parameters based on all available data and provides the robust estimates in the presence of nonnormality and nonindependence of observations.30, 31
Latent growth curve models can create random intercepts and random slopes to depict a different trajectory for each case over time,32 which can not only model the intraindividual and interindividual variations by creating the latent variables but also it permits exploration of the consequences of change.33 The trajectory of cognition change across time was modeled with 2 latent variables. The latent intercept growth factor represented the initial level of cognition, while the latent slope growth factor reflected the rate of the cognition change. Considering the 3 times follow‐up, we modeled with the specified linear LGCM. A series of models were set to add the potential factors incrementally to identify the changes of the effects childhood SES on mid‐life and late‐life cognition.
The following indices were used to assess the goodness of model fit: χ2statistic, Tucker‐Lewis index ≥ 0.95, comparative fix index ≥ 0.95, standardized root mean square residual ≤ 0.05, and root mean square error of approximation ≤ 0.08, with 90% confidence interval ≤ 0.08.34
3. RESULTS
Table 1 presents the baseline characteristics of the participants in 2011. Finally, there are 10 533 individuals involved in our analyses, including 5980 respondents aged 45–59, while 4553 aged 60–90. The mean (SD) age of the middle‐aged and older groups were 52.37 (4.41) years and 66.89 (5.74) years, respectively. Distributions of childhood SES and health conditions were summarized in Table 2.
Table 1.
Baseline characteristics of the sample in 2011
| Characteristic | Total, N = 10 533 | Aged 45–59, N = 5980 | Aged 60–90 years, N = 4553 | Statistics | P |
|---|---|---|---|---|---|
| Age, mean ± SD | 58.64 ± 8.77 | 52.37 ± 4.41 | 66.89 ± 5.74 | −146.64 | <.001 |
| Gender | 52.29 | <.001 | |||
| Male, % (ref.) | 5017 (47.6) | 2660 (44.5) | 2348 (51.6) | ||
| Female, % | 5522 (52.4) | 3319 (55.5) | 2203 (48.4) | ||
| Marital status | 495.83 | <.001 | |||
| Current married, % (ref.) | 8962 (85.1) | 5338 (89.3) | 3624 (79.6) | ||
| Divorced/separated, % | 531 (5.0) | 373 (6.2) | 158 (3.5) | ||
| Widowed, % | 958 (9.1) | 227 (3.8) | 731 (16.1) | ||
| Never married, % | 82 (0.8) | 42 (0.7) | 40 (0.9) | ||
| Hukou status | 34.10 | <.001 | |||
| Agricultural Hukou, % (ref.) | 8567 (81.4) | 4980 (83.3) | 3587 (78.8) | ||
| Nonagricultural Hukou, % | 1964 (18.6) | 999 (16.7) | 965 (21.2) | ||
| Number of chronic disease | 213.19 | <.001 | |||
| 0, % (ref.) | 6743 (64) | 4172 (69.8) | 2571 (56.5) | ||
| 1–2, % | 3351 (31.8) | 1636 (27.4) | 1715 (37.7) | ||
| 3 or more, % | 437 (4.2) | 171 (2.9) | 266 (5.9) | ||
| Current smoker | 53.78 | <.001 | |||
| Yes, % (ref.) | 4129 (39.2) | 2162 (36.2) | 1967 (43.2) | ||
| No, % | 6403 (60.8) | 3817 (63.8) | 2586 (56.8) | ||
| Alcohol consumption | 19.34 | <.001 | |||
| More than once a month, % (ref.) | 2696 (25.6) | 1537 (25.7) | 1159 (25.5) | ||
| Less than once a month, % | 807 (8.5) | 516 (8.6) | 291 (6.4) | ||
| None of these, % | 6030 (63.2) | 3927 (65.7) | 2103 (68.2) | ||
| Education in adulthood | 685.88 | <.001 | |||
| No schooling, % (ref.) | 2305 (21.9) | 1032 (17.3) | 1273 (28.0) | ||
| Primary or less than, % | 4430 (42.1) | 2168 (36.3) | 2262 (49.7) | ||
| Middle school, % | 2506 (23.8) | 1822 (30.5) | 684 (15.0) | ||
| High school, % | 1094 (10.4) | 841 (14.1) | 253 (5.6) | ||
| College, university, or high, % | 194 (1.8) | 115 (1.9) | 79 (1.7) | ||
| Food expenditure of past week, median (IQR) | 100 (150.0) | 112 (150.0) | 100 (160.0) | −10.92 | <.001 |
| Other expenditure last month, median (IQR) | 180 (240.0) | 220 (258.0) | 140 (203.0) | −22.62 | <.001 |
Abbreviation: IQR, interquartile range.
Table 2.
Distributions of childhood SES and health conditions
| Characteristic | Aged 45–59 years | Aged 60–90 years | Statistics | P | ||
|---|---|---|---|---|---|---|
| N = 5980 | % | N = 4553 | % | |||
| Childhood SES | ||||||
| Maternal occupation | 1.97 | .16 | ||||
| Farming (ref.) | 5048 | 94.2 | 3736 | 94.9 | ||
| Nonfarming | 308 | 5.8 | 200 | 5.1 | ||
| Paternal occupation | 18.30 | <.001 | ||||
| Farming (ref.) | 4641 | 80.9 | 3500 | 84.2 | ||
| Nonfarming | 1095 | 19.1 | 655 | 15.8 | ||
| Maternal education | 203.07 | <.001 | ||||
| No schooling (ref.) | 4956 | 86.5 | 4145 | 95.0 | ||
| Capable of read or writing | 773 | 13.5 | 217 | 5.0 | ||
| Paternal education | 99.85 | <.001 | ||||
| No schooling (ref.) | 3050 | 55.4 | 2733 | 65.4 | ||
| Capable of read or writing | 2458 | 44.6 | 1444 | 34.6 | ||
| Self‐evaluated financial status | 8.38 | .07 | ||||
| A lot better off than them (ref.) | 56 | 0.9 | 40 | 0.9 | ||
| Somewhat better off than them | 461 | 7.7 | 335 | 7.4 | ||
| Same as them | 3096 | 51.8 | 2327 | 51.4 | ||
| Somewhat worse off than them | 983 | 16.4 | 687 | 15.1 | ||
| A lot worse off than them | 1374 | 23.0 | 1141 | 25.1 | ||
| Self‐evaluated childhood health status | 11.98 | .02 | ||||
| Much healthier than others (ref.) | 1050 | 17.6 | 693 | 15.3 | ||
| Somewhat healthier | 1072 | 18.0 | 878 | 19.4 | ||
| About average | 3073 | 51.5 | 2389 | 52.7 | ||
| Somewhat less healthy | 467 | 7.8 | 245 | 7.6 | ||
| Much less healthy | 305 | 5.1 | 225 | 5.0 | ||
| Childhood residence | 0.46 | .50 | ||||
| Village | 5503 | 92.0 | 4173 | 91.7 | ||
| City/town | 476 | 8.0 | 379 | 8.3 | ||
Abbreviation: SES, socioeconomic status.
Distributions of cognition by gender among middle‐aged and older were showed in the Figure 1. The data showed curvilinear changes of cognition in 45–59 years old group, with slight increase in 2013 and subsequent decline in 2015. While older respondents exhibited lower initial cognition and steeper cognitive decline in comparison to younger counterparts across time. Males exhibited better cognition in comparison to their counterparts with lower cognition across time in both 2 age groups.
Figure 1.
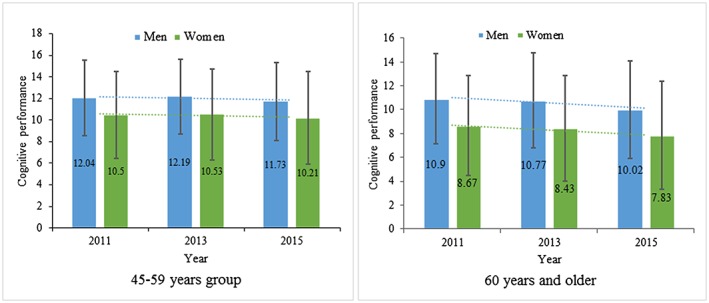
Distributions of cognitive performance by gender among middle‐aged and older Chinese adults from CHARLS during 2011–2015 [Colour figure can be viewed at http://wileyonlinelibrary.com]
3.1. LGCMs for adults aged 45–59
The estimates for the 4 established models and their detailed fit indices among the middle‐aged adults from CHARLS were summarized in Table 3. With the incremental predictors included in models subsequently, the model fitness improved obviously and the final model with all predictors showed a satisfactory goodness of fit.
Table 3.
Estimates of the intercept and slope of cognition for the 4 established LGCMs in the middle‐aged group
| Characteristic | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Predictors of intercept of cognition | ||||||||
| Initial status | 11.50 | <.001 | ||||||
| Childhood SES | ||||||||
| Maternal occupation | −0.25 | .42 | −0.33 | .26 | −0.32 | .26 | ||
| Paternal occupation | 0.73 | <.001 | 0.56 | <.001 | 0.43 | <.001 | ||
| Maternal education | 0.26 | <.001 | 0.17 | <.001 | 0.17 | <.001 | ||
| Paternal education | 0.17 | <.001 | 0.12 | <.001 | 0.10 | <.001 | ||
| Self‐evaluated childhood financial status | −0.29 | <.001 | −0.29 | <.001 | −0.22 | <.001 | ||
| Self‐evaluated childhood health status | −0.16 | <.001 | −0.15 | <.001 | −0.13 | <.001 | ||
| Childhood residence | 1.73 | <.001 | 0.77 | <.001 | 0.69 | <.001 | ||
| Demographics | <.001 | <.001 | ||||||
| Age | −0.13 | <.001 | −0.08 | <.001 | ||||
| Gender | −1.74 | <.001 | −1.28 | <.001 | ||||
| Hukou status | 1.49 | <.001 | 1.32 | <.001 | ||||
| Marital status | −0.94 | <.001 | −0.66 | <.001 | ||||
| Current smoker | 0.06 | .69 | 0.10 | .46 | ||||
| Alcohol consumption | −0.02 | .81 | −0.00 | .95 | ||||
| Number of chronic diseases | 0.10 | .14 | −0.13 | .042 | ||||
| Adulthood SES | ||||||||
| Education | 0.30 | <.001 | ||||||
| Food expenditure of last week | 0.14 | <.001 | ||||||
| The total expenditure (except for food) last month | 0.28 | <.001 | ||||||
| Predictors of rate of change | ||||||||
| Slope | −0.16 | <.001 | ||||||
| Childhood SES | ||||||||
| Maternal occupation | 0.27 | .08 | 0.28 | .07 | 0.28 | .08 | ||
| Paternal occupation | 0.07 | .38 | 0.06 | .45 | 0.06 | .47 | ||
| Maternal education | 0.02 | .43 | 0.01 | .68 | 0.02 | .61 | ||
| Paternal education | 0.03 | .09 | 0.03 | .12 | 0.02 | .20 | ||
| Self‐evaluated childhood financial status | 0.00 | .98 | 0.00 | .99 | 0.01 | .87 | ||
| Self‐evaluated childhood health status | −0.03 | .20 | −0.03 | .20 | −0.03 | .22 | ||
| Childhood residence | −0.22 | .12 | −0.24 | .09 | −0.20 | .16 | ||
| Demographics | ||||||||
| Age | −0.02 | <.001 | −0.01 | .04 | ||||
| Gender | 0.03 | .72 | 0.11 | .19 | ||||
| Hukou status | 0.03 | .07 | 0.05 | .52 | ||||
| Marital status | 0.14 | .10 | 0.14 | .10 | ||||
| Current smoker | 0.00 | .98 | 0.01 | .91 | ||||
| Alcohol consumption | 0.01 | .77 | 0.01 | .89 | ||||
| Number of chronic diseases | 0.05 | .16 | 0.04 | .26 | ||||
| Adulthood SES | ||||||||
| Education | −0.05 | <.001 | ||||||
| Food expenditure of last week | −0.07 | .01 | ||||||
| The total expenditure (except for food) last month | −0.02 | .47 | ||||||
| Fit indices | ||||||||
| AIC | 94 146.54 | 73 185.75 | 72 478.10 | 71 049.62 | ||||
| BIC | 94 200.11 | 73 327.66 | 72 710.28 | 71 339.43 | ||||
| RMSER | 0.08 (0.06, 0.10) | 0.03 (0.02, 0.04) | 0.02 (0.02, 0.03) | 0.02 (0.02, 0.03) | ||||
| CFI | 0.99 | 0.99 | 0.99 | 0.99 | ||||
| TLI | 0.98 | 0.98 | 0.98 | 0.98 | ||||
| SRMR | 0.02 | 0.01 | 0.00 | 0.01 | ||||
| P (chi‐ square test) | <.001 | <.001 | <.001 | <.001 | ||||
Abbreviations: AIC, Akaike information criterion; BIC, Bayesian information criterion; CFI, comparative fit index; LGCMs, latent growth curve models; RMSER, relative root mean square error; SES, socioeconomic status; SRMR, standardized root mean square residual; TLI, Tucker‐Lewis index.
Model 1 describes the changes of cognitive performance without any predictors. The baseline cognition in middle‐aged group was 11.50 (P < .001). The rate of cognition changes was −0.16 (P < .001), showing a typical decrease in the average rate of change in cognition across time. Additionally, the P values of variances for the intercept and slope in cognition were less than .01, suggesting that there were strong interindividual differences in both initial status and rate of change for cognition.
We added the childhood SES and health status in model 2 and found that except for the mother's occupation, other indicators of childhood SES and health status were all associated with mid‐life cognition, but did not affect the decline of cognition. Participants who had higher childhood SES, including higher self‐evaluated financial status, higher parents' education attainments, living in city or town, fathers engaged in nonfarming work, and better health, were associated with the better mid‐life cognitive performance. Childhood SES and health status explained 10.5% of the interindividual variance in initial status of cognition and 2.2% of the variance in the rate of decline.
We added the demographic covariates in model 3, resulting in the explained intercept variance and slope variance increased 27.7% and 5.5%, respectively. The indicators of childhood SES were still associated with baseline cognition, although these effects were slightly attenuated. Childhood SES still had no effects on cognition decline. Younger respondents exhibited better initial cognition and slower cognitive decline in comparison to older respondents across time. Compared with the respondents who were female, single with agricultural hukou status, their counterparts had better cognition at baseline.
We included all the predictors and covariates into LGCM in model 4. The explained intercept and slope variance were slightly improved 38.7% and 12.7%. The effects of the childhood SES and the demographic covariates on cognition showed stable. Each measures of adult SES was associated with initial status of cognition, with higher SES generally predicting better mid‐life cognitive function. Additionally, compared with participants with lower adult SES, individuals who had higher education and spent more on food predicted slower rate of cognition decline.
3.2. LGCMs for adults aged 60–90
Table 4 presents the estimates for the 4 established LGCMs and the detailed models fit indices in older group. Similarly to the younger group, model 5 showed that both P values of the intercept (10.12) and slope (−0.45) were ≤.001, indicating a lower intercept of cognition and a steeper rate of decline in comparison to those of middle‐aged group. Although most of results were similar, some differences were also identified in 2 cohorts. In older group, adults baseline cognition was only associated with the paternal education, self‐evaluated childhood SES, and health status, regardless of the parental occupation, maternal education, and childhood residence, which are different from the results of middle‐aged group.
Table 4.
Presents the estimates for the 4 established LGCMs and the detailed model fit indices in older group
| Characteristic | Model 5 | Model 6 | Model 7 | Model 8 | ||||
|---|---|---|---|---|---|---|---|---|
| β | P | β | P | β | P | β | P | |
| Predictors of intercept of cognition | ||||||||
| Initial status | 10.12 | <.001 | ||||||
| Childhood SES | ||||||||
| Maternal occupation | 0.72 | .06 | 0.53 | .14 | 0.43 | .19 | ||
| Paternal occupation | 0.70 | <.001 | 0.32 | .15 | 0.20 | .32 | ||
| Maternal education | 0.42 | <.001 | 0.25 | .02 | 0.06 | .60 | ||
| Paternal education | 0.34 | <.001 | 0.31 | <.001 | 0.17 | <.001 | ||
| Self‐evaluated childhood financial status | −0.24 | <.001 | −0.28 | <.001 | −0.15 | <.001 | ||
| Self‐evaluated childhood health status | −0.31 | <.001 | −0.27 | <.001 | −0.23 | <.001 | ||
| Childhood residence | 1.78 | <.001 | 0.56 | <.001 | 0.23 | .40 | ||
| Demographics | ||||||||
| Age | −0.15 | <.001 | −0.07 | <.001 | ||||
| Gender | −2.40 | <.001 | −1.16 | <.001 | ||||
| Hukou status | 2.03 | <.001 | 0.83 | <.001 | ||||
| Marital status | −0.42 | .01 | −0.16 | .31 | ||||
| Current smoker | 0.24 | .14 | 0.10 | .50 | ||||
| Alcohol consumption | 0.14 | .08 | 0.14 | .04 | ||||
| Number of chronic diseases | 0.03 | .71 | 0.00 | .99 | ||||
| Adulthood SES | ||||||||
| Education | 0.72 | <.001 | ||||||
| Food expenditure of last week | 0.04 | .55 | ||||||
| The total expenditure (except for food) last month | 0.31 | <.001 | ||||||
| Predictors of rate of change | ||||||||
| Slope | −0.45 | <.001 | ||||||
| Childhood SES | ||||||||
| Maternal occupation | 0.08 | .69 | 0.07 | .73 | 0.16 | .45 | ||
| Paternal occupation | 0.16 | .17 | 0.12 | .29 | 0.11 | .32 | ||
| Maternal education | −0.02 | .43 | −0.01 | .80 | −0.00 | .97 | ||
| Paternal education | −0.03 | .96 | −0.01 | .63 | −0.03 | .27 | ||
| Self‐evaluated childhood SES | −0.10 | .71 | 0.01 | .79 | 0.02 | .66 | ||
| Self‐evaluated childhood financial status | 0.04 | .19 | 0.05 | .15 | 0.07 | .30 | ||
| Childhood residence | 0.15 | .36 | 0.04 | .84 | −0.20 | .16 | ||
| Demographics | ||||||||
| Age | −0.04 | <.001 | −0.03 | <.001 | ||||
| Gender | −0.06 | .49 | 0.07 | .46 | ||||
| Hukou status | 0.17 | .04 | 0.10 | .24 | ||||
| Marital status | 0.06 | .46 | 0.05 | .55 | ||||
| Current smoker | 0.05 | .54 | 0.04 | .62 | ||||
| Alcohol consumption | −0.01 | .77 | −0.02 | .70 | ||||
| Number of chronic diseases | 0.03 | .40 | 0.03 | .39 | ||||
| Adulthood SES | ||||||||
| Education | 0.08 | <.001 | ||||||
| Food expenditure of last week | 0.00 | .97 | ||||||
| The total expenditure (except for food) last month | 0.07 | .06 | ||||||
| Fit indices | ||||||||
| AIC | 72 906.79 | 52 179.91 | 51 211.19 | 49 392.73 | ||||
| BIC | 72 958.18 | 52 314.05 | 51 430 | 49 666.40 | ||||
| RMSER | 0.09 (0.06,0.11) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.03) | ||||
| CFI | 0.99 | 0.99 | 0.99 | 0.99 | ||||
| TLI | 0.98 | 0.98 | 0.98 | 0.98 | ||||
| SRMR | 0.02 | 0.01 | 0.00 | 0.01 | ||||
| P (chi‐square test) | <.001 | <.001 | <.001 | <.001 | ||||
Abbreviations: AIC, Akaike information criterion; BIC, Bayesian information criterion; CFI, comparative fit index; LGCMs, latent growth curve models; RMSER, relative root mean square error; SES, socioeconomic status; SRMR, standardized root mean square residual; TLI, Tucker‐Lewis index.
Additionally, after adding all the predictors in the model 8, we found a better explanation of intercept variance and slope variance in elder group (53.9% and 14.3%) than those of in younger group (38.7% and 12.7%).
4. DISCUSSION
First, the results across a 5‐year follow‐up documented that although the trajectories of cognition decline were different, there was an overall decline in cognition among middle‐aged and older Chinese adults. Second, consistent with prior studies, higher childhood SES did has positive effect on cognitive performance in both age groups, while these effects were attenuated as adult SES and demographic factors adjusted. Additionally, the effects of childhood SES on baseline cognition varied from different age groups. Finally, we found no support for the association between childhood SES and the decline of cognition function in 2 age groups.
Childhood SES as one of the most frequently investigated childhood factors was often measured by fathers' educational attainment, fathers' occupation, and childhood financial status. A few studies have also included mothers' education/occupation as the indicators of childhood SES. Consistent with the our results, emerging evidence has been found to support the positively links between childhood SES and mid‐life and late‐life cognitive function in the United States, United Kingdom, and other western counties.35, 36, 37, 38 Data reported in the United States have documentated that higher childhood SESs measured by parental education and father's occupation were positively related to global cognitive function among older adults aged 50 and older.18, 39, 40 Additionally, after controlling for the adult SES and demographic factors, our results showed that higher childhood SESs, including higher parental education attainments and paternal occupation, higher self‐evaluated financial status, better health status in childhood, and living in city/or town before 16 years old, were associated with better baseline cognitive performance in younger cohort, while only paternal education attainment, self‐evaluated financial status, and health status had effects on the initial cognition status in older cohort. These different effects of childhood SES among 2 age groups can be explained by the different historical events, lifestyles they experienced. China has undergone dramatic social and economic developments after 1949, the year when the People's Republic of China was established. For the older cohort who grew up during the early half of the 20th century may experienced civil wars, famine, and malnutrition in their childhood and had less opportunities to be educated in comparison to the younger cohort.6 These cohort differences may lead to great impacts on the relationship of childhood SES with mid‐life or late‐life cognition.
Our findings also suggested that childhood SES was not a contributor to the later‐life cognitive decline in younger or older cohorts, which is consistent with the study conducted in Taiwan.41 It has suggested that the effects of childhood SES measured by paternal education and occupation could be largely explained by adult SES and that disadvantageous SES in adulthood further exacerbated declines in late‐life cognitive functioning. Our study also found that in comparison to childhood SES, adult SES can provide more explanation of intercept variance and slope variance than childhood SES in both middle‐aged and older groups. Adult SES is the main contributor to the baseline cognition, and the adult education is main driver to the decline in 2 age groups, which is in line with previous studies.42, 43 Food expenditure indicator is associated with the cognition decline in middle‐aged group but is not suitable for older group. The results of the latest research suggested that later‐life income was the only primary driver of rate of cognition change.22 However, in their study, they only used the 2 indicators to reflect the early adult SES and did not test these effects by age groups, leading to the poor comparability between their results and ours.
Although the direct influence of poor childhood SES is small, it is indeed one of the modifiable risk factors that contribute to cognitive performance. Increasing literature has indicated that opportunities for getting a higher educational attainment and better health status, especially among the children who were in lower SES environment, can bring much adult SES benefits, lower morbidity rate, and less medical care in later‐life adulthood, which may be relative to the better cognition status and slower cognition decline.18 Our results highlight the importance of public health implications that even small benefits are achievable in preventing cognitive impairment in later life through maintaining or improving a higher SES in early life.
The strengths of this study included its longitudinal design, and its large, nationally representative sample, ensuring reliable inferences to be made among middle‐aged and older Chinese population. Most of studies concerning this topic were based on cross‐sectional design, few cohort was conducted in China. To our best knowledge, this is the first longitudinal study in the China investigating the contributions of childhood SES to cognition and its rate of change using the LGCMs. This method enables us to permit exploration of the consequences of change. Further, using the cognitive scale, rather than a measure of cognitive impairment, we can capture social differences in cognition at the general representative Chinese population. Moreover, most studies on childhood SES and cognitive outcomes have only focused on educational attainment as the measure of SES,44 neglected the influence of other social childhood conditions, such as parents' occupation and childhood financial status. To reflect the childhood SES comprehensively, we included more indicators to assess it. Additionally, we investigated the life course SES effects on 2 age groups and found that indicators of childhood SES indeed have many different effects on these 2 groups.
Although we conducted the data analysis using the advanced analytical methods, some attention should be paid when explaining our results. Firstly, the indices of childhood SES and health status were all collected through their retrospective self‐report, which might introduce recall bias and influence the estimation of the childhood SES for cognition. Secondly, due to limitations of the data, childhood cognition status, relative biomarkers, and anthropometry were not measured, which may limit the study to illuminate the mechanism linking lower childhood SES and cognition impairment. Specially, the 3 measures in a 5‐year period made it impossible to scrutinize nonlinear relationships between childhood SES and cognition, we could only simply assume it in linear association. Indeed, previous study had suggested that model fit for the freely estimated or quadratic models did not provide any substantially precise enhancement than linear models.18 In addition, the lack of a third cognition testing visit at the baseline limited the study to examination of the practices effects on cognitive performance, which can mask and underestimate the cognition decline in younger cohorts. However, a recent study have suggested that after controlling the impact of practice effects and other covariates, their results provide strong, longitudinal evidence of cognitive aging in midlife women.45 Consistent with their results, our results showed an overall decline cognition in younger group (Table 3). Last but not least, although this study revealed cognitive decline as a whole across a 5‐year period of observation, suggested associations must be interpreted cautiously because they were generated at an interval that might not be long enough to discover the obvious cognitive decline. Hence, from a life course perspective, a long‐period longitudinal study should be well designed to reinvestigate the association of childhood SES and cognitive function.
5. CONCLUSIONS
Although the effects of socioeconomic conditions during childhood seem to be, in part, modified by adult SES, the linkage of adverse environment in early‐life and worse cognitive function seems to be independent of covariates during the life course. Supporting evidence was found, suggested that it is adult SES, not childhood SES related to cognition decline, particularly when covariates are considered. The study suggests that interventions in early life focused on improving SES or health status might have positive impact on baseline cognition.
CONFLICT OF INTREREST
None declared.
ACKNOWLEDGEMENTS
The data collection was supported by the Behavioral and Social Research Division of the National Institute on Aging of the National Institute of Health (grants 1‐R21‐AG031372‐01, 1‐R01‐AG037031‐01, and 3‐R01AG037031‐03S1) the Natural Science Foundation of China (grants 70773002, 70910107022, and 71130002), the World Bank (contracts 7145915 and 7159234), and Peking University. The sponsor had no role in this study design, data analysis, data interpretation, or the writing of the report. We acknowledge all the participants in the survey design and data collection and the CHARLS research team for collecting high quality, nationally representative data, and for making the data public.
Sha T, Yan Y, Cheng W. Associations of childhood socioeconomic status with mid‐life and late‐life cognition in Chinese middle‐aged and older population based on a 5‐year period cohort study. Int J Geriatr Psychiatry. 2018;33:1335–1345. 10.1002/gps.4930
REFERENCES
- 1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112‐2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2015 Neurological Disorders Collaborator Group . Global, regional, and national burden of neurological disorders during 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology. 2017;16:877‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shipley BA, Der G, Taylor MD, Deary IJ. Cognition and all‐cause mortality across the entire adult age range: health and lifestyle survey. Psychosom Med. 2006;68(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 4. Obisesan TO, Gillum RF. Cognitive function, social integration and mortality in a U.S. national cohort study of older adults. BMC Geriatr. 2009;9(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and meta‐analysis. Alzheimers & Dement. 2013;9(1):63‐75. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Z, Liu J, Li L, Xu H. The long arm of childhood in China: early‐life conditions and cognitive function among middle‐aged and older adults. J Aging Health. 2018;30(8):1319‐1344. 10.1177/0898264317715975. [DOI] [PubMed] [Google Scholar]
- 7. Jia J, Wang F, Wei C, et al. The prevalence of dementia in urban and rural areas of China. Alzheimers & Dement. 2014;10(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 8. Ding D, Zhao Q, Guo Q, et al. The Shanghai Aging Study: study design, baseline characteristics, and prevalence of dementia. Neuroepidemiology. 2014;43(2):114‐122. [DOI] [PubMed] [Google Scholar]
- 9. McEniry M. Early‐life conditions and older adult health in low‐ and middle‐income countries: a review. J Dev Orig Health Dis. 2013;4(01):10‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobayashi LC, Glymour MM, Kahn K, et al. Childhood deprivation and later‐life cognitive function in a population‐based study of older rural South Africans. Soc Sci Med. 2017;190:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hughes TM, Sink KM. Hypertension and its role in cognitive function: current evidence and challenges for the future. Am J Hypertens. 2016;29(2):149‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Radford K, Delbaere K, Draper B, et al. Childhood stress and adversity is associated with late‐life dementia in aboriginal Australians. Am J Geriatr Psychiatry. 2017;25(10):1097‐1106. [DOI] [PubMed] [Google Scholar]
- 13. Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol. 2001;30(2):256‐263. [DOI] [PubMed] [Google Scholar]
- 14. Chen WY. On the relationship between economic conditions around the time of birth and late life cognitive abilities: evidence from Taiwan. Econ Hum Biol. 2016;22:126‐139. [DOI] [PubMed] [Google Scholar]
- 15. Whalley LJ, Dick FD, McNeill G. A life‐course approach to the aetiology of late‐onset dementias. Lancet Neurol. 2006;5(1):87‐96. [DOI] [PubMed] [Google Scholar]
- 16. Everson‐Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am J Epidemiol. 2003;158(11):1083‐1089. [DOI] [PubMed] [Google Scholar]
- 17. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta‐analysis. BMC Public Health. 2014;14(1):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonzalez HM, Tarraf W, Bowen ME, Johnson‐Jennings MD, Fisher GG. What do parents have to do with my cognitive reserve? Life course perspectives on twelve‐year cognitive decline. Neuroepidemiology. 2013;41(2):101‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turrell G, Lynch JW, Kaplan GA, et al. Socioeconomic position across the lifecourse and cognitive function in late middle age. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):S43‐S51. [DOI] [PubMed] [Google Scholar]
- 20. Marmot M, Shipley M, Brunner E, Hemingway H. Relative contribution of early life and adult socioeconomic factors to adult morbidity in the Whitehall II study. J Epidemiol Community Health. 2001;55(5):301‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Melrose RJ, Brewster P, Marquine MJ, et al. Early life development in a multiethnic sample and the relation to late life cognition. J Gerontol B Psychol Sci Soc Sci. 2015;70(4):519‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marden JR, Tchetgen EJ, Kawachi I, Glymour MM. Contribution of socioeconomic status at 3 life‐course periods to late‐life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol. 2017;186(7):805‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plassman BL, Williams JW Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med. 2010;153(3):182‐193. [DOI] [PubMed] [Google Scholar]
- 24. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yi Z, Gu D, Land KC. The association of childhood socioeconomic conditions with healthy longevity at the oldest‐old ages in China. Demography. 2007;44(3):497‐518. [DOI] [PubMed] [Google Scholar]
- 26. Haas SA. The long‐term effects of poor childhood health: an assessment and application of retrospective reports. Demography. 2007;44(1):113‐135. [DOI] [PubMed] [Google Scholar]
- 27. Lei X, Sun X, Strauss J, Zhang P, Zhao Y. Depressive symptoms and SES among the mid‐aged and elderly in China: evidence from the China Health and Retirement Longitudinal Study national baseline. Soc Sci Med. 2014;120:224‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sha T, Cheng W, Yan Y. Prospective associations between pulse pressure and cognitive performance in Chinese middle‐aged and older population across a 5‐year study period. Alzheimers Res Ther. 2018;10(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen H, Mui AC. Factorial validity of the Center for Epidemiologic Studies Depression Scale short form in older population in China. Int Psychogeriatr. 2014;26(1):49‐57. [DOI] [PubMed] [Google Scholar]
- 30. Wu W, West SG, Taylor AB. Evaluating model fit for growth curve models: integration of fit indices from SEM and MLM frameworks. Psychol Methods. 2009;14(3):183‐201. [DOI] [PubMed] [Google Scholar]
- 31. Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods. 2001;6(4):330‐351. [PubMed] [Google Scholar]
- 32. Bollen K, Curran P. Latent Curve Models: A Structural Equation Perspective. A JOHN WILEY & SONS: Hoboken, New Jersey; 2006. [Google Scholar]
- 33. Preacher KJ, Wichman AL, MacCallum RC, Briggs NE. Latent growth curve modeling. London: SAGE Publications; 2008. [Google Scholar]
- 34. Kline R. Principles and Practice of Structural Equation Modeling. New York: The Guilford Press; 2010. [Google Scholar]
- 35. Horvat P, Richards M, Malyutina S, et al. Life course socioeconomic position and mid‐late life cognitive function in Eastern Europe. J Gerontol B Psychol Sci Soc Sci. 2014;69(3):470‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fors S, Lennartsson C, Lundberg O. Childhood living conditions, socioeconomic position in adulthood, and cognition in later life: exploring the associations. J Gerontol B Psychol Sci Soc Sci. 2009;64:750‐757. [DOI] [PubMed] [Google Scholar]
- 37. Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE. Life‐course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. Am J Epidemiol. 2011;173(10):1148‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karlamangla AS, Miller‐Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am J Epidemiol. 2009;170(3):331‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo Y, Waite LJ. The impact of childhood and adult SES on physical, mental, and cognitive well‐being in later life. J Gerontol B Psychol Sci Soc Sci. 2005;60(2):S93‐S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glymour MM, Tzourio C, Dufouil C. Is cognitive aging predicted by one's own or one's parents' educational level? Results from the three‐city study. Am J Epidemiol. 2012;175(8):750‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chiao C, Botticello A, Fuh JL. Life‐course socio‐economic disadvantage and late‐life cognitive functioning in Taiwan: results from a national cohort study. Int Health. 2014;6(4):322‐330. [DOI] [PubMed] [Google Scholar]
- 42. Lyketsos CG, Chen LS, Anthony JC. Cognitive decline in adulthood: an 11.5‐year follow‐up of the Baltimore Epidemiologic Catchment Area study. Am J Psychiatry. 1999;156(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 43. Evans DA, Beckett LA, Albert MS, et al. Level of education and change in cognitive function in a community population of older persons. Ann Epidemiol. 1993;3(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 44. Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015‐2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. evidence for cognitive aging in midlife women: study of womens health across the nation. PLoS One. 2017;3(12):e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]


