Abstract
The BD FACSVia™ system is a novel flow cytometer with improved workflow efficiencies. To evaluate the HLA‐B27 application developed on the BD FACSVia system utilizing the BD™ HLA‐B27 kit, we conducted a concordance study at three centers to compare with the BD FACSCalibur™ system. Prepared donor samples (n = 594) were analyzed on both the BD FACSVia and BD FACSCalibur for the HLA‐B27 assay. Adjudication of HLA‐B27 discordant results was performed using the reverse sequence‐specific oligonucleotide (rSSO) DNA typing method (LABType® SSO, One Lambda). On the BD FACSVia system 80 B27 positive, 499 B27 negative and 15 “Inconclusive” samples were observed. The corresponding BD FACSCalibur results were 73 B27 positive, 502 B27 negative and 19 “gray zone” samples. The overall concordance of HLA‐B27 determination was 98% between the two systems with seven more positives identified on BD FACSVia as compared to BD FACSCalibur. The equivocal zone between positive and negative on BD FACSVia (named “Inconclusive”) and on BD FACSCalibur (named “gray zone”) is due to antibody cross reactivity of HLA‐B27 clone GS145.2. One negative sample verified with the rSSO DNA method was reported as HLA‐B27 positive by the BD FACSVia system leading to a false positive result. Our study demonstrated concordance results between the BD FACSVia system and BD FACSCalibur. Intersite reproducibility of BD HLA‐B27 assay remained within the limits of acceptability. © 2018 The Authors. Cytometry Part B: Clinical Cytometry published by Wiley Periodicals, Inc. on behalf of International Clinical Cytometry Society
Keywords: HLA‐B27 typing, flow cytometry, concordance study, interlaboratory reproducibility
INTRODUCTION
HLA‐B27 is a class I antigen molecule of the major histocompatibility complex. It is well documented that HLA‐B27 is a genotype common to a number of rheumatic inflammatory disorders, such as ankylosing spondylitis (AS), Reiter's syndrome, acute anterior uveitis, as well as inflammatory bowel disease 1, 2, 3, 4, 5, 6. HLA‐B27 is strongly associated with AS, a systemic inflammatory disease that affects the spine and the sacroiliac and peripheral joints. The heart, bowels, and skin may also be involved in AS. Approximately, 90% of people with AS express the HLA‐B27 antigen compared to only 8% of healthy individuals.
Although DNA‐based molecular genotyping methods are available to determine the presence of the HLA‐B27 gene 7, 8, flow cytometric HLA‐B27 screening on peripheral blood lymphocytes using the HLA‐B27 monoclonal antibody is very widely used in clinical diagnostic laboratories due to its simplicity, cost effectiveness, and a short turnaround time 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20. A confounding feature of flow cytometric HLA‐B27 typing is antibody cross‐reactivity caused by the fact that the HLA‐B27 antigen shares common epitopes with a large HLA‐B7 cross‐reacting group (CREG) 21. The BD HLA‐B27 kit includes HLA‐B27 antibody clone GS145.2. Previous studies have shown that this monoclonal antibody mainly cross reacts with HLA‐B7, followed by other antigens such as B16, B17, B22, B37, B42 22, 23, 24, 25, resulting in false positive HLA‐B27 results using flow cytometry. In such cases, alternate methods, such as DNA‐based molecular typing technology are needed to further clarify HLA‐B27 status.
The HLA‐B27 assay developed on the BD FACSVia system1 is an algorithm‐driven application utilizing the IVD BD HLA‐B27 kit for routine HLA‐B27 screening. To verify performance of the HLA‐B27 clinical assay on the BD FACSVia system, we conducted a concordance and interlaboratory reproducibility study at three sites versus the BD FACSCalibur system. The three study centers were BloodCenter of Wisconsin (Milwaukee, WI), University of Southern California (Los Angeles, CA) and MedLab of BD Life Sciences (San Jose, CA). The MedLab site was not involved in study management, database management or subject selection. This site participated in the clinical trial in the same way as an outside site by following the study protocol and guidelines of good clinical practice (GCP). Therefore, the MedLab site had no impact on potential bias in study results. This performance evaluation was performed under the GCP guidelines and the US Code of Federal Regulations.
MATERIALS AND METHODS
Study Design
Concordance study
Whole blood specimens from individual donors were enrolled and were prepared using the BD HLA‐B27 kit. Samples were then run on the BD FACSVia and BD FACSCalibur side‐by‐side to determine the HLA‐B27 outcome on each system. In cases where HLA‐B27 status on the BD FACSVia was different from that of the BD FACSCalibur, specimens were sent to a molecular testing lab in the BloodCenter of Wisconsin to determine HLA‐B27 using the PCR‐rSSO method (LABType® SSO, One Lambda).
Interlaboratory reproducibility
Whole blood specimens from two donors (HLA‐B27 positive and negative) were shipped to each of the three study centers per study day. Each donor specimen was processed at each site using BD HLA‐B27 reagent to generate five stained replicates and run once per day on the BD FACSVia system for six nonconsecutive days.
Specimen Enrollment and Processing for the Concordance Study
Five hundred and ninety four specimens (594) were enrolled from 594 donors in K2 EDTA or Sodium Heparin anticoagulant and stored at room temperature (20° to 25°C) before processing. The site institutional review board or ethics committee reviewed and approved the study protocol before proceeding to site initiation of the investigational activity. Remnant whole blood specimens were enrolled, or samples were obtained from a qualified vendor/blood donor program. Remnant specimens are defined as human specimens no longer being used in a clinical test, which otherwise would have been discarded as biological waste. Subject's protected health information was deidentified from laboratory testing staff before enrollment. Demographic information of the sample cohort was not collected because the purpose of the study was to demonstrate substantial equivalence between the two flow cytometers in the evaluation of HLA‐B27 phenotype expression. As directed by the BD HLA‐B27 kit package insert, all whole blood samples in the concordance study were stained/processed/fixed within 48 hours from blood draw and analyzed on the BD FACSCalibur and BD FACSVia systems within 24 hours of staining/processing/fixing. The storage temperature for the stained/processed/fixed samples were 2° to 8°C.
Specimen Shipping and Enrollment for the Interlaboratory Reproducibility Study
The fresh whole blood from two donors were drawn in K2 EDTA anticoagulant tubes in the morning and kept at room temperature (20° to 25°C). The whole blood tubes were shipped on the same day in the afternoon through overnight delivery to each of the three study centers. The anticoagulated whole blood was not processed/fixed before shipment and was kept at ambient temperature during shipping. Upon arriving at study sites on the next day, the whole blood was processed at the individual study center and analyzed on a BD FACSVia flow cytometer. The same two donors were drawn and their whole blood samples were shipped to study sites on November 2, 9, 11, 16, 18, 23, and 30, 2015. Flow cytometric analysis was performed next day for each sample on the BD FACSVia system at all sites. As directed by the BD HLA‐B27 kit package insert, whole blood samples from the two donors were stained/processed/fixed within 48 hours from blood draw and analyzed on the BD FACSVia system within 24 hours of staining/processing/fixing. The storage temperature for the stained/processed/fixed samples were 2° to 8°C.
Daily Instrument Setup and QC
The BD FACSVia flow cytometer contains a blue (488 nm) and a red laser (640 nm) with four fixed voltage fluorescence detectors. The wavelengths of optical filters are 533/30 nm (FL1), 585/40 nm (FL2), >670 nm Long Pass (FL3), and 675/25 nm (FL4). To set up the BD FACSVia system, operators used BD Cytometer Setup and Tracking (CS&T) beads and BD FACSVia clinical software to generate an Instrument QC report. During instrument QC, BD FACSVia clinical software verified that CS&T bright beads were placed within the target fluorescence channels on the instrument. Fluorescence compensation was optimized based on the daily median fluorescence intensity values of the bright beads. The bright bead robust coefficient of variation (rCV) was also measured during setup for each fluorescence channel and compared with preset QC criteria. Instrument sensitivity was calculated automatically during the CS&T setup process and subjected to predefined specifications. For quality control of optics, electronics, and fluidics of the system, a “Pass” on the Instrument QC report had to be obtained. HLA‐B27 assay setup was subsequently performed using BD FACSVia clinical software to place the BD HLA‐B27 calibration beads on the bead suffix (target value, in units of log median fluorescence (LMF) for 256 channels full scale). An HLA‐B27 setup report with passing results was generated for each study day.
To set up the BD FACSCalibur instrument, BD CalibriteTM beads and BDTM HLA‐B27 calibration beads were prepared per the reagent instructions for use and run on the BD FACSCalibur instrument using BD FACSCompTM software. Optimized photomultiplier tube (PMT) and compensation values were generated for the BD HLA‐B27 assay by BD FACSComp software. Both the BD FACSComp QC report and BD HLA‐B27 setup report were obtained with passing results on each study day.
HLA‐B27 Determination
Samples were prepared according to the manufacturer's instructions included with the BD HLA‐B27 kit. Three production lots of the BD HLA‐B27 kit were used in this study to prepare fluorescently labeled samples for cytometric analysis. For each specimen, a total of two sample tubes stained with BD HLA‐B27 reagent were prepared for acquisition, with one tube for each system. On the BD FACSVia system, optimized instrument settings were pre‐set by the software test definition for the BD HLA‐B27 assay. HLA‐B27 acquisition and analysis was performed by BD FACSVia clinical software through automated algorithms. On the BD FACSCalibur instruments, the HLA‐B27 assay was automatically accomplished using BD HLA‐B27 software. On the BD FACSVia system, a PE (FL2 detector) versus side scatter (SSC) dot plot was first used by the software algorithm to identify the cluster of events with a uniformly bright CD3‐positive fluorescent signal. After the predefined minimum number of CD3 T‐lymphocytes or time parameter was met as stopping criteria, the software algorithm began analysis for the events falling within the CD3 T‐lymphocyte gate. The LMF intensity of the HLA‐B27 FITC signal (FL1) was calculated for the CD3 T‐lymphocytes and compared to the predetermined decision marker. Gating strategy of the BD HLA‐B27 assay on the BD FACSVia and BD FACSCalibur systems is presented in Figure 1.
Figure 1.
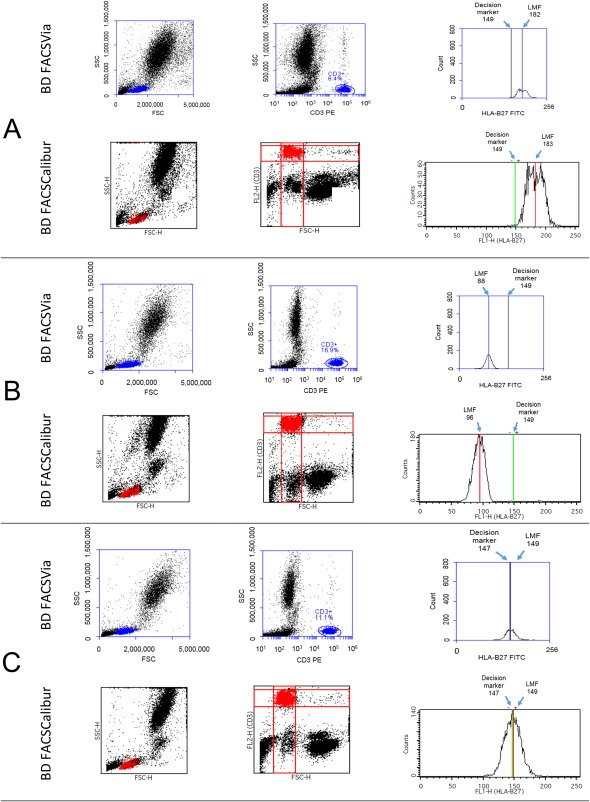
Gating strategy used by the software algorithm of BD HLA‐B27 assay on the BD FACSVia and BD FACSCalibur systems. On the BD FACSVia, initial gating of CD3+ T‐cells was placed in the CD3 PE (FL2) vs.SSC plot. On the BD FACSCalibur, initial gating of CD3+ T‐cells was placed in the FL2 (CD3) vs.FSC plot. LMF of HLA‐B27 FITC was obtained from the interval gate placed on all CD3+ T‐cells in the HLA‐B27 FITC (FL1) histogram. LMF was further compared to the predetermined decision marker using BD HLA‐B27 software algorithm to generate HLA‐B27 outcome. (A) Panel: HLA‐B27 positive sample (B) Panel: HLA‐B27 negative sample (C) Panel: Gray zone (Inconclusive) sample. In each panel, the same donor sample was analyzed using the same lot of BD HLA‐B27 kit on both the BD FACSCalibur and BD FACSVia systems. The decision marker of each reagent lot was set by the suffix on the reagent vial for HLA‐B27 FITC/CD3 PE.
The predetermined HLA‐B27 decision marker is a property of the BD HLA‐B27 reagent and is set by the suffix on the reagent vial for HLA‐B27 FITC/CD3 PE. The reagent suffix (decision marker) is in the unit of LMF and needs to be manually entered into the BD FACSCalibur and BD FACSVia clinical software before sample acquisition. The predetermined decision marker remains the same on the BD FACSCalibur and the BD FACSVia systems. To utilize the same decision marker on both instruments, the mathematical algorithm for the BD HLA‐B27 assay on the BD FACSVia system converts its LMF measurement scale to be the same as that on the BD FACSCalibur. This ensures that HLA‐B27 determination obtained on the BD FACSVia system is equivalent to the BD FACSCalibur.
On both flow cytometers, samples with HLA‐B27 LMF less than the cutoff were defined as negative. Samples with HLA‐B27 LMF more than the cutoff plus 10 LMF channels were defined as positive. Samples that fell in the range of cutoff ≤ LMF ≤ cutoff +10 were called “gray zone” on the BD FACSCalibur and reported as “Inconclusive” on the BD FACSVia. In this study, discordant HLA‐B27 results observed on the BD FACSCalibur vs.BD FACSVia were further verified using the reverse sequence‐specific oligonucleotide (rSSO) DNA typing method on a Luminex 100 (or 200) instrument (LABType® SSO, Catalog number RSSO1B, One Lambda, A Thermo Fisher Scientific Brand).
Statistical Analysis
Data of the interlaboratory reproducibility study was analyzed with the SAS® v9.3 program (SAS, Cary, NC), Analyse‐it® software (Analyse‐it, Leeds, UK), and Excel software (Microsoft®, Redmond, WA). A total of 30 acquisition runs (6 days × 5 replicates per run per day) were performed on the BD FACSVia system per study site for each donor (HLA‐B27 positive and negative) to evaluate precision. The standard deviation (SD) with 95% upper confidence limit (CL) of HLA‐B27 LMF was calculated to determine reproducibility.
RESULTS
Concordance Study
Table 1 shows the definition of concordance between the BD FACSVia system and BD FACSCalibur as well as study results. Overall percent concordance was calculated as 100*{(a + e + i)/n} for the five hundred ninety‐four (594) donor specimens enrolled at three study centers. Using the BD HLA‐B27 kit, 98% concordance in the determination of HLA‐B27 was achieved between the BD FACSVia system and the BD FACSCalibur instrument.
Table 1.
Definition and Results of Concordance Study
| Comparative method (BD HLA‐B27 kit on BD FACSCalibur) | |||||
|---|---|---|---|---|---|
| Positive | Gray zone | Negative | BD FACSVia row total | ||
| Test method (BD HLA‐B27 kit on BD FACSVia) | Positive | a = 72 | b = 8 | c = 0 | a + b + c = 80 |
| Inconclusive | d = 1 | e = 11 | f = 3 | d + e + f = 15 | |
| Negative | g = 0 | h = 0 | i = 499 | g + h + i = 499 | |
| BD FACSCalibur column total | a + d + g = 73 | b + e + h = 19 | c + f + i = 502 | n = 594 | |
Note: (a) Overall concordance was calculated as 100*{(a + e + i)/n} = 100*582/594 = 98%. (b) The five hundred and ninety four (594) samples were from 594 donors.
There were 12 discordant results between the two systems, as shown in Table 1 (b = 8, d = 1, f = 3), which required further testing using the rSSO DNA typing method. HLA‐B27 results for the 12 samples are summarized in Table 2. Seven more positive samples were identified by the BD FACSVia system compared to the BD FACSCalibur, as confirmed by the molecular testing method. One sample was observed with LMF over the “Inconclusive” zone by one channel resulting in false‐positive on the BD FACSVia system (Table 2).
Table 2.
Results of HLA‐B27 Using rSSO DNA Typing Method
| Discordant sample | BD FACSCalibur | BD FACSVia | Molecular test results |
|---|---|---|---|
| 1 | Gray Zone | Positive | Negative |
| 2 | Gray Zone | Positive | Positive |
| 3 | Gray Zone | Positive | Positive |
| 4 | Gray Zone | Positive | Positive |
| 5 | Gray Zone | Positive | Positive |
| 6 | Gray Zone | Positive | Positive |
| 7 | Gray Zone | Positive | Positive |
| 8 | Gray Zone | Positive | Positive |
| 9 | Negative | Inconclusive | Negative |
| 10 | Negative | Inconclusive | Negative |
| 11 | Negative | Inconclusive | Negative |
| 12 | Positive | Inconclusive | Positive |
Interlaboratory Reproducibility Study
The LMF value of HLA‐B27 FITC was measured to evaluate interlaboratory reproducibility of the BD HLA‐B27 assay. One lot of BD HLA‐B27 reagent kit was used in this study. On each study day, each site collected 5 replicates of LMF of HLA‐B27 FITC for a sample. Each site continued to analyze LMF of HLA‐B27 for the same donor for a total of 6 days using whole blood that was freshly drawn and shipped overnight for each study day. Each site therefore obtained six 6 sets of repeatedly measured LMF data points (a total of 30 LMF data points) from each donor on the BD FACSVia system. Summary statistics results are shown in Table 3 for the three study sites.
Table 3.
Summary Statistics for the Interlaboratory Reproducibility of BD FACSVia System
| LMF statistics of HLA‐B27 FITC per study site | ||||||||
|---|---|---|---|---|---|---|---|---|
| Donor type |
Study Site |
N | Mean | SD | CV% | Minimum | Median | Maximum |
|
HLA‐B27 negative |
BCW | 30 | 137.13 | 1.87 | 1.36 | 134.00 | 137.00 | 141.00 |
| MED | 30 | 136.13 | 1.36 | 1.00 | 134.00 | 136.00 | 138.00 | |
| USC | 30 | 134.40 | 1.73 | 1.29 | 130.00 | 134.00 | 138.00 | |
| HLA‐B27 positive | BCW | 30 | 173.00 | 1.62 | 0.94 | 170.00 | 173.00 | 176.00 |
| MED | 30 | 173.73 | 1.51 | 0.87 | 172.00 | 173.00 | 177.00 | |
| USC | 30 | 172.23 | 2.36 | 1.37 | 167.00 | 173.00 | 178.00 | |
Note: (a) BCW (BloodCenter of Wisconsin), USC (University of Southern California), MED (MedLab of BD Life Sciences). (b) CV% was calculated as SD/mean*100.
Total LMF reproducibility of a HLA‐B27 donor sample is represented by the standard deviation (SD) of total LMF calculated using combined data from all study sites. The ability to precisely measure LMF ensures reliable comparison of LMF with the HLA‐B27 cutoff. Variables affecting total LMF reproducibility are site (including operator and instrument), day, and within‐run variation. For each donor, the total mean LMF and standard deviation (SD) with upper 95% confidence limit (CL) were calculated using combined data from three sites to determine total interlaboratory reproducibility and within‐run repeatability, as shown in Table 4. The total interlaboratory reproducibility of the BD FACSVia system was ≤ 2.4 LMF channels, while within‐run repeatability was ≤ 1 LMF channel.
Table 4.
Precision Evaluation of the BD FACSVia System
| Donor type | Source of variation | Total LMF mean | SD with upper 95% CL |
|---|---|---|---|
| HLA‐B27 negative | Within‐run repeatability | 135.9 | 0.9 (1.0) |
| Total interlaboratory reproducibility | 135.9 | 2.1 (2.4) | |
| HLA‐B27 positive | Within‐run repeatability | 173.0 | 0.9 (1.0) |
| Total interlaboratory reproducibility | 173.0 | 2.0 (2.3) |
Note: (a) Combined data from three sites were used in the calculation of total LMF mean with SD (95% CL). (b) Total interlaboratory reproducibility included variables of within‐run, day and site/operator/instrument.
DISCUSSION
The determination of HLA‐B27 antigen status using flow cytometry is routinely performed in clinical laboratories in screening for different inflammatory rheumatic diseases, such as AS, which is strongly associated with the presence of the HLA‐B27 allele 1, 2. Although DNA molecular testing is the most accurate determination method, monoclonal antibody‐based flow cytometry technology is still the common screening method for HLA‐B27 typing because of its high throughput and relatively low cost 18. Cross‐reactivity of the HLA‐B27 antibody renders a small percentage of indeterminate results in the flow cytometric method. A “gray zone” (Inconclusive) above the decision marker was established on both systems to discriminate cross‐reactive samples. Other reference techniques are necessary to confirm “Inconclusive” or “gray zone” samples.
In our multicenter concordance study, we compared the BD HLA‐B27 assay on the new BD FACSVia system with the BD FACSCalibur instrument using the BD HLA‐B27 kit. We did not use another technique as a gold standard to predetermine HLA‐B27 status before testing on BD FACSCalibur and BD FACSVia systems. The same HLA‐B27 monoclonal antibody reagent was used on both systems to stain and prepare donor whole blood specimens using the lyse/wash sample preparation method. The overall concordance between the two systems was 98%. On the BD FACSCalibur flow cytometer, 19 of the total 594 samples (3.2%) fell in the “gray zone” with indeterminate results. Five hundred and two (502) samples were HLA‐B27 negative, while 73 samples were HLA‐B27 positive. On the BD FACSVia system, 15 of the total 594 samples (2.5%) were reported as “Inconclusive.” Four hundred and ninety‐nine (499) samples were HLA‐B27 negative, while 80 were HLA‐B27 positive.
There were 12 discordant samples (2.0% of the total samples, Table 2) between the BD FACSCalibur and BD FACSVia systems. Discordance occurred mainly around the border lines of negative vs.“gray zone” or “Inconclusive” and positive vs.“gray zone” or “Inconclusive”. The 12 samples were further verified using the rSSO DNA typing method (BloodCenter of Wisconsin, Milwaukee, WI). Seven of the eight HLA‐B27 positive samples determined on the BD FACSVia system (and “gray zone” on the BD FACSCalibur) were confirmed as HLA‐B27 positive by the molecular typing method. One of the eight was confirmed as HLA‐B27 negative by the molecular typing method, resulting in one false positive outcome on the BD FACSVia system. Four “Inconclusive” determinations reported on the BD FACSVia system [and negative 3 or positive 1 on the BD FACSCalibur] were confirmed by the molecular typing method as either HLA‐B27 negative or positive, matching the BD FACSCalibur determinations. None of the HLA‐B27 positive samples determined by the molecular typing method was identified as negative by either system. Overall, the new BD FACSVia system demonstrated substantial equivalence to the BD FACSCalibur instrument.
Our study showed that the BD‐FACSVia system reported seven more positive samples and one false positive (LMF of a HLA‐B27 negative sample above the “Inconclusive” zone) than the BD‐FACSCalibur in 594 total donors. BD‐HLA‐B27 antibody (clone GS145.2) showed some cross‐reactivity with other HLA‐B types which was mitigated by an equivocal zone implemented in the BD HLA‐B27 assay. Although majority of the cross‐reactivity samples fell in the “gray zone” (“Inconclusive” on the BD‐FACSVia system), we and other investigators had observed a small number of HLA‐B27 negative samples with LMF above this equivocal zone. Due to the fact that prevalence and distribution of HLA‐B antigen cross‐reactivity can vary 20, 22, we recommend laboratories to confirm this “gray zone” (“Inconclusive”) by performing their own studies.
How to cite this article: Zeng Y, Hiti A, Moranville S, Vicent G, Chavira S, Indig M de A, Graminske S, Boerner A, Schmidt A, Oreizy F, Chen A, Saleminik M, Mosqueda F, Lin A and Judge K. Human HLA‐B27 Typing Using the BD™ HLA‐B27 Kit on the BD FACSVia™ System: A Multicenter Study. Cytometry Part B 2018; 94B: 651–657.
Conflict of Interest: Seven authors are employees of BD Life Sciences.
Note
The HLA‐B27 assay on the BD FACSViaTM System is not available for sale in the US. Only FL1 and FL2 channels on the FACSViaTM instrument are cleared for in vitro diagnostic use in the US. FL3 and FL4 channels are for research use only. The FACSViaTM System with HLA‐B27 assay is CE marked to the European IVD Directive 98/79/EC.
LITERATURE CITED
- 1. Brewerton DA, Hart FD, Nicholls A, Caffrey M, James DCO, Sturrock RD. Ankylosing spondylitis and HLA 27. Lancet 1973;301:904–907. [DOI] [PubMed] [Google Scholar]
- 2. Lopez‐Larrea C, Gonzalez‐Roces S, Alvarez V. HLA‐B27 structure, function, and disease association. Curr Opin Rheumatol 1996;8:296–308. [DOI] [PubMed] [Google Scholar]
- 3. Reveille JD. An update on the contribution of the MHC to AS susceptibility. Clin Rheumatol 2014;33:749–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kiratiseavee S, Brent LH. Spondyloarthropathies: Using presentation to make the diagnosis. Cleve Clin J Med 2004;71:184–206. [DOI] [PubMed] [Google Scholar]
- 5. Cauli A, Dessole G, Fiorillo MT, Vacca A, Mameli A, Bitti P, Passiu G, Sorrentino R, Mathieu A. Increased level of HLA‐B27 expression in ankylosing spondylitis patients compared with healthy HLA‐B27‐positive subjects: a possible further susceptibility factor for the development of disease. Rheumatology 2002;41:1375–1379. [DOI] [PubMed] [Google Scholar]
- 6. Poddubnyy DA, Marker‐Hermann E, Kaluza‐Schilling W, Zeidler H, Braun J, Listing J, Sieper J, Rudwaleit M. Relation of HLA‐B27, tumor necrosis factor‐ promoter gene polymorphisms, and T cell cytokine production in ankylosing spondylitis – a comprehensive genotype‐phenotype analysis from an observational cohort. J Rheumatol 2011;38:2436–2441. [DOI] [PubMed] [Google Scholar]
- 7. Wagner T, Oberkanins C, Weinmayr B, Helmberg W, Kury F, Lanzer G. HLA‐B*27 typing by group‐specific hybridization in microtiter plates. Tissue Antigens 1998;52:175–178. [DOI] [PubMed] [Google Scholar]
- 8. Duangchanchot M, Puapairoj C, Romphruk A, Kongmaroeng C, Leelayuwat C, Romphruk AV. HLA‐B*27 subtypes in Northern and Northeastern Thais, Karens, and Bamars determined by a high‐resolution PCR‐SSP technique. Tissue Antigens 2009;73:590–594. [DOI] [PubMed] [Google Scholar]
- 9. Hulstaert F, Albrecht J, Hannet I, Lancaster P, Buchner L, Kunz J, Falkenrodt A, Tongio M, De Keyser F, Veys EM, et al. An optimized method for routine HLA‐B27 screening using flow cytometry. Cytometry 1994;18:21–29. [DOI] [PubMed] [Google Scholar]
- 10. Lingenfelter B, Fuller TC, Hartung L, Hunter J, Wittwer C. HLA‐B27 screening by flow cytometry. Cytometry 1995;22:146–149. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds WM, Evans PR, Wilson PJ, Wong WM, Darke C, Smith JL. Automated routine HLA‐B27 typing by flow cytometry. J Immunol Methods 1996;197:1–5. [DOI] [PubMed] [Google Scholar]
- 12. Chou SJ, Lai NS, Su JP, Wu JL, Lan JL. Two color analysis of HLA‐B27 antigen by flow cytometer—a comparative study by conventional microlymphocytotoxicity, DNA genotyping polymerase chain reaction and flow cytometric measurement. J Clin Lab Anal 1997;11:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ulrich G. Use of flow cytometry for HLA B27 phenotyping: study of a HLA B7/HLA B27 double marker technique. Allerg Immunol (Paris) 1997;29:11–14. [PubMed] [Google Scholar]
- 14. Coates E, Darke C. Routine HLA‐B27 typing by flow cytometry: differentiation of the products of HLA‐B*2702, B*2705 and B*2708. Eur J Immunol 1998;25:29–37. [DOI] [PubMed] [Google Scholar]
- 15. Bonnaud G, Aupetit C, Preux PM, Cogné M, Drouet M. Optimization of HLA‐B27 testing by association of flow cytometry and DNA typing. Clin Rheumatol 1999;18:23–27. [DOI] [PubMed] [Google Scholar]
- 16. Levering WHBM, Wind H, Granger V, Sintnicolaas K, Hooijkaas H, Reilly JT, Gratama JW, Barnett D. Long‐term stabilized blood samples as controls for flow cytometric HLA‐B27 screenings: a feasibility study. Cytometry B Clin Cytom 2008;74:169–181. [DOI] [PubMed] [Google Scholar]
- 17. Seo BY, Won DII. Flow cytometric human leukocyte antigen‐B27 typing with stored samples for batch testing. Ann Lab Med 2013;33:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Monneret G, Seffert O, Debard AL, Gutowski MC, Couprie N, Larbre JP, Tebib J, Bienvenu J. Standardization and automation of HLA B27 typing by flow cytometry: validation and comparison with microlymphocytotoxicity. Ann Biol Clin (Paris) 2000;58:461–466. [PubMed] [Google Scholar]
- 19. Darke C, Coates E. One‐tube HLA‐B27/B2708 typing by flow cytometry using two “Anti‐HLA‐B27” monoclonal antibody reagents. Cytometry B Clin Cytom 2010;78:21–30. [DOI] [PubMed] [Google Scholar]
- 20. Seipp MT, Erali M, Wies RL, Wittwer C. HLA‐B27 typing: evaluation of an allele‐specific PCR melting assay and two flow cytometric antigen assays. Cytometry B Clin Cytom 2005;63B:10–15. [DOI] [PubMed] [Google Scholar]
- 21. Tambur AR, Claas FHJ. HLA epitopes as viewed by antibodies: what is it all about? Am J Transplant 2015;15:1148–1154. [DOI] [PubMed] [Google Scholar]
- 22. Levering WHBM, Wind H, Sintnicolaas K, Hooijkaas H, Gratama JW. Flow cytometric HLA‐B27 screening: cross‐reactivity patterns of commercially available anti‐HLA‐B27 monoclonal antibodies with other HLA‐B antigens. Cytometry B Clin Cytom 2003;54B:28–38. [DOI] [PubMed] [Google Scholar]
- 23. Levering WHBM, van den Beemd R, te Marvelde JG, van Beers WAM, Hooijkaas H, Sintnicolaas K, Gratama JW. External Quality Assessment of flow cytometric HLA‐B27 typing. Cytometry 2000;42:95–105. [PubMed] [Google Scholar]
- 24. Hoffmann JJML, Janssen WCM. HLA‐B27 phenotyping with flow cytometry: further improvement by multiple monoclonal antibodies. Clin Chem 1997;43:1975–1981. [PubMed] [Google Scholar]
- 25. Neumüller J, Schwartz DWM, Dauber E, Mayr WR. Evaluation of four monoclonal antibodies against HLA‐B27 for their reliability in HLA‐B27 typing with flow cytometry (FC): Comparison with the classic microlymphocytotoxic test (MLCT). Cytometry 1996;26:209–215. [DOI] [PubMed] [Google Scholar]


