Abstract
4vHPV vaccination has been tested in randomized controlled trials under almost ideal conditions, and studies of real‐life use have compared outcome between vaccinated and unvaccinated women from the same birth cohort and mostly before screening age. Here we present the first—to our knowledge—evaluation of the impact of the 4vHPV vaccination in real life without selection bias in the reported data. The study has been carried out by comparing the results after first cervical screening between an HPV‐vaccinated and an unvaccinated birth cohort, consisting of women born in Denmark in 1993 and 1983, respectively. Cytology data covering an 8‐year period, from the age of 15 (age of HPV‐vaccination) to age 23 (age of invitation to first cervical screening), were retrieved from the Danish National Pathology Register. Abnormal cytology, defined as atypical squamous cell of undetermined significance and worse (ASCUS+) was detected in 9.4% of women born in 1993 as compared with 9.0% of women born in 1983; RR = 1.04 (95% CI 0.96–1.12), p = .29. Detection of high‐grade squamous intraepithelial lesion (HSIL) was statistically significantly lower in the 1993 than in the 1983 cohort, RR = 0.6 (95% CI 0.5–0.7), p < .0001, while the opposite pattern was seen for ASCUS RR = 1.4 (95% CI 1.2–1.6), p < .0001. The decrease in HSIL means that more women can be spared referral for colposcopy and biopsy. The increase of ASCUS could be explained by transition from conventional to liquid‐based cytology, but this observation requires further monitoring.
Keywords: HPV vaccination, human papillomavirus, cervical screening, cervical cytology
Short abstract
What's new?
In women without prior exposure to human papillomavirus (HPV) infection, quadrivalent HPV (4vHPV) vaccine is associated with a reduction in high‐grade squamous intraepithelial lesions (HSILs) and atypical squamous cells of undetermined significance (ASCUS). In this study, involving HPV‐vaccinated and unvaccinated birth cohorts of women in Denmark, the authors evaluated the impact of HPV vaccination on these same cytological abnormalities. The findings show that women who received 4vHPV vaccine as girls experienced no significant change in ASCUS+ at their first cytology. Incidence of HSILs, however, was significantly lower in vaccinated women compared with women who did not receive the vaccine.
The first human papillomavirus (HPV) vaccine was marketed in 2006, and its use disseminated rapidly in high income countries. Marketing of the HPV vaccine followed randomized controlled trials showing a close to complete protection against vaccine‐type‐related high‐grade cervical intraepithelial neoplasia (CIN2+) in women vaccinated as HPV‐naïve.1, 2 The trials evaluated the efficacy of the HPV vaccine under almost perfect conditions. Now, the first birth cohorts of women HPV vaccinated as girls, and therefore vaccinated when most of them were HPV naïve, approach screening age. This gives the opportunity to evaluate the effectiveness of the HPV vaccine in routine health care provided to the general population.
In Denmark, free HPV vaccination was introduced in October 2008 for girls born in 1993 to 1995. Since 2009, HPV vaccination of girls turning 12 years has been part of the child vaccination program.3 The quadrivalent HPV‐vaccine (4vHPV) Gardasil (Merck) was used in the Danish vaccination program from 2008 to 2015.4 The coverage was high during the first years of the vaccination program; amongst girls born in 1993 to 1995 around 80% had first dose, and 70% were fully vaccinated.5 A woman in Denmark is invited to cervical screening from the age of 23 years.6 The first birth cohort of Danish women offered free HPV vaccination as girls thus entered the cervical screening program in 2016.
The first indication of a reduced risk of cervical cancer in HPV‐vaccinated women will be a decrease in detection of cytological abnormalities. We report here on the outcome of this first screening round of women born in 1993. The objective was to determine the impact of the introduction of HPV vaccination on cytological abnormalities. The predefined hypothesis was that women offered HPV vaccination as girls had a better protection against developing cellular changes and subsequently a lower risk of abnormal cytology than women from unvaccinated birth cohorts.
Material and Methods
Study population
We conducted a population‐based cohort study in Denmark using national health register data. In Denmark, approval by the Danish Data Inspective Agency (SUND‐2016–22) serves as ethical approval of register‐based studies without contact with patients.
Since 1986, all Danish women turning 23 years have been invited to cervical screening.7 The screening sample is taken by the general practitioner (GP), and analyzed for cytological abnormalities at centralized pathology laboratories. Up until 2002, all laboratories used conventional, manually read cytology. Transition to liquid‐based cytology (LBC) and imaging‐assisted reading started in 2002, spread gradually, and these technologies have since 2015 been used in all laboratories.8, 9, 10 Since 1997, reporting to the National Pathology Register (NPR) has been mandatory for hospital laboratories, but it only became mandatory for private pathologists in 2005.11 Since 2000, cytology samples from all Denmark, except Copenhagen County, have been reported in hospital laboratories, while Copenhagen County predominantly used private pathologists.12 To ensure complete ascertainment of cytology results, we excluded the Copenhagen County from the study.
We studied two closed cohorts. First, women born in 1993, and living in Denmark all the time between 1 January 2009 and 1 April 2017 were included. This cohort had been offered free HPV‐vaccination at the age of 15 from October 2008, and was first invited to screening in 2016. Second, women born in 1983, and living in Denmark all the time between 1 January 1999 and 1 April 2007 were included. This cohort had never been offered free HPV vaccination, and was first invited to screening in 2006. Women from both birth cohorts living in or moving to Copenhagen County were excluded. End‐of‐study on 1 April 2017 was based on the availability of screening data for the 1993 birth cohort. By 1 April 2017, women born in December 1993 and invited to screening in December 2016 would have had at least three months to be screened following their invitation. The pseudo‐date of 1 April 2007 was used for the 1983 cohort to ensure equal follow‐up time for the two cohorts. In this study, exposure is defined as the offer of free HPV vaccination.
Data sources and outcomes
In Denmark, a unique personal identification number is assigned at birth or immigration to all persons, making it possible to link data from different registers and to ensure complete follow‐up. Data on vital status, emigration and addresses were retrieved from the Central Population Register. Data on cervical cytologies was retrieved from the NRP, where a sample was registered at the date it was received at the laboratory (Supporting Information, A). To ensure inclusion of samples analyzed by private pathologists, we supplemented with data from the National Health Services Register (NHSR; Supporting Information, B). As this register is based on reimbursement of services, it does not include information on diagnoses, and date of reimbursement is registered instead of date of service. Data on HPV‐vaccination status were retrieved from NHSR, which includes special codes for reimbursement of HPV vaccination within the child vaccination program. Data on HPV vaccination outside the program were retrieved from the Prescription Register, which includes information on all prescribed and purchased drugs in Denmark (Supporting Information, C).
Our primary outcome was diagnosis at first cytology. Secondary outcomes included proportion of women with a first cytology (any cytology), and age at first cytology. In Denmark, SNOMED13 is used in coding of pathology specimens. It is mandatory to assign both a T‐code for topography and an M‐code for morphology. Cervical cytologies are defined by T‐codes starting with T8X3*, see Supporting Information. For morphology coding, the Bethesda classification14 was introduced in 2007 in Denmark and implemented gradually. Therefore, especially for the 1983 birth cohort, cytologies were coded using former classification systems. These former codes were converted to Bethesda codes using the conversion table in the Danish Quality Assurance of the Cervical Cancer Screening Program15 supplemented with recoding by an expert pathologist (author LGL; Supporting Information D). In the analysis, cytology was divided into five categories: (i) negative for intraepithelial lesion or malignancy (NILM); (ii) atypical squamous cells of undetermined significance (ASCUS) (in the analysis the small group of atypical glandular cells (AGC) was merged with ASCUS); (iii) low‐grade squamous intraepithelial lesion (LSIL); (iv) high‐grade squamous intraepithelial lesion (HSIL) (in the analysis the small group of atypical squamous cells cannot exclude HSIL (ASCH) was merged with HSIL); and (v) unsatisfactory sample. In case of more than one M‐code per sample, a hierarchy of diagnoses was applied where the most severe diagnosis was chosen. A code of unsatisfactory was overruled by any diagnosis. ASCUS+ was used for ASCUS and any more severe diagnosis. Missing was assigned to samples analyzed by private pathologists and with no diagnostic code.
Age at first cytology was calculated from the date of birth and the date of receiving the sample at the laboratory or reimbursement date in case of samples with missing diagnostic code. Age at HPV vaccination was calculated from the date of birth and the date on reimbursement for first HPV vaccination within the child vaccination program and date of purchase for self‐paid vaccination outside the program. We defined HPV‐vaccinated women as those having had at least one dose of the HPV vaccine.
Data on geographical distribution of the two cohorts were tabulated from our retrieved data by study start for each of the cohorts. Data on proportion of women with at least high‐school degree at the age of 22 years were retrieved from public statistics.16 We searched the literature for data on self‐reported age at sexual debut. The most precise data were for women born 1960–198717 and 1983–1997,18 respectively. Data on proportion of girls sexually active at the age of 15 and on daily smoking at the age of 15 were retrieved from the School Health Surveys.19, 20
Statistical analyses
The study was conducted as a national, register‐based cohort study including all eligible women. No power calculation was therefore performed prior to the initiation of the study.
Indication for cytology is not reported in the NPR, but women screened before the age of 23 years are screened on their own or their doctor's initiative either as opportunistic screening or based on symptoms. Hence, results were stratified by age at first cytology. To avoid selection bias, our primary comparison was between the entire 1993 and the entire 1983 cohorts. However, in a subanalysis results for the 1993 birth cohort were stratified also by HPV‐vaccination status. Unsatisfactory cytology is more frequent in conventional cytology than in LBC.21 We therefore restricted the comparison between the two cohorts of first cytology to women with a first satisfactory sample.
Because we operated with closed cohorts of women with full follow‐up over the entire study period, incidence proportions (IP) were calculated with corresponding Clopper–Pearson 95% confidence intervals (CI). Relative risks (RR) for the 1993 versus the 1983 birth cohorts were calculated using χ 2 test with 95% CI. p values of <0.05 were considered to be statistically significant. Pseudo‐anonymized register data were accessed via the research service of Statistics Denmark. SAS statistical software version 9.4 (TS1M3) was used for the analysis.
Results
Study population
In total, 26,331 women were born in 1983 and lived in Denmark on January 1, 1999; of these, 6,870 women were excluded from the study mainly because they lived in/moved to the Copenhagen County (Table 1 and Supporting Information, Figures E and F). In total, 34,140 women were born in 1993 and lived in Denmark on 1 January 2009, and of these, 8,662 women were excluded for the same reasons as for the 1983 cohort. The percentage of excluded women was similar, 26% for 1983 and 25% for 1993. The two study populations included 19,461 and 25,478 women, respectively. The 1983 cohort was smaller than the 1993 cohort. In the beginning of the 1980s, the birth rate was in generally lower than in the 1990s probably due to the economic crisis, and in 1983, the birth rate hit a historical nadir.22
Table 1.
Characteristics of study population
| Characteristics | Birth cohort 1983 | Birth cohort 1993 |
|---|---|---|
| Number of womena | 26,331 | 34,140 |
| ‐Excluded because living in or moving to Copenhagen County | 4,507 (17%) | 6,029 (18%) |
| ‐Excluded for other reasons | 2,363 (9%) | 2,633 (8%) |
| Number of included women | 19,461 | 25,478 |
| Region of residence at study startb | ||
| Northern Denmark | 2,647 (14%) | 3,378 (13%) |
| Central Denmark | 5,389 (28%) | 7,011 (27%) |
| Southern Denmark | 5,346 (27%) | 6,786 (27%) |
| Zealand | 3,268 (17%) | 4,260 (17%) |
| Capital | 2,811 (14%) | 4,043 (16%) |
| High‐school degreec | 52% | 57% |
| Age of sexual debutd | 16 years | 16 years |
| Sexually active at age 15e | Not available | 37% |
| Smoking daily at age 15f | 21% | 10% |
| HPV‐vaccinatedg | 0% | 92% |
Number of women living in Denmark January 1, 1999/2009.
Proportion of number of included women.
Statistics Denmark: Proportion of women with at least high‐school degree at age 22.
Self‐reported sexual debut age for women born 1960–1987 (median) and 1983–1997 (mean).
Self‐reported sexual activity at age 15 years for girls born in 1995.
Self‐reported smoking status at age 15 years for girls born in 1983 and 1995, respectively.
HPV‐vaccination status at 1 April 2007 and 2017, respectively.
The distributions of women across the Danish regions were fairly similar for the two birth cohorts, and so were the proportions of women with at least a high‐school degree at the age of 22 years. The proportion of 15‐year old girls smoking daily decreased from 21% to 10%. The average age at sexual debut was 16 years for both cohorts. It should though be taken into account that the available data covered a broad range of birth cohorts. In the 1993 cohort, 37% of the girls reported to be sexually active at the age of 15 years.
In women born in 1993, 86% had been HPV‐vaccinated ≤15 years, and 6% above this age (Table 2). These women were all vaccinated with 4vHPV apart from three women vaccinated with the bivalent HPV vaccine (2vHPV). The vast majority were vaccinated through the child vaccination program, while only a minority had purchased the vaccine themselves; 1381 women out of 23,330 vaccinated women. The mean age of vaccination was 14.9 years. The proportion of women with any cytology was fairly similar in the two cohorts; 63% versus 61%; but cytology before age of invitation to screening was more common in the 1983 than in the 1993 cohort, 27% versus 19%. While overall 38% of the vaccinated women in the 1993 cohort had no cytology, this was the case for 55% of the unvaccinated women.
Table 2.
Age at first cytology and HPV vaccination in the study population
| Age at first cytology, years | 1983 cohort |
1993 cohort Age at first HPV vaccination, years |
||||
|---|---|---|---|---|---|---|
| <15 | 15 | >15 | Nonvaccinated | Total | ||
| <20 | 1,771 (9%) | 259 | 1,012 | 106 | 144 | 1,521 (6%) |
| 20–22 | 3,587 (18%) | 531 | 2,359 | 158 | 245 | 3,293 (13%) |
| ≥23a | 6,946 (36%) | 1,427 | 8,118 | 559 | 582 | 10,686 (42%) |
| No cytology | 7,157 (37%) | 1,766 | 6,412 | 623 | 1,177 | 9,978 (39%) |
| Total | 19,461 (100%) | 3,983 (16%) | 17,901 (70%) | 1,446 (6%) | 2,148 (8%) | 25,478 (100%) |
A few women turned 24 years before having their first cytology result.
Cervical cytology
The proportion of unsatisfactory samples and samples missing pathology diagnoses decreased from 6% in the 1983 cohort to 1.2% in the 1993 cohort (Table 3 and Fig. 1). In both cohorts, 60% of women had a satisfactory first sample. The mean age of first cytology was 21.7 years for women born in 1983 versus 22.1 years for women born in 1993. Among these women, largely the same proportions, 9.0% in the 1983 cohort and 9.4% in the 1993 cohort, had an abnormal cytology defined as ASCUS+; RR = 1.04 (95% CI 0.96–1.12), p = 0.29. However, the risk of having HSIL was statistically significantly lower in the 1993 than in the 1983 cohort, RR = 0.6 (95% CI 0.5–0.7), p < 0.0001.
Table 3.
Result of first cervical cytology in women offered HPV vaccination (1993) and women not offered HPV vaccination (1983)
| 1983, N = 19,461 | 1993, N = 25,478 | 1993 vs. 1983 | |||
|---|---|---|---|---|---|
| Birth cohort | Number of women with first samples, % | Excl. unsatisfactory and missing, % (CI) | Number of women with first samples, % | Excl. unsatisfactory and missing, % (CI) | RR (CI) Excl. unsatisfactory and missing |
| Any cytology |
12,304 63.2% |
11,564 59.4% |
15,500 60.8% |
15,315 60.1% |
0.96 (0.95–0.98)d
p < 0.0001 |
| Diagnosis of first cytology | |||||
| NILM |
10,523 85.5% |
10,523 91.0% (90.5–91.5) |
13,879 89.5% |
13,879 90.6% (90.1–91.1) |
0.996 (0.989–1.003) p = 0.29 |
| ASCUSa |
347 2.8% |
347 3.0% (2.7–3.3) |
644 4.2% |
644 4.2% (3.9–4.5) |
1.4 (1.2–1.6) p <0.0001 |
| LSIL |
486 4.0% |
486 4.2% (3.8–4.6) |
622 4.0% |
622 4.1% (3.8–4.4) |
1.0 (0.9–1.1) p = 0.56 |
| HSILb |
208 1.7% |
208 1.8% (1.6–2.1) |
170 1.1% |
170 1.1% (1.0–1.3) |
0.6 (0.5–0.7) p < 0.0001 |
| ASCUS+ |
1041 8.5% |
1041 9.0% (8.5–9.5) |
1436 9.3% |
1436 9.4% (8.9–9.8) |
1.04 (0.96–1.12) p = 0.29 |
| Unsatisfactory |
568 4.6% |
‐ |
185 1.2% |
‐ | ‐ |
| Missingc |
172 1.4% |
‐ |
0 0% |
‐ | ‐ |
Defined as ASCUS and AGC.
Defined as HSIL and ASCH.
Sample analyzed by private pathologist.
RR for any cytology computed including unsatisfactory and missing diagnosis.
Figure 1.
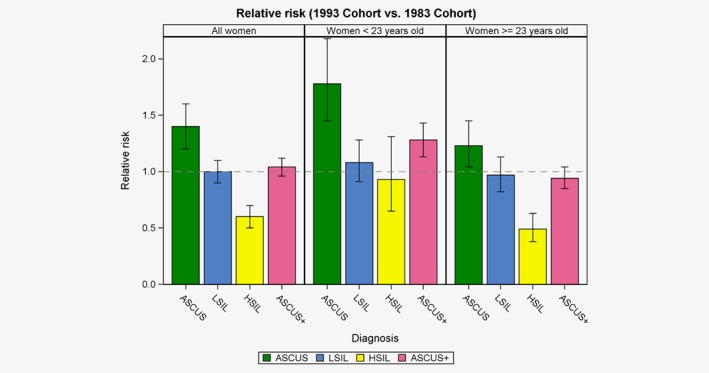
Detection of cervical abnormalities at first cytology result in an HPV‐vaccinated cohort (1993) compared with a non‐HPV‐vaccinated cohort (1983) in Denmark. Relative risk and 95% confidence intervals. [Color figure can be viewed at http://wileyonlinelibrary.com]
Among women with a first cytology before the age of 23 years, the risk of an ASCUS+ result was more frequent in the 1993 than in the 1983 cohort; the percentages being 11.8% and 9.3%, respectively; RR= 1.28 (95% CI 1.13–1.43), p < 0.001 (Table 4 and Fig. 1). This excess risk derived predominantly from ASCUS; RR = 1.78 (95% CI 1.45–2.18). In these women examined at young age, the proportions with HSIL were almost similar; RR = 0.93 (95% CI 0.65–1.31), p = 0.67. In women first examined at regular screening age, the risk of an ASCUS+ result was almost the same in the two cohorts; RR = 0.94 (95% CI 0.85–1.04), p = 0.24. The overall decline in proportion of women with HSIL derived almost entirely from this oldest age group; RR = 0.49 (95% CI 0.38–0.63), p <0.0001.
Table 4.
Age‐stratified results of first cytology, excl. unsatisfactory and missing
| Birth cohort | 1983 | 1993 | RR 1993 vs. 1983 (CI) |
|---|---|---|---|
| Age <23 years at first cytology | |||
| Any cytology | 4,957 | 4,705 |
0.72 (0.70 – 0.75) p < 0.0001 |
| Diagnosis of first cytology | |||
| NILM |
4497 90.7% (89.9–91.5) |
4148 88.2% (87.2–89.1) |
0.97 (0.95–0.98), p <0.0001 |
| ASCUS |
144 2.9% (2.5–3.4) |
243 5.2% (4.6–5.8) |
1.78 (1.45–2.18), p <0.0001 |
| LSIL |
250 5.0% (4.4–5.7) |
256 5.4% (4.8–6.1) |
1.08 (0.91–1.28), p = 0.38 |
| HSIL |
66 1.3% (1.0–1.7) |
58 1.2% (0.9–1.6) |
0.93 (0.65–1.31), p = 0.67 |
| ASCUS+ |
460 9.3% (8.5–10.1) |
557 11.8% (10.9–12.8) |
1.28 (1.13–1.43) p < 0.0001 |
| Age ≥23 years at first cytology | |||
| Any cytology | 6,607 | 10,610 |
1.23 (1.20 – 1.26) p < 0.0001 |
| Diagnosis of first cytology | |||
| NILM |
6026 91.2% (90.5–91.9) |
9731 91.7% (91.2–92.2) |
0.94 (0.85–1.04) p = 0.24 |
| ASCUS |
203 3.1% (2.7–3.5) |
401 3.8% (3.4–4.2) |
1.23 (1.04–1.45) p = 0.01 |
| LSIL |
236 3.6% (3.1–4.0) |
366 3.4% (3.1–3.8) |
0.97 (0.82–1.13) p = 0.67 |
| HSIL |
142 2.1% (1.8–2.5) |
112 1.1% (0.9–1.3) |
0.49 (0.38–0.63) p < 0.0001 |
| ASCUS+ |
581 8.8% (8.1–9.5) |
879 8.3% (7.8–8.8) |
0.94 (0.85–1.04) p = 0.24 |
The HPV‐vaccination coverage in our 1993 cohort was much higher than the 78% officially reported.5 We therefore made two sensitivity analyses. First, we included Copenhagen County under the assumption that this county might have lower vaccination coverage than the rest of the country, but this was not the case. Second, our analysis was based on women born in 1993 and present in Denmark from January 1, 2009 to April 1, 2017. If we included instead all women born in 1993, present in Denmark in 2016, and vaccinated within the child vaccination program, we reach a vaccination coverage of 77.7%, similar to the officially reported coverage based on the same data. The difference between our 91.6% and the official coverage of 78% can then be explained by population movements, where some vaccinated women left Denmark over the follow‐up period and some unvaccinated women entered Denmark. We furthermore included self‐purchased vaccine in calculation of coverage.
Among the 15,315 women in the 1993 cohort with a first satisfactory cytology result, 13,543 were HPV‐vaccinated at age ≤15 years; 815 at age >15 years, and 957 were unvaccinated (Table 5). The risk of ASCUS+ was lower in early vaccinated than in unvaccinated women; RR = 0.78 (95% CI 0.65–0.93), p = 0.008. This pattern was seen for all subdiagnoses, but the numbers for unvaccinated women were small. The late vaccinated women had the same risk of ASCUS+ as the unvaccinated women; RR = 1.09 (95% CI 0.85–1.40), p = 0.5, suggesting that a larger proportion of HPV non‐naïve women is present in this group.
Table 5.
Results of first cytology for 1993 birth cohort stratified by HPV vaccination status, excl. unsatisfactory and missing
| Cytology diagnosis |
Vaccinated ≤15a
N = 13,543 (88.4%) |
Vaccinated >15a
N = 815 (5.3%) |
Unvaccinated N = 957 (6.3%) |
≤15 vs. unvaccinated RR (CI), p |
>15 vs. unvaccinated RR (CI), p |
|---|---|---|---|---|---|
| NILM | 12,321 (91.0%) | 712 (87.4%) | 846 (88.4%) |
1.03 (1.00–1.05) p = 0.008 |
0.99 (0.95–1.02) p = (0.50) |
| ASCUS | 552 (4.1%) | 44 (5.4%) | 48 (5.0%) |
0.81 (0.61–1.08) p = 0.16 |
1.08 (0.72–1.60) p = 0.71 |
| LSIL | 531 (3.9%) | 42 (5.1%) | 49 (5.1%) |
0.77 (0.58–1.02) p = 0.07 |
1.00 (0.67–1.50) p =0.97 |
| HSIL | 139 (1.0%) | 17 (2.1%) | 14 (1.5%) |
0.70 (0.41–1.21) p = 0.20 |
1.43 (0.71–2.87) p = 0.32 |
| ASCUS+ | 1222 (9.0%) | 103 (12.6%) | 111 (11.6%) |
0.78 (0.65–0.93) p = 0.008 |
1.09 (0.85–1.40) p = 0.50 |
Defined as at least one dose (22 women had first cytology before or on the date of the first HPV vaccination).
Discussion
Principal findings
In this study, we determined the impact of HPV vaccination on cytology results in the Danish cervical screening program at the latest at age 23 years, where women are first invited to screening. We compared results from a vaccinated cohort of women born in 1993 with results from an unvaccinated cohort of women born in 1983. The two cohorts had fairly similar cervical cancer risk profiles apart from a decrease in the proportion of daily smokers.
The introduction of HPV vaccination did not affect attendance to screening; in both cohorts around 60% of women had been screened at the latest at age 23 years. There was no difference between the two cohorts in proportion of women with ASCUS+; this being around 9% in both cohorts. There was, however, a statistically significant drop of 40%, from 1.8% to 1.1%, in the proportion of women with HSIL.
In the interpretation of these findings four issues have to be considered: HPV‐vaccination coverage; prevalence of sexual activity at age 15; prevalence of smoking at age 15; and transition from conventional cytology to LBC. The efficacy of 4vHPV was tested in the FUTURE randomized controlled trials. In these trials, the risk of an ASCUS+ cytology result was reduced by 17.1%.23 The study included women with no history of genital warts or abnormal cytology; seronegative, HPV‐DNA negative for HPV 6, 11, 16, 18 and for 10 other high risk HPV‐types, and cytology normal at recruitment. Some differences between the settings may explain why this decrease in ASCUS+ was not seen in our data. First, vaccination coverage was not 100% but 92% in our 1993 cohort. Second, not all women in our 1993 cohort were HPV‐naïve at time of vaccination; 37% reported to be sexually active at the age of 15 years, and we will assume that all women vaccinated later were sexually active, and that sexually active women had a 46% prevalence of high risk HPV DNA.24 Then 21% (=((0.37 × 0.86) + 0.06 + 0.08) × 0.46) of our 1993 cohort would have been HPV non‐naïve. But with these reservations we should still have seen a decrease in ASCUS+ of about 13% (= 1 − ((0.79 × 0.83) + 0.21)).
The lower smoking prevalence in the 1993 than in the 1983 cohort should if anything increase protection against ASCUS+ in the younger cohort. In a Danish study, smoking was associated with a hazard ratio of 1.32 for CIN3+ in high‐risk HPV‐positive women.25 If we assume that the same hazard ratio would be applicable to the impact of smoking in the risk of HSIL, the decrease in smoking prevalence from 21% in the 1983 birth cohort to 10% in the 1993 birth cohort would have affected the risk of HSIL only marginally; from 1.8% to 1.7%. It is therefore clear that the observed decrease to 1.1% cannot be explained by the difference in smoking between the two birth cohorts.
The change from conventional cytology to LBC might, however, have worked in the opposite direction. SurePath LBC provides a better protection against cervical cancer than conventional cytology.26 In Denmark, conventional cytology has been replaced mainly by SurePath LBC, and the transition was associated with a considerable increase in the proportion of ASCUS+ in young women.8, 9 It is, however, not possible to quantify the possible impact of this change in technology. Cytology samples for the women born in 1983 and included in this study were taken with gradually increasing numbers from 1998 to 2006. Cytology samples for women born in 1993 were taken from 2008 to 2016. For both birth cohorts, many laboratories participated in the analysis of the samples, and the laboratories’ transition to LBC took place at different points in time between 2002 and 2015. This transition may therefore have overshadowed a possible effect of HPV vaccination on occurrence of ASCUS+. The FUTURE studies showed a 44.5% (95% CI 4.3%–68.6%) decrease in HSIL in the intervention arm as compared with the control arm.23 Our result of a 40% (95% CI (30%–50%) decrease in HSIL is in line with the FUTURE result.
Furthermore, the FUTURE study showed high efficacy of the quadrivalent vaccine against genital warts.23 In Australia, the incidence of genital warts decreased following the introduction of the quadrivalent vaccine.27 A decrease was seen also in Denmark from 2009.28 In principle, further indication of vaccine effectiveness might be obtained from data on the national time trend in detection of ASCUS. Such data have, however, been reported only since 2013,29, 30 showing a relative stable proportion about 3% with some variation across the Danish regions.
Strengths and limitations
Our study was based on entire birth cohorts offered or not‐offered HPV vaccination, and on the outcome of cytology after all women had been invited to the screening program. We thus avoided the selection bias from comparing vaccinated with unvaccinated women, and the register data ensured complete follow‐up.
The study design included a temporal comparison of screening results 10 years apart. The results could therefore be confounded by changes in background risk over time. However, the two cohorts had fairly similar cervical cancer risk profiles apart from a decrease in smoking from the 1983 to the 1993 cohort, and this decrease may be a possible confounding to be taken into account. We had more women with unsatisfactory samples in the 1983 birth cohort than in the 1993 birth cohort. Conventional cytology samples may be unsatisfactory due to blood and/or inflammation, and women with unsatisfactory samples are recommended for retesting. However, in this study, we included for simplicity only first cytology samples. Blood and/or inflammation are eliminated from LBC samples resulting in a lower proportion of unsatisfactory samples.
Transition from conventional cytology to SurePath LBC may in part explain the lack of an effect of HPV vaccination on occurrence of ASCUS+. We have no Danish data for the positive predictive value of HSIL for cervical intraepithelial neoplasia (CIN)2+. Changes from the 1983 to the 1993 birth cohorts are unlikely, as the pathology departments have followed the same diagnostic procedures throughout the study period, but a possible diagnostic drift in HSIL associated with the transition from conventional cytology to LBC cannot be excluded. Screening coverage was only 60% in our 1993 cohort, but so it was in the 1983 cohort. In cervical screening, the risk profile of nonresponders differs from that of responders,31 and in our 1993 data, 94% of the screened women were HPV vaccinated as compared with 88% of the nonscreened women. Our results might therefore slightly overestimate the effect of HPV vaccination. Somewhat surprisingly, the risk of HSIL was not statistically significantly decreased in vaccinated as compared with nonvaccinated women in the 1993 birth cohort. This might be due to herd immunity as overall 92% of the birth cohort was vaccinated, as also suggested by virology observations from Scotland.32, 33 However, the number of non‐vaccinated women was small, and we do not know that sexual activity before or at the age of 15 years was equally distributed between vaccinated and nonvaccinated women. It should be taken into account also that while 62% of the vaccinated women in the 1993 birth cohort were screened, this was the case for only 45% of the nonvaccinated women, as a similar observation reported previously from Scotland34 and the USA,35 although in different study designs. The follow‐up time was limited; thus, only 3 months for women invited to screening in December 1993. The exclusion of Copenhagen County may limit generalizability, but does not affect the comparison between the two cohorts.
Other studies
The early impact of HPV vaccination on screening outcome in Denmark has previously been reported for HPV‐vaccinated versus unvaccinated women born in 1993–1994 to be a 60% (95% CI 44%–71%) decrease in atypia or worse.36 This study was, however, undertaken in a highly selected population, as only 1.3% of the cohort had been screened at the time of the investigation.
A systematic review of the real‐world experiences of vaccination with 4vHPV concluded that the estimates of the vaccine “effectiveness generally corresponded to vaccine efficacy from clinical trials”.37 It should though be taken into account that most of the reviewed studies were prone to selection bias as they compared vaccinated with unvaccinated women and mostly reported on cervical abnormalities observed prior to normal screening age. A study from Sweden thus reported a 63% (95% CI 51%–72%) decline in cervical intraepithelial neoplasia (CIN)2+ in women vaccinated when they were ≤16 years based on their screening outcome at the age of 13–22 years.38, 39 This is a larger decline than seen in the FUTURE studies,23 and it could probably be due to the selective character of study data as invitation to screening starts only at the age of 23 years in Sweden.
In Australia, screening has been recommended from the age of 18 or two years after start of sexual intercourse. A comparison of women vaccinated when they were ≤16 years with unvaccinated women reported a decline in any high‐grade histological abnormality of 38% (95% CI 12%–56%),40 which is in line with the FUTURE finding.23 The study showed a decline in high‐grade cytology of 36% (95% CI 5%–57%) and an insignificant decline in low‐grade cytology of 17% (95% CI 3%–33%); consistent with our results for HSIL and LSIL.
Scotland is another country with an early start of screening age, and this country started vaccination with the bivalent HPV vaccine in 2008. A comparison between women born in 1988; 99.95% unvaccinated; and in 1993; 22.98% unvaccinated; showed a decline of 54% (95% CI 47%–61%) in detection of CIN2+ at the first cervical screen at age 20 or 21.41 Given that vaccination coverage was partially only, this finding is in line with the results of the PATRICIA trial.42 Like our study, the Scottish study avoided selection bias by comparing birth cohorts instead of comparing vaccinated and unvaccinated women; and by using screening outcomes after start of the regular screening age.
Implications
In Denmark, women with HSIL are referred for colposcopy with biopsy.43 The observed reduction in risk of HSIL following HPV vaccination implies that more than one third of the previously referred women can be spared this procedure and health care resources can be saved. Among the generations born in the early 2000s, Denmark experienced a considerable drop in HPV vaccination coverage due to concern over possible side effects,5 despite no evidence for a causal association.44 Once this concern has settled, the fact that HPV vaccination has the immediate beneficial effect of sparing women from referral to colposcopy may add to the incentive for vaccination.
Future research
In this study, we focused on the very first effect of HPV vaccination detectable after women vaccinated as girls entered the screening program. The further follow‐up of the cohorts will focus on impact of vaccination on CIN and conization. The lack of an impact of HPV vaccination on detection of ASCUS was to some extent a puzzle although the transition from conventional cytology to LBC is likely to have contributed. HPV triage of ASCUS is not undertaken in women below the age of 30, but it would be of interest to explore the HPV‐type distribution in ASCUS samples from the HPV‐vaccinated women.
Conclusion
We presented data from a 4vHPV‐vaccinated cohort compared with an unvaccinated cohort from the time when they had all been invited into the organized screening program. The observed decrease in HSIL was a promising outcome of the HPV vaccination.
Roles and Responsibilities
LHT and EL were responsible for the idea for the study and the collection of the register data. LHT, EL and GN contributed to the design of the study. LHT, EL, GN and LGL contributed to the analyses and interpretation of the data. LHT is first author, but EL, GN and LGL contributed to the preparation of the manuscript and all named authors have approved the final version of the manuscript.
Conflicts of Interest
EL, LHT and LGL received test kits from Roche free of charge for a randomized controlled trial. EL has participated in meetings with Roche with fees paid to the University of Copenhagen. EL had participated in one meeting organized by the HPV Prevention and Control Board and in one meeting organized by GSK; both without payment. GN has no conflict of interest relevant for this study to report.
Funding
The study was financially supported by Kirsten and Freddy Johansen's Fund. Funding played no role in the study or manuscript.
Transparency Declaration
All authors affirm that the manuscript is an honest, accurate and transparent account of the study being reported. No important aspects of the study have been omitted.
Supporting information
Supporting Information
References
- 1. Garland SM, Hernandez‐Avila M, Wheeler CM, for the Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators , et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356:1928–43. [DOI] [PubMed] [Google Scholar]
- 2. The FUTURE II study group . Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. N Engl J Med 2007; 356:1915–27. [DOI] [PubMed] [Google Scholar]
- 3. Sander BB, Rebolj M, Valentiner‐Brandth P, et al. Introduction of human papillomavirus vaccination in Nordic countries. Vaccine 2012; 30:1425–33. [DOI] [PubMed] [Google Scholar]
- 4. Andersen PH. Statens Serum Institut; EPI‐NEWS No. 49, 2015. https://www.ssi.dk/English/News/EPI-NEWS/2015/No%2049%20-%202015.aspx
- 5.State Serum Institute, Vaccine coverage (In Danish). https://www.ssi.dk/Smitteberedskab/Sygdomsovervaagning/VaccinationSurveillance.aspx?xaxis=Cohort&vaccination=5&sex=0&landsdel=100&show=&datatype=Vaccination&extendedfilters=True#HeaderText [Accessed 9 November 2017]
- 6.Screening for livmoderhalskræft – anbefalinger, 2012. Sundhedsstyrelsen (In Danish). https://sundhedsstyrelsen.dk/~/media/B1211EAFEDFB47C5822E883205F99B79.ashx
- 7. Lynge E, Rygaard C, Baillet MV, et al. Cervical screening at crossroads. Apmis 2014;122:667–73. [DOI] [PubMed] [Google Scholar]
- 8. Barken SS, Rebolj M, Lynge E, et al. Outcomes in cervical screening using various cytology technologies: what's age got to do with it?. Eur J Cancer Prev 2013;22:367–73. [DOI] [PubMed] [Google Scholar]
- 9. Rask J, Lynge E, Franzmann M, et al. Impact of technology on cytology outcome in cervical cancer screening of young and older women. Int J Cancer 2014; 134:2168–79. [DOI] [PubMed] [Google Scholar]
- 10.Dansk Kvalitetsdatabase for Livmoderhalskræftscreening (DKLS) årsrapport 2015. (In Danish). https://www.sundhed.dk/content/cms/82/4682_dkls-%C3%A5rsrapport-2015.pdf
- 11.Patobank/Historik (In Danish). http://www.patobank.dk/index.php?ID=16&lang=da [Accessed 2 November 2017]
- 12.Screening for livmoderhalskræft – anbefalinger, 2007. Sundhedsstyrelsen (In Danish). https://www.sst.dk/~/media/2C40A729A6D8452984626577FFD2C752.ashx
- 13.Patobank/SNOMED (In Danish). http://www.patobank.dk/index.php?ID=18&lang=da http://www.patobank.dk/index.php?ID=1&lang=da [Accessed 9 November 2017]
- 14. Solomon D, Davey D, Kurman R, Forum Group Members , et al. Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002;287:2114–9. [DOI] [PubMed] [Google Scholar]
- 15.Dansk Kvalitetsdatabase for Livmoderhalskræftscreening (DKLS) årsrapport 2013. (In Danish). https://www.sundhed.dk/content/cms/82/4682_dkls-%C3%A5rsrapport-august_vers3-8_final.pdf
- 16.Statistibanken.dk, Statistics Denmark. https://www.statistikbanken.dk/statbank5a/default.asp?w=1239 [Accessed 16 October 2017]
- 17. Olesen TB, Jensen KE, Munk C, et al. Liva – population survey of female sexual habits. Ugeskr Laeger 2010;172:3254–9. In Danish. [PubMed] [Google Scholar]
- 18. Jørgensen MJ, Maindal HT, Christensen KS, et al. Sexual behavior among young Danes aged 15–29 years: a cross‐sectional study of core indicators. Sex Transm Infect 2015;91:171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasmussen M, Due P, Holstein BE. Skolebørnsundersøgelsen 1998: Sundhed, sundhedsvaner og sociale forhold (In Danish). Institut for Folkesundhedsvidenskab, Københavns Universitet, 2000.
- 20. Rasmussen M, Due P, Skolebørnsundersøgelsen, 2010. (In Danish). Statens Institut for Folkesundhed, Syddansk Universitet, 2011. http://www.hbsc.dk/downcount/HBSC-Rapport-2010.pdf
- 21. Beerman H, van Dorst EBL, Kuenen‐Boumeester V, et al. Superior performance of liquid‐based versus conventional cytology in a population‐based cervical screening program. Gynecol Oncol 2009;112:572–6. [DOI] [PubMed] [Google Scholar]
- 22. Virlev L. Færre siger ja til hinanden under økonomisk krise (In Danish). Publication from Statistics Denmark, May 25th 2011. http://www.dst.dk/da/Statistik/bagtal/2011/2011-05-25-vielser [Accessed 2 November 2017]
- 23. Munoz N, Kjaer SK, Sigurdsson K, et al. Impact of human papillomavirus (HPV)‐6/11/16/18 vaccine on all HPV‐associated genital diseases in young women. J Natl Cancer Inst 2010;102:325–39. [DOI] [PubMed] [Google Scholar]
- 24. Kjær SK, Munk C, Junge J, et al. Carcinogenic HPV prevalence and age‐specific type distribution in 40,382 women with normal cervical cytology, ASCUS/LSIL, HSIL or cervical cancer: what is the potential for prevention? Cancer Causes Control 2014;25:179–89. [DOI] [PubMed] [Google Scholar]
- 25. Jensen KE, Schmiedel S, Frederiksen K, et al. Risk for cervical intraepithelial neoplasia grade 3 or worse in relation to smoking among women with persistent human papillomavirus infection. Cancer Epidemiol Biomarkers Prev 2012;21:1949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rozemeijer K, Naber SK, Penning C, et al. Cervical cancer incidence after normal cytological sample in routine screening using SurePath, ThinPrep, and conventional cytology: population based study. BMJ 2017;356:j504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith MA, Liu B, McIntyre P, et al. Trends in genital warts by socioeconomic status after the introduction of the national HPV vaccination program in Australia: analysis of national hospital data. BMC Infect Dis 2016;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bollerup S, Baldur‐Felskov B, Blomberg M, et al. Significant reduction in the incidence of genital warts in young men 5 years into the Danish Human Papillomavirus vaccination program for girls and women. Sex Transm Dis 2016;43:238–42. [DOI] [PubMed] [Google Scholar]
- 29. Lynge E, Andersen B, Christensen J, et al. Cervical screening in Denmark ‐ a success followed by stagnation. Acta Oncol 2018;57:354–61. [DOI] [PubMed] [Google Scholar]
- 30.Dansk Kvalitetsdatabase for Livmoderhalskræftscreening – Årsrapport 2016. 2017; Version 6. [in Danish]
- 31. Kristensson JH, Sander BB, von Euler‐Chelpin M, et al. Predictors of non‐participation in cervical screening in Denmark. Cancer Epidemiol 2014;38:174–80. [DOI] [PubMed] [Google Scholar]
- 32. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7‐year cross‐sectional study. Lancet Infect Dis 2017;17:1293–302. [DOI] [PubMed] [Google Scholar]
- 33. Cameron RL, Kavanagh K, Pan J, et al. Human papillomavirus prevalence and herd immunity after introduction of vaccination program, Scotland, 2009–2013. Emerg Infect Dis 2016;22:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palmer TJ, McFadden M, Pollock KG, et al. HPV immunisation and increased uptake of cervical screening in Scottish women; observational study of routinely collected national data. Br J Cancer 2016;114:576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paynter CA, Van Treeck BJ, Verdenius I, et al. Adherence to cervical cancer screening varies by human papillomavirus vaccination status in a high‐risk population. Prev Med Rep 2015;2:711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baldur‐Felskov B, Dehlendorff C, Munk C, et al. Early impact of human papillomavirus vaccination on cervical neoplasia – Nationwide Follow‐up of Young Danish Women. J Natl Cancer Inst 2014;106:djt460. [DOI] [PubMed] [Google Scholar]
- 37. Garland SM, Kjaer SK, Munoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real‐world experience. Clin Infect Dis 2016;63:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herweijer E, Sundström K, Ploner A, et al. Quadrivalent HPV vaccine effectiveness against high‐grade cervical lesions by age at vaccination: a population‐based study. Int J Cancer 2016;138:2867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herweijer E, Sundström K, Ploner A, et al. Erratum. Int J Cancer 2017;141:E1–4. [DOI] [PubMed] [Google Scholar]
- 40. Brotherton JML, Malloy M, Budd AC, et al. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: observational cohort of young women in Australia. Papillomavirus Res 2015;1:59–73. [Google Scholar]
- 41. Cameron RL, Kavanagh K, Watt DC, et al. The impact of bivalent HPV vaccine on cervical intraepithelial neoplasia by deprivation in Scotland: reducing the gap. J Epidemiol Community Health 2017;71:954–60. [DOI] [PubMed] [Google Scholar]
- 42. Lehtinen M, Paavonen J, Wheeler CM, HPV PATRICIA Study Group , et al. Overall efficacy of HPV‐16/18 AS04‐adjuvant vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4‐year end‐of‐study analysis of the randomised, double‐blind PATRICIA trial. Lancet Oncol 2012;13:89–99. [DOI] [PubMed] [Google Scholar]
- 43. Noehr B, Frydshou D, Kahn HS, et al. Udredning, behandling og kontrol af cervical dysplasia (In Danish), DSOG, 2012. http://gynobsguideline.dk/hindsgavl/Cervixdysplasi2012.pdf
- 44. European Medicines Agency . EMA/762033/2015. Pharmacovigilance risk assessment committee. Assessment report. Review under article 20 of regulation (EC) no 726/2004. Human papillomavirus (HPV) vaccines, 11 November 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vaccines_20/Opinion_provided_by_Committee_for_Medicinal_Products_for_Human_Use/WC500197129.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


