Abstract
Background
Lifestyle interventions to reduce weight and increase activity may preserve higher-order cognitive abilities in overweight/obese adults with type 2 diabetes (T2D).
Methods
Adults (N = 5,084) with T2D who enrolled in a randomized clinical trial of a 10-year intensive lifestyle intervention (ILI) compared with diabetes support and education were queried at baseline and repeatedly during follow-up for complaints about difficulties in memory, problem-solving, and decision-making abilities.
Results
For those without baseline complaints, assignment to ILI was associated with lower odds that complaints would emerge during follow-up for decision-making ability (odds ratio [OR]=0.851, [95% CI, 0.748,0.967], p=0.014), and, among individuals who were not obese, lower odds that complaints would emerge about problem-solving ability (OR=0.694 [0.510,0.946]). No cognitive benefits from ILI were seen for individuals with baseline complaints about cognitive abilities. ILI may have exacerbated the severity of complaints about problem-solving ability during follow-up among individuals with baseline complaints and cardiovascular disease (OR=2.949 [1.378,6.311]).
Conclusions
A long-term multidomain ILI may reduce the likelihood that complaints about difficulties in higher-order cognitive abilities will emerge in T2D adults without pre-existing complaints. Among those with pre-existing complaints, the ILI did not prevent increases in complaint severity.
Keywords: Behavioral intervention, Type 2 diabetes mellitus, Self-reported cognitive ability
Type 2 diabetes (T2D) increases adults’ risk for developing cognitive deficits (1). Individuals are aware of these changes: those with diabetes report greater rates of difficulties in cognitive abilities than age-matched controls (2,3). Domains associated with complex cognitive abilities, such as memory, executive function, and processing speed, are among those that are affected (4). These are important for every day functioning and, for those with diabetes, are critical to its management (5–7). Older adults who report declines in cognitive abilities are at increased risk for progression to dementia (8,9), and for markers of Alzheimer’s disease such as gray matter volume loss (10–12), cerebral hypoperfusion (13), and amyloid deposition (14,15), even in the absence of objective cognitive deficits. Self-assessed difficulties in cognitive abilities are included as a diagnostic criterion for Alzheimer’s dementia (16), highlighting their importance.
More than 80% of U.S. adults with T2D are overweight or obese (17) and the majority are sedentary (18). Mid-life obesity and sedentary lifestyle are risk factors for cognitive decline (19,20). Therefore, behavioral intervention to counteract these factors may be expected to protect cognitive abilities in T2D.
We have recently reported the legacy of a 10-year behavioral weight loss intervention on objective measures of cognitive function (ie, scores from cognitive function tests) among adults with T2D mellitus within the context of a large randomized controlled clinical trial, the Action for Health in Diabetes (Look AHEAD) (21–23). The intensive lifestyle intervention (ILI) to decrease energy intake and increase physical activity appeared to have a differential impact on cognitive function depending on individual’s weight and history of cardiovascular disease at baseline. For those who were overweight [body mass index (BMI) 25–29 kg/m2] but not obese, and for those with no history of cardiovascular disease, random assignment to ILI compared to a control condition appeared to leave a legacy (8–13 years post-randomization) of slightly better cognitive function. In contrast, assignment to the intervention appeared to leave a legacy of slightly worse cognitive function for those with Class 3 obesity (BMI ≥ 40 kg/m2) or a history of cardiovascular disease. A limitation of these prior reports, however, is that no objective assessments of cognitive function were obtained until at least 8 years after ILI began, which raises concerns about the potential of differential follow-up and leaves unanswered what differences may have emerged earlier during the intervention.
Although the Look AHEAD trial did not objectively assess cognitive function until late during follow-up, complaints about difficulties with cognitive abilities were queried throughout. This manuscript makes use of these self-reports to examine whether ILI reduced the odds that these complaints emerged throughout follow-up, beginning at Year 1, and for those complaining of some difficulties at baseline, whether it reduced the odds that the severity of complaints increased. We also examine whether the differential intervention effects on cognitive function among subgroups based on obesity and cardiovascular disease history observed for cognitive test beginning in year 8 were seen earlier in these complaints. Because cognitive ability and depression are linked in diabetes (24) and ILI-reduced depressive symptoms (25), we also assess the degree to which any intervention effects on cognitive complaints were attenuated with covariate adjustment for a measure of depressive symptom severity in sensitivity analyses.
Methods
The design and methods of Look AHEAD have been published previously (26), as have its CONSORT diagram and primary results (27). It was a randomized controlled trial that recruited 5,145 individuals (during 2001–2004) who were overweight or obese and had T2D. Enrollees were aged 45–76 years, with BMI > 25 kg/m2 (>27 kg/m2 if on insulin), glycated hemoglobin (HbA1c) < 11%, systolic/diastolic blood pressure < 160/100 mmHg, and triglycerides < 600 mg/dL. Prior to enrollment, each prospective participant completed a 2-week run-in during which they were required to successfully record information daily about diet and physical activity. Each met with a behavioral psychologist or interventionist to confirm that they understood intervention requirements and to exclude those with significant issues (eg, depression or alcohol abuse) that might impair adherence. These requirements likely screened out individuals with major cognitive deficits. Participants provided informed consent. Local Institutional Review Boards approved protocols.
Interventions
Participants were randomly assigned with equal probability to the multidomain ILI or a control condition of diabetes support and education (DSE). ILI included diet modification and physical activity designed to induce weight loss to average ≥7% at year 1 and maintain this over time (28). ILI participants were assigned a daily calorie goal (1200–1800 based on initial weight), with <30% of total calories from fat (<10% from saturated fat) and ≥15% of total calories from protein. The physical activity goal was ≥175 min/week through activities similar in intensity to brisk walking.
DSE participants were invited to attend three group sessions each year focused on diet, physical activity, and social support (29). They did not receive specific diet, activity, or weight goals or information on behavioral strategies.
Interventions were terminated September, 2012. The mean (range) length of intervention for both ILI and DSE participants we study was 9.8 (8.4, 11.1) years.
Self-Assessed Cognitive Ability
Participants were queried about difficulties with three cognitive abilities at baseline and repeatedly over time. The Health Utilities Index, a measure of preference-weighted quality of life (30,31), included two questions related to memory and problem-solving that define its cognition utility function (32):
How would you describe your ability to remember things during the past 4 weeks?
1 = Able to remember most things
2 = Somewhat forgetful
3 = Very forgetful
4 = Unable to remember anything at all
How would you describe your ability to think and solve day-to-day problems, during the past 4 weeks?
1 = Able to think clearly and solve problems
2 = Had a little difficulty
3 = Had some difficulty
4 = Had a great deal of difficulty
5 = Unable to think or solve problems
The Health Utilities Index was administered at baseline and at annual visits through 10–13 years of planned follow-up, depending on the timing of randomization.
The Beck Depression Index-II (BDI-2), a measure of the severity of depressive symptoms (33), included the following query about decision-making in reference to the prior 7 days:
1 = I make decisions about as well as I ever could.
2 = I put off making decisions more than I used to.
3 = I have greater difficulty in making decisions than before.
4 = I can’t make decisions at all anymore.
BDI-2 questionnaires were administered at baseline, annually through year 4, again at year 8, and once between years 10 and 13, depending on the timing of randomization, following the termination of the ILI.
All questionnaires to register these cognitive complaints were collected and processed by staff that was unaware of intervention assignment.
Baseline assessment of risk factors for cognitive decline
Other self-reported characteristics and conditions were assessed using standardized questionnaires. Blood pressure was measured in duplicate using a Dinamap Monitor Pro 100 automated device. Blood specimens were collected after a 12-h fast and analyzed using standardized laboratory procedures for HbA1c. Weight was obtained using digital scales (27). Baseline history of cardiovascular disease was based on self-report of prior myocardial infarction, coronary artery bypass, angioplasty/stent procedures, peripheral vascular disease, stroke, stable angina, and classes I/II heart failure. Prescription medications were brought to annual clinic visits for recording.
Statistical Analysis
Baseline characteristics of intervention groups were compared with chi-squared and t-tests. Our primary goals were, in parallel analyses, to compare the burden over time of complaints about cognitive difficulties for individuals free of baseline complaints and the burden over time of increased severity in complaints for those with baseline complaints. Because of this, we analyzed the prevalence of cognitive complaints over follow-up, rather than adopting approaches based on times-to-events or Markov models. Odds ratios for the prevalence of complaints about cognitive ability over time for those with no baseline complaints were assessed using generalized estimating equations with logit parameters and covariate adjustment for sex, education, and baseline age. For those with baseline complaints, odds ratios for the prevalence of more severe complaints than reported at baseline were similarly assessed. To examine whether associations were attributable to general effects on depressive symptoms, we included the BDI-2 scores minus the contribution of the decision-making item as a covariate in supporting analyses. Based on evidence that the effect of the Look AHEAD intervention varied depending on baseline obesity status and cardiovascular disease history (21–23), we examined whether there was similar evidence for these with the cognitive complaints, using formal tests for interactions.
Results
Of the 5,145 Look AHEAD enrollees, 5,084 (98.8%) provided at least one self-assessment of difficulties in cognitive ability during follow-up. Table 1 portrays the distributions of their risk factors for cognitive decline and the three self-reported complaints at baseline. Overall, 23% of participants reported at least some difficulty with memory, 12% reported some difficulty with problem-solving, and 16% reported some difficulty with decision-making at baseline. Spearman correlations among the three ordinal measures were all highly significant (p < .0001): r = 0.42 for memory versus problem-solving complaints, r = 0.22 for memory versus decision-making complaints, and r = 0.31 for problem-solving versus decision-making complaints.
Table 1.
Characteristics at the Time of Enrolment of Participants Who Had Follow-up Self-Reports of Cognitive Abilities: Mean (Standard Deviation), Percent, or N (Percent)
| DSE N = 2544 |
ILI N = 2540 |
p-value | |
|---|---|---|---|
| Age | 58.85 (6.88) | 58.58 (6.81) | .15 |
| Female | 59.8% | 59.3% | .76 |
| Race/Ethnicity | |||
| African-American | 15.7% | 15.5% | |
| American Indian | 5.0% | 5.1% | .96 |
| Hispanic | 13.2% | 13.2% | |
| Non-Hispanic White | 63.4% | 63.0% | |
| Other, multiple | 2.8% | 3.1% | |
| Education | |||
| High school | 51.2% | 49.9% | |
| College graduate | 21.1% | 22.4% | .60 |
| Post college | 18.7% | 19.1% | |
| Other | 9.1% | 8.6% | |
| BMI, kg/m2 | 36.0 (5.8) | 35.9 (6.0) | .24 |
| HbA1c, % | 7.31 (1.20) | 7.25 (1.14) | .07 |
| Insulin use, missing = 178 | 16.4% | 15.5% | .38 |
| Diabetes duration, years | 6.85 (6.45) | 6.77 (6.64) | .67 |
| Hypertension | 82.7% | 83.7% | .34 |
| Smoking | |||
| Never | 50.9% | 49.6% | |
| Former | 44.9% | 45.8% | .57 |
| Current | 4.3% | 4.7% | |
| History of cardiovascular disease | 13.4% | 14.2% | .43 |
| Beck Depression Inventory 2 | 5.33 (0.09) | 5.51 (0.10) | .17 |
| Memory, Missing = 13 | |||
| 1 = Able to remember most things | 1952 (76.9%) | 1969 (77.7%) | |
| 2 = Somewhat forgetful | 549 (21.6%) | 530 (20.9%) | .70 |
| 3 = Very forgetful | 35 (1.4%) | 35 (1.4%) | |
| 4 = Unable to remember anything | 1 (0.0%) | 0 (0.0%) | |
| Problem-Solving, Missing = 14 | |||
| 1 = No difficulty | 2227 (87.8%) | 2228 (88.0%) | |
| 2 = Little difficulty | 250 (9.8%) | 239 (9.4%) | |
| 3 = Some difficulty | 53 (2.1%) | 59 (2.3%) | .79 |
| 4 = Great deal of difficulty | 7 (0.3%) | 4 (0.2%) | |
| 5 = Unable | 1 (0.0%) | 2 (0.1%) | |
| Decision-Making, Missing=1 | |||
| 1 = As well as ever | 2140 (84.2%) | 2126 (83.7%) | |
| 2 = Put off making decisions | 356 (14.0%) | 344 (13.5%) | .09 |
| 3 = Greater difficulty than before | 47 (1.8%) | 70 (2.8%) | |
| 4 = Unable | 0 (0.0%) | 0 (0.0%) | |
Supplemental Table 1 summarizes changes in weight by intervention assignment. Significant differences in percent weight changes from baseline between intervention groups were maintained over time for the full cohort and for subgroups defined by baseline obesity and cardiovascular disease history. However, the magnitude of differences tended to decline over time.
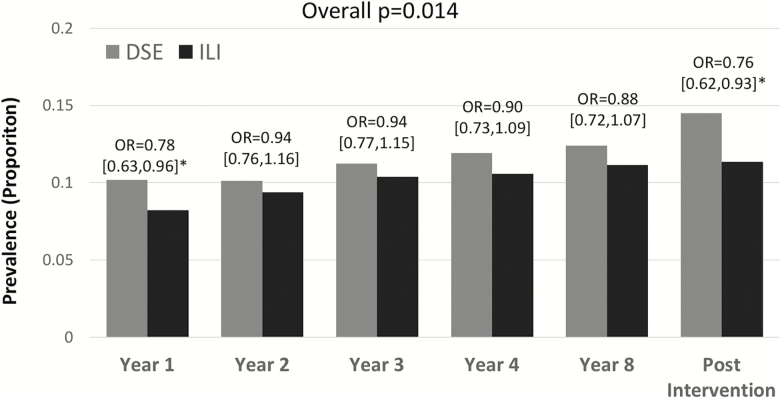
Self-reported complaints were collected at year 1 from 98.5% of participants; 82.3% provided at least one assessment 10 or more years from baseline. Table 2 lists odds ratios between intervention groups for cognitive complaints among participants with no complaint about each ability at baseline, with covariate adjustment for age, education, and sex. There were not significant differences between intervention groups for the incidence of complaints about memory and problem-solving ability. However, random assignment to ILI compared with DSE was associated with significantly lower odds for complaints about difficulties in decision-making: odds ratio 0.851 (p = .014). Figure 1 portrays the prevalence of decision-making complaints over time for those without complaints at baseline, by intervention assignment. This prevalence increased over time in both groups, however at each time, it was greater among DSE participants.
Table 2.
Odds Ratio Over Time for Complaints About Difficulties in Cognitive Abilities: Participants with No Complaints at Baseline (Covariate Adjustment for Sex, Education, and Baseline Age)
| Odds Ratio [95% Confidence Interval] Interaction p-Value |
|||
|---|---|---|---|
| Memory | Problem-Solving | Decision-Making | |
| Overall | (N = 3867) 0.978 [0.876,1.093] p = .699 |
(N = 4387) 0.948 [0.841,1.069] p = .387 |
(N = 4207) 0.851 [0.748,0.967]* p = .014 |
| Obesity Level† | |||
| Nonobese | (N = 542) 0.925 [0.683,1.225] |
(N = 629) 0.694 [0.510,0.946]* |
(N = 620) 0.635 [0.449,0.898]* |
| Obese | (N = 3325) 0.985 [0.874,1.110] p = .707 |
(N = 3758) 1.001 [0.878,1.140] p = .033 |
(N = 3587) 0.894 [0.779,1.027] p = .072 |
| History of CVD‡ | |||
| No | (N = 3359) 0.962 [0.853,1.084] |
(N = 3797) 0.952 [0.834,1.086] |
(N = 3644) 0.841 [0.732,0.967]* |
| Yes | (N = 508) 1.076 [0.807,1.436] p = .477 |
(N = 590) 0.903 [0.677,1.206] p = .746 |
(N = 563) 0.898 [0.640,1.258] p = .728 |
Notes: *95% confidence interval excludes 1.
†Overweight is BMI <30 kg/m2; Obese is BMI ≥ 30 kg/m2.
‡CVD is cardiovascular disease based on self-report of prior myocardial infarction, coronary artery bypass, angioplasty/stent procedures, peripheral vascular disease, stroke, stable angina, and classes I/II heart failure.
Figure 1.
Prevalence of complaints about decision-making ability over time by intervention assignment for participants with no baseline complaints. Included are prevalence rates and covariate (age, sex, education) adjusted odds ratios with 95% confidence intervals to compare intervention groups. Post-intervention assessments were made during years 10–13.
Table 2 includes separate analyses for participants stratified by baseline obesity and cardiovascular disease history. There were no differences between intervention groups for the emergence of complaints about memory for subgroups based on obesity status or cardiovascular disease history. Differences between intervention groups in the emergence of complaints about problem-solving varied according to obesity status (p = .033). For participants who were initially overweight, the odds ratio [95% confidence interval] for problem-solving complaints was 0.694 [0.510,0.946]; for participants who were initially obese, it was 1.001 [0.878,1.140]. Similarly, for the emergence of decision-making complaints, intervention effects were marginally stronger among overweight participants (p = .072): odds ratios 0.635 [0.449,0.898] and 0.894 [0.779,1.027], respectively. Differences between intervention groups in the emergence of complaints about cognitive abilities did not vary markedly by cardiovascular disease history.
Table 3 examines whether the severity of cognitive complaints across the ordinal categories increased during follow-up among those who reported at least some difficulty at baseline. Overall, there were no significant differences between intervention groups for the increasing severity of any of the three cognitive complaints. This finding was consistent regardless of baseline obesity status. However, there was evidence that odds of increasing severity of complaints problem-solving ability were greater among ILI compared to DSE participants with cardiovascular disease history: odds ratio 2.949 [1.378,6.331], with interaction p = .007. Supplementary Figure 1 portrays the prevalence of increased severity in complaints about problem-solving ability over time between intervention groups for participants with cardiovascular disease history. Differences between groups emerged early and were maintained over time.
Table 3.
Odds Ratio Over Time for Increased Severity of Cognitive Complaints Among Participants with Baseline Complaints (Covariate Adjustment for Sex, Education, and Baseline Age)
| Odds Ratio [95% Confidence Interval] Interaction p-Value |
|||
|---|---|---|---|
| Memory | Problem-Solving* | Decision-Making | |
| Overall | (N = 1127) 1.012 [0.754,1.359] p = .935 |
(N = 604) 1.102 [0.798,1.522] p = .555 |
(N = 800) 1.273 [0.862,1.880] p = .225 |
| Obesity Level† | |||
| Nonobese | (N = 193) 1.082 [0.530,2.212] |
(N = 105) 1.357 [0.650,2.832] |
(N = 120) 0.903 [0.281,2.902] |
| Obese | (N = 935) 1.000 [0.724,1.383] p = .844 |
(N = 499) 1.054 [0.737,1.508] p = .545 |
(N = 680) 1.321 [0.871,2.002] p = .548 |
| History of CVD‡ | |||
| No | (N = 951) 0.952 [0.689,1.316] |
(N = 510) 0.922 [0.648,1.310] |
(N = 677) 1.241 [0.817,1.886] |
| Yes | (N = 177) 1.319 [0.669,2.603] p = .394 |
(N = 94) 2.949 [1.378,6.311]§ p = .007 |
(N = 123) 1.362 [0.492,3.770] p = .868 |
Notes: *The three participants who reported they were unable to solve problems at baseline were excluded from this analysis in that they could not progress to a more severe category.
†Overweight is BMI <30 kg/m2; Obese is BMI ≥ 30 kg/m2.
‡CVD is cardiovascular disease based on self-report of prior myocardial infarction, coronary artery bypass, angioplasty/stent procedures, peripheral vascular disease, stroke, stable angina, and classes I/II heart failure.
§95% confidence interval excludes 1.
We examined whether intervention group differences in cognitive complaints among those without baseline complaints (Supplementary Table 2) might be accounted for by differences in depressive symptoms, as measured by adjusted BDI-2 score (after subtracting the contribution of self-reported decision-making ability from this score). We included mean BDI-2 scores across follow-up as a covariate in analyses. This attenuated the odds ratio for decision-making complaints in the overall sample: odds ratio 0.941 [0.818,1.082], p = 0.394 (Supplementary Table 2). Similarly, including interactions between the mean adjusted BDI-2 scores and obesity attenuated interactions between intervention assignment and obesity for complaints about problem-solving (p = .088) and decision-making (p = .508) abilities.
We also examined whether the interaction observed between cardiovascular disease history and the odds of increasing severity of problem-solving complaints among participants with baseline complaints might be explained by adjusted BDI-2 score. Including adjusted BDI-2 as an interaction term did not attenuate interactions between intervention assignment and cardiovascular disease history on the increasing severity of problem-solving complaints (adjusted OR = 2.510; interaction p = .013).
Supplementary Table 3 examines associations that the severity of cognitive complaints most distal from randomization (mean 10.7 years post-randomization) had with the first objective measures (collected from years 8 to 13, mean 10.4 years). Presented are both mean standardized cognitive function tests scores for participants grouped by self-report of no difficulty versus at least some difficulty and Spearman correlations between reported difficulty severity and test scores. All relationships were highly significant (p < .002) and in the expected direction, however relationships were not strong.
Discussion
In a large cohort of individuals with T2D mellitus, a multidomain ILI to limit dietary energy intake, improve diet, and increase physical activity reduced the incidence of complaints about decision-making ability, with some evidence that this benefit was greatest among individuals who were overweight but not obese. It also appeared to lower the incidence of complaints about problem-solving ability, but only among individuals who were initially nonobese. The ILI appeared to have no overall impact on the development of complaints about memory ability.
Among individuals who reported some difficulties in cognitive ability at baseline, ILI appeared to offer no benefits towards reducing rates at which complaints became more severe. In fact, ILI may have differentially increased the severity of complaints about problem-solving ability among individuals with cardiovascular disease history.
Complaints about cognitive abilities were significantly (but not strongly) related to later scores from objective tests of cognitive function, providing some internal validation that the findings on cognitive complaints may resonate with Look AHEAD findings from the cognitive assessments, which were only administered later during follow-up.
Maintaining Cognitive Abilities
Decision-making and problem-solving are complex tasks requiring higher order cognitive functioning. Within Look AHEAD, self-reported abilities in decision-making and problem-solving were most highly correlated with measures of executive function and processing speed, although correlations were modest. ILI appeared to reduce the incidence of complaints about decision-making ability and the rate that they occurred throughout follow-up, beginning in year 1. While subgroup differences did not reach statistical significance (p = .072), there was some evidence that benefits for self-reported decision-making ability were most evident for participants who were initially nonobese. These results are consistent with the intervention benefits seen at years 8–9 postrandomization in processing speed (interaction p = .008) and executive function (interaction p = .11), which were limited to nonobese participants (21,23). The incidence of complaints about problem-solving ability also appeared to be slowed by ILI, but only for nonobese participants.
Together, these findings suggest that multidomain lifestyle intervention may provide some cognitive benefits within 1 year, which may be maintained long-term, among adults with T2D with high cognitive functioning. These included fewer complaints about decision-making and problem-solving abilities and were most strong for, and possibly limited to, those who were nonobese. The Look AHEAD ILI improved many risk factors for cognitive deficits, including markers of diabetes and blood pressure control and levels of lipids and inflammation markers, symptoms of depression, and sleep apnea severity (24), which may have fostered these benefits.
Colombe and Kramer, in a meta-analysis of short-term interventions targeting increased aerobic activity, reported general benefits across four cognitive domains, with the largest benefits for executive function (34). The multidomain (diet, physical activity, risk factor management, and cognitive training) FINGER randomized controlled clinical trial found cognitive benefits over 2 years for higher order cognitive functions (executive function and processing speed), but not memory, in adults with normal cognition at increased risk for cognitive declines (35). Baker et al. found that a 6-month aerobic exercise intervention in adults with newly diagnosed diabetes or prediabetes increased executive function but not memory (32). Siervo et al. conducted a meta-analysis of short-term (≤2 years) clinical trials of weight loss interventions in general cohorts (ie, not limited to those with diabetes) and found evidence for modest benefits in executive function and memory domains (36). These studies generally support findings from the Look AHEAD intervention for cognitive abilities, predominantly those related to higher-order functions. However, Espeland et al. (37) reported that a 2-year intervention to increase physical activity, leg strength, flexibility, and balance in sedentary older adults benefited the memory of individuals with diabetes, but not executive functioning or processing speed.
ILI did not prevent further worsening of cognitive complaints among those with baseline complaints. Of concern, it may have exacerbated the severity of complaints about problem-solving for those with cardiovascular disease history. This finding is consistent with the objective cognitive assessments conducted later in Look AHEAD, which found that intervention was associated with relatively worse performance on tests of executive function and processing speed among individuals with cardiovascular disease history (22). It also resonates with findings that, compared with the control condition, cerebral blood flow was lower among obese intervention participants who had relatively poorer cognitive functioning and thus, perhaps, increased levels of vascular disease (38).
Our findings are strengthened by randomization, standardized intervention, extended follow-up, and robust retention. Some limitations should be noted. As volunteers, participants may not align with general clinical populations. Screening procedures may have excluded those with baseline cognitive impairment. The single-item queries for complaints about difficulties in cognitive ability are not validated measures and the severity of these complaints did not correlate highly with objective cognitive function measures, which were not available at baseline. However, the two HUI items comprise its cognition score (39) and the individual BDI item on decision-making has been analyzed separately elsewhere (eg, (40)). While our analyses were motivated by Look AHEAD publications of its objective cognitive function assessments, we have not controlled type 1 error across inferences and thus findings may be considered exploratory.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work and the Action for Health in Diabetes are supported through the following cooperative agreements from the National Institutes of Health: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. The following federal agencies have contributed support: National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Center on Minority Health and Health Disparities; Office of Research on Women’s Health; the Centers for Disease Control and Prevention; and the Department of Veterans Affairs. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources.
Additional support was received from the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); Frederic C. Bartter General Clinical Research Center (M01RR01346); and the Wake Forest Alzheimer’s Disease Core Center (P30AG049638-01A1).
The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Acknowledgements
Conflict of interest
M. A. E. is a member of the Editorial Board for this journal. No other authors have conflicts to declare.
References
- 1. Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry. 2011;11:108. doi: 10.1186/1471-244X-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matsuzawa T, Takata T, Yokono K, et al. A warning index used in prescreening for Alzheimer’s disease, based on self-reported cognitive deficits and vascular risk factors for dementia in elderly patients with type 2 diabetes. Int J Alzheimers Dis. 2012;2012:124215. doi: 10.1155/2012/124215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20:278–291. doi: 10.1017/S1355617713001483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. King DK, Glasgow RE, Toobert DJ, et al. Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care. 2010;33:751–753. doi: 10.2337/dc09-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Zgibor J, Matthews JT, Charron-Prochownik D, Sereika SM, Siminerio L. Self-monitoring of blood glucose is associated with problem-solving skills in hyperglycemia and hypoglycemia. Diabetes Educ. 2012;38:207–218. doi: 10.1177/0145721712440331 [DOI] [PubMed] [Google Scholar]
- 7. Tomlin A, Sinclair A. The influence of cognition on self-management of type 2 diabetes in older people. Psychol Res Behav Manag. 2016;9:7–20. doi: 10.2147/PRBM.S36238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand. 2014;130:439–451. doi: 10.1111/acps.12336 [DOI] [PubMed] [Google Scholar]
- 10. Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 11. Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch Gen Psychiatry. 2011;68:845–852. doi: 10.1001/archgenpsychiatry.2011.80 [DOI] [PubMed] [Google Scholar]
- 13. Scheef L, Spottke A, Daerr M, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d [DOI] [PubMed] [Google Scholar]
- 14. Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Harten AC, Visser PJ, Pijnenburg YA, et al. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 16. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nguyen NT, Nguyen XM, Lane J, Wang P. Relationship between obesity and diabetes in a US adult population: findings from the National Health and Nutrition Examination Survey, 1999–2006. Obesity Surg. 2011;21:351–355. doi: 10.1007/s11695-010-0335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan PW. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128 [DOI] [PubMed] [Google Scholar]
- 19. Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iso-Markku P, Waller K, Vuoksimaa E, et al. Midlife physical activity and cognition later in life: a prospective twin study. J Alzheimers Dis. 2016;54:1303–1317. doi: 10.3233/JAD-160377 [DOI] [PubMed] [Google Scholar]
- 21. Espeland MA, Rapp SR, Bray GA, et al. ; Action for Health In Diabetes (Look AHEAD) Movement and Memory Subgroup; Look AHEAD Research Group. Long-term impact of behavioral weight loss intervention on cognitive function. J Gerontol A Biol Sci Med Sci. 2014;69:1101–1108. doi: 10.1093/gerona/glu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rapp SR, Luchsinger JA, Baker LD, et al. Effect of a long-term intensive lifestyle intervention on cognitive function: action for health in diabetes study. J Am Geriatr Soc. 2017;9:146–152. doi: 10.1111/jgs.14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Espeland MA, Luchsinger JA, Baker LD, et al. ; Look AHEAD Study Group. Effect of a long-term intensive lifestyle intervention on prevalence of cognitive impairment. Neurology. 2017;88:2026–2035. doi: 10.1212/WNL.0000000000003955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shin N, Hill-Briggs F, Langan S, Payne JL, Lyketsos C, Golden SH. The association of minor and major depression with health problem-solving and diabetes self-care activities in a clinic-based population of adults with type 2 diabetes mellitus. J Diabetes Complications. 2017;31:880–885. doi: 10.1016/j.jdiacomp.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubin RR, Peyrot M, Gaussoin SA, et al. ; Look AHEAD Research Group. Four-year analysis of cardiovascular disease risk factors, depression symptoms, and antidepressant medicine use in the Look AHEAD (Action for health in diabetes) clinical trial of weight loss in diabetes. Diabetes Care. 2013;36:1088–1094. doi: 10.2337/dc12-1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Look AHEAD Research Group. Look AHEAD design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. [DOI] [PubMed] [Google Scholar]
- 27. The Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. New Eng J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The Look AHEAD Research Group. The Look AHEAD Study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Look AHEAD Research Group. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8:320–329. doi: 10.1177/1740774511405858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cox CL, Lensing S, Rai SN, Hinds P, Burghen E, Pui CH. Proxy assessment of quality of life in pediatric clinical trials: application of the health utilities index 3. Qual Life Res. 2005;14:1045–1056. [DOI] [PubMed] [Google Scholar]
- 31. Feeny D, Furlong W, Torrance GW, et al. Multiattribute and single-attribute utility functions for the health utilities index mark 3 system. Med Care. 2002;40:113–128. [DOI] [PubMed] [Google Scholar]
- 32. Baker LD, Frank LL, Foster-Schubert K, et al. Aerobic exercise improves cognition for older adults with glucose intolerance, a risk factor for Alzheimer’s disease. J Alzheimers Dis. 2010;22:569–579. doi: 10.3233/JAD-2010-100768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- 34. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430 [DOI] [PubMed] [Google Scholar]
- 35. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 36. Siervo M, Arnold R, Wells JC, et al. Intentional weight loss in overweight and obese individuals and cognitive function: a systematic review and meta-analysis. Obes Rev. 2011;12:968–983. doi: 10.1111/j.1467-789X.2011.00903.x [DOI] [PubMed] [Google Scholar]
- 37. Espeland MA, Lipska K, Miller ME, et al. ; LIFE Study Investigators. Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. J Gerontol A Biol Sci Med Sci. 2017;72:861–866. doi: 10.1093/gerona/glw179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Espeland MA, Luchsinger JA, Neiberg RH, et al. ; Action for Health in Diabetes Brain Magnetic Resonance Imaging Research Group. Long term effect of intensive lifestyle intervention on cerebral blood flow. J Am Geriatr Soc. 2018;66:120–126. doi: 10.1111/jgs.15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang P, Hire D, Espeland MA, et al. ; Look AHEAD Research Group. Impact of intensive lifestyle intervention on preference-based quality of life in type 2 diabetes: results from the Look AHEAD trial. Obesity (Silver Spring). 2016;24:856–864. doi: 10.1002/oby.21445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohr DC, Goodkin DE, Likosky W, Beutler L, Gatto N, Langan MK. Identification of beck depression inventory items related to multiple sclerosis. J Behav Med. 1997;20:407–414. PMID: 9298438. [DOI] [PubMed] [Google Scholar]
- 41. Espeland MA, Carmichael O, Hayden K, et al. ; Action for Health In Diabetes Brain Magnetic Resonance Imaging (Look AHEAD Brain) and Action for Health Movement and Memory Ancillary Study Research Groups. Long-term impact of weight loss intervention on changes in cognitive function: exploratory analyses from the action for health in diabetes randomized controlled clinical trial. J Gerontol A Biol Sci Med Sci. 2018;73:484–491. doi: 10.1093/gerona/glx165 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.