Abstract
Background
Aging is a multidimensional process with a remarkable interindividual variability. This study is focused on identifying groups of population with similar aging patterns, and to define the health trajectories of these groups. Sociodemographic and health determinants of these trajectories are also identified.
Methods
Data from the English Longitudinal Study of Aging (ELSA) and the Health and Retirement Study (HRS) were used. A set of self-reported health items and measured tests were used to generate a latent health metric by means of a Bayesian multilevel IRT model, assessing the ability of the metric to predict mortality. Then, a Growth Mixture Model (GMM) was conducted in each study to identify latent classes and assess health trajectories. Kaplan–Meier survival curves were obtained for each class and a multinomial logistic regression was used to identify determinants of these trajectories.
Results
The health score generated showed an adequate ability to predict mortality over 10 years in ELSA (AUC = 0.74; 95% CI: 0.72, 0.75) and HRS (AUC = 0.74; 95% CI: 0.73, 0.75). By means of GMM, four latent classes were identified in ELSA and five in HRS. Chronic conditions, no qualification and low level of household wealth were associated to the classes which showed a higher mortality in both studies.
Conclusion
The method based on the creation of a common metric of health and the use of GMM to identify similar patterns of aging, allows for the comparison of trajectories of health across longitudinal surveys. Multimorbidity, educational level, and household wealth could be considered as determinants associated to these trajectories.
Keywords: Health trajectories, Healthy aging, Longitudinal surveys, Health metrics, Latent classes
Worldwide life expectancy has considerably increased over the last five decades (1), with an expected increment of people aged 60 years or above from 11.2% in 2011 up to a 22% in 2050 (2). However, there are inconsistent findings on whether this increase on the life span is accompanied by a better health status (3–5). There is also a dramatic shift toward increased burden of noncommunicable diseases (6). The World Health Organization’s World Report on Ageing and Health (7) as a result argues that to get a true sense of the dynamics of aging, the focus should be put on health trajectories in order to optimize well-being and health gains from interventions. Therefore, healthy aging has important implications, since multimorbidity and disability in the elderly population has a remarkable impact in society and economy (8,9).
Aging is a multidimensional process that implies a gradual accumulation of molecular and cellular damage over time, which results in a progressive decline in physical and mental capacities (10), increasing the risk for illness and death (11). Nonetheless, there are individual differences in the way people age (12,13). Consequently, developing health metrics to quantify health levels of individuals in a way that could be aggregated to the population levels is a major challenge in understanding healthy aging trajectories (14).
According to the International Classification of Functioning (15) and the World Health Organization’s conceptualization of health status for measurement (14), a measure of health requires quantifying the functional ability of an individual. The definition comprises the individuals’ intrinsic capacity and its interactions with their environment. Health status can be measured either through self-reported items capturing the presence of difficulties in a given domain of functioning, or using measured tests of capacity, such as cognitive tasks, walking speed, or grip strength (16,17).
To provide a definition of successful aging is a complex task, as mentioned in a systematic review (18). Moreover, although health declines with age, individuals do not have identical rates and timing of decline. Adopting a life course approach can help to understand why different people and populations age differently (19–21). Trajectories on health can have a step-ladder pattern or show a precipitous decline at a determined point in time.
The present study aims at identifying groups of people with varying patterns of health trajectories, their determinants, and sociodemographic patterns associated with those trajectories. A common metric of health, utilizing data from the English Longitudinal Study of Ageing (ELSA) (22) and the Health and Retirement Study (HRS), is used to compare these trajectories (23).
Methods
Sample and Study Design
Data from the first 7 waves of the ELSA (2002–2014), and the first 11 waves of the HRS (1992–2012) were used in this paper. Both studies are biannual, longitudinal, and focused on adults aged 50 years and over, considering nationally representative samples from the English and the U.S. populations, respectively. Specifics of the ELSA sample, study design, and data collection are available at the ELSA project website (https://www.elsa-project.ac.uk/). All participants in ELSA have given informed consent. Ethical approval for all the ELSA waves was granted from the National Research Ethics Service (MREC/01/2/91). Participants in HRS provided verbal consent to participate and an informed consent document. Collection and production of HRS data was based on the requirements from the University of Michigan’s Institutional Review Board (IRB). Further details of the study design and sampling procedure are available on the HRS website (hrsonline.isr.umich.edu).
Measures
A set of 45 items were initially identified in the ELSA baseline, comprising self-reported health questions related to impairments in body functions, limitations in Activities of Daily Living (ADLs), and limitations in Instrumental Activities of Daily Living (IADLs); and also a set of measured tests covering cognitive functioning and walking speed. A full description of the 45 items is provided elsewhere (17). Thirty of these 45 items were identified in at least one of the HRS waves and were identified as common items to anchor the scale. The statistical model considered for creating the metric of health allows for the inclusion of the anchoring items as well as the additional items in ELSA but not in HRS. Original questions in ELSA and HRS varied in the number of response categories. Self-reported health questions comprised between two and five response options and some category labels were slightly different in ELSA and HRS. For this reason, a previous harmonization effort was carried out in the present study: after examining the content and potentially different response categories in each question across waves and studies, the original items were coded according to the presence or absence of difficulties (coded as 0 and 1, respectively). For measured tests, the sample was considered separately in each wave of each study and the lower quartile of each distribution determined in each case. Then, the original variable was dichotomized in each wave of each study indicating presence (values lower and equal to the 25th percentile of the distribution, coded 0) and absence (values higher than the 25th percentile of the distribution, coded 1) of difficulties. Higher values in the latent health score obtained from these items indicate a better health status.
Sociodemographic variables as gender, age, formal qualification, ethnicity, and household wealth, were also used in the statistical analysis. Formal qualification was defined in both surveys as having a degree or certificate recognized by the English or the U.S. education system, respectively. Household wealth was measured as the respondent’s net value of total wealth (including second home) less all debt. Participants were also asked if a doctor had ever told them that they are suffering from any of the diseases that were included in a list of chronic conditions, and the presence of chronic conditions was categorized in 0, 1, or 2+. Ethnicity was considered only in HRS, since the heterogeneity observed, and comprised four categories: whites, African American, Hispanic, and others. On the other hand, this variable was not included in the specific analyses carried out in ELSA, since the homogeneity of the ELSA sample in terms of ethnicity (the percentage of whites at ELSA baseline was 97.0%).
For mortality in ELSA, data from participants who provided informed consent to linkage to the National Health Service Central Register at baseline were used, and the mortality status was updated at February 2012; while in HRS mortality was determined by matching study records to the National Death Index and using information from the household members participating in the study.
Statistical Analysis
A common metric of health was created simultaneously for ELSA and HRS waves, using a Bayesian multilevel Item Response Theory (IRT) method. All parameters were simultaneously estimated using the Markov Chain Monte Carlo approach (24). Based on the concepts of anchor items and specific-study items, the procedure described in Caballero et al. (17) was employed, considering “study” (HRS/ELSA) as level-variable and transforming the latent score into a 0–100 scale, with higher values indicating a better health status.
Before running the IRT model, the measurement invariance of the 30 common items was tested across both studies. A sequential approach was considered, testing the goodness-of-fit of four nested models which represented respectively configural, metric, strong and strict measurement invariance. A detailed description of the procedure is provided in the Supplementary Material (Appendix 1).
A mixed-effect multilevel regression model was carried out to assess whether the metric is sensitive to aging. Additional details about the statistical approach employed to create the above-mentioned common metric of health and the mixed-effect multilevel regression model, are provided as Supplementary Material (Appendix 1).
The ability of the metric of health to predict mortality over ten years was assessed using Receiver Operating Characteristics Curves and adjusting by gender. The mortality analysis was separately carried out for ELSA and HRS, taking health status at 2002 as the predictor in both cases and using the mortality status in 2012 as the outcome. A total of 11,906 participants in ELSA Wave 1 and 12,652 participants in HRS Wave 6, both interviewed in 2002, were considered.
Healthy aging trajectories were then analyzed separately for each study. A Growth Curve Mixture Modeling (GMM) (25) framework was used to identify a finite set of homogeneous groups based on health trajectories across waves in each study. To decide the optimum number of subgroups/classes, the Sample-size Adjusted Bayesian Information Criteria (SABIC), as well as the Lo, Mendell, and Rubin likelihood test (LMR-LRT) were used. The appropriate number of classes to be used in the GMM was based on a Latent Class Growth Analysis (LCGA) model previously implemented. LCGA is a special type of GMM, whereby the variance and covariance estimates for the growth factors within each class are assumed to be fixed to zero. Additional criteria used to select the final model with the corresponding number of classes were: (i) successful convergence; (ii) entropy values over 0.70; (iii) no less than 1% of total sample in a class; and (iv) average of the posterior probabilities of class membership over 0.70. Class membership was based on the highest average of the posterior probability. Growth parameters of each class were estimated, fixing interclass variances of intercepts and slopes to be equal to avoid possible convergence problems.
Based on sociodemographic variables, a multinomial logistic regression analysis was conducted to examine the likelihood of being in each class previously determined in the GMM. In ELSA and HRS, the modal class (the class with the largest sample size) was used as the reference category. In order to conduct a mortality analysis and assess survival rates associated to each class, survival curves by class were generated using Kaplan–Meier estimates. Survival rates across time were considered as the outcome in the Kaplan–Meier curve.
The overall sample (n = 55,684) who participated in any of the waves of ELSA or HRS, was considered in the Bayesian multilevel IRT analysis conducted for creating the common metric of health and the mixed-effect multilevel regression model. All the waves were considered for these analyses. On the other hand, the general profile associated to healthy aging trajectories was assessed considering, in each study, the subset of participants interviewed at baseline (n = 11,906 in ELSA and n = 12,648 in HRS). Data management, descriptive analyses and multilevel models were implemented in Stata (26). GMM analyses were carried out in Mplus (27), and the sirt package (28) in R(29) was employed to conduct the analysis based on the Bayesian multilevel IRT approach.
Results
A total of 55,684 subjects participated in at least one wave of either ELSA or HRS studies. A combined data set was created, with 18,396 participants (54.5% of women) from ELSA and 37,288 participants (56.2% of women) from HRS. In ELSA, a total of 360 subjects from the 11,906 who participated at baseline (3.02%) did not provide informed consent to linkage to the National Health Service Register and were excluded from the mortality analysis. Participants who provided this consent in ELSA were significantly older than those who did not (64.11 ± 10.84 vs 62.04 ± 10.62; t (11,904) = 3.57; p < .001), while significant differences between both groups were not found in terms of gender (χ2 [1] = 2.45; p = .12) nor formal qualification (χ2 [1] = 0.50; p = .48).
A latent score on health was created across the ELSA and HRS data. The Expected-A-Posteriori (EAP) reliability was 0.86. The 30 common items identified showed strict measurement invariance across both studies. Specific details about the measurement invariance are shown in the Supplementary Material (Appendix 1, Table S1). The metric of health was sensitive to aging, as also shown in the Supplementary Material (Table S2). The metric showed also an adequate ability to predict mortality in ELSA (AUC = 0.74; 95% CI: 0.72, 0.75) and HRS (AUC = 0.74; 95% CI: 0.73, 0.75), with higher scores associated with a lower mortality over 10 years.
Identifying Health Trajectories in the ELSA Study
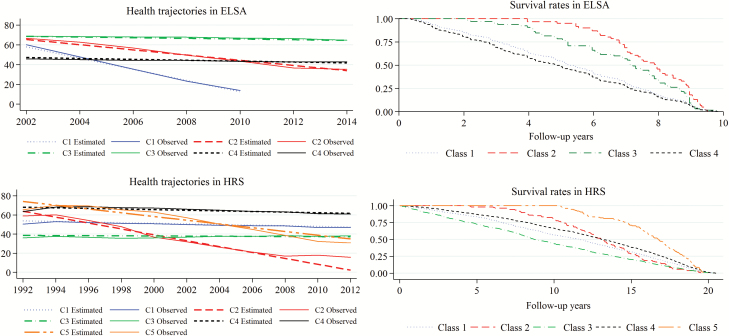
According to the criteria for determining the number of classes, a four-class GMM was considered for identifying groups of subjects in ELSA based on health trajectories (Figure 1). A modal class was detected (Class 3), comprising a 63% of the total sample. Significant decreasing trends (p < .001) in health across time were found for all classes (Supplementary Table S3). Class 1 showed the strongest decreasing trend, although comprised the smallest proportion of subjects. The general profile at ELSA baseline for each class is showed in Table 1.
Figure 1.
Health trajectories in ELSA and HRS studies, and survival curves by class. Note: Since the small sample size of people from ELSA Class 1 who continued in the study after the first five waves, health trajectories for this class are shown for the period 2002–2010. The observed trajectories were based on the mean scores on health status observed, while the estimated trajectories were based on the mean values on health status predicted by the GMM model. ELSA = English Longitudinal Study of Aging; GMM = Growth Mixture Model; HRS = Health and Retirement Study.
Table 1.
Baseline General Profile of the Four Classes Identified in the GMM in the ELSA Study
| Class 1 | Class 2 | Class 3 | Class 4 | p | Effect Size | |
|---|---|---|---|---|---|---|
| Number of subjects | 81 | 340 | 7,491 | 3,994 | - | - |
| Age, mean (SD) | 74.59 (11.36) | 69.76 (10.19) | 60.72 (9.28) | 69.58 (10.98) | <.001 | 0.44 |
| Male, n (%) | 37 (45.68) | 161 (47.35) | 3543 (47.30) | 1,502 (37.61) | <.001 | 0.09 |
| Mean health score at ELSA baseline (SD) | 57.88 (10.76) | 63.25 (7.68) | 65.20 (8.69) | 43.78 (8.50) | <.001 | 1.17 |
| Formal qualification, n (%) | 48 (59.26) | 177 (52.06) | 5,133 (68.52) | 1,637 (40.99) | <.001 | 0.26 |
| Belonging to the 1st-2nd quintile of household wealth (in Pounds), n (%) | 35 (43.21) | 151 (44.41) | 2,195 (29.30) | 2,999 (57.65) | <.001 | 0.27 |
| Number of chronic conditions | <.001 | 0.31 | ||||
| 0, n (%) | 43 (53.09) | 170 (50.00) | 4,552 (60.77) | 792 (19.83) | ||
| 1, n (%) | 22 (27.16) | 136 (40.00) | 2,343 (31.28) | 1,772 (44.37) | ||
| 2+, n (%) | 16 (19.75) | 34 (10.00) | 596 (7.96) | 1,430 (35.80) |
Note: Cramer’s V was used as effect size measure in the comparisons across categorical variables, while Cohen’s f was used as effect size measure in the comparisons across continuous variables.
ELSA = English Longitudinal Study of Aging; GMM = Growth Mixture Model.
Considering the modal class as reference, the population was older in the remaining classes as it can be seen in the multinomial logistic regression model (Table 3). When comparing with the modal class, Class 4 (the second largest group) was associated with multimorbidity (odds ratio [OR] = 1.93; 95% confidence interval [CI]: 1.57, 2.38), less formal qualification (OR = 0.78; 95% CI: 0.67, 0.91) and a lower level of household wealth (OR = 1.74; 95% CI: 1.49, 2.03). Kaplan–Meier curves associated to classes 1 and 4 showed the highest mortality rates during the follow-up (Figure 1).
Table 3.
Multinomial Logistic Regression Models for Predicting Classes Identified in ELSA and HRS
| Variables | ELSA | HRS | |||||
|---|---|---|---|---|---|---|---|
| Class 1 (n = 81) | Class 2 (n = 340) | Class 4 (n = 3,994) | Class 1 (n = 3,942) | Class 2 (n = 116) | Class 3 (n = 1,161) | Class 5 (n = 247) | |
| OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | OR (95 % CI) | |
| Age | 1.15*** (1.12, 1.18) | 1.09*** (1.08, 1.11) | 1.07*** (1.06, 1.08) | 1.02*** (1.01, 1.03) | 1.12*** (1.09, 1.16) | 1.02** (1.01, 1.04) | 1.10*** (1.07, 1.13) |
| Health at baseline | 0.92 (0.89, 0.95) | 1.00 (0.99,1.01) | 0.72*** (0.71, 0.73) | 0.85*** (0.83, 0.84) | 0.95*** (0.93, 0.97) | 0.68*** (0.67, 0.69) | 1.03** (1.01, 1.04) |
| Formal qualification (ref. no) | 1.49 (0.93, 2.40) | 0.85*** (0.67, 0.91) | 0.78*** (0.67, 0.91) | 0.54*** (0.48,0.62) | 0.64* (0.42, 0.97) | 0.37*** (0.30, 0.46) | 0.61** (0.45, 0.83) |
| Gender (ref. male) | 1.19 (0.76, 1.87) | 1.10 (0.88,1.38) | 1.21** (1.04, 1.41) | 1.21** (1.09, 1.36) | 0.89 (0.59, 1.35) | 1.33** (1.07, 1.64) | 1.08 (0.82, 1.42) |
| Belonging to the 1st-2nd quintile of household wealth (ref. no) | 1.54 (0.97, 2.45) | 1.66*** (1.32, 2.10) | 1.74*** (1.49, 2.03) | 1.73*** (1.55, 1.95) | 2.77*** (1.84, 4.17) | 2.81*** (2.25, 3.50) | 1.30 (0.97, 1.74) |
| Number of chronic conditions (ref. 0) | |||||||
| 1 | 0.65 (0.38, 1.11) | 1.38** (1.09, 1.76) | 1.50*** (1.27, 1.77) | 1.73*** (1.53, 1.96) | 1.01 (0.62, 1.66) | 2.69*** (1.91, 3.80) | 1.04 (0.78, 1.40) |
| 2+ | 1.57 (0.85, 2.90) | 1.36 (0.92, 2.02) | 1.93*** (1.57, 2.38) | 3.82*** (3.33, 4.37) | 3.33*** (2.11, 5.24) | 10.53*** (7.60, 14.58) | 1.51* (1.06, 2.14) |
| Ethnicity (ref. whites) | |||||||
| African-American | - | - | - | 1.20** (1.04, 1.39) | 1.87** (1.17, 2.99) | 1.42** (1.10, 1.84) | 1.22 (0.85, 1.76) |
| Hispanic | - | - | - | 1.34** (1.10, 1.63) | 1.95* (1.07, 3.55) | 1.60** (1.16, 2.21) | 1.23 (0.78, 1.94) |
| Other | - | - | - | 1.09 (0.76, 1.56) | 2.39 (0.92, 6.23) | 1.51 (0.79, 2.89) | 0.82 (0.30, 2.25) |
Note: Full multinomial logistic regression model coefficients in the ELSA (reference category = Class 3; n = 7,491) and HRS (reference comparison category = Class 4, n = 7,182) studies. Ethnicity variable was considered only in the HRS study.
CI = Confidence interval; ELSA = English Longitudinal Study of Aging; HRS = Health and Retirement Study; OR = Odds ratio.
Identifying Health Trajectories in the HRS Study
Five classes were identified in HRS (Figure 1). Supplementary Table S4 displays the estimated growth parameters for each class. Results revealed a modal class (Class 4), comprising a 57% of the total sample. Decreasing significant trends in health scores across time were found for four out of the five classes. Class 3, which presented the worse health status at baseline (lowest mean intercept), had associated a nonsignificant (p = .115) slope mean. The general profile at HRS baseline is showed in Table 2 for each class.
Table 2.
Baseline General Profile of the Five Classes Identified in the GMM in the HRS Study
| Class 1 | Class 2 | Class 3 | Class 4 | Class 5 | p | Effect Size | |
|---|---|---|---|---|---|---|---|
| Number of subjects | 3942 | 116 | 1,161 | 7,182 | 247 | - | - |
| Age, mean (SD) | 55.76 (5.59) | 58.80 (6.84) | 56.24 (5.67) | 54.68 (5.58) | 57.60 (6.31) | <.001 | 0.13 |
| Male, n (%) | 1,552 (39.37) | 71 (61.21) | 441 (37.98) | 6,655 (50.89) | 146 (59.11) | <.001 | 0.12 |
| Mean health score at HRS baseline (SD) | 48.57 (7.80) | 56.98 (9.24) | 35.10 (8.35) | 60.90 (7.97) | 62.28 (7.48) | <.001 | 1.05 |
| Formal qualification, n (%) | 2,565 (65.07) | 71 (61.21) | 520 (44.79) | 5,927 (82.53) | 172 (69.64) | <.001 | 0.27 |
| Belonging to the 1st-2nd quintile of household wealth (in Dollars), n (%) | 1,963 (49.80) | 67 (57.76) | 858 (73.90) | 2,082 (28.99) | 89 (36.03) | <.001 | 0.29 |
| Ethnicity | <.001 | 0.10 | |||||
| Whites, n (%) | 2,685 (68.22) | 64 (55.17) | 623 (53.75) | 5,572 (77.62) | 174 (70.45) | ||
| African American, n (%) | 744 (18.90) | 31 (26.72) | 325 (28.04) | 924 (12.87) | 43 (17.41) | ||
| Hispanic, n (%) | 426 (10.82) | 16 (13.79) | 182 (15.70) | 525 (7.31) | 26 (10.53) | ||
| Other, n (%) | 81 (2.06) | 5 (4.31) | 29 (2.50) | 158 (2.20) | 4 (1.62) | ||
| Number of chronic conditions | <.001 | 0.33 | |||||
| 0, n (%) | 859 (21.79) | 37 (31.90) | 74 (6.37) | 3,772 (52.52) | 113 (45.75) | ||
| 1, n (%) | 1,373 (34.83) | 30 (25.86) | 238 (20.50) | 2,466 (34.34) | 83 (33.60) | ||
| 2+, n (%) | 1,710 (43.38) | 49 (42.24) | 849 (73.13) | 944 (13.14) | 51 (20.65) |
Note: Cramer’s V was used as effect size measure in the comparisons across categorical variables, while Cohen’s f was used as effect size measure in the comparisons across continuous variables.
ELSA = English Longitudinal Study of Aging; GMM = Growth Mixture Model; HRS = Health and Retirement Study.
In HRS, the modal class (Class 4) was associated with younger people with formal qualification and without chronic conditions, according to the results obtained in the multinomial logistic regression (Table 3). When comparing with Class 4 (reference category), multimorbidity was associated with belonging to Class 1 (OR = 3.82; 95% CI: 3.33, 4.37) and specially Class 3 (OR = 10.53; 95% CI: 7.60, 14.58). In general terms, Class 3, which had the highest percentage of people with chronic conditions, showed the highest rate of mortality (Figure 1). On the other hand, Class 5 (with the highest health score at baseline) showed the highest rates of survival across time.
Discussion
This article provides a methodological approach to identify a finite set of homogeneous population groups based on health trajectories across time. Before assessing health trajectories, a common latent metric of health was jointly estimated using nationally representative samples from the English and U.S. populations. The conceptualization of this health metric is based on intrinsic capacity and functional ability, and summarizes the underlying health status of an individual as an overall latent composite of different domains of human functioning (14). The metric of health employed in the present article is based on functioning domains as Walking, Sight, Hearing, Balance, Dizziness, Memory, Orientation in time, Cognition, Pain, Energy, Sleep, Incontinence, Mobility, and limitations in ADLs and IADLs (17). The metric also showed an adequate ability to predict mortality, and it was sensitive to aging, according to the analyses conducted.
The creation of a latent health metric after using information from self-reported items and measured tests, is a procedure which has already been implemented in other researches either to compare health status across studies (16), to compare health status across waves of the same study (17), or to identify relevant factors related to health status within a single study (30–32). The present article is set in the context of the Ageing Trajectories of Health: Longitudinal Opportunities and Synergies (ATHLOS) project (http://athlosproject.eu/), assessing health trajectories and sociodemographic determinants in longitudinal surveys.
When measuring healthy aging trajectories, the remarkable variability in the aging process between individuals (12) is a key challenge. In that regard, the GMM-based methodology presented in this study allows to: (i) determine a specific number of latent classes that age similarly; and (ii) estimate healthy aging trajectories of these groups (25). This information from class membership could be used to identify factors and determinants of healthy aging trajectories.
According to the results obtained from the multinomial logistic regression model, systematic relationships were detected in terms of health status at baseline, formal qualification, and household wealth across the different classes identified in ELSA and HRS. The modal classes identified in both studies showed a slight decline trend on health across waves and were associated to higher levels of formal qualification and household wealth. These findings are consistent with previous results, where years of education and income were positively related to a better health status (16,33–35). Education and family income have been also found to be relevant factors to understand the variations in health trajectories when people age (36). Moreover, our results showed that health status at baseline was strongly related to a better health status across waves in both studies, which highlights the importance of reaching young elderly with a good health status.
The presence of chronic conditions was another factor systematically related to those classes with worse health status and stronger health decline across time in both ELSA and HRS. Specifically, multimorbidity showed a strong association with belonging to those classes, which is consistent with previous results conducted in United States (37) and English (17) populations, which revealed a negative association between multimorbidity and health.
Results from the Kaplan–Meier survival curves suggested differences in mortality rates across classes identified in the GMM in both ELSA and HRS studies. Regarding the general sociodemographic profile of the classes with the highest mortality rates in both studies, these classes had lower levels of formal qualification and household wealth. Moreover, they also presented a worse health status at baseline, as well as a higher prevalence of chronic conditions. These findings are consistent with previous results, where years of education and income were positively related to a better health status (33–35).
It is worth noticing that the class with worst health status (ie, lowest health scores at baseline and accelerated aging decline) in the HRS study comprised the largest proportions of African American and Hispanic populations. These ethnic differences in aging are consistent with previous research that revealed significant differences in health between whites and non-whites (38–40). In that regard, some studies suggest that the differences in health between white and non-white populations could be associated with ethnic differences in the use of health care services (41,42).
The method proposed in this paper has been implemented in a data set comprising two nationally representative samples focused on people aged 50 years and over, which allows for generalizing conclusions to other samples of the same populations. Moreover, the methodological approach implemented in this article has two important contributions. First, it allows for the simultaneous estimation of a common health metric for different longitudinal studies, based on a set of self-reported health questions and measured tests that may vary across studies. Second, the generated latent health score can be used to identify aging trajectories within each study, which allows for analyzing latent health changes across time in different homogenous groups of the general population.
Some limitations of the present study should be considered. First of all, ELSA and HRS have a different number of waves, and the follow-up time varies between the two studies. Moreover, both studies are mainly focused on population aged 50 years and over; although a smaller comparison sample of adults aged 18–49 years has been included in both studies, there is not enough information in relation to younger cohorts. In that regard, further research should be carried out focusing on other population age groups, especially exploring differences among healthy individuals in their pace of aging (43,44) and considering also that the relationship between education and health declines with age (45). In addition, the small sample size in some of the latent classes identified by the GMM approach requires caution when interpreting and generalizing results of these trajectories. Other potential limitation could be the presence of a different set of items in both studies (45 in ELSA, while only 30 of these items were available in some of the HRS waves); however, one of the advantages of the Bayesian multilevel IRT approach considered is that can deal with items varying across studies and waves (17). In terms of predictive ability, the metric of health obtained has shown a good performance. The use of the approach proposed is especially relevant in the context of the ATHLOS project, where self-reported health items and measured tests can vary across waves and studies.
In conclusion, a methodological approach to assess trajectories of health in longitudinal studies has been proposed in the present article, allowing for the analysis of determinants of these trajectories. A common metric of health was created, allowing for the inclusion of study-specific items. This metric was sensitive to aging and showed a good performance to predict mortality, providing a reliable measure of health and enabling the identification of a finite number of homogeneous classes based on the health scores obtained across the different waves of longitudinal studies. In general terms, the classes with lower health scores at baseline and stronger decline trends on health showed the highest rates on mortality in the English and the U.S. populations. The presence of chronic conditions, the lack of formal qualification, and a low level of household wealth were associated to a worse health trajectory.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the Ageing Trajectories of Health: Longitudinal Opportunities and Synergies (ATHLOS) project. The ATHLOS project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 635316. The first seven ELSA waves have been funded jointly by U.K. government departments and the National Institute on Aging, in the United States. The HRS is funded by the National Institute on Aging (U01 AG009740) and the Social Security Administration and is performed at the Institute for Social Research, University of Michigan. Javier de la Fuente work is supported by the FPU predoctoral grant (FPU16/03276) from the Spanish Ministry of Education, Culture and Sport.
Acknowledgments
The authors thank the ATHLOS Consortium for useful discussions.
Conflict of Interest
None reported.
References
- 1. Passarino G, De Rango F, Montesanto A. Human longevity: genetics or lifestyle? It takes two to tango. Immun Ageing. 2016;13:12. doi:10.1186/s12979-016-0066-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. United Nations. World Economic and Social Survey 2013. 2013. doi:10.1016/j.compind.2010.10.001 [Google Scholar]
- 3. Jagger C, Gillies C, Moscone F, et al. . Inequalities in healthy life years in the 25 countries of the European Union in 2005: a cross-national meta-regression analysis. Lancet. 2008;372:2124–2131. doi:10.1016/S0140-6736(08)61594–9 [DOI] [PubMed] [Google Scholar]
- 4. Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older Americans: National health and nutrition examination surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100:100–107. doi:10.2105/AJPH.2008.157388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stewart ST, Cutler DM, Rosen AB. US trends in quality-adjusted life expectancy from 1987 to 2008: combining national surveys to more broadly track the health of the nation. Am J Public Health. 2013;103:78–87. doi:10.2105/AJPH.2013.301250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO. Global Status Report on Noncommunicable Diseases 2010. 2011. doi:ISBN 978 92 4 156422 9 [Google Scholar]
- 7. WHO. World Report on Ageing and Health. 2015. [Google Scholar]
- 8. United Nations, Department of Economic and Social Affairs, Population Division World Population Prospects: The 2012 Revision, Highlights and Advance Tables. Working Paper No. ESA/P/WP.228; 2013. [Google Scholar]
- 9. Ensrud KE, Lui L-Y, Langsetmo L, et al. . Effects of mobility and multimorbidity on inpatient and post-acute health care utilization. J Gerontol A Biol Sci Med Sci. 2018; 73:1343–1349. doi:10.1093/gerona/glx128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beard JR, Officer A, de Carvalho IA, et al. . The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi:10.1016/S0140-6736(15)00516-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller RA. Biology of aging and longevity. Hazzard’s Geriatric Medicine and Gerontology; 2009:Chapter 1. doi:10.1001/jama.2009.1561 [Google Scholar]
- 12. Steves CJ, Spector TD, Jackson SH. Ageing, genes, environment and epigenetics: what twin studies tell us now, and in the future. Age Ageing. 2012;41:581–586. doi:10.1093/ageing/afs097 [DOI] [PubMed] [Google Scholar]
- 13. Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69:640–649. doi:10.1093/gerona/glt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salomon JA, Mathers CD, Chatterji S, Sadana R, Üstün TB, Murray CJL. Chapter 26: Quantifying individual levels of health: definitions, concepts, and measurement issues. Health Systems Performance Assessment; 2003:301–318. http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=26367362&lang=es&site=ehost-live. [Google Scholar]
- 15. World Health Organization. The international classification of functioning, disability and health. World Heal Organ. 2001;18:237. doi:10.1097/01.pep.0000245823.21888.71 [Google Scholar]
- 16. Cieza A, Oberhauser C, Bickenbach J, et al. . The English are healthier than the Americans: really?Int J Epidemiol. 2015;44:229–238. doi:10.1093/ije/dyu182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caballero FF, Soulis G, Engchuan W, et al. . Advanced analytical methodologies for measuring healthy ageing and its determinants, using factor analysis and machine learning techniques: the ATHLOS project. Sci Rep. 2017;7:43955. doi:10.1038/srep43955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cosco TD, Prina AM, Perales J, Stephan BC, Brayne C. Operational definitions of successful aging: a systematic review. Int Psychogeriatr. 2014;26:373–381. doi:10.1017/S1041610213002287 [DOI] [PubMed] [Google Scholar]
- 19. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. doi:10.1093/ije/31.2.285 [PubMed] [Google Scholar]
- 20. Hsu HC, Jones BL. Multiple trajectories of successful aging of older and younger cohorts. Gerontologist. 2012;52:843–856. doi:10.1093/geront/gns005 [DOI] [PubMed] [Google Scholar]
- 21. Franco OH, Karnik K, Osborne G, Ordovas JM, Catt M, van der Ouderaa F. Changing course in ageing research: the healthy ageing phenotype. Maturitas. 2009;63:13–19. doi:10.1016/j.maturitas.2009.02.006 [DOI] [PubMed] [Google Scholar]
- 22. Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the english longitudinal study of ageing. Int J Epidemiol. 2013;42:1640–1648. doi:10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the health and retirement study (HRS). Int J Epidemiol. 2014;43:576–585. doi:10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fox J-P, Glas CAW. Bayesian estimation of a multilevel irt model using Gibbs sampling. Psychometrika. 2001;66:271–288. doi:10.1007/BF02294839 [Google Scholar]
- 25. Ram N, Grimm KJ. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev. 2009;33:565–576. doi:10.1177/0165025409343765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. StataCorp. Stata Statistical Software: Release 14. 2015. 2015. doi:10.2307/2234838 [Google Scholar]
- 27. Muthén LK, Muthén BO.. Mplus User’s Guide. Sixth Edition; 2011. doi:10.1111/j.1600-0447.2011.01711.x [Google Scholar]
- 28. Robitzsch A. sirt: Supplementary Item Response Theory Models https://cran.rproject org/web/packages/sirt/sirt.pdf. 2016.
- 29. R Development Core Team R: a language and environment for statistical computing. Vienna Austria: R Foundation for Statistical Computing; 2016. https://doi.org/10.1038/sj.hdy.6800737 [Google Scholar]
- 30. Bostan C, Oberhauser C, Stucki G, Bickenbach J, Cieza A. Which environmental factors are associated with lived health when controlling for biological health? - a multilevel analysis. BMC Public Health. 2015;15:508. doi:10.1186/s12889-015-1834-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hosseinpoor AR, Williams JS, Jann B, et al. . Social determinants of sex differences in disability among older adults: a multi-country decomposition analysis using the World Health Survey. Int J Equity Health. 2012;11:52. doi:10.1186/1475-9276-11-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hosseinpoor AR, Stewart Williams J, Amin A, Araujo de Carvalho I, Beard J, Boerma T, … Chatterji S. Social determinants of self-reported health in women and men: understanding the role of gender in population health. PLoS ONE. 2012;7(4):e34799 https://doi.org/10.1371/journal.pone.0034799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avendano M, Jürges H, Mackenbach JP. Educational level and changes in health across Europe: longitudinal results from SHARE. J Eur Soc Policy. 2009;19:301–316. doi:10.1177/1350506809341512 [Google Scholar]
- 34. Kahn RS, Wise PH, Kennedy BP, Kawachi I. State income inequality, household income, and maternal mental and physical health: cross sectional national survey. BMJ. 2000;321:1311–1315. doi:10.1136/bmj.321.7272.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McLeod CB, Lavis JN, Mustard CA, Stoddart GL. Income inequality, household income, and health status in Canada: a prospective cohort study. Am J Public Health. 2003;93:1287–1293. doi:10.2105/ajph.93.8.1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim J, Durden E. Socioeconomic status and age trajectories of health. Soc Sci Med. 2007;65:2489–2502. doi:10.1016/j.socscimed.2007.07.022 [DOI] [PubMed] [Google Scholar]
- 37. Wei MY, Kabeto MU, Langa KM, Mukamal KJ. Multimorbidity and physical and cognitive function: performance of a new multimorbidity-weighted index. J Gerontol A Biol Sci Med Sci. 2017;73(2):225–232. https://doi.org/https://doi.org/10.1093/gerona/glx114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pereira CCA, Palta M, Mullahy J, Fryback DG. Race and preference-based health-related quality of life measures in the United States. Qual Life Res An Int J Qual Life Asp Treat Care Rehabil. 2011;20:969–978. doi:10.1007/s11136-010-9813-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sudano JJ, Baker DW. Explaining US racial/ethnic disparities in health declines and mortality in late middle age: the roles of socioeconomic status, health behaviors, and health insurance. Soc Sci Med. 2006;62:909–922. doi:10.1016/j.socscimed.2005.06.041 [DOI] [PubMed] [Google Scholar]
- 40. Wu C, Smit E, Sanders JL, Newman AB, Odden MC. A modified healthy aging index and its association with mortality: the National Health and Nutrition Examination Survey, 1999–2002. J Gerontol A Biol Sci Med Sci. 2017;72(10):1437–1444. https://doi.org/10.1093/gerona/glw334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowen ME, Gonzalez HM. Racial/ethnic differences in the relationship between the use of health care services and functional disability: the Health and Retirement Study (1992–2004). Gerontologist. 2008;48:659–667. doi:10.1093/geront/48.5.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57 (Suppl 1):108–145. doi:10.1177/1077558700574006 [DOI] [PubMed] [Google Scholar]
- 43. Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. J Gerontol A Biol Sci Med Sci. 2017;72:210–215. doi:10.1093/gerona/glw191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Santoni G, Marengoni A, Calderón-Larrañaga A, et al. . Defining health trajectories in older adults with five clinical indicators. J Gerontol A Biol Sci Med Sci. 2017;72:1123–1129. doi:10.1093/gerona/glw204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huisman M, Kunst AE, Bopp M, et al. . Educational inequalities in cause-specific mortality in middle-aged and older men and women in eight western European populations. Lancet. 2005;365:493–500. doi:10.1016/S0140-6736(05)17867-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.