Abstract
Background
Lifestyle interventions have been shown to improve physical function over the short term; however, whether these benefits are sustainable is unknown. The long-term effects of an intensive lifestyle intervention (ILI) on physical function were assessed using a randomized post-test design in the Look AHEAD trial.
Methods
Overweight and obese (body mass index ≥ 25 kg/m2) middle-aged and older adults (aged 45–76 years at enrollment) with type 2 diabetes enrolled in Look AHEAD, a trial evaluating an ILI designed to achieve weight loss through caloric restriction and increased physical activity compared to diabetes support and education (DSE), underwent standardized assessments of performance-based physical function including a 4- and 400-m walk, lower extremity physical performance (expanded Short Physical Performance Battery, SPPBexp), and grip strength approximately 11 years postrandomization and 1.5 years after the intervention was stopped (n = 3,783).
Results
Individuals randomized to ILI had lower odds of slow gait speed (<0.8 m/s) compared to those randomized to DSE (adjusted OR [95% CI]: 0.84 [0.71 to 0.99]). Individuals randomized to ILI also had faster gait speed over 4- and 400-m (adjusted mean difference [95% CI]: 0.019 [0.007 to 0.031] m/s, p = .002, and 0.023 [0.012 to 0.034] m/sec, p < .0001, respectively) and higher SPPBexp scores (0.037 [0.011 to 0.063], p = .005) compared to those randomized to DSE. The intervention effect was slightly larger for SPPBexp scores among older versus younger participants (0.081 [0.038 to 0.124] vs 0.013 [−0.021 to 0.047], p = .01).
Conclusions
An intensive lifestyle intervention has modest but significant long-term benefits on physical function in overweight and obese middle-aged and older adults with type 2 diabetes.
ClinicalTrials.gov Identifier
Keywords: Diabetes, Weight loss, Physical function, Mobility
Limitations in physical function predict future disability resulting in dependency and greater healthcare costs, morbidity, and mortality (1,2). Obesity is a strong predictor of limitations in physical function, operating through both direct (eg, biomechanical changes) and indirect (eg, obesity-related comorbidities) pathways (3,4). Type 2 diabetes further accelerates declines in physical function and increases the risk of disability (5,6).
Lifestyle interventions have been shown to improve physical function over the short term. In middle aged and older adults who were overweight or obese, participation in weight loss and exercise interventions lasting up to 18 months improved performance-based physical function measures (7–12). In the Look AHEAD trial, overweight or obese middle aged and older adults with type 2 diabetes randomized to an intensive lifestyle intervention for weight loss had slower declines in self-reported physical function over 4 and 8 years of follow-up compared to those randomized to a diabetes support and education control (13,14). However, reductions in skeletal muscle mass that accompany weight loss may lead to muscle weakness (15), resulting in impaired physical function and disability (16). Thus, whether potential benefits of lifestyle change on performance-based physical function are sustainable over the long-term is unknown.
This study examined the effect of a long-term intensive lifestyle intervention (ILI) designed to achieve and maintain weight loss through caloric restriction and increased physical activity in middle-aged and older overweight and obese adults with diabetes on performance-based measures of physical function assessed approximately 11 years after randomization compared to a diabetes support and education (DSE) control group in Look AHEAD. We hypothesized that individuals randomized to ILI would have better performance-based physical function 11 years postrandomization compared to DSE. The consistency of the findings was also examined in pre-specified subgroups defined according to baseline age, gender, and body mass index (BMI).
Methods and Procedures
Look AHEAD was a multi-center, randomized controlled trial designed to test the effects of an intensive lifestyle intervention designed to achieve and maintain a 7% weight loss on cardiovascular morbidity and mortality (17). In brief, Look AHEAD recruited 5,145 individuals with type 2 diabetes between August 2001 and April 2004 who were 45–76 years of age and had a BMI ≥25 kg/m2 (≥27 kg/m2 if on insulin), HbA1c <11%, systolic blood pressure <160 mmHg, diastolic blood pressure <100 mmHg, and triglycerides <600 mg/dl.
Intervention
At enrollment into the Look AHEAD trial, participants were randomly assigned by center to an intensive lifestyle intervention (ILI) or a Diabetes Support and Education (DSE) control condition. ILI participants were given an individual weight loss goal of ≥10% and physical activity goal of ≥50 min/wk in the first month and ≥175 min/wk by the end of 6 months (18). In Phase 1, ILI participants were seen weekly for the first 6 months and three times per month for the next 6 months using a combination of group and individual sessions. In Phase II (Years 2–4), participants had one in-person individual session and a minimum of one additional contact by phone, mail or email per month with a goal of weight maintenance or reversal of weight regain. In Phase III (Years 5+), participants were encouraged to continue individual monthly sessions (a minimum of two individual sessions per year were required) and refresher campaigns with a goal of prevention of weight regain. DSE participants were invited to three group sessions focused on diet, physical activity, or social support each year for the first 4 years and one session annually thereafter (19). DSE participants did not receive information on behavioral strategies.
Acting on the recommendation of the trial’s Data and Safety Monitoring Board, the intervention was stopped due to futility on September 14, 2012 (20). At that time, participants had been in the intervention for up to 11 years (median 9.6 years). All Look AHEAD participants who were alive when the intervention was stopped were invited to join a follow-up observational study to determine the longer term effects of the intervention on a number of outcomes.
Physical Function Measures
The Look AHEAD study assessed performance-based physical function at all sites between August 2013 and December 2014, approximately 11.4 years (range, 9.5–13.2 years) after randomization and 1.6 years (range, 1.0–2.3 years) after the intervention was stopped. Clinic staff masked to intervention assignment conducted all physical function measures following centralized training and certification.
The Short Physical Performance Battery (SPPB) was administered to assess lower extremity physical function (21). The SPPB consists of standing balance tasks (ability to stand with the feet together in side-by-side, semi- and full-tandem positions for 10 s each), a 4-m walk to assess usual gait speed, and time to complete 5 repeated chair stands. Each of the three performance measures is assigned a score ranging from 0 (inability to perform the task) to 4 (the highest level of performance) and summed to create an SPPB score ranging from 0 to 12 (best). The SPPB was modestly expanded (SPPBexp) to minimize ceiling effects of the SPPB when used in well-functioning populations by increasing the holding time of the standing balance tasks to 30 s and adding a single leg stand (22). The SPPBexp component scores are calculated as the ratio of observed performance to the best possible performance and summed to provide a continuous score ranging from 0 to 3 (higher scores indicative of better performance).
Usual gait speed over 400 m was measured on a 20-m course (23); due to insufficient space, four clinics used a 10-m course. Participants were instructed to walk at their usual pace and time to complete the 400-m walk was recorded. Participants who were wheelchair-bound or dependent on a walker or quad cane, had a cardiovascular disease (CVD) event in the past 3 months, or whose blood pressure was >170/100 mmHg were excluded from testing. 400-m gait speed was calculated for those participants who completed the walk (3,026 out of 3,384 who attempted the walk).
Grip strength (kg) was measured twice in each hand using an isometric Hydraulic Hand Dynamometer (Jamar, Bolingbrook, IL). The maximum force from two trials for the stronger hand was used in the analyses.
Mobility disability was adjudicated by committee and defined as definite (unable to complete the 400-m walk in 15 min or less, 4-m gait speed <0.44 m/s if the 400-m walk was not attempted, wheelchair/walker/quad cane dependent, or self-report of being unable to walk in past 4 weeks; n = 497), probable (4-m gait speed ≥0.44 but <0.8 m/s if the 400-m walk was not attempted or if neither the 400-m nor the 4-m walk was attempted due to safety concerns; n = 108), possible (400-m walk not attempted due to pain or cardiovascular or orthopedic contraindications; n = 127), or absent (400-m walk completed in less than 15 min; n = 3,014). Mobility limitation was undeterminable in 85 participants. Three separate mobility disability definitions were examined: (a) definite disability versus no, probable, possible disability, or dead; (b) definite disability or dead versus no, probable, or possible disability; (c) probable, possible, definite disability, or dead versus no disability. Participants were also classified as having slow gait speed (4-m gait speed < 0.8 m/s), impaired lower extremity function (SPPB score ≤ 9), and impaired grip strength (<26 kg in men, <16 kg in women).
Potential Risk Factors for Physical Limitations
Self-reported characteristics and conditions were assessed using standardized questionnaires at baseline. A maximal graded exercise test was administered and cardiorespiratory fitness estimated in metabolic equivalents (METS) (24). The 36-item Short Form Health Survey (SF-36), which measures eight health domains including physical functioning, was used as a measure of health status, with domain subscale scores ranging from 0 to 100 (higher scores indicating better functioning or well-being) (25). Height was measured in duplicate using a stadiometer. Clinic staff masked to intervention assignment collected annual measures of weight throughout the trial using a digital scale. The Paffenbarger Physical Activity Index was collected in a subset of participants at baseline and Year 1 and 4 and in all participants at Year 8 and the observational follow-up visit (26).
Statistical Analyses
Unadjusted comparisons between groups were done using chi-square tests for proportions and t-tests or ANOVA for continuous variables measured at baseline and follow-up. Logistic regression was used to compare categorical physical performance measures and analysis of covariance (ANCOVA) was used to compare continuous physical performance measures among intervention groups adjusted for age, gender, race/ethnicity, clinic site, and baseline BMI, CVD history, and SF-36 Physical Functioning score. To account for selection bias potentially caused by dropout, death and missing outcomes, the conditional probability to be included in the analysis sample for all randomized participants was calculated based on their baseline characteristics (age, gender, race/ethnicity, clinic site, and baseline BMI, HbA1c %, hypertension, CVD history, cardiorespiratory fitness, and SF-36 physical functioning score). Then a sensitivity analysis was performed that included the calculated conditional probability as an additional covariate in the logistic regression or ANCOVA models described above. The consistency of the intervention effect on each outcome across pre-specified subgroups defined by baseline age (<60 vs ≥60 years), gender, and BMI (< 30 vs ≥30 kg/m2) was examined by adding intervention group by subgroup interactions to the adjusted models and examining the effect across subgroups when p for interaction was <0.10. All analyses were performed in SAS 9.4 (Cary, NC).
Results
At baseline (2001–2004), 5,145 participants were randomized to either ILI (n = 2,570) or DSE (n = 2,575) in the Look AHEAD trial. Of those, 4,033 were still active approximately 11 years after randomization and 1.5 years after the intervention ended; 524 were deceased; and 588 had dropped out. Of the 4,033 active participants, 3,979 consented to continued follow-up and 3,783 (95%) had at least one physical function measure; 74% of those originally randomized in the Look AHEAD trial.
Participants with physical function data who were included in these analyses (n = 3,783) were younger (58.1 vs 60.4 years, p < .0001); less likely to be male (39% vs 45%, p < .0001) or have a history of cardiovascular disease (12% vs 20%, p < .0001) at baseline; were more likely to be African American (16% vs 13%, p < .0001); had a lower baseline BMI (35.8 vs 36.3 kg/m2, p = .02); and had higher baseline cardiorespiratory fitness (7.3 vs 6.8 METS, p < .0001) compared to the original Look AHEAD participants who were excluded from the analyses due to death, loss-to-follow-up, or lacking all physical function data (n = 1,362). Furthermore, participants included in these analyses had a higher self-reported SF-36 Physical Functioning score at baseline compared to those who were excluded (48.7 vs 47.7, p < .0001). There was no difference in the distribution of intervention assignment between those who had physical performance data and the original Look AHEAD cohort. The risk factor distribution in the analysis sample was balanced between intervention groups (Table 1), except a greater percentage of ILI participants had a cardiorespiratory fitness level ≥7.5 METS (43% vs 40%, p = .02) compared to DSE participants.
Table 1.
Characteristics at the Time of Enrollment by Intervention Assignment: The Look AHEAD Study
| Original Randomized Sample | Analytical Sample | ||||
|---|---|---|---|---|---|
| Intensive Lifestyle Intervention (ILI) | Diabetes Support and Education (DSE) | Intensive Lifestyle Intervention (ILI) | Diabetes Support and Education, DSE) | p-Value* | |
| N | 2,570 | 2,575 | 1,902 | 1,881 | |
| Age, years | 58.6 ± 6.8 | 58.9 ± 6.9 | 58.0 ± 6.5 | 58.3 ± 6.6 | .28 |
| ≥60 years | 1,090 (42.4%) | 1,125 (43.7%) | 738 (38.8%) | 761 (40.5%) | .30 |
| Female gender | 1,526 (59.4%) | 1,537 (59.7%) | 1,152 (60.6%) | 1,164 (61.9%) | .41 |
| Race | .95 | ||||
| African American/Black (not Hispanic) | 400 (15.6%) | 404 (15.7%) | 308 (16.2%) | 315 (16.7%) | |
| White | 1,621 (63.1%) | 1,631 (63.3%) | 1,164 (61.2%) | 1,152 (61.2%) | |
| Hispanic | 340 (13.2%) | 340 (13.2%) | 260 (13.7%) | 248 (13.2%) | |
| Other/mixed | 208 (8.1%) | 200 (7.8%) | 170 (8.9%) | 166 (8.8%) | |
| BMI, kg/m2 | 35.9 ± 6.0 | 36.0 ± 5.8 | 35.7 ± 5.9 | 36.0 ± 5.8 | .08 |
| BMI ≥ 30 kg/m2 | 2,167 (84.3%) | 2,213 (85.9%) | 1,585 (83.3%) | 1,605 (85.3%) | .09 |
| Prior cardiovascular disease | 365 (14.2%) | 347 (13.5%) | 236 (12.4%) | 204 (10.8%) | .13 |
| Cardiorespiratory fitness, METS | 7.2 ± 1.9 | 7.2 ± 2.0 | 7.4 ± 2.0 | 7.3 ± 2.0 | .54 |
| SF-36 Physical Functioning Score | 48.5 ± 7.8 | 48.4 ± 8.0 | 48.7 ± 7.8 | 48.7 ± 7.8 | .96 |
BMI = body mass index; METS = metabolic equivalent; SF-36 = Short Form Health Survey.
*Mean ± SD or number (%) with t-test or chi-square to evaluate the distribution across intervention groups in the analysis sample.
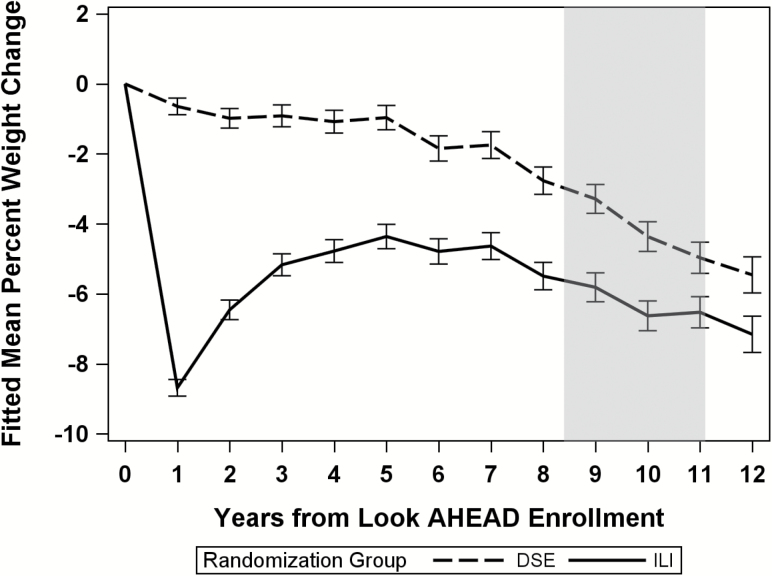
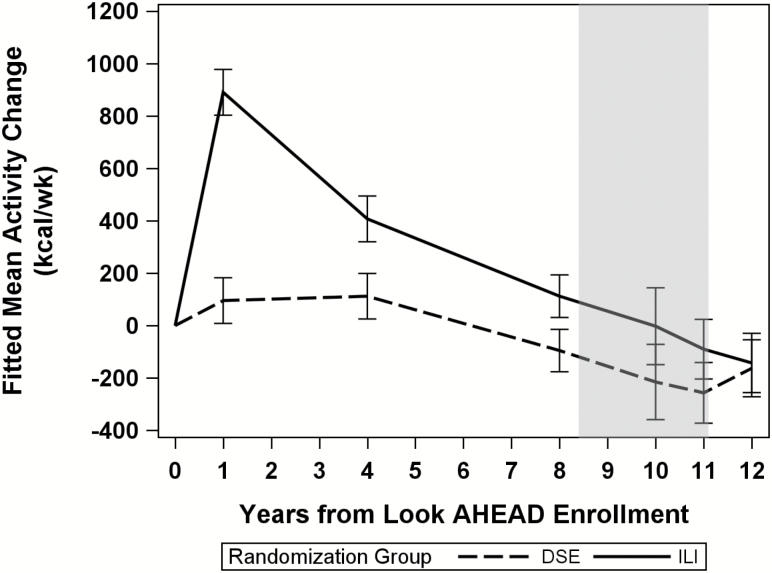
The ILI intervention produced substantial differences in weight loss (p < .0001) and physical activity (p < .001) compared to DSE. Differences were largest after the first year of intervention, but remained through the end of the intervention (September 2012) (Figure 1 and 2). ILI participants lost a mean (SE) 8.7% (0.13%) of their weight at Year 1 and maintained a 6.0% (0.2%) mean weight loss through the end of the intervention. In contrast, weight loss in the DSE participants was 0.6% (0.1%) at Year 1 and 3.4% (0.2%) at the end of the intervention. Change in self-reported physical activity from baseline also differed by intervention group at year 1 (mean [SD]: 893.1 [42.6] vs 99.5 [42.5] kcals/wk for ILI vs DSE, p < .0001) and at Year 8 (mean [SD]: 112.9 [39.6] vs −96.0 [39.5] kcals/wk for ILI vs DSE, p = .0002).
Figure 1.
Percent weight change since randomization by intervention group: the Look AHEAD study. Shaded area designates end of the intensive lifestyle intervention which varies depending on enrollment date.
Figure 2.
Change in physical activity since randomization by intervention group: the Look AHEAD study. Shaded area designates end of the intensive lifestyle intervention which varies depending on enrollment date.
The physical function data were collected an average of 11.4 years after randomization (range, 9.5–13.2 years). Table 2 shows the unadjusted frequencies and means of the physical function tests grouped by intervention assignment and the adjusted odds ratios and mean differences (95% CI). In unadjusted analyses, ILI participants had faster 4- and 400-m gait speed and higher SPPBexp scores and a smaller proportion of ILI participants had slow gait speed (<0.8 m/s) compared to DSE participants. After adjusting for age, gender, race/ethnicity, clinic, and baseline SF-36 physical function score, CVD history, and BMI, ILI participants had lower odds of slow gait speed compared to DSE participants; however, there were no associations between intervention assignment and mobility disability or impaired lower extremity function or grip strength. ILI participants also had faster gait speed over 4- and 400-m and higher SPPBexp scores. Results were similar when time to complete the 400-m walk was imputed based on distance and time walked for the 354 participants who were unable to complete the walk (data not shown). To determine whether 4-m gait speed was driving the observed association with SPPBexp score, the other two SPPBexp components were also examined; ILI participants had greater standing balance times and faster chair stand pace (adjusted mean difference [95% CI]: 1.87 [0.33 to 3.41] and 0.007 [0.000 to 0.014] seconds, respectively). Analyses using the conditional probability of being included in the analytical sample to adjust for attrition and nonparticipation yielded similar estimated intervention effects overall and within all subgroups (data not shown).
Table 2.
Unadjusted Means and Frequencies by Intervention Assignment and Adjusted Odds Ratios (95% CI) or Mean Differences (95% CI) for Physical Function by Treatment Group at 11 Year Follow-Up: The Look AHEAD Study
| Measures of Physical Function | Treatment Group | Unadjusted Treatment Group p-Value | Adjusted* Treatment Group, Odds Ratios or Mean Differences (95% CI) | Adjusted Treatment Group p-Value | |||
|---|---|---|---|---|---|---|---|
| ILI | DSE | ||||||
| N | N (%) or Mean ± SD | N | N (%) or Mean ± SD | ||||
| Mobility disability | |||||||
| Definite mobility disability | 2,134 | 237 (11.1) | 2,137 | 260 (12.2) | .28 | 0.96 (0.78 to 1.17) | .66 |
| Definite mobility disability or death | 2,134 | 487 (22.8) | 2,137 | 535 (25.0) | .09 | 0.92 (0.79 to 1.07) | .28 |
| Definite/probable/possible mobility disability or death | 2,134 | 607 (28.4) | 2,137 | 650 (30.4) | .16 | 0.95 (0.82 to 1.10) | .50 |
| Slow gait speed (<0.8 m/s) | 1,832 | 386 (21.1) | 1,806 | 441 (24.4) | .02 | 0.84 (0.71 to 0.99) | .04 |
| Impaired lower extremity function (SPPB ≤9) | 1,781 | 742 (41.7) | 1,731 | 772 (41.7) | .98 | 1.02 (0.88 to 1.18) | .79 |
| Impaired grip strength (<26 kg men; <16 kg women) | 1,761 | 252 (14.3) | 1,732 | 265 (15.3) | .41 | 1.08 (0.89 to 1.32) | .43 |
| Gait speed | |||||||
| 4-m gait speed (m/s) | 1,832 | 0.95 ± 0.21 | 1,806 | 0.93 ± 0.21 | .0008 | 0.019 (0.007 to 0.031) | .002 |
| 400-m gait speed (m/s)† | 1,532 | 1.00 ± 0.19 | 1,494 | 0.97 ± 0.19 | <.0001 | 0.023 (0.012 to 0.034) | <.0001 |
| Lower extremity physical performance | |||||||
| SPPB score (range, 0−12) | 1,781 | 9.4 ± 2.4 | 1,731 | 9.3 ± 2.4 | .10 | 0.105 (−0.036 to 0.245) | .14 |
| SPPBexp score (range, 0−3) | 1,855 | 1.50 ± 0.46 | 1,817 | 1.46 ± 0.46 | .003 | 0.037 (0.011 to 0.063) | .005 |
| Grip strength (kg) | 1,761 | 27.0 ± 9.9 | 1,732 | 26.5 ± 9.7 | .14 | 0.307 (−0.115 to 0.729) | .15 |
SPPB = short physical performance battery; SPPBexp = expanded short physical performance battery.
*Adjusted for age, gender, race/ethnicity, clinic, and baseline SF-36 physical functioning, cardiovascular disease, and body mass index, and whether or not an alternate (10-m) course was used (400-m gait speed mean difference only). OR: DSE is reference group. Mean differences: ILI–DSE.
†Results shown are for participants who completed the 400-m walk only.
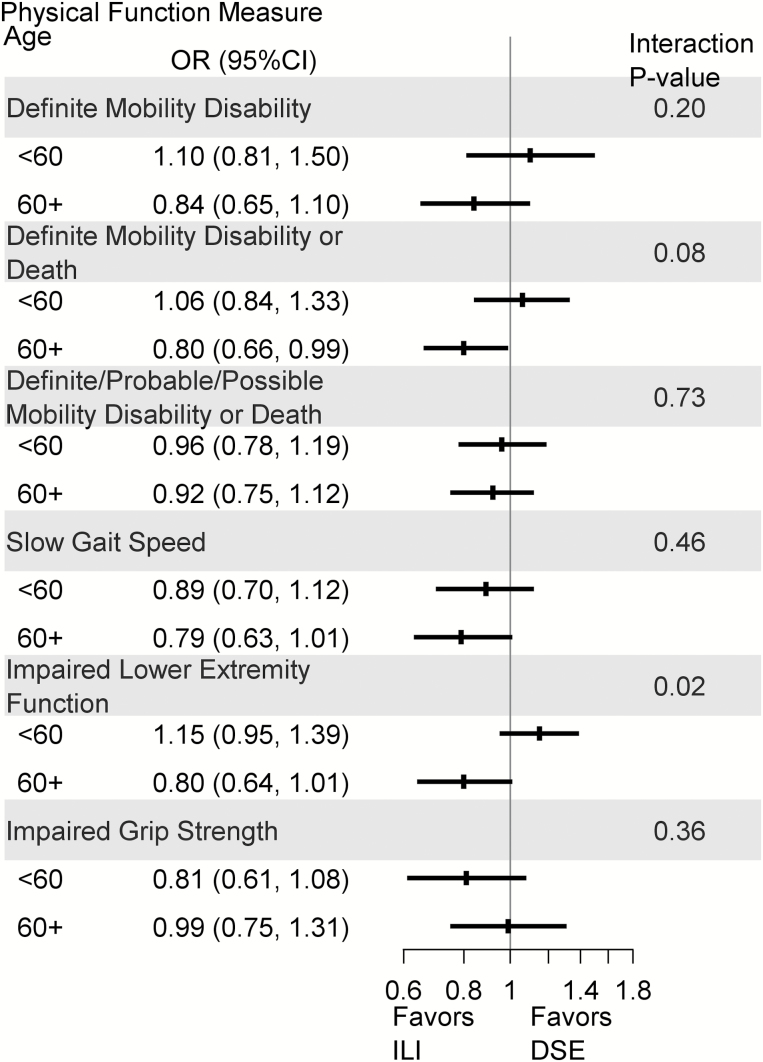
The intervention effect appeared to vary by baseline age, gender, and BMI for some physical function outcomes (p for interactions < .10); thus, estimates (95% CI) of the associations between the intervention and physical function were obtained for age, gender, and BMI subgroups (Supplementary Tables 1–3). For those who were ≥60 years at randomization, ILI participants had a lower odds of definite mobility disability or death (adjusted OR [95% CI]: 0.80 [0.66 to 0.98]) and impaired lower extremity function (adjusted OR [95% CI]: 0.81 [0.65 to 1.02]) (Figure 3), and higher SPPB (adjusted mean difference [95% CI]: 0.393 [0.161 to 0.625]) and SPPBexp (adjusted mean difference [95% CI]: 0.081 [0.038 to 0.124]) scores (Supplementary Table 1) than DSE participants; however, there were no significant differences by intervention arm among those <60 years at randomization. For gender, male ILI participants had higher SPPBexp scores compared to male DSE participants (adjusted mean difference [95% CI]: 0.070 [0.028–0.112]; Supplementary Table 2). For those with a BMI <30 kg/m2 at baseline, ILI participants had a lower odds of slow gait speed compared to DSE participants (adjusted OR [95% CI]: 0.56 [0.35 to 0.90]; Supplementary Table 3).
Figure 3.
Forest plot of mobility disability, slow gait speed, and impaired lower extremity function and grip strength by age and intervention assignment: the Look AHEAD study. Adjusted for gender, race/ethnicity, clinic, and baseline SF-36 physical functioning, cardiovascular disease, and body mass index.
Discussion
Overweight and obese middle aged and older adults with type 2 diabetes who were randomized to a long-term intensive lifestyle intervention were less likely to have slow gait speed (<0.8 m/s) approximately 11 years postrandomization and 1.5 years after the intervention ended compared to those randomized to a diabetes support and education control group. Individuals randomized to the intervention also had faster gait speed over both short and long distances and better lower extremity function as assessed by a performance-based physical function test. Grip strength, an indicator of general upper body strength, did not differ between the intervention groups, suggesting that weight loss did not lead to declines in strength despite anticipated declines in lean mass. For some physical function outcomes, the intervention effect was slightly larger among older participants in particular.
Previous trials showing performance-based functional benefits of lifestyle interventions in middle aged and older persons who were overweight or obese have been of shorter duration (5–18 months) (7–12). In Look AHEAD with approximately 11 years of follow-up, ILI participants’ mean gait speed (at usual pace) was approximately 0.02 m/s better than DSE participants and among those aged 60 years and older at baseline, the SPPB score was approximately 0.4 points higher. These findings are consistent with those observed at the 8-year follow-up in a small subset of the Look AHEAD trial (27). For physical function, differences of 0.03–0.08 m/s in gait speed and 0.3–0.8 points on the SPPB have been reported to represent small albeit clinically meaningful differences (28–30). Furthermore, ILI participants were less likely to have a slow gait speed (<0.8 m/s), which has been associated with increased risk of disability and mortality (31,32), compared to DSE participants.
However, some physicians are reluctant to recommend weight loss in older adults due to concerns regarding the functional consequences of the loss of lean mass (33,34). According to the sarcopenia hypothesis, lower lean mass leads to weakness (16). Body composition was assessed through the 8-year follow-up in four of the Look AHEAD sites (n = 1,019) (35). Weight loss in DSE participants was comprised almost entirely of lean mass. In ILI participants, weight loss was comprised of both fat and lean mass with ILI participants having significantly lower lean mass at 8-year follow-up than DSE participants; there was, however, no significant interaction with age. Nevertheless, for several of the performance-based physical function measures, there appeared to be a benefit among older (≥60 years at baseline), but not younger, ILI participants compared to DSE participants. No differences in upper extremity strength were observed between ILI and DSE participants in either the younger or older subgroups. The lack of an association between changes in lean mass and strength has also been observed in other short-term weight loss trials (36,37).
This study has notable strengths and limitations. Although these analyses are based on a post-test design, the comparisons are based on randomization assignment, thereby accounting for potential unmeasured confounders between the groups. Retention approximately 11 years post-randomization was excellent, exceeding 80% of the original sample. Although losses to follow-up were associated with predictors of impaired physical function at baseline, sensitivity analyses applying statistical techniques to account for differential participation based on baseline characteristics provided similar results. However, since the analyses are based on those who returned for the observational follow-up visit, this should not be considered an intention-to-treat analysis. Furthermore, multiple comparisons were made on several measures of physical function which may increase the probability of Type I error, thus, caution should be used when interpreting the p-values. The intervention was successful in achieving sustained long-term weight loss in a substantial proportion of participants providing a unique opportunity to examine the long-term benefits of randomization to weight loss on physical function. DSE participants also lost weight over the latter part of the trial which may have attenuated differences observed in physical function. Intentionality of weight loss was not assessed, however. While the intervention was also successful in increasing physical activity in the early phases of the trial, group differences in change in physical activity had diminished by the observational follow-up visit. Physical function was only measured postrandomization and not at baseline so the extent to which change in weight was associated with change in physical function cannot be assessed. Furthermore, had physical function been measured earlier in the study when there was greater separation of weight loss between the two groups, greater differences in physical function may have been observed. As eligible volunteers for the Look AHEAD trial, these results may not be generalizable to other overweight or obese populations without diabetes.
In conclusion, overweight and obese middle aged and older adults with diabetes who were randomized to a long-term intensive lifestyle intervention for weight loss were less likely to have slow gait speed and had small, albeit significant, benefits in gait speed and lower extremity function approximately 11 years later and 1.5 years after the intervention ended. Differences in upper body strength were not observed between the randomized groups. Thus, despite the anticipated declines in lean mass with weight loss, physical function was not negatively impacted. Intentional weight loss through dietary modification and increased physical activity may be useful in preventing or delaying the onset of impaired physical function and mobility disability in overweight and obese middle aged and older individuals with type 2 diabetes.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
This work was supported by the National Institutes of Health through cooperative agreements with the National Institute of Diabetes and Digestive and Kidney Diseases: DK57136, DK57149, DK56990, DK57177, DK57171, DK57151, DK57182, DK57131, DK57002, DK57078, DK57154, DK57178, DK57219, DK57008, DK57135, and DK56992. Additional funding was provided by the National Heart, Lung, and Blood Institute; National Institute of Nursing Research; National Institute on Aging; National Center on Minority Health and Health Disparities; NIH Office of Research on Women’s Health; and the Centers for Disease Control and Prevention. This research was supported in part by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Indian Health Service (I.H.S.) provided personnel, medical oversight, and use of facilities. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the I.H.S. or other funding sources. Additional support was received from The Johns Hopkins Medical Institutions Bayview General Clinical Research Center (M01RR02719); the Massachusetts General Hospital Mallinckrodt General Clinical Research Center and the Massachusetts Institute of Technology General Clinical Research Center (M01RR01066); the Harvard Clinical and Translational Science Center (RR025758-04); the University of Colorado Health Sciences Center General Clinical Research Center (M01RR00051) and Clinical Nutrition Research Unit (P30 DK48520); the University of Tennessee at Memphis General Clinical Research Center (M01RR0021140); the University of Pittsburgh General Clinical Research Center (GCRC) (M01RR000056), the Clinical Translational Research Center (CTRC) funded by the Clinical & Translational Science Award (UL1 RR 024153) and NIH grant (DK 046204); the VA Puget Sound Health Care System Medical Research Service, Department of Veterans Affairs; and the Frederic C. Bartter General Clinical Research Center (M01RR01346). The following organizations have committed to make major contributions to Look AHEAD: FedEx Corporation; Health Management Resources; LifeScan, Inc., a Johnson & Johnson Company; OPTIFAST® of Nestle HealthCare Nutrition, Inc.; Hoffmann-La Roche, Inc.; Abbott Nutrition; and Slim-Fast Brand of Unilever North America.
Supplementary Material
Acknowledgments
The investigators thank the Look AHEAD Study participants and the investigative staff at each clinical site for their contributions. A complete listing of the Look AHEAD investigators/personnel can be found in the Supplementary Materials.
Writing Group: Denise K. Houston,1 Rebecca H. Neiberg,2 James O. Hill,3 John M. Jakicic,4 Karen C. Johnson,5 Edward W. Gregg,6 Van S. Hubbard,7 Michael E. Miller,2 F. Xavier Pi-Sunyer,8 W. Jack Rejeski,9 Rena R. Wing,10 and Stephen B. Kritchevsky1 for the Look AHEAD Research Group
1Department of Internal Medicine, Section on Gerontology and Geriatric Medicine, Winston-Salem, North Carolina, 2Department of Biostatistical Sciences, Wake Forest School of Medicine, Winston-Salem, North Carolina; 3University of Colorado Denver School of Medicine, Aurora, Colorado; 4Department of Health and Physical Activity, University of Pittsburgh, Pittsburgh, Pennsylvania; 5Department of Preventive Medicine, University of Tennessee Health Science Center, Memphis, Tennessee; 6Centers for Disease Control, Atlanta, Georgia; 7National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland; 8Columbia University Medical Center, New York, New York; 9Department of Health and Exercise Science, Wake Forest University, Winston-Salem, North Carolina; 10Brown University and Miriam Hospital, Providence, Rhode Island.
Author Contributions: R.H.N. performed the data analysis. D.K.H., M.E.M., R.H.N., and S.B.K. interpreted the data analysis. DKH drafted the manuscript. All authors reviewed and edited the manuscript. Please see the online Supplementary Materials for a complete list of the authors recognized for the contribution of funding, conception and design of the Look AHEAD Study, and acquisition of data.
Conflict of Interest Statement
None declared.
References
- 1. Guralnik JM, Fried LP, Salive ME. Disability as a public health outcome in the aging population. Annu Rev Public Health. 1996;17:25–46. doi:10.1146/annurev.pu.17.050196.000325 [DOI] [PubMed] [Google Scholar]
- 2. Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. [DOI] [PubMed] [Google Scholar]
- 3. Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev. 2010;11:568–579. doi:10.1111/j.1467-789X.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 4. Jensen GL, Hsiao PY. Obesity in older adults: relationship to functional limitation. Curr Opin Clin Nutr Metab Care. 2010;13:46–51. doi:10.1097/MCO.0b013e32833309cf [DOI] [PubMed] [Google Scholar]
- 5. Figaro MK, Kritchevsky SB, Resnick HE, et al. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29:2039–2045. doi:10.2337/dc06-0245 [DOI] [PubMed] [Google Scholar]
- 6. Park SW, Goodpaster BH, Strotmeyer ES, et al. ; Health, Aging, and Body Composition Study Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30:1507–1512. doi:10.2337/dc06-2537 [DOI] [PubMed] [Google Scholar]
- 7. Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–1510. doi:10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- 8. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi:10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rejeski WJ, Brubaker PH, Goff DC Jr, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171:880–886. doi:10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Messier SP, Mihalko SL, Legault C, et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi:10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101:991–999. doi:10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi:10.1001/jama.2015.17346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rejeski WJ, Ip EH, Bertoni AG, et al. ; Look AHEAD Research Group Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–1217. doi:10.1056/NEJMoa1110294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rejeski WJ, Bray GA, Chen SH, et al. ; Look AHEAD Research Group Aging and physical function in type 2 diabetes: 8 years of an intensive lifestyle intervention. J Gerontol A Biol Sci Med Sci. 2015;70:345–353. doi:10.1093/gerona/glu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65 years and older: a review of the controversy. Exp Gerontol. 2013;48:1054–1061. doi:10.1016/j.exger.2013.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S. [DOI] [PubMed] [Google Scholar]
- 17. Ryan DH, Espeland MA, Foster GD, et al. ; Look AHEAD Research Group Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. [DOI] [PubMed] [Google Scholar]
- 18. Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wesche-Thobaben JA. The development and description of the comparison group in the Look AHEAD trial. Clin Trials. 2011;8:320–329. doi:10.1177/1740774511405858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 22. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–M649. [DOI] [PubMed] [Google Scholar]
- 23. Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. [DOI] [PubMed] [Google Scholar]
- 24. Jakicic JM, Egan CM, Fabricatore AN, et al. ; Look AHEAD Research Group Four-year change in cardiorespiratory fitness and influence on glycemic control in adults with type 2 diabetes in a randomized trial: the Look AHEAD Trial. Diabetes Care. 2013;36:1297–1303. doi:10.2337/dc12-0712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 26. Paffenbarger RS Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108: 161–175. [DOI] [PubMed] [Google Scholar]
- 27. Houston DK, Leng X, Bray GA, et al. ; Action for Health in Diabetes (Look AHEAD) Movement and Memory Ancillary Study Research Group A long-term intensive lifestyle intervention and physical function: the Look AHEAD Movement and Memory Study. Obesity (Silver Spring). 2015;23:77–84. doi:10.1002/oby.20944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi:10.1111/j.1532-5415. 2006.00701.x [DOI] [PubMed] [Google Scholar]
- 29. Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perera S, Studenski S, Newman A, et al. ; Health ABC Study Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (Health ABC study). J Gerontol A Biol Sci Med Sci. 2014;69:1260–1268. doi:10.1093/gerona/glu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71:63–71. doi:10.1093/gerona/glv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sørensen TI. Weight loss causes increased mortality: pros. Obes Rev. 2003;4:3–7. [DOI] [PubMed] [Google Scholar]
- 34. Morley JE, Chahla E, Alkaade S. Antiaging, longevity and calorie restriction. Curr Opin Clin Nutr Metab Care. 2010;13:40–45. doi:10.1097/MCO.0b013e3283331384 [DOI] [PubMed] [Google Scholar]
- 35. Pownall HJ, Bray GA, Wagenknecht LE, et al. ; Look AHEAD Research Group Changes in body composition over 8 years in a randomized trial of a lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2015;23:565–572. doi:10.1002/oby.21005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:866–871. [DOI] [PubMed] [Google Scholar]
- 37. Marsh AP, Shea MK, Vance Locke RM, et al. Resistance training and pioglitazone lead to improvements in muscle power during voluntary weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:828–836. doi:10.1093/gerona/gls258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.