Abstract
Background
We describe the recruitment of participants for Strategies to Reduce Injuries and Develop Confidence in Elders (STRIDE), a large pragmatic cluster randomized trial that is testing the effectiveness of a multifactorial intervention to prevent serious fall injuries. Eligible persons were 70 years or older, community-living, and at increased risk for serious fall injuries. The modified goal was to recruit 5,322 participants over 20 months from 86 primary care practices within 10 diverse health care systems across the United States.
Methods
The at-risk population was identified using two distinct but complementary screening strategies that included three questions administered centrally via the mail (nine sites) or in the clinic (one site), while recruitment was completed centrally by staff at Yale.
Results
For central screening, 226,603 letters mailed to 135,118 patients yielded 28,719 positive screens (12.7% of those mailed and 46.5% of the 61,729 returned). In the clinic, 22,537 screens were completed, leading to 5,732 positive screens (25.4%). Of the 34,451 patients who screened positive for high risk of serious fall injuries, 31,872 were sent a recruitment packet and, of these, 5,451 (17.1%) were enrolled over 20 months (mean age: 80 years; 62% female). The participation rate was 34.0% among eligible patients. The enrollment yields were 3.6% (vs 5% projected) for each patient screened centrally, despite multiple screens, and 10.5% (vs 33.9% projected) for each positive clinic screen.
Conclusions
Despite lower-than-expected yields, the STRIDE Study exceeded its modified recruitment goal. If the STRIDE intervention is found to be effective, the two distinct strategies for identifying a high-risk population of older persons could be implemented by most health care systems.
Keywords: Recruitment, Randomized clinical trials, Serious fall injuries
The recruitment of older persons, particularly those who are frail or otherwise vulnerable, for large multisite pragmatic trials poses many challenges, such as reconciling competing clinical and research priorities (1,2). We recently completed enrollment for the Strategies to Reduce Injuries and Develop Confidence in Elders (STRIDE) Study, a large 40-month pragmatic cluster randomized trial set in 86 primary care practices within 10 diverse health care systems (ie, clinical sites) across the United States (3). The aim of STRIDE is to determine the effectiveness of an evidence-based, patient-centered multifactorial intervention to prevent serious fall injuries in an at-risk population of community-living older persons.
Based on formal sample size calculations (3), STRIDE set an initial goal of enrolling 6,000 participants aged 75 years or older over 18 months. A unique feature of STRIDE was centralized recruitment, with two distinct but complementary screening strategies to identify an at-risk population. The current report describes the procedures that allowed us to exceed a modified goal of enrolling 5,322 participants aged 70 years or older over 20 months. We highlight the successes and lessons learned from the STRIDE recruitment effort, report enrollment yields by clinical site, age, and sex, and provide baseline characteristics of the 5,451 participants enrolled and cost estimates for the two screening strategies. The information provided should assist other investigators who are planning or conducting large pragmatic trials of vulnerable older persons, particularly trials in different health care systems.
Methods
STRIDE is being conducted at 10 US health care systems (Supplementary Material A) and was approved by a Central Institutional Review Board at Brigham and Women’s Hospital. All participants provided verbal informed consent or assent (with proxy consent), and all study materials and interviews were available in English and Spanish.
The study design for STRIDE, including selection of the eligible primary care practices, has been previously described (3). The 86 practices were randomized to intervention or control group using covariate-based, stratified, constrained randomization to balance practice characteristics within and across the 10 health care systems. Participants within these practices had to be ≥70 years and at increased risk for serious fall injuries based on a “Yes” response to ≥1 of 3 screening questions: (a) have you fallen and hurt yourself in past year? (b) have you fallen ≥2 times in past year?, and (c) are you afraid that you might fall because of balance or walking problems (4–6)? Persons were excluded if they did not receive primary care at the assigned practice; planned to move out of the area in the coming year; resided in a nursing home; were enrolled in hospice or reported being too ill to participate; did not speak English or Spanish; or were not capable of providing informed consent (or assent) because of impairments in cognition/hearing and did not have a proxy.
Study Recruitment Goals
Based on formal sample size estimates, the initial goal was to enroll 6,000 participants over 18 months from 86 practices at the 10 clinical sites (Supplementary Table 1). Because initial enrollment was slower than projected, the goal was modified to 5,322 participants over 20 months, with maximum follow-up extended from 36 to 40 months to maintain 90% power. Although the original plan was to end recruitment for a specific practice after its enrollment target had been reached, this was not possible due to slower enrollment at other practices. The Data Coordinating Center (DCC) monitored screening and recruitment activities and provided the Screening, Recruitment and Retention Committee, which met monthly via conference call, with a set of weekly reports to track progress on critical benchmarks.
Screening
Based on earlier pilot testing (3), the primary strategy was central screening. Nine of the 10 clinical sites provided the DCC with contact information for age-eligible patients in their participating practices. These patient lists were updated biannually during the recruitment period. The Yale Recruitment and Assessment Center (RAC) sent patients a letter addressed from their primary care practice asking them to complete a self-addressed, prepaid screening postcard, which included the three previously described questions, and mail it back to the RAC. Because the sample of age-eligible patients was largely fixed, additional screening postcards were sent to non-responders who did not return the prior postcard within 75 days. Patients who were not at increased risk for serious fall injuries, hereafter referred to as screen-negative, could subsequently be rescreened after a minimum of 9 months.
Screening at the remaining site (Reliant Medical Group), which opted not to provide a patient list, was completed by practice staff during routine primary care visits. The screening questions were embedded within the electronic health record (EHR) work flow, as a part of standard vital signs, and the results of positive screens (and total number of screens performed) were regularly transmitted to the DCC electronically along with the patients’ contact information.
Centralized Recruitment
Patients who were at increased risk for serious fall injuries, hereafter referred to as screen-positive, were sent a recruitment packet by the RAC. The packet included an invitation letter from their primary care practice, recruitment letter from STRIDE, study brochure, consent/privacy information sheet (Supplementary Material B), initial monthly fall calendar (7), and opt-out postcard. The recruitment letter indicated that they could opt out from being contacted about the study by returning the self-addressed, prepaid postcard to the RAC within 2 weeks. Patients who did not opt out were called by a RAC recruiter (up to five attempts, including at least one evening and one weekend call). Because randomization of practices occurred prior to participant enrollment, recruitment staff were kept masked to practice assignment to reduce potential bias.
During the telephone interview, the recruiter reviewed the purpose of the study, answered any questions and, after obtaining verbal consent, completed the six-item Callahan cognitive screener, which has comparable diagnostic test characteristics as the Mini-Mental State Examination (8). After confirming that the patient did not meet any exclusion criteria, the recruiter collected baseline data on sociodemographic characteristics, height and weight, physical activity, self-rated health, chronic conditions, and use of mobility aid.
Patients found to have significant cognitive impairment, defined as ≥4 errors on the six-item screener, were required to have a proxy/caregiver willing to provide consent, facilitate adherence to the study protocol (including completing the assessments), and assist with implementing the intervention as needed.
Strategies to Enhance Screening and Recruitment
At the outset of the trial, the lower age limit was 75 years. Because the pool of available patients was not sufficient given the lower than projected screen-to-enrollment rate, the age limit was lowered incrementally to 73 and then 70. In collaboration with the STRIDE Local and National Patients’ and Stakeholders’ Committees, which represented the perspectives of patients and other stakeholders (9), the following strategies were implemented to further enhance screening and recruitment: (a) adding (i) small, nonfinancial incentives, such as bookmarks, (ii) site-specific patient and/or local physician story card to accompany the screening postcards, and (iii) a signed notecard from Martha Stewart (http://marthastewart.com), highlighting the importance of fall prevention, to the recruitment packets; (b) modifying the screening and recruitment letters and consent form to improve clarity, reduce redundancy, and enhance appeal; (c) raising study visibility through site-specific activities, eg, local press releases, posting flyers and distributing information cards at participating practices, in-services for practice clinicians, etc.; (d) using cell phones with area codes local to the specific trial sites by RAC recruiters to reduce the number of “no contacts”; (e) allowing sites to contact screen-positive patients who could not be reached by the RAC to determine their potential interest in the study; (f) encouraging sites to provide the RAC with names of screen-positive patients who had initially declined to participate but changed their mind after discussion with their primary care provider or after receiving more information about the study; and (g) having recruitment calls made by trained (and masked) staff at two of the central sites with low enrollment.
Tracking Costs
Direct screening and recruitment costs for personnel and non-personnel were estimated for the central and clinic-based screening strategies.
Statistical Analyses
The yields for enrollment were calculated as cumulative incidence curves, with enrollment as the outcome and other “terminal” events, such as opt outs and not eligible, as competing events. These curves are analogous to traditional survival curves for time-to-event outcomes. Screens that “timed out” (ie, had an ambiguous status or were pending at the close of enrollment because the screen had not been returned, recruitment packet had not been mailed, etc.), were also evaluated as competing events, but with an arbitrary event time of 600 days. Since all enrollments and true competing events occurred within 578 days, this “work around” protected the integrity of the curves. The “timed-out” screens were not censored because their enrollment status was known. Baseline characteristics were calculated for all participants and subsequently compared between those aged 70–74 and 75 or older given the change in age eligibility.
Results
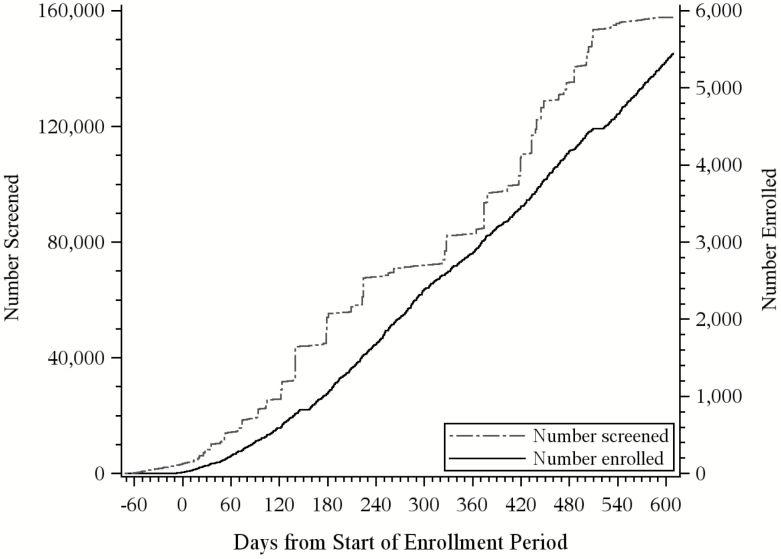
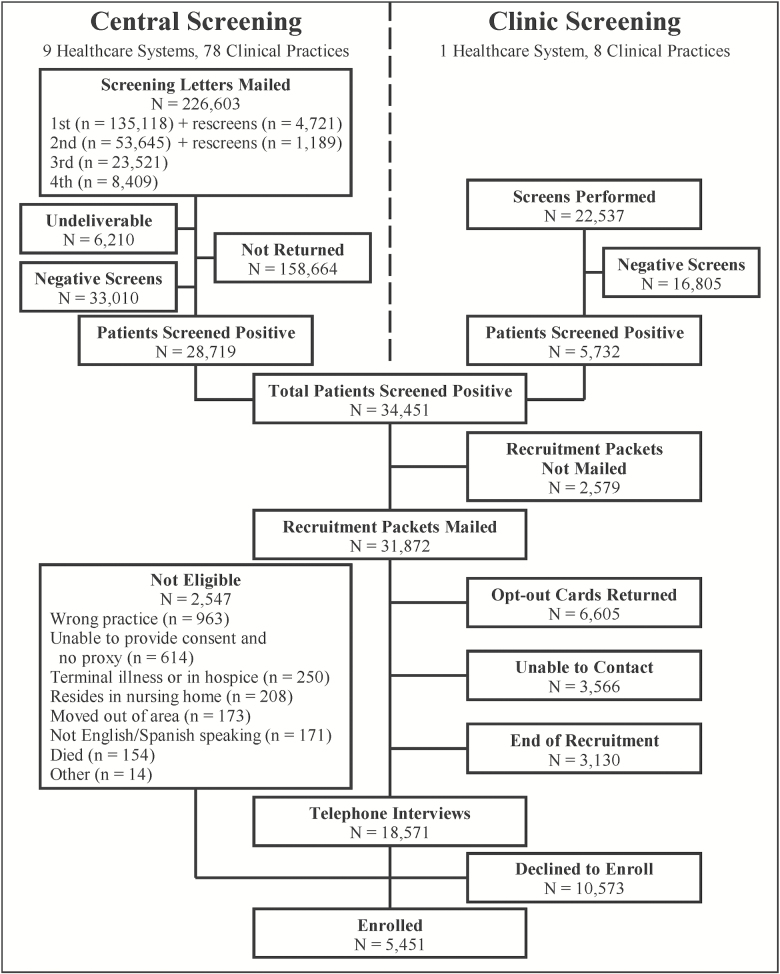
In total, 5,451 participants were enrolled over 20 months (through March 31, 2017). As shown in Figure 1, enrollment started slowly but accelerated after about 6 months, corresponding to increases in the number of persons screened. The recruitment flow from screening to enrollment is summarized in Figure 2. For central screening, 226,603 letters were mailed to 135,118 patients, leading to 28,719 positive screens (12.7% of those mailed and 46.5% of the 61,729 returned). In the clinic, 22,537 screens were completed, leading to 5,732 positive screens (25.4%). Of the 34,451 patients who screened positive for high risk of serious fall injuries, 31,872 were sent a recruitment packet and, of these, 5,451 (17.1%) were ultimately enrolled. The yield increased to 19.0% after omission of the 3,130 patients who were not contacted because recruitment had ended. The most common reasons for exclusion during the telephone interview were assignment to the wrong practice, unable to provide consent, and terminal illness. Of the 16,024 patients who were eligible, 34.0% agreed to participate.
Figure 1.
Number of persons screened and enrolled over time. The formal start of enrollment, corresponding to 0 days on the x-axis, was August 3, 2015, although a small number of participants were enrolled earlier from Reliant Medical Group. Because only positive screens were returned, the 22,537 screened for Reliant (of the 157,655 total) will not all represent distinct persons. Screening ended on February 28, 2017, and enrollment ended on March 31, 2017.
Figure 2.
STRIDE Study screening-to-enrollment funnel. Recruitment packets were not mailed to 3,130 patients because recruitment had ended.
More detailed information about screening and recruitment by clinical site is provided in Tables 1 and 2, respectively. For central screening, the prevalence of positive screens varied from 40.2% at Pittsburgh to 58.8% at Mount Sinai, with an overall rate of 46.5% (Table 1). This rate was considerably lower for the 5,910 rescreens (19.5%) than for the 220,693 first-completed screens (48.1%). Across all of the 10 sites, the number of patients enrolled varied from 369 at Mercy Health Network to 735 at Partners Health care (Table 2). Among patients having a telephone interview, the percent enrolled varied from 21.9 at Reliant Medical Group to 49.5 at Michigan.
Table 1.
Screening to Identify Patients at High Risk for Serious Fall Injuries by Clinical Sitea
| Clinical Site | Practices | Age-Eligible Patients | First Screen | Second Screen | Third Screen | Fourth Screen | First Rescreen | Second Rescreen | Screens Returned | Positive Screens (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Central Screening | ||||||||||
| Essential Health | 8 | 18,660 | 18,393 | 6,173 | 2,226 | 0 | 368 | 125 | 9,366 | 4,190 (44.7) |
| HealthCare Partners | 8 | 11,651 | 11,514 | 5,714 | 3,351 | 2,531 | 1,104 | 283 | 6,686 | 3,167 (47.4) |
| Johns Hopkins Medicine | 11 | 19,837 | 18,944 | 6,409 | 2,022 | 0 | 92 | 28 | 7,697 | 3,661 (47.6) |
| Mercy Health Network, U Iowa | 8 | 12,498 | 11,755 | 6,367 | 3,244 | 2,369 | 1,036 | 212 | 6,497 | 2,849 (43.9) |
| Michigan Medicine, U Michigan | 5 | 9,575 | 8,078 | 3,797 | 1,364 | 608 | 804 | 239 | 5,675 | 2,530 (44.6) |
| Mount Sinai Health System | 9 | 18,025 | 16,047 | 7,469 | 3,376 | 0 | 53 | 20 | 5,114 | 3,008 (58.8) |
| Partners HealthCare | 12 | 24,053 | 22,609 | 7,069 | 2,912 | 283 | 0 | 0 | 7,842 | 3,547 (45.2) |
| U Pittsburgh Medical Center | 8 | 12,181 | 11,534 | 5,606 | 2,449 | 1,156 | 963 | 163 | 7,587 | 3,050 (40.2) |
| U Texas Medical Branch | 9 | 25,004b | 16,244 | 5,041 | 2,577 | 1,462 | 301 | 119 | 5,265 | 2,717 (51.6) |
| Overall | 78 | 151,484 | 135,118 | 53,645 | 23,521 | 8,409 | 4,721 | 1,189 | 61,729 | 28,719 (46.5) |
| Clinic Screening | ||||||||||
| Reliant Medical Groupc | 8 | 22,490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 5,732 (25.4) |
| Total | 86 | 173,974 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 34,451c |
Note: aAs described in the Methods, screening postcards were resent to non-responders who did not return the prior postcard within 75 days. Screen-negative patients could subsequently be rescreened after a minimum of 9 months.
bAs shown in Supplementary Table 3, 11,445 (45.8%) of these patients were in a single practice, explaining the disparity with the much lower number of first screens.
cGiven the operational differences between central and clinic screening, a rate for total positive screens was not calculated.
Table 2.
Recruitment of Screen Positive Patients at High Risk for Serious Fall Injuries by Clinical Site
| Clinical Site | Recruitment Packets Mailed | Telephone Interview | Enrolled | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Totala | Opted Out | Unable to Contact | End of Recruitment | Total | Ineligible | Declined Participation | Total | % of Eligibleb | |
| Essential Health | 3,732 | 1,052 | 391 | 313 | 1,976 | 332 | 1,182 | 462 | 28.1 |
| HealthCare Partners | 3,141 | 644 | 429 | 431 | 1,637 | 222 | 996 | 419 | 29.6 |
| Johns Hopkins Medicine | 3,209 | 646 | 443 | 500 | 1,620 | 252 | 748 | 620 | 45.3 |
| Mercy Health Network, U Iowa | 2,832 | 762 | 224 | 293 | 1,553 | 190 | 994 | 369 | 27.1 |
| Michigan Medicine, U Michigan | 2,033 | 383 | 219 | 205 | 1,226 | 118 | 559 | 549 | 49.5 |
| Mount Sinai Health System | 2,971 | 440 | 235 | 323 | 1,973 | 358 | 1,111 | 504 | 31.2 |
| Partners HealthCare | 3,079 | 506 | 296 | 346 | 1,931 | 258 | 938 | 735 | 43.9 |
| Reliant Medical Group | 5,336 | 1,093 | 711 | 350 | 3,182 | 437 | 2,143 | 602 | 21.9 |
| U Pittsburgh Medical Center | 2,852 | 605 | 346 | 281 | 1,620 | 151 | 819 | 650 | 44.2 |
| U Texas Medical Branch | 2,687 | 474 | 272 | 88 | 1,853 | 229 | 1,083 | 541 | 33.3 |
| Overall | 31,872 | 6,605 | 3,566 | 3,130 | 18,571 | 2,547 | 10,573 | 5,451 | 34.0 |
Note: aValues are lower than the number of positive screens (in Table 1) because enrollment had ended before all recruitment packets could be mailed.
bThe denominator includes the number of participants with a telephone interview minus the number found to be ineligible.
As shown in Supplementary Figure 1, the yield for return of the (central) screening postcards (left panel) varied considerably by number and type of screening attempt, with the highest rates for the first rescreen and first screen and lowest rates for the third and fourth screens. The pattern for the enrollment yield (right panel) was very similar, except that the highest rate was observed for the first screen rather than the first rescreen, and the yield for the second rescreen was comparable to that of the third screen.
The enrollment yields from time of first screening are shown in Supplementary Figure 2 for the central sites. Values were considerably higher for Michigan (6.8%) and Pittsburgh (5.6%) than for the other seven sites, which had yields ranging in a narrow band from 2.5% for Essential Health to 3.6% for HealthCare Partners. The corresponding yields according to age and sex are provided in Supplementary Table 2. Values were highest for persons aged 75–79 and 80–84 years and lowest for those aged 70–74 and 90+ years. The yield was modestly higher for women than men.
The number of participants enrolled for each practice is shown in Supplementary Table 3. These values varied considerably for each of the sites, largely reflecting differences in the number of age-eligible patients within each practice. The range of values was least wide for Michigan (94–125), HealthCare Partners (31–70), and Reliant (51–99). The greatest number of participants were enrolled at the Partners PH3 practice (n = 199), while the fewest were enrolled at the Partners PH2 and PH8 practices (n = 10 each). As shown at the bottom of the table, 82.1% of the 4,849 patients enrolled from the central sites were from the first screen, while 13.3% were from the second screen.
Table 3 provides the baseline characteristics of the 5,451 participants. Among all participants, the mean age was nearly 80 years, 62% were female, 13% were Black or Hispanic, and about half had at least a college education. Self-rated health was very good to excellent for a minority of participants, the mean number of chronic conditions was greater than 2, and a substantial proportion of participants used a mobility aid. The most common positive screening question for fall injuries was fear of falling, followed by a fall with injury in the past year. About 41% of participants had two or more positive screening questions. Participants aged 70–74 years, representing about a fifth of the sample, generally had more favorable characteristics than those 75 years or older.
Table 3.
Baseline Characteristics of Study Participantsa
| Characteristic | Overall N = 5,451 |
Age 70–74 N = 1,037 |
Age 75+ N = 4,414 |
p Valueb |
|---|---|---|---|---|
| Age (years), mean | 79.7 ± 5.8 | 72.4 ± 1.4 | 81.5 ± 5.0 | <.001 |
| Female, n (%) | 3,379 (62.0) | 680 (65.6) | 2,699 (61.1) | .008 |
| Race, n (%) | .124 | |||
| White/Caucasian | 4,965 (91.1) | 927 (89.4) | 4,038 (91.5) | |
| Black/African American | 292 (5.4) | 68 (6.6) | 224 (5.1) | |
| Asian | 70 (1.3) | 11 (1.1) | 59 (1.3) | |
| Other/mixed | 91 (1.7) | 23 (2.2) | 68 (1.5) | |
| Refused/DK/missing | 33 (0.6) | 8 (0.8) | 25 (0.6) | |
| Hispanic/Latino ethnicity, n (%) | 407 (7.5) | 97 (9.4) | 310 (7.0) | .010 |
| Education, n (%) | <.001 | |||
| Less than high school graduate | 325 (6.0) | 40 (3.9) | 285 (6.5) | |
| High school graduate or equivalent | 920 (16.9) | 116 (11.2) | 804 (18.2) | |
| Some college or equivalent | 1,356 (24.9) | 272 (26.2) | 1,084 (24.6) | |
| College graduate | 1,056 (19.4) | 198 (19.1) | 858 (19.4) | |
| Post graduate | 1,789 (32.8) | 410 (39.5) | 1,379 (31.2) | |
| Refused/DK/missing | 5 (0.1) | 1 (0.1) | 4 (0.1) | |
| Lives alone, n (%) | 2,269 (41.6) | 371 (35.8) | 1,898 (43.0) | <.001 |
| Difficulty meeting monthly payments on bills: somewhat, very, completely, n (%) | 786 (14.4) | 170 (16.4) | 616 (14.0) | .044 |
| No regular physical activity in past month, n (%) | 1,121 (20.6) | 179 (17.3) | 942 (21.3) | .003 |
| Body mass index (kg/m2), mean | 27.6 ± 5.6 | 28.9 ± 6.3 | 27.3 ± 5.3 | <.001 |
| Self-rated health, n (%) | .208 | |||
| Excellent | 581 (10.7) | 128 (12.3) | 453 (10.3) | |
| Very good | 1,772 (32.5) | 347 (33.5) | 1,425 (32.3) | |
| Good | 2,071 (38.0) | 373 (36.0) | 1,698 (38.5) | |
| Fair or poor | 1,020 (18.7) | 187 (18.0) | 833 (18.9) | |
| Refused/DK/missing | 7 (0.1) | 2 (0.2) | 5 (0.1) | |
| Chronic conditions, mean | 2.1 ± 1.3 | 2.0 ± 1.3 | 2.1 ± 1.3 | <.001 |
| Hypertension, n (%) | 3,522 (64.6) | 612 (59.0) | 2,910 (65.9) | <.001 |
| Myocardial infarction, n (%) | 550 (10.1) | 67 (6.5) | 483 (10.9) | <.001 |
| Congestive heart failure, n (%) | 384 (7.0) | 55 (5.3) | 329 (7.5) | .015 |
| Stroke, n (%) | 425 (7.8) | 56 (5.4) | 369 (8.4) | .001 |
| Cancer, n (%) | 1,399 (25.7) | 251 (24.2) | 1,148 (26.0) | .231 |
| Diabetes, n (%) | 1,114 (20.4) | 228 (22.0) | 886 (20.1) | .169 |
| Chronic lung disease, n (%) | 798 (14.6) | 172 (16.6) | 626 (14.2) | .049 |
| Parkinson’s disease, n (%) | 140 (2.6) | 25 (2.4) | 115 (2.6) | .722 |
| Hip fracture, n (%) | 251 (4.6) | 25 (2.4) | 226 (5.1) | <.001 |
| Fracture since age 50, n (%) | 1,794 (32.9) | 336 (32.4) | 1,458 (33.0) | .698 |
| Arthritis, n (%) | 1,122 (20.6) | 218 (21.0) | 904 (20.5) | .698 |
| Errors on Callahan cognitive screen, n (%) | <.001 | |||
| None | 4,203 (77.1) | 912 (87.9) | 3,291 (74.6) | |
| One | 854 (15.7) | 106 (10.2) | 748 (16.9) | |
| Two or three | 234 (4.3) | 14 (1.4) | 220 (5.0) | |
| Four or more | 13 (0.2) | 1 (0.1) | 12 (0.3) | |
| Not administeredc | 147 (2.7) | 4 (0.4) | 143 (3.2) | |
| Proxy interview, n (%) | 162 (3.0) | 5 (0.5) | 157 (3.6) | <.001 |
| Use of mobility aid or nonambulatory, n (%) | ||||
| Inside home | 980 (18.0) | 114 (11.0) | 866 (19.6) | <.001 |
| Outside home | 1,823 (33.4) | 218 (21.0) | 1,605 (36.4) | <.001 |
| Screening questions for fall injuries, n (%) | ||||
| Fell two or more times in past year | 1,911 (35.1) | 383 (36.9) | 1,528 (34.6) | .160 |
| Fell and hurt self in past year | 2,120 (38.9) | 435 (41.9) | 1,685 (38.2) | .025 |
| Afraid of falling because of balance or walking problems | 4,679 (85.8) | 857 (82.6) | 3,822 (86.6) | .001 |
| No. positive fall screening questions, n (%) | .728 | |||
| One | 3,205 (58.8) | 599 (57.8) | 2,606 (59.0) | |
| Two | 1,233 (22.6) | 238 (23.0) | 995 (22.5) | |
| Three | 1,013 (18.6) | 200 (19.3) | 813 (18.4) | |
| Eligible based on fear of falling alone, n (%) | 2,625 (48.2) | 463 (44.6) | 2,162 (49.0) | .012 |
Note: aAll means are expressed ± SD; because of time constraints, data were not collected on medications.
bFor statistical comparisons, the t-test was used for continuous variables, while the chi-square test was used for dichotomous and categorical variables.
cInterview was completed entirely by the proxy.
The costs for screening and recruitment are provided in Supplementary Table 4. On a per enrollee basis, the total costs were about 16% higher for central versus clinic screening. This modest difference was due primarily to higher non-personnel costs.
Discussion
In the STRIDE Study, 5,451 community-living persons, aged 70 or older, who were at high risk for serious fall injuries, were recruited from 86 primary care practices within 10 diverse health care systems across the United States for a pragmatic cluster randomized trial. The study exceeded its modified goal of enrolling 5,322 participants over 20 months, but several modifications to the original protocol were required because of lower-than-expected recruitment yields. Participants tended to be highly educated, with modest representation of Blacks and Hispanics, but had high prevalence of chronic conditions, diminished self-reported health, and use of mobility aids, reflecting their heightened risk for serious fall injuries. The study demonstrated the feasibility of two distinct screening strategies (central and clinic-based) that could be implemented by most health care systems if the STRIDE multifactorial intervention is found to be effective in preventing serious fall injuries.
The initial STRIDE goal was to enroll 6,000 participants over 18 months. We had estimated that about 5% of patients screened centrally would be enrolled, based on pilot testing, but the observed yield, despite multiple screens, was only 3.6% (Supplementary Table 2). This lower-than-expected yield was primarily due to lower return of the screening postcard (30% observed vs 45% estimated), higher opt out (21% observed vs 12% estimated), and higher refusal among eligible screen-positive patients having a telephone interview (66% observed vs 34% estimated). Despite falling short of these original estimates, we achieved 90% of our initial enrollment goal. The study was able to maintain 90% power because maximum follow-up was extended from 36 to 40 months.
Because the number of age-eligible patients was largely fixed after randomization of the practices, several modifications of the protocol were implemented, including mailing additional screening postcards to non-responders and rescreening patients who were previously screen-negative. Although the corresponding enrollment yields were considerably lower than that of the initial screen, the additional screens and re-screens, together with the other protocol modifications, allowed us to exceed our modified enrollment goal. The low yields, however, led us to relax our goal of enrolling a comparable number of participants in each practice within a health care system (or clinical site), as specified in the original sample size calculations (3). The yields were highest for Michigan and Pittsburgh, which allowed these two sites to exceed their original enrollment goals by 36% and 8%, respectively. The only other central site that met its enrollment goal was Partners HealthCare, which had a large number of age-eligible patients, reducing the need for the less efficient repeated screens and rescreens. Although the enrollment yield was much lower for patients aged 70–74 years than those 75 years or older, the large pool of these “young-old” patients led to a sizeable number of enrolled participants.
The large number of patients screened positive at Reliant, the only clinic-based site, also allowed its overall enrollment goal to be met, despite a lower-than-expected yield (10.5% observed vs 33.9% estimated), and facilitated enrollment of a generally comparable number of patients at each of its eight practices. The number of screening postcards (central strategy) and recruitment packets (both strategies) were adjusted on an on-going basis in an attempt to balance several competing needs, including: minimizing disparities in the number of patients enrolled per practice, achieving site-specific and overall enrollment goals, and avoiding a large infusion of newly enrolled patients at a specific site given site- and practice-specific constraints on the number of new intervention patients that could be evaluated per unit of time.
Among screen-positive patients who were deemed eligible during the telephone interview, only 34% agreed to participate in the trial. The challenges of recruiting older persons for clinical research, particularly those who are frail or otherwise vulnerable, are well known (1). Because other large multisite trials of older persons, such as LIFE Study (10), Testosterone Trials (2) and ASPREE (11), had a more complex set of screening and recruitment procedures characterized by sequential assessments of eligibility, it is difficult to compare participation rates. After initial telephone screening, these trials, in contrast to STRIDE, recruited potential participants in person. In addition, according to the STRIDE interviewers, many screen-positive patients did not consider themselves at risk for serious fall injuries. Nearly half of the participants were at high risk based on the fear of falling screening question, while the remainder had two or more falls or a fall injury in the past year. About a third had experienced a fracture since age 50, while nearly 5% had a prior hip fracture. These results, coupled with the high prevalence of mobility aids, suggest that the study population, which had a mean age of 80, should have a high incidence of serious fall injuries over the projected 2.5-year (median) follow-up period (4–6). It was not possible to compare baseline characteristics between patients who agreed to participate in the trial and those who did not, since data were not available on the latter group.
On a per enrollee basis, the total costs of the two screening strategies were generally comparable, suggesting that decisions about how best to identify a high-risk group of older patients for implementation of the STRIDE intervention, should it be shown effective, could be based on other considerations, which might differ from one health care system to another. In theory, the “reach” of the central (or population-based) strategy might be greater than that of the clinic-based strategy since it is not tethered to any health care utilization, but in practice the reach of the two strategies may be comparable since the population of older patients seen in most primary care settings is likely enriched for fall risk (6). For the central strategy, the costs were higher for the subsequent screens and rescreens, relative to the first screen, because of the lower enrollment yields, but multiple screens were needed to achieve the desired sample size. For both strategies, the total cost per enrollee was less than half that of the LIFE Study (10), which included face-to-face screening and assessment, in addition to telephone screening, both of which were site-specific rather than centralized.
Because of selective return of the screening postcards, it is not possible to directly compare the prevalence of positive screens at the population- versus clinic-based levels. Patients from racial/ethnic minority groups or with low socioeconomic status or cognitive impairment may be less likely to respond to screening postcards, providing one explanation for the relatively high educational attainment, modest representation of Blacks and Hispanics, and low prevalence of significant cognitive impairment in STRIDE. After completion of study recruitment, Reliant Medical Group has continued clinic-based screening, suggesting that the associated personnel cost might be subsumed under routine intake activities as part of providing optimal geriatric care.
The lessons learned from STRIDE can help to inform the design and implementation of other large pragmatic cluster randomized trials, especially those that are conducted within different health care systems. Before randomization of practices or other groups of individuals to the relevant interventions, it would be advisable to have a substantial excess of potential participants to account for lower-than-expected enrollment yields. We increased the pool of potential participants after randomization by lowering age eligibility, but this change could diminish power by reducing the overall outcome rate given the more favorable baseline characteristics of the younger participants. An alternative approach would have been to randomize a larger number of practices and to hold some in reserve pending the early recruitment results. Our relatively low participation rate suggests the need for sites to better educate the target population, before and during the trial, about the risk and consequences of the condition serving as the basis for intervention. The anecdotal reports from our interviewers are supported by prior research showing that lack of awareness of fall morbidity and preventability is a significant impediment to implementing fall risk assessment and management in clinical practice (12). Real-world implementation of the STRIDE intervention will likely require aggressive educational efforts and outreach by insurers and health care systems. Finally, the development, modification, and implementation of our screening and recruitment procedures were greatly facilitated by input from the patient and stakeholder group, which helped to craft the recruitment packets and reviewed other relevant patient materials.
In summary, despite lower-than-expected yields, the STRIDE Study successfully enrolled 5,451 community-living persons, aged 70 or older, at high risk for serious fall injuries from 86 primary care practices within 10 diverse health care systems across the United States. To identify a high-risk population, the study demonstrated the feasibility of two distinct screening strategies that could be implemented by most health care systems if the STRIDE multifactorial intervention is found to be effective in preventing serious fall injuries. The lessons learned from STRIDE should assist other investigators who are planning or conducting large pragmatic trials of vulnerable older persons, particularly trials in different health care systems.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
The STRIDE study was funded primarily by the Patient Centered Outcomes Research Institute (PCORI), with additional support from the National Institute on Aging (NIA) at NIH. Funding is provided and the award managed through a cooperative agreement (5U01AG048270) between the NIA and the Brigham and Women’s Hospital. Additional support was provided by the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342), Yale Center for Clinical Investigation (National Center for Advancing Translational Sciences Award UL1TR000142), Boston Claude D. Pepper Older Americans Independence Center at Brigham and Women’s Hospital (P30AG013679) and Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences Award UL1TR001102). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or PCORI. Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging.
Conflict of Interest
Dr. Gill serves on the Editorial Board of the Journal of Gerontology: Medical Sciences.
Clinicaltrials.gov identifier: NCT02475850
References
- 1. Mody L, Miller DK, McGloin JM, et al. . Recruitment and retention of older adults in aging research. J Am Geriatr Soc. 2008;56:2340–2348. doi:10.1111/j.1532-5415.2008.02015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cauley JA, Fluharty L, Ellenberg SS, et al. . Recruitment and screening for the Testosterone Trials. J Gerontol A Biol Sci Med Sci. 2015;70: 1105–1111. doi:10.1093/gerona/glv031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhasin S, Gill TM, Reuben DB, et al. . Strategies to reduce injuries and develop confidence in elders (STRIDE): a cluster-randomized pragmatic trial of a multifactorial fall injury prevention strategy: design and methods. J Gerontol A Biol Sci Med Sci. 2018; 73:1053–1061. doi:10.1093/gerona/glx190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jennings LA, Reuben DB, Kim SB, et al. . Targeting a high-risk group for fall prevention: strategies for health plans. Am J Manag Care. 2015;21:e519–e526. [PMC free article] [PubMed] [Google Scholar]
- 5. Ganz DA, Kim SB, Zingmond DS, et al. . Effect of a falls quality improvement program on serious fall-related injuries. J Am Geriatr Soc. 2015;63:63–70. doi:10.1111/jgs.13154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tinetti ME, Kumar C. The patient who falls: “it’s always a trade-off”. JAMA. 2010;303:258–266. doi:10.1001/jama.2009.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hannan MT, Gagnon MM, Aneja J, et al. . Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am J Epidemiol. 2010;171:1031–1036. doi:10.1093/aje/kwq024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi:10.1097/01.MLR.0000024610.33213.C8 [DOI] [PubMed] [Google Scholar]
- 9. Frank L, Basch E, Selby JV, Patient-centered outcomes research I. The PCORI perspective on patient-centered outcomes research. JAMA. 2014;312:1513–1514. doi:10.1001/jama.2014.11100 [DOI] [PubMed] [Google Scholar]
- 10. Marsh AP, Lovato LC, Glynn NW, et al. . Lifestyle interventions and independence for elders study: recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi:10.1093/gerona/glt064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McNeil JJ, Woods RL, Nelson MR, et al. . Baseline characteristics of participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J Gerontol A Biol Sci Med Sci. 2017;72:1586–1593. doi:10.1093/gerona/glw342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baker DI, King MB, Fortinsky RH, et al. . Dissemination of an evidence-based multicomponent fall risk-assessment and -management strategy throughout a geographic area. J Am Geriatr Soc. 2005;53:675–680. doi:10.1111/j.1532-5415.2005.53218.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.