Abstract
Rationale
αvβ3 integrins play an important role in angiogenesis and cell migration of tumours. They are highly expressed on activated endothelial cells of newly formed blood vessels. Here, we compare the targeting characteristics of four 68Ga-labelled multimeric cyclic RGD-based tracers in an αvβ3 integrin-expressing tumour model and in a tumour model where αvβ3 integrin is expressed solely on the neovasculature.
Methods
Female BALB/c nude mice were subcutaneously injected with SK-RC-52 (αvβ3 integrin-positive) or FaDu (αvβ3 integrin-negative) tumour cells. 68Ga-labelled DOTA-E-[c(RGDfK)]2 TRAP-(RGD)3, FSC-(RGD)3, or THP-(RGD)3 were intravenously administered to the mice (0.5 nmol per mouse, 10-15 MBq), followed by microPET/CT imaging and ex vivo biodistribution studies one hour post injection. Nonspecific uptake of the tracers in both models was determined by co-injecting an excess of unlabelled DOTA-E-[c(RGDfK)]2 (50 nmol) along with the radiolabelled tracers.
Results
Imaging and biodistribution data showed specific uptake in the tumours for each tracer in both tumour models. Tumour uptake of 68Ga-FSC-(RGD)3 was significantly higher than that of 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, and 68Ga-THP-(RGD)3, in the SK-RC-52 model, but not in the FaDu model, where 68Ga-FSC-(RGD)3 showed significantly higher tumour uptake than 68Ga-TRAP-(RGD)3. Most importantly, differences were also observed in normal tissues and in tumour-to-blood ratios.
Conclusion
All tracers showed sufficient targeting of αvβ3 integrin expression to allow tumour detection. While the highest tumour uptake was found for 68Ga-FSC-(RGD)3 and 68Ga-THP-(RGD)3 in the SK-RC-52 and FaDu model, respectively, tumour-to-blood ratio and uptake in normal tissues should also be considered in selecting the most optimal tracer for specific diagnostic or therapeutic applications with radiolabelled RGD peptides.
Introduction
Molecular imaging of αvβ3 integrin expression with SPECT (Single-Photon Emission Computed Tomography) or PET (Positron Emission Tomography) is a promising technique that allows for imaging of tumour angiogenesis. αvβ3 integrins are transmembrane proteins, recognizing the arginine-glycine-aspartate (RGD) sequence of extracellular matrix proteins. αvβ3 integrins are expressed on the tumour cell membrane of several solid tumours, thereby modulating tumour growth and metastases [1]. During the process of angiogenesis, activated αvβ3 integrins are expressed on endothelial cells of newly formed vessels, inducing gene-expression and cytoskeleton changes and regulating cell growth and survival of tumour cells [2]. αvβ3 integrins are studied as targets for tumour-targeted therapies [3], RGD-drug conjugates [4], and as a target for molecular imaging agents to further characterize tumours and predict and/or assess response of anti-angiogenic cancer therapies. [5].
A series of RGD analogues for in vivo imaging of αvβ3 integrins have been developed with different approaches to obtain ligands with a high in vivo stability, high affinity for αvβ3 integrins, and optimal pharmacokinetics [6]. It has been shown that cyclization of linear RGD peptides and multimerization improved the binding affinity of RGD ligands for αvβ3 integrins [7–10]. For wider adoption of PET and SPECT imaging of αvβ3 integrin expression in the clinic, radiolabelled ligands require straightforward, robust, and efficient labelling protocols. The most recently developed ligands can be radiolabelled very efficiently (>90%) and rapidly (<15 minutes) with gallium-68 (68Ga), making them suited ligands for clinical applications. Because of the excellent physical characteristics of 68Ga (short half-life (68 minutes), high positron yield (89%)), and the availability of GMP-compliant 68Ge/68Ga generators, 68Ga-labelled RGD peptides can now be considered as tracers for PET imaging of angiogenesis.
Major efforts have been made in developing stable 68Ga-labelled RGD peptides and several different monomeric 68Ga-binding bifunctional chelators were considered [11, 12], of which 68Ga-NOTA-RGD was already studied in lung cancer- and lymphoma patients [11]. However, dimeric, trimeric, and tetrameric RGD analogues have shown higher specific accumulation in αvβ3 integrin expressing tumours compared to their monomeric counterparts [7–10]. Four recently developed multimeric 68Ga-labelled RGD peptides, with good in vivo imaging characteristics, are 68Ga-DOTA-E-[c(RGDfK)]2 [7, 8], 68Ga-TRAP-(RGD)3 [13, 14], 68Ga-FSC-(RGD)3 [15, 16], and 68Ga-THP-(RGD)3 [17]. These four multimeric 68Ga-labelled αvβ3 integrin-targeted tracers have been used in preclinical studies to investigate the in vitro, in vivo, and ex vivo targeting characteristics and their potential for clinical application [8, 13–17]. However, all four RGD peptides have been tested in separate experiments using different murine tumour models with high levels of αvβ3 integrin expression. To directly compare the imaging characteristics of these 68Ga-labelled RGD peptides, here we assessed the in vivo and ex vivo behaviour in both αvβ3 integrin-positive (SK-RC-52) and αvβ3 integrin-negative (FaDu, αvβ3 integrin expression only on neovasculature) human xenograft tumour models in the same experimental set-up and under standardized conditions.
Materials and Methods
Synthesis and Radiolabelling RGD Peptides
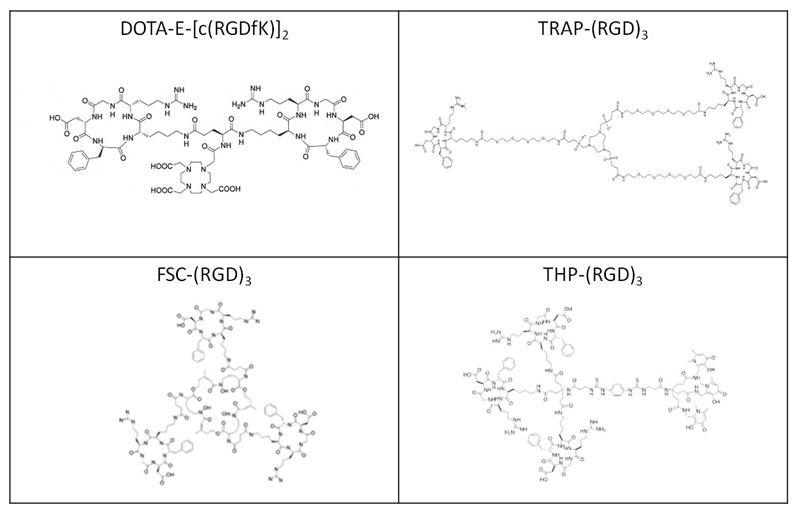
The RGD peptides DOTA-E-[c(RGDfK)]2 (Molecular weight 1704.8 g/mol), TRAP-(RGD)3 (Molecular weight 3145.0 g/mol), FSC-(RGD)3 (Molecular weight 2782.3 g/mol), and THP-(RGD)3 (Molecular weight 3720.6 g/mol) were synthesized as described in previous studies [7, 8, 13–17]. The structures of the four RGD peptides are shown in figure 1. The four RGD peptides were labelled with 68Ga eluted from a GG100 TiO2-based 68Ge/68Ga generator (Eckert & Ziegler, Berlin, Germany) eluted with 0.1 M HCl. Three fractions of 1 mL were collected and the second fraction (fraction with the highest activity) was used for labelling the peptides.
Figure 1.
Structures of unlabelled DOTA-E-[c(RGDfK)]2 (upper left), TRAP-(RGD)3 (upper right), FSC-(RGD)3 (lower left), and 68Ga-THP-(RGD)3 (lower right).
68Ga-labelled DOTA-E-[c(RGDfK)]2 (6 µg) and TRAP-(RGD)3 (11 µg) were prepared by mixing the RGD peptide (1 μg/μL), 68Ga (700 μL, 142-168 MBq), and 1.0 M HEPES buffer (88 µL) for 20 minutes (DOTA-E-[c(RGDfK)]2) or 5 minutes (TRAP-(RGD)3) at 95°C. 68Ga-labelled FSC-(RGD)3 (10 µg) and THP-(RGD)3 (13 µg) were prepared by mixing the RGD peptide (1 μg/μL), 68Ga-eluate (700 μL, 105-196 MBq), and 150 µL 0.5 M NaOAc, in an Eppendorf LoBind™ tube for 15 minutes at room temperature.
All tracers were further diluted to 15 MBq/0.3 ml in 0.1 M phosphate buffered saline (PBS) with 0.5% Bovine Serum Albumin (BSA) at pH (pH 7.4) for in vivo studies.
Quality Control
Instant thin-layer chromatography (ITLC) analysis of all four 68Ga-labelled RGD peptides was performed with silica gel chromatography strips (Agilent Technologies), using 0.1M NH4OAc/0.1M EDTA (1:1 v/v) as mobile phase. The strips were analyzed using a Fujifilm BAS-1800II Scanner (Fuji Photo Film Co., Tokyo, Japan) and Aida Software (version 4.21, Raytest GmbH, Straubenhardt, Germany).
The radiochemical purities were also determined by reverse-phase high-performance liquid chromatography (RP-HPLC) on the Agilent 1100 system (Agilent Technologies, Palo Alto, CA, USA) using a C18 column (RX-C18, 4.6×250 mm, Zorbax) eluted with a gradient mobile phase [0–5 min 97% buffer A, 5–15 min 97% buffer A to 0% buffer A (buffer A = 0.1% trifluoroacetic acid (TFA) in H2O, buffer B = B=0.1% TFA in acetonitrile)] at 1 mL/min. The radioactivity of the eluate was monitored using an in-line NaI radiodetector. Elution profiles were analyzed using Gina Star software (version 5.9, Raytest GmbH, Straubenhardt, Germany).
Solid-Phase αvβ3 Integrin Binding Assay
The IC50 of DOTA-E-[c(RGDfK)]2, TRAP-(RGD)3, FSC-(RGD)3, and THP-(RGD)3 for binding αvβ3 integrin was determined using a solid-phase competitive binding assay, with 111In-labelled DOTA-E-[c(RGDfK)]2 (6 MBq/µg) as the read-out tracer. The binding assays were carried out as described previously [8]. 111In-labelled DOTA-E-[c(RGDfK)]2 was prepared by adding 29.9 µL (16 MBq) 111InCl3 solution to 1 μL peptide dissolved in 60 µL 0.5M MES buffer (2-(N-morpholino)ethanesulfonic acid), pH 5.5, and was further purified on an Oasis® HLB (1 cm3, 30 mg) cartridge (Waters, Milford, MA, USA). Radiochemical purity was determined by ITLC.
Two 96-well vinyl microtiter plates (Corning B.V., Schiphol-Rijk, The Netherlands) were coated with a solution of purified human αvβ3 integrin (150 ng/mL, 100 μL/well) (Chemicon International, Temecula, CA, USA) in coating buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2 and 1 mM MnCl2). Non-specific binding was assessed by blocking with 1% BSA in coating buffer (200 μL/well). The appropriate peptide dilution (3 μM to 5x10-5 μM, 50 μL/well) and 111In-DOTA-E-[c(RGDfK)]2 (50 μL/well) was added. After incubation, wells were washed and cut out of the plates. The amount of radioactivity in each well was counted in the gamma counter (PerkinElmer Inc, Waltham, MA, USA), along with 1% standards. For each tracer, triplicate measurements were performed.
Cell Lines and Animal Studies
The in vivo behaviour of 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, or 68Ga-THP-(RGD)3 was determined in two different tumour models in athymic mice. First, mice were used with subcutaneous SK-RC-52 human renal cell carcinoma xenografts (tumour cells expressing αvβ3 integrins on both tumour cell membrane and neovasculature; αvβ3 integrin-positive tumours). Second, to study the RGD uptake in tumour neovasculature only, mice were used with subcutaneous FaDu human squamous cell carcinomas (αvβ3 integrin expressed solely on neovasculature; αvβ3 integrin-negative tumours [18]). Both tumour cell lines were obtained from the American Type Culture Collection (Molsheim Cedex, France) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, BRL Life Sciences Technologies, The Netherlands), supplemented with 1% glutamine (Gibco) and 10% fetal calf serum (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands) (RPMI+) at 37°C in a humidified atmosphere with 5% CO2. Before subcutaneous inoculation, tumour cells were washed in PBS, harvested with 0.25% trypsin (5 minutes at 37°C in a humidified atmosphere with 5% CO2), counted, spun down (5 minutes at 1000xg) and resuspended to the appropriate concentration with RPMI+. Mice were injected subcutaneously in the left flank with SK-RC-52 cells (2x106 cells in 0.2 mL RPMI+ per mouse) or FaDu cells (4.5x106 cells in 0.2 mL RPMI+ per mouse). Tumours were allowed to grow until they reached a volume of approximately 200 mm3 as determined by caliper measurements in three dimensions.
The animal studies were approved by The Animal Welfare Body of the Radboud University Medical Centre Nijmegen and were performed in accordance with the Dutch legislation (Dutch Act on Animal Experimentation). Female athymic BALB/c nude mice (6- to 8-week old BALB/c nu/nu/Foxn1nu, Janvier, n=64) acclimatized for seven days after transportation to the animal facility. The mice were housed in filter-topped cages (3 or 5 mice/cage) in a specific pathogen-free unit under standard laboratory conditions (day-night rhythm, 12-12 h; room temperature, 20-24 °C; relative humidity, 50-60 %) and had free access to water and animal chow (Snif Voer, Soest, The Netherlands).
Biodistribution studies and MicroPET/CT Imaging
Mice with subcutaneous SK-RC-52 xenografts (4 groups, 5 mice/group) or with subcutaneous FaDu xenografts (4 groups, 5 mice/group) were intravenously injected with 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, or 68Ga-THP-(RGD)3 (0.5 nmol, 15 MBq in 0.3 ml) in the tail vein of awake mice. Mice were warmed with a heat lamp for several minutes prior to injection. To study the non-αvβ3 integrin-mediated localization of the four 68Ga-labelled RGD peptides, separate groups of mice (8 groups, 3 mice/group) were co-injected with a 50 nmol (86 µg) excess of unlabelled DOTA-E-[c(RGDfK)]2.
All mice were euthanized by CO2/O2-asphyxiation one hour after injection and tissue samples were dissected and weighed. Additionally, salivary glands of mice bearing FaDu xenografts were dissected and weighed. The uptake of the radiolabelled RGD peptides was calculated as the fraction of injected dose, as determined by gamma counter whilst simultaneously measuring 1% standards, per gram of tissue (%ID/g).
MicroPET/CT scans were obtained directly after euthanasia (2-3 mice/group). Mice were positioned in supine position in the MicroPET/CT scanner (Inveon, Siemens Preclinical Solutions, Knoxville, TN, USA), with an intrinsic spatial resolution of 1.5 mm. Mice were consecutively scanned for 20, 30, or 40 minutes to acquire PET data. For anatomical localisation a CT scan was acquired directly after PET, with a spatial resolution of 113 μm (80 kV, 500 μA, exposure time 300 ms).
PET data were reconstructed using Inveon Acquisition Workplace software version 2.04 (Siemens Preclinical Solutions, Knoxville, TN, USA), using an ordered subset expectation maximisation 3-D (OSEM3D), with a matrix of 256×256×161, pixel size of 0.40×0.40×0.796 mm3, 16 OSEM3D subsets and 2 iterations. Post reconstruction filtering was performed using 3D Gaussian filter kernel with full width half maximum of 1.5 mm. CT data were reconstructed using a modified Feldkamp algorithm with the same reconstruction software. The resulting matrix was 768x786 pixels with 512 transverse slices (voxel size of 110.5×110.5×110.5 µm3). PET and CT images were fused for anatomical reference using automatic rigid registration software available at the workstation. Before visualization of tracer accumulation within tumour tissue and other organs, the registration was visually checked.
Statistical analysis
The IC50-values of the four 68Ga-labelled RGD peptides were calculated using a non-linear regression model in GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA, USA). Data from the biodistribution studies were expressed as mean percentage injected dose per gram (%ID/g) ± standard deviation (SD). Differences in tracer uptake among the four 68Ga-labelled RGD peptides in the tumour, blood, and salivary glands (as an example of relevant normal tissue) were determined in SPSS Statistics Version 22.0.0.1 (IBM, Armonk, New York) using a one-way ANOVA followed by post hoc test Bonferroni correction to correct for multiple comparisons. The level of significance was set to p<0.05.
Results
Radiolabelling
Labelling efficiency of all four compounds was >95% labelling efficiency as determined with ITLC and HPLC. Mean specific activities after radiolabelling were 49.1 MBq/nmol (68Ga-DOTA-E-[c(RGDfK)]2), 42.3 MBq/nmol (68Ga-TRAP-(RGD)3), 55.2 MBq/nmol (68Ga-FSC-(RGD)3), and 42.6 MBq/nmol (68Ga-THP-(RGD)3). Retention times in the HPLC elution profiles ranged from 11.9 to 14.9 minutes.
Competitive binding assay (IC50)
The IC50-values of DOTA-E-[c(RGDfK)]2, TRAP-(RGD)3, FSC-(RGD)3, and THP-(RGD)3 for αvβ3 integrin were determined in a competitive binding assay. Binding to αvβ3 integrin of 111In-labelled DOTA-E-[c(RGDfK)]2 was determined in the presence of increasing concentrations of unlabelled peptide (Supplementary figure 1). The IC50-value of dimeric DOTA-E-[c(RGDfK)]2 (3.8 ± 0.7 nM) was significantly lower than that of the trimeric compounds TRAP-(RGD)3 (IC50 = 10.0 ± 1.8 nM, p=0.005), FSC-(RGD)3, (IC50 = 9.0 ± 1.6 nM, p=0.006), and THP-(RGD)3 (IC50 = 11.4 ± 1.9 nM, p=0.003). There was no statistically significant difference among the IC50 of the trimeric compounds (p=0.7).
Biodistribution studies
The biodistribution profiles one hour after injection of 68Ga-labelled DOTA-E-[c(RGDfK)]2, TRAP-(RGD)3, FSC-(RGD)3, and THP-(RGD)3 in mice with subcutaneous SK-RC-52 and FaDu tumours are summarized in figure 2. The experiments were carried out in two sessions within four consecutive days.
Figure 2.
Biodistribution of 68Ga-labelled RGD peptides in BALB/c nude mice (n=5 per group) with subcutaneous growing SK-RC-52 xenograft tumours (A) or FaDu tumours (B). Tissues and organs were dissected one hour post injection. Values are expressed as mean percentage injected dose per gram ± standard deviation (mean %ID/g ± SD). Peptide dose was 0.5 nmol/mouse. Significant differences in tumour uptake between the different tracers are marked with an asterisk (p<0.05 (*), p<0.01 (**), and p<0.001 (***)).
In the SK-RC-52 model, mean tumour uptake of 68Ga-FSC-(RGD)3 (12.5±2.5 %ID/g) was significantly higher compared to that of 68Ga-DOTA-E-[c(RGDfK)]2 (5.3±1.9 %ID/g, p<0.0005), 68Ga-TRAP-(RGD)3 (4.4±0.8 %ID/g, p<0.0005), and 68Ga-THP-(RGD)3 (5.3±0.6 %ID/g, p<0.0005), whereas in the FaDu model, tracer uptake of 68Ga-FSC-(RGD)3 (1.9±0.3 %ID/g) was only significantly higher compared to 68Ga-TRAP-(RGD)3 (1.0±0.2 %ID/g, p=0.026) and was not significantly different from 68Ga-DOTA-E-[c(RGDfK)]2 (1.6±0.5 %ID/g, p=1.0) and 68Ga-THP-(RGD)3 (2.2±0.7 %ID/g, p=1.0). The difference in tumour uptake between 68Ga-TRAP-(RGD)3 and 68Ga-THP-(RGD)3 was also significantly different (p=0.002) in the FaDu xenograft tumour model.
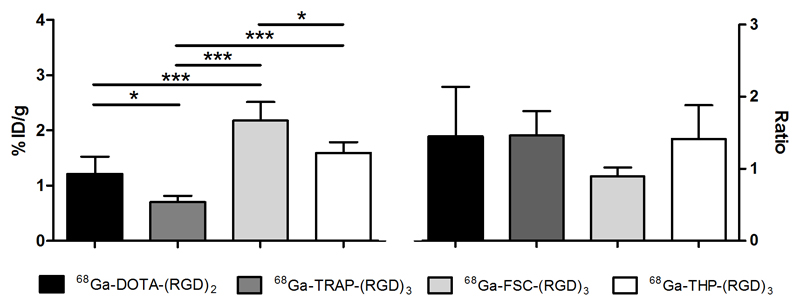
Co-injection of an excess of unlabelled DOTA-E-[c(RGDfK)]2 with radiolabelled RGD peptides resulted in significantly lower tracer uptake in both the SK-RC-52 and FaDu tumours, demonstrating the receptor-specific uptake of each of the four radiolabelled RGD peptides (Supplementary figure 2 and 3). The tumour-to-blood ratio (TBR) of 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, and 68Ga-THP-(RGD)3 in the SK-RC-52 model were 24.4±16.0, 38.5±3.8, 30.7±8.1, and 13.5±1.5 respectively, which are markedly higher than the TBR of in the FaDu model (5.9±2.9, 5.8±0.9, 3.5±0.6, and 6.8±2.8, respectively). In the SK-RC-52 tumour model, TBR between 68Ga-TRAP-(RGD)3 and 68Ga-THP-(RGD)3 was significantly different (p=0.012). No statistically significant differences among the TBR of the other RGD peptides were observed (p>0.05). Whereas individual differences between the four RGD tracers in SK-RC-52 tumour-to-tissue ratios were observed, comparable FaDu tumour-to-tissue ratios between the four tracers were found (table 1). As an example of a relevant normal tissue, tracer uptake in salivary glands showed statistically significant differences between 68Ga-DOTA-E-[c(RGDfK)]2 (1.2 ± 0.3 %ID/g), 68Ga-TRAP-(RGD)3 (0.7 ± 0.1 %ID/g), 68Ga-FSC-(RGD)3 (2.2 ± 0.3 %ID/g), and 68Ga-THP-(RGD)3 (1.6 ± 0.2 %ID/g) in the FaDu model (figure 3).
Table 1.
Tumour-to-normal tissue ratios for 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, and 68Ga-THP-(RGD)3 in BALB/c nude mice bearing SK-RC-52 and FaDu xenografts.
| Tumour-to-tissue ratio | 68Ga-DOTA-E-[c(RGDfK)]2 | 68Ga-TRAP-(RGD)3 | 68Ga-FSC-(RGD)3 | 68Ga-THP-(RGD)3 | ||||
|---|---|---|---|---|---|---|---|---|
| SK-RC-52 | FaDu | SK-RC-52 | FaDu | SK-RC-52 | FaDu | SK-RC-52 | FaDu | |
| Blood | 24.4±16.0 | 5.9±2.9 | 38.5±3.8 | 5.8±0.9 | 30.7±8.1 | 3.5±0.6 | 13.5±1.5 | 6.8±2.8 |
| Muscle | 14.7±3.0 | 4.6±2.3 | 20.9±3.6 | 4.8±1.2 | 17.2±3.2 | 3.6±0.6 | 11.3±1.7 | 4.1±1.2 |
| Heart | 12.2±5.4 | 4.1±2.1 | 15.3±2.3 | 4.4±1.4 | 12.4±3.2 | 2.4±0.4 | 7.7±1.7 | 3.4±1.2 |
| Lung | 4.5±2.0 | 1.9±0.9 | 4.8±0.9 | 1.5±0.4 | 4.8±0.9 | 0.9±0.1 | 2.2±0.5 | 1.2±0.4 |
| Liver | 2.5±0.8 | 0.8±0.5 | 3.0±0.5 | 0.8±0.2 | 2.5±0.2 | 0.8±0.1 | 1.3±0.3 | 0.6±0.2 |
| Spleen | 2.7±0.8 | 0.9±0.4 | 3.0±0.5 | 0.9±0.2 | 2.6±0.5 | 0.6±0.06 | 1.6±0.4 | 0.6±0.2 |
| Pancreas | 14.3±4.2 | 3.8±1.7 | 17.3±3.7 | 4.6±1.0 | 17.9±3.5 | 3.0±0.4 | 10.9±2.4 | 4.5±1.6 |
| Stomach | 2.7±1.3 | 0.7±0.2 | 3.0±0.7 | 0.8±0.2 | 2.6±0.3 | 0.6±0.1 | 1.7±0.3 | 0.7±0.3 |
| Duodenum | 1.5±0.9 | 0.4±0.04 | 1.4±0.4 | 0.3±0.07 | 1.5±0.4 | 0.4±0.08 | 0.9±0.3 | 0.4±0.2 |
| Kidneys | 1.7±0.6 | 0.5±0.2 | 1.1±0.1 | 0.5±0.1 | 1.9±0.3 | 0.4±0.05 | 0.6±0.1 | 0.3±0.08 |
Figure 3.
Tracer uptake (mean %ID/g ± SD) and tumour-to-tissue ratios of the four 68Ga-labelled RGD peptides in salivary glands of BALB/c nude mice with subcutaneously growing FaDu xenograft tumours, one hour post injection. Significant differences in tracer uptake in the salivary glands are marked with an asterisk (p<0.05 (*) and p<0.005 (***)).
MicroPET/CT Images
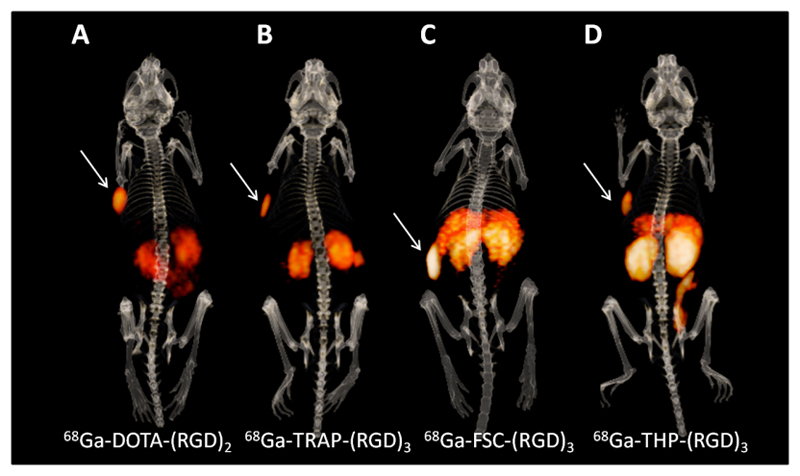
The microPET/CT images acquired one hour post injection were in line with the biodistribution data. MicroPET/CT images clearly visualized SK-RC-52 tumours, but also showed high uptake in kidneys and intestines (figure 4). Despite the high uptake in normal tissues, uptake of all four tracers was visible in the FaDu tumours, with signal intensities in line with the biodistribution studies (supplementary figure 4).
Figure 4.
Typical examples of 3-dimensional microPET/CT images one hour post injection of BALB/c nude mice with subcutaneously bearing SK-RC-52 xenograft tumours in the left flank, indicated with white arrows. Mice were injected with 10-20 MBq, 0.5 nmol 68Ga-DOTA-E-[c(RGDfK)]2 (A), 68Ga-TRAP-(RGD)3 (B), 68Ga-FSC-(RGD)3 (C), or 68Ga-THP-(RGD)3 (D).
Discussion
This study systematically compared the in vivo targeting characteristics of four 68Ga-labelled multimeric RGD peptides: one dimeric peptide (68Ga-DOTA-E-[c(RGDfK)]2) and three trimeric peptides (68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, and 68Ga-THP-(RGD)3). We demonstrated receptor-specific uptake of all tracers and showed that αvβ3 integrin expression could be imaged by the four RGD peptides not only in an αvβ3 integrin-positive tumour model (figure 4), but also in a tumour xenograft model in which αvβ3 was only expressed on the tumour vasculature (FaDu) (supplementary figure 4), providing the potential for imaging of low-level integrin expression.
So far, different strategies have been considered to develop RGD-based peptides with optimal imaging characteristics. Previously reported experiments with multimeric RGD peptides including 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, and 68Ga-THP-(RGD)3, showed improved tumour targeting compared to monomeric RGD peptides [8, 14, 16, 17]. In this head-to-head comparison differences in absolute tumour uptake amongst multimeric compounds were observed, with highest tumour uptake for 68Ga-FSC-(RGD)3 in SK-RC-52 tumours and 68Ga-THP-(RGD)3 in FaDu tumours. However, our study also indicated that enhanced tumour uptake of 68Ga-FSC-(RGD)3 and 68Ga-THP-(RGD)3 did not result in improved imaging quality in terms of enhanced tumour-to-normal tissue ratios. Concomitant tracer accumulation in non-target organs, like spleen, kidneys, liver, and duodenum was shown to be αvβ3 integrin receptor-mediated (supplementary figure 2), presumably due to αvβ3 integrin expression on endothelial cells in these organs [18–20]. These findings are in line with previously published data [8, 19].
Whereas in vivo studies with RGD peptides mainly focus on high affinities for αvβ3 integrin, fast blood clearance, and in vivo stability, successful clinical translation is essentially dependent on high contrast images with low uptake in non-target organs. Quantification of tracer uptake in neovasculature may suffer from increased αvβ3 integrin-mediated uptake in both tumour and non-tumour tissues. Furthermore, adequate interpretation of PET images may be hampered by uptake in background tissues. For example, in patients with head and neck cancer, imaging angiogenesis in tumour lesions could be interfered by uptake in the salivary glands (figure 3). Enhanced tracer accumulation of 68Ga-FSC-(RGD)3 and 68Ga-THP-(RGD)3 in salivary glands was observed, with both tracers showing two-fold higher uptake values as compared to 68Ga-DOTA-E-[c(RGDfK)]2 and 68Ga-TRAP-(RGD)3. Therefore, for selection of the best RGD-based tracer to image αvβ3 integrin expressing tumours, tracer uptake in background tissues should be considered. For example, in their study in patients with squamous cell carcinoma of the head and neck, Beer et al. showed that PET/CT images could be interpreted adequately and tumour uptake in the oral cavity could be accurately determined quantitatively using 18F-Galacto-RGD, despite the uptake of the tracer in the salivary glands [21].
In the current study, the in vivo tracer behaviour was shown to be different in the two human xenograft models used: a significantly higher tumour accumulation in mice with SK-RC-52 tumours was found for 68Ga-FSC-(RGD)3, whereas in the FaDu model only significantly lower uptake for 68Ga-TRAP-(RGD)3 was observed. The exact mechanism leading to these differences is unknown. It has been hypothesized that the enhanced tumour uptake of multimeric RGD tracers is due to simultaneous binding of more than one RGD unit to αvβ3 integrins. However, this is unlikely due to limited spacer length between the RGD units [22, 23]. Hypothetically, the differential αvβ3 integrin expression levels in the two models may explain the variation tumour uptake. At the higher αvβ3 expression levels in the SK-RC-52 model, affinity enhancement due to statistical rebinding of multimeric RGD tracers might play a more important role [24]. In addition, their pharmacokinetic behaviour may be influenced by the structural arrangement of the conjugated RGD peptides (e.g. distance between the RGD units) and the rigidity of its chelator. Nevertheless, the precise effect of the structure of RGD peptide conjugates on its binding behaviour to αvβ3 integrins in various tumour models remains to be elucidated.
Molecular imaging of αvβ3 integrin expression with radiolabelled RGD peptides has been studied in several clinical applications. RGD PET/CT has allowed imaging in tumours and their metastases and has provided supporting diagnostic information on tumour angiogenesis, in addition to 18F-FDG PET/CT imaging [25–28]. Radiolabelled RGD analogues have also been studied as tools to monitor therapy response, both preclinically [5] and clinically [28–30]. With the addition of 90Y- and 177Lu, the RGD peptides might even be considered for therapeutic applications [31, 32], although the specific uptake in various normal tissues might limit the activity dose that can be safely administered.
Conclusions
In this study differences in the in vivo targeting characteristics of 68Ga-DOTA-E-[c(RGDfK)]2, 68Ga-TRAP-(RGD)3, 68Ga-FSC-(RGD)3, and 68Ga-THP-(RGD)3 were found. The highest tumour uptake was found for 68Ga-FSC-(RGD)3 and 68Ga-THP-(RGD)3 in the SK-RC-52 and FaDu model, respectively. By considering chemical structure, binding affinity, and tracer uptake in tumour tissue as well as (surrounding) normal tissue, the most optimal radiopharmaceutical can be selected for specific diagnostic or therapeutic applications with radiolabelled RGD peptides.
Supplementary Material
Acknowledgments
The authors would like to thank Iris Lamers-Elemans, Kitty Lemmens-Hermans, Bianca Lemmers-van de Weem, and Maikel School of the animal facility for technical assistance during the experiments.
References
- 1.Brakebusch C, et al. Integrins in invasive growth. J Clin Invest. 2002;109(8):999–1006. doi: 10.1172/JCI15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 3.Katsamakas S, et al. RGD-mediated delivery of small-molecule drugs. Future Med Chem. 2017;9(6):579–604. doi: 10.4155/fmc-2017-0008. [DOI] [PubMed] [Google Scholar]
- 4.Dal Corso A, et al. alphavbeta3 Integrin-Targeted Peptide/Peptidomimetic-Drug Conjugates: In-Depth Analysis of the Linker Technology. Curr Top Med Chem. 2016;16(3):314–29. doi: 10.2174/1568026615666150701114343. [DOI] [PubMed] [Google Scholar]
- 5.Terry SY, et al. Can 111In-RGD2 monitor response to therapy in head and neck tumor xenografts? J Nucl Med. 2014;55(11):1849–55. doi: 10.2967/jnumed.114.144394. [DOI] [PubMed] [Google Scholar]
- 6.Haubner R, Maschauer S, Prante O. PET radiopharmaceuticals for imaging integrin expression: tracers in clinical studies and recent developments. Biomed Res Int. 2014;2014:871609. doi: 10.1155/2014/871609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dijkgraaf I, et al. Improved targeting of the alpha(v)beta (3) integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging. 2007;34(2):267–73. doi: 10.1007/s00259-006-0180-9. [DOI] [PubMed] [Google Scholar]
- 8.Dijkgraaf I, et al. PET imaging of alphavbeta(3) integrin expression in tumours with (6)(8)Ga-labelled mono-, di- and tetrameric RGD peptides. Eur J Nucl Med Mol Imaging. 2011;38(1):128–37. doi: 10.1007/s00259-010-1615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poethko T, et al. Improved tumor uptake, tumor retention and tumor/background ratios of pegylated RGD-multimers. Journal of Nuclear Medicine. 2003;44(5):46p–46p. [Google Scholar]
- 10.Thumshirn G, et al. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chemistry. 2003;9(12):2717–25. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 11.Kim JH, et al. Whole-body distribution and radiation dosimetry of (68)Ga-NOTA-RGD, a positron emission tomography agent for angiogenesis imaging. Cancer Biother Radiopharm. 2012;27(1):65–71. doi: 10.1089/cbr.2011.1061. [DOI] [PubMed] [Google Scholar]
- 12.Knetsch PA, et al. [68Ga]NODAGA-RGD for imaging alphavbeta3 integrin expression. Eur J Nucl Med Mol Imaging. 2011;38(7):1303–12. doi: 10.1007/s00259-011-1778-0. [DOI] [PubMed] [Google Scholar]
- 13.Notni J, et al. TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chemistry. 2011;17(52):14718–22. doi: 10.1002/chem.201103503. [DOI] [PubMed] [Google Scholar]
- 14.Notni J, Pohle K, Wester HJ. Be spoilt for choice with radiolabelled RGD peptides: preclinical evaluation of (6)(8)Ga-TRAP(RGD)(3) Nucl Med Biol. 2013;40(1):33–41. doi: 10.1016/j.nucmedbio.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Knetsch PA, et al. [(68)Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging alphavbeta3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nucl Med Biol. 2015;42(2):115–22. doi: 10.1016/j.nucmedbio.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhai C, et al. Comparison of Ga-68-Labeled Fusarinine C-Based Multivalent RGD Conjugates and [Ga]NODAGA-RGD-In Vivo Imaging Studies in Human Xenograft Tumors. Mol Imaging Biol. 2016 doi: 10.1007/s11307-016-0931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imberti C, et al. Enhancing PET Signal at Target Tissue in Vivo: Dendritic and Multimeric Tris(hydroxypyridinone) Conjugates for Molecular Imaging of alphavbeta3 Integrin Expression with Gallium-68. Bioconjug Chem. 2017;28(2):481–495. doi: 10.1021/acs.bioconjchem.6b00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terry SY, et al. Imaging integrin alphavbeta3 on blood vessels with 111In-RGD2 in head and neck tumor xenografts. J Nucl Med. 2014;55(2):281–6. doi: 10.2967/jnumed.113.129668. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, et al. microPET of tumor integrin alphavbeta3 expression using 18F-labeled PEGylated tetrameric RGD peptide (18F-FPRGD4) J Nucl Med. 2007;48(9):1536–44. doi: 10.2967/jnumed.107.040816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Max R, et al. Immunohistochemical analysis of integrin alpha vbeta3 expression on tumor-associated vessels of human carcinomas. Int J Cancer. 1997;71(3):320–4. doi: 10.1002/(sici)1097-0215(19970502)71:3<320::aid-ijc2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Beer AJ, et al. [18F]galacto-RGD positron emission tomography for imaging of alphavbeta3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(22 Pt 1):6610–6. doi: 10.1158/1078-0432.CCR-07-0528. [DOI] [PubMed] [Google Scholar]
- 22.Kiessling LL, Pohl NL. Strength in numbers: non-natural polyvalent carbohydrate derivatives. Chem Biol. 1996;3(2):71–7. doi: 10.1016/s1074-5521(96)90280-x. [DOI] [PubMed] [Google Scholar]
- 23.Liu S. Radiolabeled cyclic RGD peptides as integrin alpha(v)beta(3)-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009;20(12):2199–213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, et al. microPET imaging of glioma integrin {alpha}v{beta}3 expression using (64)Cu-labeled tetrameric RGD peptide. J Nucl Med. 2005;46(10):1707–18. [PubMed] [Google Scholar]
- 25.Beer AJ, et al. Comparison of integrin alphaVbeta3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med. 2008;49(1):22–9. doi: 10.2967/jnumed.107.045864. [DOI] [PubMed] [Google Scholar]
- 26.Zheng K, et al. 68Ga-NOTA-PRGD2 PET/CT for Integrin Imaging in Patients with Lung Cancer. J Nucl Med. 2015;56(12):1823–7. doi: 10.2967/jnumed.115.160648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mi B, et al. Pilot Prospective Evaluation of (18)F-Alfatide II for Detection of Skeletal Metastases. Theranostics. 2015;5(10):1115–21. doi: 10.7150/thno.12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen SH, et al. RGD-K5 PET/CT in patients with advanced head and neck cancer treated with concurrent chemoradiotherapy: Results from a pilot study. Eur J Nucl Med Mol Imaging. 2016;43(9):1621–9. doi: 10.1007/s00259-016-3345-1. [DOI] [PubMed] [Google Scholar]
- 29.Luan X, et al. 18F-alfatide PET/CT may predict short-term outcome of concurrent chemoradiotherapy in patients with advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2016;43(13):2336–2342. doi: 10.1007/s00259-016-3505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, et al. Can an 18F-ALF-NOTA-PRGD2 PET/CT Scan Predict Treatment Sensitivity to Concurrent Chemoradiotherapy in Patients with Newly Diagnosed Glioblastoma? J Nucl Med. 2016;57(4):524–9. doi: 10.2967/jnumed.115.165514. [DOI] [PubMed] [Google Scholar]
- 31.Shi J, et al. Potential therapeutic radiotracers: preparation, biodistribution and metabolic characteristics of 177Lu-labeled cyclic RGDfK dimer. Amino Acids. 2010;39(1):111–20. doi: 10.1007/s00726-009-0382-0. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, et al. Two (9)(0)Y-labeled multimeric RGD peptides RGD4 and 3PRGD2 for integrin targeted radionuclide therapy. Mol Pharm. 2011;8(2):591–9. doi: 10.1021/mp100403y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.