Abstract
The mechanism behind chromatid break formation is as yet unclear, although it is known that DNA double-strand breaks (DSBs) are the initiating lesions. Chromatid breaks formed in cells in the G2-phase of the cell-cycle disappear (‘rejoin’) as a function of time between radiation exposure and cell fixation. However, the kinetics of disappearance of chromatid breaks does not correspond to those of DSB rejoining, leading us to seek alternative models. We have proposed that chromatid breaks could be formed indirectly from DSB and that the mechanism involves topoisomerase IIα. In support of this hypothesis we have recently shown that frequencies of radiation-induced chromatid breaks are lower in two variant human promyelocytic leukaemic cell lines with reduced topoisomerase IIα expression. Here we report that suppression of topoisomerase IIα in human hTERT-RPE1 cells, either by its abrogation using specific siRNA or by inhibition of its catalytic activity with the inhibitor ICRF-193, causes a reduction in frequency of chromatid breaks in radiation-exposed cells. The findings support our hypothesis for the involvement of topoisomerase IIα in the formation of radiation-induced chromatid breaks, and could help explain inter-individual variation in human chromosomal radiosensitivity; elevation of which has been linked with cancer susceptibility.
Keywords: Radiosensitivity, Topoisomerase IIα, Chromatid breaks, G2-assay
1. Introduction
Variation in the response of humans to ionising radiation is exemplified by the range of frequencies of metaphase chromatid breaks observed in PHA-stimulated peripheral blood T-lymphocytes from different individuals [1,2], exposed to low-dose irradiation in the G2-phase of the cell cycle. Besides this wide spread in chromatid ‘radiosensitivity’ amongst different, apparently normal individuals, a striking increase in chromatid break frequency in lymphocytes following irradiation has been observed in 40% or more of breast and other cancer cases as compared with groups of normal non-cancer individuals [1–13]. Furthermore, it has been found that first-degree relatives of breast cancer cases show an elevated chromatid break phenotype, indicating heritability of the trait and the possibility that chromatid radiosensitivity could be a marker of breast cancer susceptibility [4,14,15]. In order to clarify the reasons for such variation in chromosomal radiosensitivity it is first necessary to understand the mechanisms involved in formation of chromatid breaks.
Chromatid breaks (defined here as chromatid discontinuities or terminal chromatid deletions) are classically explained by ‘breakage-first’ where a chromatid break derives from and is essentially an expanded double-strand breaks (DSB) [16]. However, more recent research has shown that the disappearance of chromatid breaks with time after irradiation is not directly related to DSB rejoining [17] although DSB rejoining does influence chromatid break frequency [18]. Our working hypothesis to explain the formation of chromatid breaks is that the DSB is an initiating event within a chromatin loop that leads to an intra- or inter-chromatid rearrangement resulting from an incomplete mis-joining of chromatin ends during the decatenation of chromatids during the G2-phase of the cell cycle [17,19,20]. The frequency of chromatid breaks arising from inter-chromatid rearrangements, generated by both radiation and restriction endonucleases (in live porated cells), is between 10% and 16% as determined by harlequin (Fluorescence Plus Giemsa) staining of chromatids [21,22]. However, the proportion of chromatid breaks resulting from intra-chromatid rearrangements is not known, but if our hypothesis is correct, could be more than 80%.
Due to the involvement of topoisomerase IIα (topo IIα) in decatenation of chromatids during the G2-phase we suspect this enzyme could be involved in, or responsible for, the intra- or inter-chromatid rearrangement process, and hence chromatid breaks. Topo IIα is known to be susceptible to a variety of poisons and inhibitors [23], and becomes error-prone in the presence of reactive oxygen species, where it can result in cleavage and exclusion of chromatin loops at the base of which topo IIα can often be found [24]. Error-proneness of topo IIα might also be enhanced by both endogenous and radiation-induced abasic sites, which are known to act as potent topo IIα poisons [25].
Topo IIα is an essential nuclear DNA-processing enzyme involved in several important cellular functions such as replication, transcription, G2 checkpoint regulation, chromosome condensation and sister chromatid decatenation [23]. The expression of topo IIα is cell-cycle dependent, upregulated in the G2-phase of the cell cycle [26] and a target for a variety of anti-cancer agents [27,28] most of which are known as topo II poisons. These poisons either enhance cleavage activity by stabilising or increasing the rate of formation of the cleavable complex [29,30] or inhibit religation [31]. In both cases a high level of protein-associated DSB occurs so that cells either undergo apoptosis [32] or produce chromosome aberrations such as translocations [33]. Other agents, e.g. ICRF-193 are classified as inhibitors, and prevent the catalytic scission of DNA by topo IIα [34]. The other topo II isoform (topo IIβ) in contrast, present in equal amounts throughout the cell cycle [26], is thought to function in DNA repair [35], and is involved in neuronal development in mice [36]. It is for these reasons that topo IIα is focussed on here.
Previously we reported that topo IIα expression level is a determinant of chromatid radiosensitivity in human promyelocytic leukaemia (HL60) cells [37]. Variant cell lines derived from the parental HL60 with low topo IIα expression by prolonged growth in mitoxantrone [38] showed reduced chromatid breakage when exposed to radiation. The frequency of chromatid breaks correlated positively (r2 = 0.93) with topo IIα expression level.
Here we report on data that extends and reinforces the evidence for the involvement of topo IIα in chromatid breaks. In these studies we have used siRNA to reduce expression of topo IIα, or treated cells with ICRF-193 to inhibit topo IIα in radiation-exposed cells to further test the hypothesis that topo IIα is involved in the formation of chromatid breaks.
2. Materials and methods
2.1. Cell line and culture
hTERT transformed retinal pigment epithelium (hTERT-RPE1) cells were obtained from the ATCC and were maintained in exponential growth in DMEM/F12 medium containing 10% foetal calf serum and antibiotics.
2.2. Lysate preparation and protein quantification
After trypsinisation, exponentially growing cells were washed twice in PBS (Gibco) and resuspended in 14 μl/106 cells sample reducing buffer (2% SDS (BDH Biochemical, Poole, UK), 100 mM DTT, 10% glycerol (BDH), 60 mM Tris pH 6.8 (BDH), 0.1% bromophenol blue). Protein was then denatured for 5 min in boiling water and stored at −20 °C.
The amount of protein in lysis buffer without bromophenol blue, was quantified using the Bio-Rad Laboratories Protein Assay in which known BSA (Promega Corporation, Southampton, UK) concentrations, namely 0–8 μg/ml, were used as reference values at a 595 nm wavelength (Hemel Hempstead, UK).
2.3. Immunoblotting
11 μg of cell lysate was run on a 7% SDS polyacrylamide gel. Proteins were transferred on to a nitrocellulose membrane (Watman, Schleicher & Schuell, Dassel, Germany) at 100 V for 1 h and checked with Ponceau red stain. Protein epitopes were blocked in 2% milk powder in PBS/Tween for 15 min. Membranes were further incubated for 2 h (or at 4 °C overnight) in either rabbit anti-topoisomerase IIα or mouse anti-β-actin (Abcam, Cambridge, UK) at 1:20,000 and 1:5000 respectively in PBS/Tween-20. After multiple washes in PBS/Tween, the membranes were incubated in either anti-mouse or anti-rabbit antibody both of which were horse-radish peroxidase-conjugated (Pierce Biotechnology, Rockford, USA) at 1:100,000 dilution. Membranes were then washed twice in PBS/Tween and the secondary antibodies detected by enhanced chemiluminescence (Millipore, Watford, UK). The amount of protein in samples was estimated using image analysis (Image J).
2.4. RT-PCR analysis of topo IIα mRNA
Total RNA was extracted (Qiagen RNeasy mini kit) from fresh hTERT-RPE1 cells incubated with or without siRNA against topo IIα for 12 h. cDNA was made (Qiagen Quantitect Reverse Transcription kit) and PCR performed (Sigma Readymix Taq PCR Reaction Mix) with primers (VH Bio) taken from literature: Topo IIα primers [39]; β-actin primers [40]. PCR samples were run on 2% agarose gels. All PCR products were amplified on the same hTERT-RPE1 cell derived cDNA.
DNA sequencing was performed by the Sequencing Service, University of Dundee, using Applied Biosystems Big-Dye Ver3.1 chemistry on an Applied Systems Model 3730 automated capillary DNA Sequencer.
2.5. Immunocytochemistry
Exponentially growing RPE cells were cytospun at 50×g, high acceleration for 7 min with an end concentration of 10,000 cells/slide (Cytospin 2, Thermo Fischer Scientific Inc, Waltham, MA, USA). After a fixative stage of 15 min in 100% cold acetone at room temperature, cells were washed twice for 15 min in PBS before being permeabilised with fresh 0.2% Triton X-100 in PBS (PBST). Cells were washed as before and blocked overnight at 4 °C in 5% BSA in PBST in a Copland jar. Primary anti-topoisomerase IIα antibody was added at a dilution of 1:500 (Abcam) in fresh 5% BSA/PBST in a moisture chamber for 1 h at room temperature before being washed. Cells were further incubated in 1:160 dilution of anti-rabbit-FITC-conjugated secondary antibody in a moisture chamber for 1 h at room temperature and in the dark. DAPI/Vectashield (Vector Laboratories Inc, Burlingame, CA, USA) was added after another washing period and slides were mounted and kept overnight in the dark at 4 °C until ready the next day for observing at 63× under a fluorescent microscope (Zeiss Axioskop, Welwyn Garden City, Hertfordshire, UK).
2.6. siRNA incubation
Cells were incubated with various concentrations of 2 separate siRNAs against topoisomerase II alpha, namely 5′-CCUUCAACUAUCUUCUUGAtt-3′ and 5′-GCUCCUAACUUCUAGUAACtt-3′, or scrambled siRNA (Ambion, Warrington, UK) and transfected into cells as described by the manufacturer (Dharmacon, Chicago, IL, USA) for 6 or 12 h.
2.7. Chromatid break analyses
Cells incubated with 0, 1, or 2 nM siRNA against topoisomerase II alpha for 6 or 12 h were irradiated with a dose of 0.3 Gy (137Cs γ-rays). These, along with unirradiated controls were incubated for 30 min before the addition of colcemid 0.1 μg/ml for 1 h.
In a different experiment ICRF-193 was added, at either 25 or 100 nM, to exponentially growing RPE cells which were then irradiated with a dose of 0.3 Gy γ-rays. These, along with unirradiated controls were incubated for 30 min before the addition of colcemid 0.1 μg/ml for 2 h.
Cells treated with either siRNA or ICRF-193 were then centrifuged and resuspended in hypotonic solution (75 mM KCl) for 7 min at 37 °C. Cells were fixed 3 times in 3:1 methanol:acetic acid (BDH). Cells were spread onto slides manually and dried by air. Slides were further stained with 10% Giemsa (BDH) in Gurr’s buffer (BDH) for 10 min. 100 metaphases were examined for chromatid breaks using oil-immersion (100×) optics (Zeiss Axioplan 2, Welwyn Garden City, Hertfordshire, UK). Chromatid breaks were defined as any chromatid discontinuity [41].
2.8. Mitotic index
Exponentially growing RPE (1 × 106) cells were incubated with various concentrations of ICRF-193 (Sigma, stock in 30% ethanol), ranging from 0 to 100 nM for a total of 2.5 h. In other cases cells were incubated with 1 nM siRNA against topo IIα for 12 h and then irradiated with γ-rays at a dose of 0.3 Gy. In either case, cells were then simultaneously incubated in 0.1 μg/ml colcemid (Gibco) for a total of either 1 (siRNA) or 2 (IRCF-193) h at 37 °C before being washed in medium by centrifugation at 250 × g. Cells were fixed in 70% ethanol for 30 min on ice. After centrifugation, cells were permeabilised and stained for phospho-histone H3 according to the manufacturer’s protocol (Cell Signalling Technology, Danvers, MA, USA). Cells were also incubated for 30 min at 37 °C in 1 mg/ml ribonuclease A in 0.5% donkey serum in PBS, centrifuged and resuspended in a solution of propidium iodide 2 μg/ml in PBS for 30 min at room temperature before being washed in PBS and analysed by FACScan (Becton-Dickinson BioSciences, Oxford, UK) with CellQuest software (San Jose, CA, USA).
3. Results
3.1. Immunoblotting following siRNA treatment
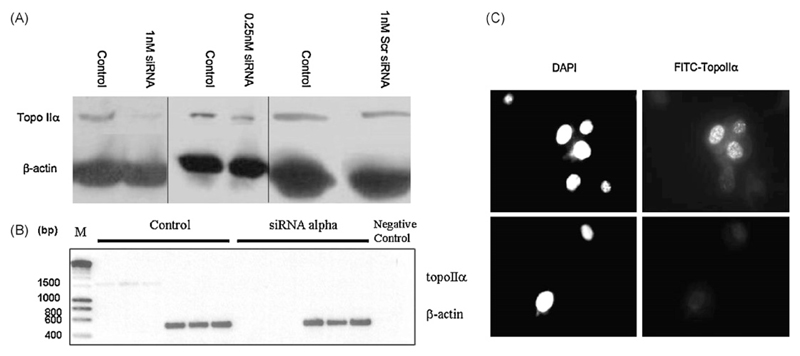
Western blot analysis of whole cell lysates from exponentially growing hTERT-RPE1 cells, 12 h following 1 nM siRNA treatment shows that topo IIα levels were decreased when normalised against β-actin levels and compared with controls (Fig. 1A, left panel). This was confirmed with a separate siRNA against a different exon of topo IIα (data not shown). Incubation with 0.25 nM siRNA (Fig. 1A, middle panel) resulted in less reduction in expression than was observed with 1 nM siRNA. Incubation with 1 nM scrambled (scr) siRNA (Fig. 1A, right panel) had no effect on topo IIα expression.
Fig. 1.
Western blots of topoisomerase IIα and β-actin in hTERT-RPE cells incubated for 12 h with 1 nM and 0.25 nM siRNA against topo IIα or 1 nM scrambled (Scr) siRNA (A). (B) RT-PCR products (reactions performed in triplicate) from cDNA extracted from control hTERT-RPE cells, and cells treated with siRNA against topo IIα (1 nM, 12 h). Negative control represents an RT-PCR reaction without cDNA and M stand for marker. (C) Immunocytochemistry with hTERT-RPE cells incubated with or without 1 nM siRNA against topo IIα for 12 h using primary anti-topo IIα antibody, and FITC-labelled secondary antibody (right panels). Nuclei (left panels) are stained with DAPI (magnification 63×).
3.2. mRNA transcription following siRNA treatment
Fig. 1B shows the products of RT-PCR on cDNA from hTERT-RPE1 cells treated with siRNA. Single bands were observed and the negative control was blank, suggesting primer specificity. Products ran at the expected MWs according to primer and mRNA sequence data. The RT-PCR analysis indicated that siRNA treatment for 12 h caused a marked reduction in mRNA transcription. This was confirmed with a separate siRNA against a different exon of topo IIα (data not shown). The product sequences showed 94.4% and 97.8% identity for forward and reverse primers against topo IIα respectively as determined by LALIGN and 95% and 98% identity for human topo IIα mRNA as determined by BLAST. Sequences showed 99.7% and 98.7% identity for forward and reverse primer respectively against β-actin as determined by LALIGN and 99% identity with human β-actin mRNA for both primers as determined by BLAST. As the primers cross intron/exon boundaries and during the mRNA extraction genomic DNA is removed, it is ensured that it is mRNA and not genomic DNA that is amplified.
3.3. Immunocytochemistry on siRNA treated cells
Immunocytochemical staining of exponentially growing cells (Fig. 1C) confirmed the reduction of topo IIα expression level, 12 h following treatment with 1 nM siRNA.
3.4. Chromatid breaks and mitotic index in irradiated cells treated with siRNA
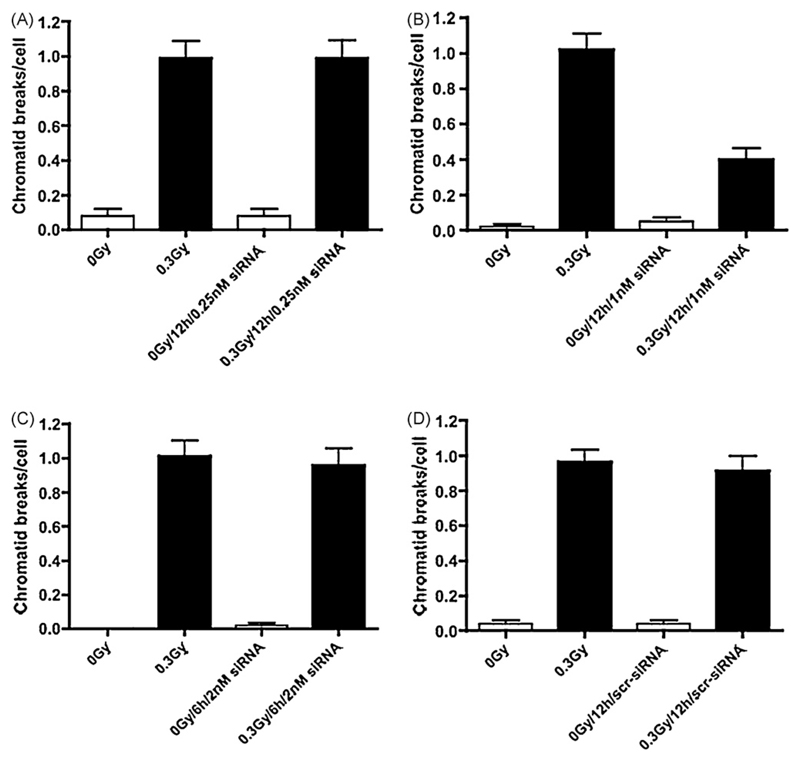
Fig. 2 shows that lowering topo IIα expression with a 12 h incubation of cells with 1 nM siRNA (panel B) in irradiated cells significantly lowered the radiation-induced chromatid break frequency (P ≤ 0.0001). This was confirmed with 1 nM of another siRNA against topo IIα (data not shown, P ≤ 0.0001). Incubation for 12 h with 0.25 nM siRNA (panel A), 2 nM siRNA for 6 h (panel C), or 1 nM scrambled for 12 h (panel D) had no significant effect on the number of chromatid breaks per irradiated cell.
Fig. 2.
Frequencies of chromatid breaks using the G2-assay in control (unirradiated) and irradiated hTERT-RPE cells incubated with or without 0.25 nM (graph A) or 1 nM siRNA (graph B) against topo IIα for 12 h or 2 nM siRNA for 6 h (graph C). Graph D shows chromatid break numbers for control or irradiated hTERT-RPE cells incubated with 1 nM scrambled siRNA for 12 h. Vertical bars represent SEM standard errors of mean values.
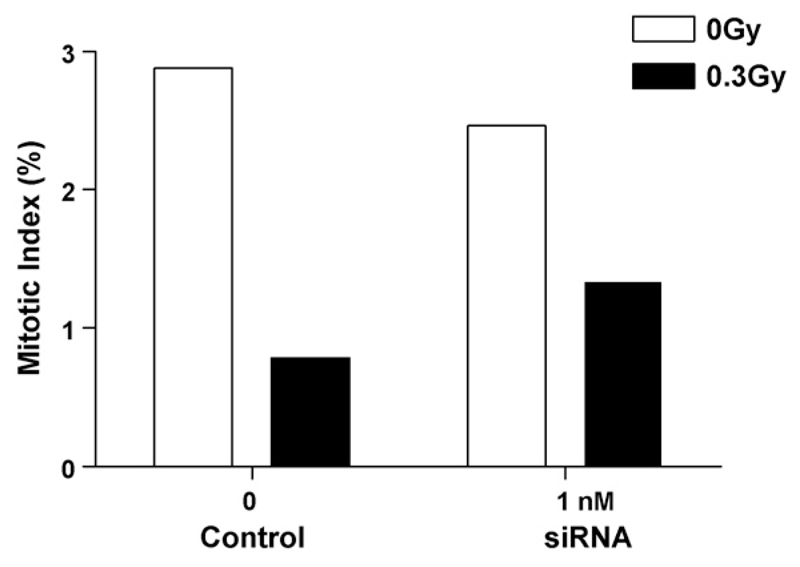
Irradiation of cells (0.3 Gy) lowered the mitotic index in hTERT-RPE1 cells as measured by FACS analysis (Fig. 3). However, the relative decrease in mitotic index from 0 to 0.3 Gy was not reduced further by siRNA incubation at 1 nM siRNA for 12 h.
Fig. 3.
The effect of 1 nM siRNA against topo IIα on mitotic index in control (unirradiated) and irradiated hTERT-RPE cells as measured by FACS analysis of fluorescently labelled anti-phospho-histone H3. 104 cells were analysed per sample.
3.5. Chromatid breaks and mitotic index in irradiated cells treated with ICRF-193
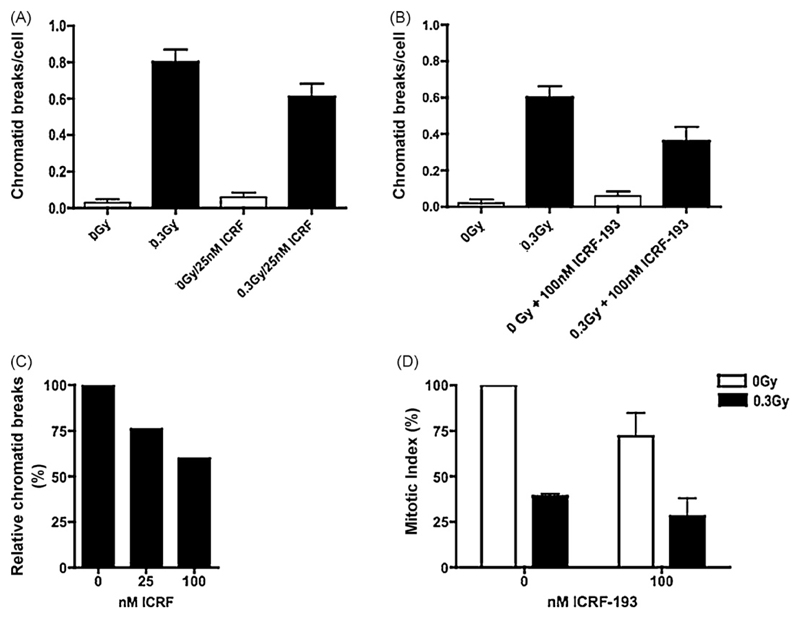
Fig. 4 shows the effect of incubation of hTERT-RPE1 cells with the topo IIα catalytic inhibitor ICRF-193 at either 25 nM (panel A) or 100 nM (panel B) on the number of chromatid breaks after exposure to 0.3 Gy of γ-rays. Combining these results (panel C) shows that increasing concentrations of ICRF-193 from 0 to 100 nM shows a negative correlation with normalised percentage chromatid break frequency. Also, panel D shows that as ICRF-193 concentration increases, the mitotic index decreases in both irradiated and non-irradiated samples.
Fig. 4.
Frequencies of chromatid breaks using the G2-assay in control (unirradiated) and irradiated hTERT-RPE cells incubated with or without 25 nM (graph A) or 100 nM (graph B) ICRF-193. Bars represent SEM standard errors of mean values. Combined results from graphs A and B have been normalised in graph C by dividing by the chromatid break frequency without ICRF-193. Graph D shows the effect of ICRF-193 on mitotic index in hTERT-RPE cells as measured by FACs analysis of anti-phospho-histone H3 fluorescently labelled cells. 104 cells were analysed per sample.
Unlike treatment with siRNA, incubation of cells with ICRF-193 following irradiation caused an additional depression of mitotic index (Fig. 4, panel D). The relative mitotic index was reduced by 61% (radiation alone), and by 71% (radiation plus 100 nM ICRF-193).
4. Discussion
The results presented here show that incubation with 2 specific siRNAs against topo IIα leads to a significantly decreased topo IIα expression and radiation-induced chromatid break frequency (Figs. 1 and 2). The results confirm and strengthen the conclusion we drew from a previous study using promyelocytic leukaemia cell variants [37]. Surprisingly, 0.25 nM siRNA against topo IIα incubation showed a similar effect to the control (scrambled) siRNAs suggesting either a threshold or a separate pool of topo IIα involved in the formation of chromatid breaks. Another reason might be the non-synchronised nature of the cells as siRNA incorporation into G2 cells might be ‘diluted’ by cells in other phases of the cell-cycle.
Both western blotting (Fig. 1A) and RT-PCR (Fig. 1B) as well as immunocytochemistry (Fig. 1C) indicate substantial knockdown of topo IIα by 0.25 and 1 nM siRNA except in control experiments (meaning?). Quantification of the knockdown using double-antibody sandwich (DAS)-ELISA analysis with anti-topo IIα antibodies showed a 72% knockdown in topo IIα expression level in cells treated with 1 nM siRNA for 12 h. The immunocytochemistry also suggests a universally spread knockdown over all cell populations perhaps indicating that 0.25 nM siRNA was not sufficient to lower expression levels in G2 cells in order to see an effect on chromatid break frequency. Immunocytochemistry also confirms previous findings of a nuclear localisation of topo IIα [42,43].
Topo IIα has previously been implicated in chromosome condensation in mitosis [44]. However, in hTERT-RPE1 cells incubated with 1 nM siRNA for 12 h no visible reduction in chromosome condensation was observed.
As a further test of the involvement of topo IIα in the formation of radiation-induced chromatid breaks, the effect of topo IIα inhibition by ICRF-193 on the frequency of chromatid breaks was tested. ICRF-193 is a potent bis(2,6)-dioxopiperazine derivative that inhibits topoisomerase II activity in a non-intercalative manner [34]. Unlike topo II poisons, such as etoposide and m-AMSA, ICRF-193 does not stabilise cleavable complex formation, but instead maintains the enzyme in a closed clamp state and thus does not usually cause DSB [45]. However, under some conditions ICRF-193 has been shown to cause DSB [46]. Here we show that the inhibitor caused a decrease in chromatid break frequency, suggesting that it does not cause DSB in this cell line at doses up to 100 nM.
We show here that incubation of cells with the topo IIα inhibitor ICRF-193 reduces chromatid break frequency (Fig. 4). This substantiates the results for the siRNA experiments and provides more evidence for the role of topo IIα in the formation of chromatid breaks. In vitro experiments suggest a different range of active IRCF-193 specific to either topoisomerase IIα or β, namely 0.32–3.2 μM and 10–100 μM respectively [47]. Taking these results into account it is likely that the in vivo range of 25 or 100 nM is specific to topo IIα only, which is supported by a previous study that also suggested that it was indeed topo IIα that was the damage-inducing isoform [35].
In order to ensure that the lowered chromatid break frequency resulting from siRNA or ICRF-193 treatments is not due to cells with high chromatid break frequencies blocking in G2, the mitotic index was measured in irradiated and control cells incubated with either siRNA against topo IIα (Fig. 3) or ICRF-193 at 100 nM (Fig. 4). In both cases, lowering of either topo IIα expression or activity with ICRF-193 decreased the mitotic index, confirming a role for topo IIα in normal G2 checkpoint function [48]. This also verifies normal checkpoint activity in the RPE cells [49]. Irradiated cells also showed a lowered mitotic index, again confirming normal checkpoint function. However, no significant lowering of mitotic index was seen in cells incubated with 1 nM siRNA against topo IIα as compared with untreated controls (Fig. 3). However, in ICRF-193-treated cells the mitotic index was lowered more (by approximately 10%) in 100 nM ICRF-193 plus 0.3 Gy (Fig. 4D) treated cells as against untreated controls. However, this does not explain the 40% decrease in chromatid break frequency in cells incubated with 100 nM ICRF-193. Previous data suggests that when using low radiation doses (0.3 Gy) as used here in the G2-assay, the minor cell-cycle block induced does not significantly affect chromatid break frequency [50].
Our results clearly show that topoisomerase IIα is involved in the formation of chromatid breaks, which we postulate could be an indirect pathway steming from an initiating DSB. The reasons why we consider that chromatid breaks do not stem directly from DSB have been rehearsed several times previously [19]. We suggest that the presence of a DSB in the vicinity of topo IIα might lead it to become error-prone in either its cleavage or religation. It is known that chromatin is arranged in large looped domains throughout both interphase and mitosis, and that topo IIα is located at the base of these megabase loops [51], we suggested that errors could occur through misjoining of chromatin strands during the decatenation process. This might then lead to either the complete loss of DNA through loop excision or loop sequence inversion which would result in a chromatid break if not completed or repaired.
Perhaps one way in which topo IIα could become error-prone in irradiated cells might be through the formation of abasic sites close to the DSB within a looped chromatin structure. It has been shown [52] that abasic sites are present at low ionising radiation doses and that they affect topo IIα in a similar way to topo IIα poisons, such as etoposide, albeit in a more potent way. Abasic sites are produced endogenously by normal cellular metabolism as well as irradiation and it is probable that not all are repaired and removed, thus increasing the formation of topo IIα-induced DSB [25].
In conclusion our data demonstrate a link between radiation-induced chromatid break frequency and topo IIα expression or activity, which together with previous findings, strongly support our hypothesis of a role for topo IIα in the formation of radiation-induced chromatid breaks. Finally we suggest that variation in topo IIα expression in stimulated human T-lymphocytes might contribute to inter-individual radiation sensentivity [1–5].
Acknowledgements
The work was supported by the Breast Cancer Campaign (2006NovSP12) and the Chief Scientist Office, Scottish Executive (CSO, CZB/4B510). SYA Terry is supported by the University of St Andrews.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- [1].Scott D, Spreadborough A, Levine E, Roberts SA. Genetic predisposition to breast cancer. Lancet. 1994;344:1444. doi: 10.1016/s0140-6736(94)90615-7. [DOI] [PubMed] [Google Scholar]
- [2].Riches AC, Bryant PE, Steel CM, Gleig A, Robertson AJ, Preece PE, Thompson AM. Chromosomal radiosensitivity in G2-phase lymphocytes identifies breast cancer patients with distinctive tumour characteristics. Br J Cancer. 2001;85:1157–11561. doi: 10.1054/bjoc.2001.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scott D, Barber JBP, Spreadborough AR, Burrill W, Roberts SA. Increased radiosensitivity in breast cancer patients: a comparison of two assays. Int J Radiat Biol. 1999;75:1–10. doi: 10.1080/095530099140744. [DOI] [PubMed] [Google Scholar]
- [4].Roberts SA, Spreadborough AR, Bulman B, Barber JBP, Evans DRG, Scott D. Heritability of cellular radiosensitivity: a marker of low penetrance predisposition genes in breast cancer? Am J Hum Genet. 1999;65:784–794. doi: 10.1086/302544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baria K, Warren C, Roberts SA, West CM, Scott D. Chromosomal radiosensitivity as a marker of predisposition to common cancers. Br J Cancer. 2001;84:892–896. doi: 10.1054/bjoc.2000.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Terzoudi GI, Jung T, Hain J, Vrouvas J, Margaritis K, Donta-Bakoyiannis C, Makropoulos V, Angelakis PH, Pantelias GE. Increased chromosomal radiosensitivity in cancer patients: the role of cdk1/cyclin-B activity level in the mechanisms involved. Int J Radiat Biol. 2000;76:607–615. doi: 10.1080/095530000138268. [DOI] [PubMed] [Google Scholar]
- [7].Papworth R, Slevin N, Roberts SA, Scott D. Sensitivity to radiation-induced chromosome damage may be a marker of genetic predisposition in young head and neck cancer patients. Br J Cancer. 2001;84:776–782. doi: 10.1054/bjoc.2000.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Buchholz TA, Wu X. Radiation-induced chromatid breaks as a predictor of breast cancer risk. Int J Radiat Oncol Biol Phys. 2001;49:533–537. doi: 10.1016/s0360-3016(00)01502-9. [DOI] [PubMed] [Google Scholar]
- [9].Baeyens A, Thierens H, Claes K, Poppe B, Messiaen L, de Ridder L, Vral A. Chromosomal radiosensitivity in breast cancer patients with a known or putative genetic predisposition. Br J Cancer. 2002;87:1379–1385. doi: 10.1038/sj.bjc.6600628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baeyens A, Broecke RVD, Makar A, Thierens H, de Ridder L, Vral A. Chromosomal radiosensitivity in breast cancer patients: influence of age of onset of the disease. Oncol Rep. 2005;13:347–353. [PubMed] [Google Scholar]
- [11].Howe OL, Daly PA, Seymour C, Ormiston W, Nolan C, Mothersill C. Elevated G2 chromosomal radiosensitivity in Irish breast cancer patients: a comparison with other studies. Int J Radiat Biol. 2005;81:373–378. doi: 10.1080/09553000500147642. [DOI] [PubMed] [Google Scholar]
- [12].Lisowska H, Lankoff A, Wieczorek A, Florek A, Kuszewski T, Góźdź S, Wojcik A. Enhanced chromosomal radiosensitivity in peripheral blood lymphocytes of larynx cancer patients. Int J Radiat Oncol Biol Phys. 2006;66:1245–1252. doi: 10.1016/j.ijrobp.2006.07.1370. [DOI] [PubMed] [Google Scholar]
- [13].De Ruyck K, de Gelder V, Van Eijkeren M, Boterberg T, De Neve W, Vral A, Thierens H. Chromosomal radiosensitivity in head and neck cancer patients: evidence for genetic predisposition? Br J Cancer. 2008;98:1723–1738. doi: 10.1038/sj.bjc.6604345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Parshad R, Price FM, Bohr VA, Cowan R, Zujewski JKA, Sanford KK. Deficient DNA repair capacity, a predisposing factor in breast cancer. Br J Cancer. 1996;74:1–5. doi: 10.1038/bjc.1996.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patel RK, Trivedi AH, Arora DC, Bhatavdekar JM, Patel DD. DNA repair proficiency in breast cancer patients and their first-degree relatives. Int J Cancer. 1997;73:20–24. doi: 10.1002/(sici)1097-0215(19970926)73:1<20::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- [16].Bender AM, Griggs HG, Bedford JS. Mechanisms of chromosomal aberration production. III. Chemicals and ionizing radiation. Mut Res. 1974;23:197–212. doi: 10.1016/0027-5107(74)90140-7. [DOI] [PubMed] [Google Scholar]
- [17].Bryant PE, Gray LJ, Peresse N. Progress towards understanding the nature of chromatid breakage. Cytogenet Genome Res. 2004;104:65–71. doi: 10.1159/000077467. [DOI] [PubMed] [Google Scholar]
- [18].Macleod RAF, Bryant PE. Similar kinetics of chromatid aberrations in X-irradiated xrs 5 and wild-type Chinese hamster cells. Mutagenesis. 1990;5:407–410. doi: 10.1093/mutage/5.4.407. [DOI] [PubMed] [Google Scholar]
- [19].Bryant PE. The signal model: a possible explanation for the conversion of DNA double-strand breaks into chromatid breaks. Int J Radiat Biol. 1998;73:243–251. doi: 10.1080/095530098142338. [DOI] [PubMed] [Google Scholar]
- [20].Bryant PE. Origin of chromosome aberrations: mechanisms. In: Obe G, Vijayalaxmi, editors. Chromosomal Alterations: Methods, Results and Importance in Human Health. Springer-Verlag; Berlin, Heidelberg: 2007. pp. 177–199. [Google Scholar]
- [21].Harvey AN, Savage JRK. Investigating the nature of chromatid breaks produced by restriction endonucleases. Int J Radiat Biol. 1997;71:21–28. doi: 10.1080/095530097144373. [DOI] [PubMed] [Google Scholar]
- [22].Mozdarani H, Liu N, Jones NJ, Bryant PE. The XRCC2 human repair gene influences recombinational rearrangments leading to chromatid breaks. Int J Radiat Biol. 2001;77:859–865. doi: 10.1080/09553000110054890. [DOI] [PubMed] [Google Scholar]
- [23].Austin CA, Marsh KL. Eukaryotic DNA topoisomerase II beta. Bioessays. 1998;20:215–226. doi: 10.1002/(SICI)1521-1878(199803)20:3<215::AID-BIES5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- [24].Li T, Chen AY, Mao Y, Wang H, Liu LF. Activation of topoisomerase II-mediated excision of chromosomal DNA loops during oxidatie stress. Genes Dev. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kingma PS, Corbett AH, Burcham PC, Marnett LJ, Osheroff N. Abasic sites stimulate double-stranded DNA cleavage mediated by topoisomerase II. J Biol Chem. 1995;270:21441–21444. doi: 10.1074/jbc.270.37.21441. [DOI] [PubMed] [Google Scholar]
- [26].Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH. Proliferation and cell cycle dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991;2:209–214. [PubMed] [Google Scholar]
- [27].Liu LF. DNA topoisomerase poisons as antitumor drugs. Annu Rev Biochem. 1989;58:351–375. doi: 10.1146/annurev.bi.58.070189.002031. [DOI] [PubMed] [Google Scholar]
- [28].Chen AY, Liu LF. DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- [29].Chen GL, Yang L, Rowe TC, Halligan BD, Tewey KM, Liu LF. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984;259:13560–13566. [PubMed] [Google Scholar]
- [30].Sabourin M, Osheroff N. Sensitivity of human type II topoisomerases to DNA damage: stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 2000;28:1947–1954. doi: 10.1093/nar/28.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Osheroff N. Effect of antineoplastic agents on the DNA cleavage/religation reaction of eukaryotic topoisomerase II: inhibition of DNA religation by etoposide. Biochemistry. 1989;28:6157–6160. doi: 10.1021/bi00441a005. [DOI] [PubMed] [Google Scholar]
- [32].McClendon AK, Osheroff N. DNA topoisomerase II, genotoxicity, and cancer. Mutat Res. 2007;623:83–97. doi: 10.1016/j.mrfmmm.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Felix CA, Kolaris CP, Osheroff N. Topoisomerase II and the etiology of chromosome translocations. DNA Rep. 2006;5:1093–1108. doi: 10.1016/j.dnarep.2006.05.031. [DOI] [PubMed] [Google Scholar]
- [34].Tanabe K, Ikegami Y, Ishida R, Andoh T. Inhibition of topoisomerase II by antitumor agents bis(2,6-dioxopiperazine) derivatives. Cancer Res. 1991;51:4903–4908. [PubMed] [Google Scholar]
- [35].Mandraju RK, Kannaapiran P, Kondapi AK. Distinct roles of topoisomerase II isoforms: DNA damage accelerating alpha, double-strand break repair promoting beta. Arch Biochem Biophys. 2008;470:27–34. doi: 10.1016/j.abb.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [36].Yang X, Li W, Prescott ED, Burden SJ, Wang JC. DNA topoisomerase IIbeta and neural development. Science. 2000;287:131–134. doi: 10.1126/science.287.5450.131. [DOI] [PubMed] [Google Scholar]
- [37].Terry SY, Riches AC, Bryant PE. A role for topoisomerase II alpha in the formation of radiation-induced chromatid breaks. Br J Cancer. 2008;99:670–674. doi: 10.1038/sj.bjc.6604514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Harker WG, Slade DL, Drake FH, Parr RL. Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II beta isoform. Biochemistry. 1991;30:9953–9961. doi: 10.1021/bi00105a020. [DOI] [PubMed] [Google Scholar]
- [39].Lang AJ, Mirski SE, Cummings HJ, Yu Q, Gerlach JH, Cole SP. Structural organization of the human TOP2A and TOP2B genes. Gene. 1998;221:255–266. doi: 10.1016/s0378-1119(98)00468-5. [DOI] [PubMed] [Google Scholar]
- [40].Xu J, Stolk JA, Zhang X, Silva SJ, Houghton RL, Matsumura M, Vedvick TS, Leslie KB, Badaro R, Reed SG. Identification of differentially expressed genes in human prostate cancer using subtraction and microarray. Cancer Res. 2000;60:1677–1682. [PubMed] [Google Scholar]
- [41].Bryant PE, Gray L, Riches AC, Steel CM, Finnon P, Howem O, Kesterton I, Vral A, Curwen GB, Smart V, Tawn EJ, et al. The G2 chromosomal radiosensitivity assay. Int J Radiat Biol. 2002;78:863–866. doi: 10.1080/09553000210144484. [DOI] [PubMed] [Google Scholar]
- [42].Davies SM, Robson CN, Davies SL, Hickson ID. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1998;263:17724–17729. [PubMed] [Google Scholar]
- [43].Ivanova EC, Donev RM, Djondjurov LP. Localisation of DNA topoisomerase IIalpha in mouse erythroleukemia cells. Mol Cells. 1999;9:309–313. [PubMed] [Google Scholar]
- [44].Uemura T, Ohkura H, Adachi Y, Morino K, Shiozaki K, Yanagida M. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell. 1987;50:917–925. doi: 10.1016/0092-8674(87)90518-6. [DOI] [PubMed] [Google Scholar]
- [45].Roca J, Ishida R, Berger JM, Andoh T, Wang JC. Antitumor bisdioxopiperazines inhibit yeast DNA topoisomerase II by trapping the enzyme in the form of a closed protein clamp. Proc Natl Acad Sci U S A. 1994;91:1781–1785. doi: 10.1073/pnas.91.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Park I, Avraham HK. Cell cycle-dependent DNA damage signaling induced by ICRF-193 involves ATM, ATR, CHK2, and BRCA1. Exp Cell Res. 2006;312:1996–2008. doi: 10.1016/j.yexcr.2006.02.029. [DOI] [PubMed] [Google Scholar]
- [47].Perrin D, van Hille B, Hill BT. Differential sensitivities of recombinant human topoisomerase IIalpha and beta to various classes of topoisomerase II-interacting agents. Biochem Pharmacol. 1998;56:503–507. doi: 10.1016/s0006-2952(98)00082-3. [DOI] [PubMed] [Google Scholar]
- [48].Anderson H, Roberge M. Topoisomerase II inhibitors affect entry into mitosis and chromosome condensation in BHK cells. Cell Growth Differ. 1996;7:83–90. [PubMed] [Google Scholar]
- [49].Lei M, Erikson RL. Plk1 depletion in nontransformed diploid cells activates the DNA-damage checkpoint. Oncogene. 2008;27:3935–3943. doi: 10.1038/onc.2008.36. [DOI] [PubMed] [Google Scholar]
- [50].Pretazzoli V, Salone B, Bosi A, Olivieri G. Variability of G2 checkpoint sensitivity to low doses of X-rays (2 cGy): correlation with G2 chromatid aberrations but not with an adaptive response. Mutagenesis. 2000;15:531–535. doi: 10.1093/mutage/15.6.531. [DOI] [PubMed] [Google Scholar]
- [51].Poljak L, Kas E. Resolving the role of topoisomerase II in chromatin structure and function. Trends Cell Biol. 1995;5:348–354. doi: 10.1016/s0962-8924(00)89068-6. [DOI] [PubMed] [Google Scholar]
- [52].Sutherland BM, Bennett PV, Sidorkina O, Laval J. Clustered DNA damages induced in isolated DNA and human cells by low doses of ionizing radiation. PNAS. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]