Abstract
The aims of this study were to calculate bone lesion absorbed doses resulting from a weight-based administration of 223Ra-dichloride, to assess the relationship between those doses and corresponding 18F-fluoride uptake and to assess the potential of quantitative 18F-fluoride imaging to predict response to treatment.
Methods
Five patients received two intravenous injections of 223Ra-dichloride, 6 weeks apart, at 110 kBq/kg whole-body weight. The biodistribution of 223Ra in metastatic lesions as a function of time after administration as well as associated lesion dosimetry were determined from serial 223Ra scans. PET/CT imaging using 18F-fluoride was performed prior to the first treatment (baseline), and at week 6 immediately before the second treatment and at week 12 after baseline.
Results
Absorbed doses to metastatic bone lesions ranged from 0.6 Gy to 44.1 Gy. For individual patients there was an average factor of 5.3 difference (range 2.5-11.0) between the maximum and minimum lesion dose. A relationship between lesion absorbed doses and serial changes in 18F-fluoride uptake was demonstrated (r2 = 0.52). A log-linear relationship was demonstrated (r2 = 0.77) between baseline measurements of 18F-fluoride uptake prior to 223Ra-dichloride therapy and changes in uptake 12 weeks after the first cycle of therapy. Correlations were also observed between both 223Ra and 18F-fluoride uptake in lesions (r = 0.75) as well as between 223Ra absorbed dose and 18F-fluoride uptake (r = 0.96).
Conclusions
There is both inter-patient and intra-patient heterogeneity of absorbed dose estimates to metastatic lesions. A relationship between 223Ra lesion absorbed dose and subsequent lesion response was observed. Analysis of this small group of patients suggests that baseline uptake of 18F-fluoride in bone metastases is significantly correlated with corresponding uptake of 223Ra, the associated 223Ra absorbed dose and subsequent lesion response to treatment.
Keywords: Treatment Planning, Absorbed dose, 223Ra, Radium, alpha emitter, 18F, Fluoride
Introduction
Prostate cancer is the most commonly diagnosed non-cutaneous malignancy in men [1]. A significant number of patients will present with metastatic disease in the bones. Hormonal therapy is used as a first line treatment but in many cases the disease will eventually cease to respond to hormonal therapies.
In these circumstances, a range of palliative treatment options for bone metastases due to castration resistant prostate cancer (CRPC) are available, including a number of beta particle emitting radionuclides that are selectively taken up in areas of increased osteoblastic activity. Examples of such molecular radiotherapy treatments include 89Sr-chloride, 153Sm-EDTMP, 186Re-HEDP and 188Re-HEDP. Due to the range of the beta particles potential irradiation of normal bone marrow is anticipated and haematological toxicity is the main side-effect of such treatments [2].
Recently, treatment with 223Ra-dichloride has become available [3]. 223Ra is an alpha emitting radionuclide with a half-life of 11.4 days which also demonstrates increased uptake in regions of high osteoblastic activity. The short range of the alpha particles emitted (< 80 μm) enables high absorbed doses to be delivered to skeletal metastases whilst limiting the absorption of radiation within the bone marrow [4, 5].
The emissions from 223Ra include photons at energies that allow gamma camera imaging [6, 7]. However patients receiving 23Ra-dichloride are typically injected with 55 kBq/kg body mass resulting in limited count statistics in the images. As a result, tomographic imaging is impractical whilst 2D whole-body 223Ra images are characterised by significant levels of Poisson noise which can limit the positive identification of lesions for analysis. Nonetheless, two recent studies have used 2D gamma camera imaging to determine 223Ra absorbed doses according to the MIRD formalism, to measure biodistribution and to calculate normal organ dosimetry in patients treated with 223Ra-dichloride [8, 9]. In addition, Carrasquillo et al [10] used 2D gamma camera imaging to demonstrate early bone uptake of 223Ra as well as small bowel excretion. 2D gamma camera images were also used by Pacilio et al [11] to calculate the absorbed dose to bone metastases in patients receiving 223Ra-dichloride therapy. Notably, this group reported a range of lesion absorbed doses with an order of magnitude difference between the lowest and highest values. Earlier studies of 223Ra therapy have already demonstrated a relationship between the activity administered to a particular cohort of patients (e.g. 25 kBq/kg vs 50 kBq/kg) and the average change in pain index or PSA level across those cohorts [12, 13]. However to date there has been no evidence of a relationship between the lesion absorbed dose and response to treatment with 223Ra-dichloride.
18F-fluoride localises to the inorganic part of the bone, particularly in areas of osteoblastic activity, and has been used extensively for PET imaging of metastatic bone disease. Several studies have demonstrated superior sensitivity and specificity of 18F-fluoride imaging in comparison to conventional bone scintigraphy [14, 15]. Fluoride ions are incorporated into the hydroxyapatite crystal structure of the bone by substitution for hydroxyl (OH) ions. The relationship between osteoblastic and osteoclastic activity is believed to determine the amount of incorporation into the bone [16], given by.
| (1) |
In common with 18F-fluoride, 223Ra localises to areas of osteoblastic activity and the hydroxyapatite crystal structure is also thought to be the target for 223Ra ions [4]. These ions are taken up into bone by ionic exchange with the calcium ions [17]. Given the common hydroxyapatite target, it can be hypothesised that pre-treatment 18F-fluoride PET imaging may provide theragnostic information regarding the response of lesions to treatment with 223Ra dichloride.
In this study the cumulated activity, the effective half-life and the range of absorbed doses delivered to a number of lesions were determined. The relationship between 223Ra absorbed doses and subsequent changes in 18F-fluoride uptake in these lesions was also investigated. To our knowledge such dose-response data for 223Ra-dichloride therapy has not previously been published. Furthermore, the potential for a single pre-treatment assessment of 18F-fluoride uptake to predict lesion response to 223Ra-dichloride treatment and to provide the basis for a surrogate dose-response relationship was investigated.
Materials and Methods
Data are presented from a post-hoc analysis of a phase I open-label clinical trial (NCT00667537) of 223Ra-dichloride in patients with bone metastases due to CRPC. Six patients with bone metastases from castration-resistant prostate cancer, as determined by 99mTc-MDP bone scintigraphy no less than 6 weeks prior to treatment, were included in this study. Of the six patients recruited, five also underwent 18F-fluoride PET/CT imaging. Only these five patients were included in this post-hoc analysis.
Patients were scheduled to receive 100 kBq/kg of 223Ra-dichloride intravenously at baseline (therapy 1) and after 6 weeks (therapy 2). Following a revision of the primary standard for radionuclide calibrators [18, 19] it was retrospectively acknowledged that in fact patients received 110 kBq/kg. 18F-fluoride PET/CT imaging was performed at baseline up to 3 weeks before the first administration of 223Ra-dichloride, at 6 weeks just prior to the second administration of 223Ra-dichloride and at 12 weeks to assess treatment response. The mean age of patients that received both 223Ra-dichloride and 18F-fluoride was 63.2 years (range, 57 to 70 years). Normal 223Ra organ dosimetry in these patients has previously been reported [8]. A description of the changes in 18F-fluoride uptake, concluding that 18F-fluoride could be used as a biomarker to monitor response to 223Ra-dichloride has also been previously reported [20] as were eligibility criteria [8, 20]. Approval for the study was obtained from the appropriate research ethics committees and the national Administration of Radioactive Substances Advisory Committee, and all patients provided written informed consent.
99mTc-MDP Imaging
Whole-body 99mTc-MDP images were acquired 3 hours after injection of 600 MBq 99mTc-MDP. Imaging was performed on a Forte gamma camera (Philips Medical Systems, Cleveland, OH, US) using a low energy high resolution collimator according to a protocol previously detailed. Whole-body views were acquired for approximately 20 minutes using a matrix size of 256 x 1024. Imaging was performed using an energy window set at 140 +/- 10% keV.
223Ra Imaging
Sets of whole-body 223Ra images were acquired in conjunction with each 223Ra-dichloride administration. Imaging was performed on a Forte gamma camera (Philips Medical Systems, Cleveland, OH, US) using a medium energy general purpose collimator according to a protocol previously detailed [6]. Whole-body and spot views were acquired for approximately 30 minutes each using matrix sizes of 256 x 1024 and 256 x 256 pixels respectively. Imaging was performed using an energy window set at 82 +/- 20% keV. The first scan was acquired within 0 – 4 h post-injection and subsequent scans were acquired at 24, 48, 96 and 144 h post-injection. All images were acquired post-void to reduce artefacts due to radioactivity in the bladder. The count rate for all measurements was sufficiently low (< 1 kcts/s over the entire spectrum counted by the camera) that no correction was required for detector dead-time.
223Ra Image Analysis & Dosimetry
Regions of interest (ROIs) around 29 metastatic bone lesions were initially delineated on the anterior and posterior 99mTc-MDP whole-body images. Lesions corresponding to visible sites of increased 223Ra uptake were selected. For superscan patients (2/5), selected whole vertebrae were delineated as were the femoral heads. In one case an increased area of uptake in the right tibia was also selected. The 99mTc images were manually registered to the 223Ra images using Hermes Hybrid Viewer (Hermes Medical, Sweden) to rigidly transform the images, thereby allowing transfer of the ROIs to the 223Ra whole-body and static images. The ROIs were then expanded in order to reduce the effect of spill-out due to partial volume effects and to limit the impact of any misregistration. Similarly to the work of Pacilio et al [11], a background correction for lesion uptake was determined from a region placed adjacent to the lesion ROI using the technique described by Buijs et al [21] whereby the background region was used to calculate an average background count per pixel. ROI delineation was performed by four observers comprising 3 experienced dosimetry physicists and 1 dual accredited consultant radiologist / nuclear medicine physician. The same consultant radiologist confirmed that all selected lesions were metastatic in nature.
Activity quantification was performed on the patient images according to either the conjugate view method or the single view effective point source method [22]. The conjugate view method was applied to those lesions situated close to a point equidistant between the anterior and posterior patient surface at the level of the lesion. The single view method was used to quantify uptake in lesions close to either the anterior or posterior surface which were not visible on the opposite view. Image counts were converted to activity using measured sensitivity and attenuation correction factors for 223Ra as previously detailed [6]. The depth of a lesion within a patient was measured on the CT scan associated with the baseline 18F-fluoride PET scan.
Metastatic bone lesion volumes were measured by applying a Fuzzy Locally Adaptive Bayesian (FLAB) segmentation algorithm, initially developed for accurate outlining of PET data for radiotherapy planning, to the corresponding lesion sites on the baseline 18F-fluoride PET images. [23].
The decay constant λ associated with the effective half-life, t1/2 eff and initial activity, A0, of 223Ra in identified lesions was calculated by fitting a single exponential to the time activity curve. The fit was constrained such that the effective half-life could not be greater than the physical half-life. Cumulated activity was calculated according to,
| (2) |
The total mean absorbed dose, D, to the target region included contributions from the decay of the daughter products of 223Ra (219Rn, 215Po, 211Pb, 211Bi, 211Po, 207Tl) The absorbed doses delivered to metastatic lesions by each isotope were calculated using self-irradiation S-factors for unit density spheres [24], scaled for an assumed bone density of 1.3 g ml-1 [25]. The absorbed dose is then given by
| (3) |
where Si is the self-irradiation S-Factor for isotope i and spherical volume Vs; and Vt is the lesion volume as determined by PET imaging. No radiation weighting factor was used to adjust the reported absorbed doses. It was assumed that the biodistribution of the daughter isotopes was the same as the 223Ra parent isotope [26].
18F-fluoride Imaging
Repeated 18F-fluoride PET imaging was performed to assess treatment response. At each imaging time point, total body (vertex to feet) PET imaging was performed 1 h following injection of 250 MBq 18F-fluoride on a Gemini PET/CT scanner (Philips Medical Systems, Cleveland, OH, US). Data were acquired for 3.5 min per bed position following a low-current (50 mAs) CT scan performed for attenuation correction and lesion localisation.
18F-fluoride Image Analysis
18F-fluoride PET scans were assessed semi-quantitatively for evidence of response at the 6 and 12-week time points compared to the baseline pre-treatment scan. In the case of one patient, the third PET scan was rejected for analysis due to technical problems with the image acquisition. FLAB derived outlines of the 29 lesions identified on 99mTc-MDP imaging were produced using an IDL routine incorporated into a Symbia.Net workflow (Siemens Healthcare, Germany). These were used to measure the SUVmean as well as the volume of each lesion. SUVmean values were corrected for partial volume effects as a function of lesion volume (Vt) using the following expression for recovery coefficient (RC) as described by Kessler et al [27] where σ is the standard deviation of a Gaussian function describing the point spread function, determined by fitting equation 4 to the measured recovery curve associated with an IEC NEMA image quality phantom filled with 18F. For the PET imaging described in this study a value of σ = 4.3 mm was used.
| (4) |
The patient body mass and lesion volumes were used to convert values of SUVmean to percentage injected activity (%IA).
| (5) |
Percentage changes in SUVmean relative to the baseline PET study were used to define the response to 223Ra-dichloride therapy on a lesion by lesion basis. Changes were measured at both 6 weeks and 12 weeks after baseline. Response to treatment after 6 weeks was plotted as a function of the 223Ra absorbed dose due to the first therapy. Response to treatment after 12 weeks was plotted as a function of the total 223Ra absorbed dose.
18F-fluoride and 223Ra Comparison
To test the hypothesis that 18F-fluoride uptake is predictive of both, 223Ra uptake and subsequent lesion response, the %IA of 18F in each lesion at baseline was compared to %IA of 223Ra at the time of the first therapy administration. Additionally, the %IA of 18F in each lesion at 6 weeks post therapy 1 was compared to %IA of 223Ra at the time of the second therapy administration.
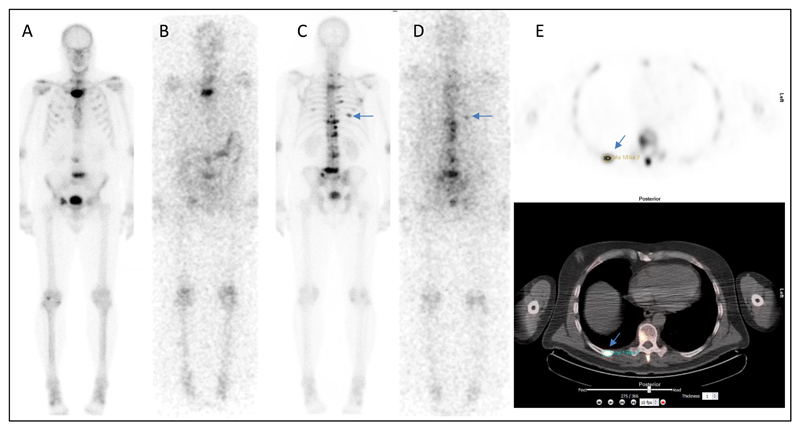
The SUVmean at baseline was compared to the calculated absorbed dose due to 223Ra uptake in lesions. In addition, response to treatment at 6 weeks and 12 weeks post 223Ra-dichloride was plotted as a function of baseline SUVmean.. Figure 1 shows an example of all imaging (223Ra WB imaging, 99mTc WB imaging and 18F-fluoride PET/CT.)
Figure 1.
Examples of acquired images for patient 2. A) and C) show 99mTc-MDP whole-body imaging. B) and D) show 223Ra WB imaging 6 days after therapy administration. Arrows indicate uptake in posterior right rib. E) 18F-Fluoride PET/CT imaging at baseline including FLAB outlining of posterior right rib lesion used for volume estimation.
Uncertainty Analysis
Data were analysed using Graphpad Prism software. The 223Ra lesion time activity curves were fitted with a mono-exponential function to estimate values for A0 and λ as well as u(A0) and u(λ), the uncertainty in those estimates, and r(A0, λ) the covariance of A0 and λ.
The overall uncertainty in the cumulated activity was calculated according to equation 6 [28]. The fractional uncertainty in the cumulated activity was used as an estimate of the fractional uncertainty in the absorbed dose estimate.
| (6) |
The uncertainties associated with the 18F-Fluoride uptake measurements were derived from the results of Lin et al [29] who reported a coefficient of variation of 6.6% for lesion SUVmean.
Statistical Analysis
The distribution of absorbed doses and the log-transform of this distribution were tested for normality using the D’Agostino-Pearson criteria. The Kruskal-Wallis test and its associated p-value were used to compare the dose distributions resulting from the measurements of different observers.
Spearman rank correlation coefficients and their associated p-values were calculated between the %IA of 18F-fluoride and 223Ra in the lesions as well as between the lesion SUVmean and the 223Ra absorbed dose. Non-linear regression was performed to fit a log-linear function to the absorbed dose-response data as well as to the relationship between baseline 18F-fluoride SUVmean and subsequent percentage changes in SUVmean following therapy. The fits were weighted by the uncertainty associated with the response measurements. Coefficients of determination were calculated for these fits.
Results
223Ra Image Analysis & Dosimetry
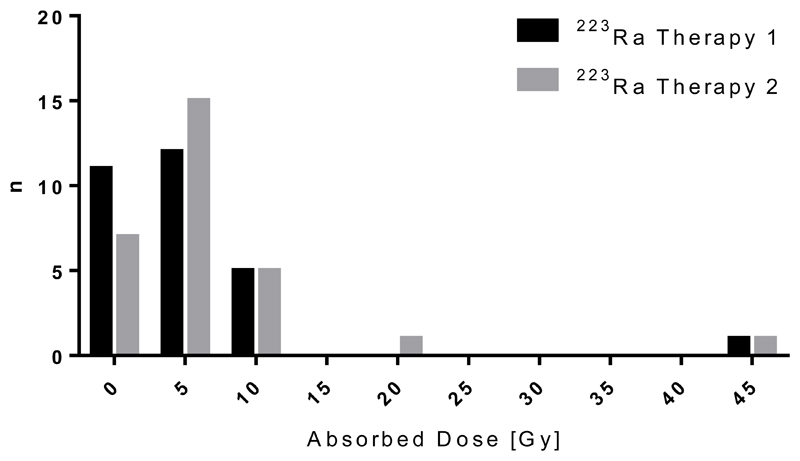
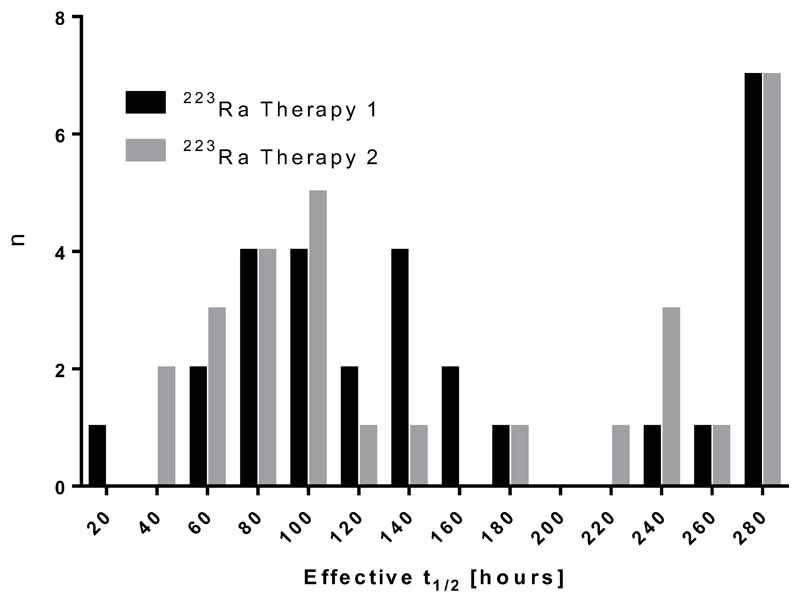
A total of 29 metastatic lesions were identified on co-registered 99mTc-MDP and 223Ra WB images. The dosimetry results for these lesions are summarised in Table 1. Absorbed doses ranged from 0.6±0.3 Gy to 44.1±16.4 Gy with a median absorbed dose of 4.1 Gy (see Figure 2). There was no significant difference in the observed absorbed doses determined from the lesion outlines of each operator (p=0.58). The log-transform of each distribution was found to be normally distributed. The median uncertainty in the lesion dose calculations (±1.0 Gy) was more than an order of magnitude less than the range of the calculated values. Approximately 80% of the absorbed dose in each lesion was due to alpha and beta particles emitted from the daughter isotopes. A full breakdown of the relative contribution of each isotope is given in Table 2. The effective half-life of 223Ra in lesions ranged from 44 hours to 274 hours (the physical half-life) with a median effective half-life of 136 hours (See Figure 3)).
Table 1. 223Ra Lesion Dosimetry.
List of lesions, their location and estimated absorbed doses. * no uncertainty estimate of effective half-life was possible where the monoexponential fit reached the constraint of 223Ra physical half-life.
| Patient number | Lesion Site | Vol [ml] | Therapy | Absorbed Dose [Gy] (223Ra and daughter isotopes) | Effective Half-life [hrs] |
|---|---|---|---|---|---|
| 1 | T5 vertebra | 5.9 | 1 | 10.2 ± 1.5 | 273.6 ± 0.0* |
| 2 | 11.3 ± 2.3 | 237.5 ± 180.9 | |||
| Left femoral head | 29.1 | 1 | 3.6 ± 0.7 | 273.6 ± 0.0* | |
| 2 | 4.7 ± 0.2 | 273.6 ± 0.0* | |||
| C4 vertebra | 8.5 | 1 | 10.9 ± 1.6 | 273.6 ± 0.0* | |
| 2 | 11.2 ± 1.2 | 273.6 ± 0.0* | |||
| Right SI joint | 6.6 | 1 | 6.2 ± 1.1 | 273.6 ± 0.0* | |
| 2 | 8.5 ± 0.4 | 181.7 ± 23.8 | |||
| S1 vertebra | 7.7 | 1 | 3.8 ± 0.6 | 273.6 ± 0.0* | |
| 2 | 4.3 ± 0.9 | 273.6 ± 0.0* | |||
| L3 vertebral process | 3.7 | 1 | 7.3 ± 3.3 | 157.0 ± 141.0 | |
| 2 | 10.0 ± 1.7 | 273.6 ± 0.0* | |||
| Left pelvis (ischeum) | 5.5 | 1 | 3.4 ± 1.0 | 273.6 ± 0.0* | |
| 2 | 4.4 ± 1.4 | 273.6 ± 0.0* | |||
| 2 | L5 vertebra | 41.2 | 1 | 6.2 ± 0.3 | 159.9 ± 23.7 |
| 2 | 4.2 ± 0.7 | 127.4 ± 47.3 | |||
| T12 vertebra | 1.2 | 1 | 43.8 ± 11.2 | 143.0 ± 100.1 | |
| 2 | 44.1 ± 16.4 | 66.6 ± 50.6 | |||
| R pelvis | 7.1 | 1 | 4.9 ± 0.8 | 83.8 ± 24.9 | |
| 2 | 17.5 ± 2.9 | 218.9 ± 198.1 | |||
| L3 vertebra | 5.6 | 1 | 10.4 ± 0.9 | 108.5 ± 22.1 | |
| 2 | 5.5 ± 0.5 | 102.6 ± 19.2 | |||
| R Rib9 | 2.8 | 1 | 6.4 ± 1.2 | 92.7 ± 36.7 | |
| 2 | 4.0 ± 0.9 | 59.5 ± 24.5 | |||
| Sternum | 18.7 | 1 | 6.0 ± 0.4 | 91.0 ± 12.2 | |
| 2 | 5.2 ± 0.2 | 91.4 ± 7.7 | |||
| 3 | Right Skull | 39.9 | 1 | 1.6 ± 0.3 | 135.7 ± 90.1 |
| 2 | 1.1 ± 0.2 | 94.6 ± 30.6 | |||
| Left Skull | 1.9 | 1 | 1.5 ± 1.0 | 84.4 ± 153.3 | |
| 2 | 1.5 ± 0.7 | 136.9 ± 162.4 | |||
| T11 vertebra | 9.2 | 1 | 4.9 ± 0.6 | 76.3 ± 23.2 | |
| 2 | 5.8 ± 1.2 | 83.8 ± 35.0 | |||
| L3 vertebra | 10.8 | 1 | 1.5 ± 0.7 | 141.2 ± 282.8 | |
| 2 | 5.3 ± 1.8 | 76.5 ± 47.8 | |||
| L4 vertebra | 22.9 | 1 | 1.7 ± 0.8 | 61.1 ± 69.7 | |
| 2 | 2.6 ± 0.3 | 71.7 ± 17.1 | |||
| Right Rib 7 | 13.9 | 1 | 4.0 ± 1.3 | 92.0 ± 41.0 | |
| 2 | 2.7 ± 0.5 | 61.0 ± 20.2 | |||
| 4 | L2 vertebra | 39.4 | 1 | 0.6 ± 0.3 | 70.9 ± 51.7 |
| 2 | 1.0 ± 0.2 | 44.4 ± 20.3 | |||
| L3 vertebra | 56.4 | 1 | 1.0 ± 0.4 | 66.8 ± 37.8 | |
| 2 | 1.0 ± 0.2 | 45.0 ± 17.1 | |||
| T12 vertebra | 32.0 | 1 | 1.5 ± 0.1 | 178.3 ± 27.7 | |
| 2 | 1.2 ± 0.2 | 94.1 ± 7.7 | |||
| L femoral head | 68.9 | 1 | 1.4 ± 0.1 | 114.4 ± 25.6 | |
| 2 | 1.1 ± 0.1 | 78.3 ± 12.0 | |||
| R femoral head | 67.5 | 1 | 1.1 ± 0.2 | 94.0 ± 31.9 | |
| 2 | 1.0 ± 0.2 | 98.3 ± 34.9 | |||
| 5 | L1 vertebra | 45.5 | 1 | 1.8 ± 0.3 | 112.0 ± 43.6 |
| 2 | 2.5 ± 0.2 | 240.7 ± 81.9 | |||
| T11 vertebra | 42.3 | 1 | 2.2 ± 0.3 | 145.5 ± 53.2 | |
| 2 | 3.0 ± 0.0 | 232.0 ± 8.8 | |||
| Right Femoral Head | 59.0 | 1 | 8.1 ± 0.5 | 273.6 ± 0.0* | |
| 2 | 7.0 ± 0.5 | 273.6 ± 0.0* | |||
| Left Femoral Head | 105.0 | 1 | 3.6 ± 0.3 | 261.5 ± 87.3 | |
| 2 | 3.4 ± 0.0 | 257.2 ± 11.8 | |||
| Right tibia | 66.8 | 1 | 5.7 ± 0.9 | 168.2 ± 86.7 | |
| 2 | 8.3 ± 1.3 | 273.6 ± 0.0* |
Figure 2.
Histogram of lesion absorbed dose. Data are shown for 29 lesions measured from 5 patients.
Table 2. Relative absorbed dose from Ra223 and daughter isotopes.
Relative contribution of each isotope to lesion absorbed dose.
| Isotope | Contribution to total lesion dose [%] |
|---|---|
| Ra-223 | 21.3 |
| Rn-219 | 24.6 |
| Ra-215 | 26.9 |
| Pb-211 | 1.6 |
| Bi-211 | 23.9 |
| Po-211 | 0.1 |
| Tl-207 | 1.7 |
Figure 3.
Histogram of lesion effective half-life. Data are shown for 29 lesions measured from 5 patients.
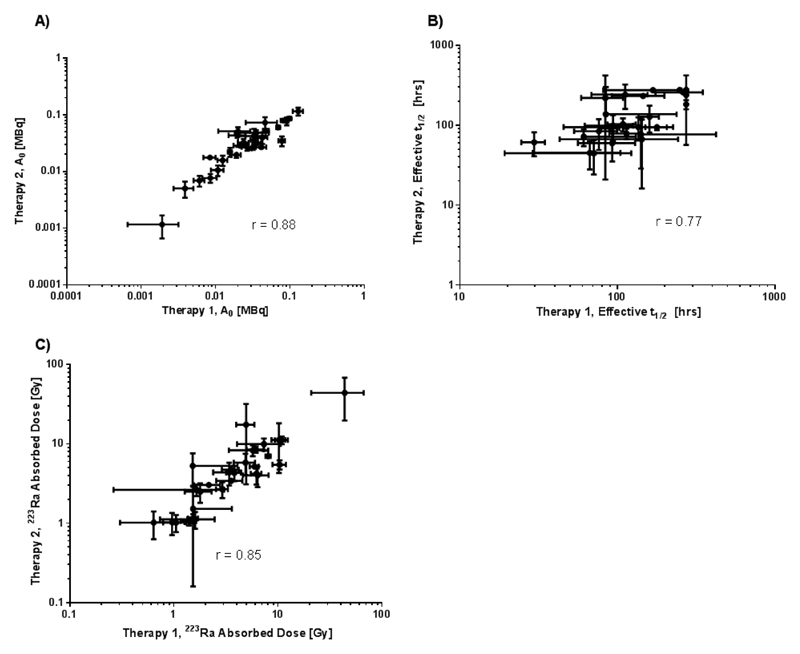
A significant high correlation was observed between lesion uptake (A0) at the time of the first therapy and the time of the second therapy 6 weeks later (r = 0.88, p < 0.0001). The relationship between the effective half-life of 223Ra measured at the two different time points was also significantly correlated (r = 0.77, p < 0.0001) as was the relationship between the lesion absorbed doses (r = 0.85, p < 0.0001). These relationships are shown in Figure 4.
Figure 4.
Relationship between estimated absorbed dose parameters for therapy 1 and therapy 2. A) Initial lesion uptake of 223Ra, B) of 223Ra lesion effective half-life and C) lesion absorbed dose.
223Ra – 18F-fluoride dose response relationship
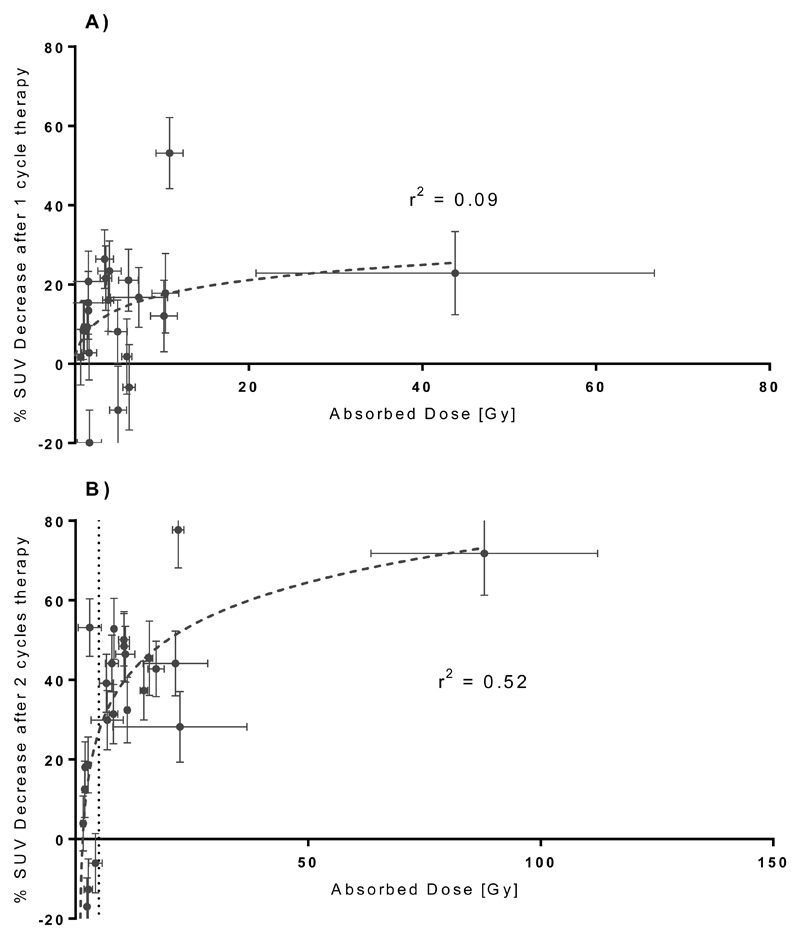
The relationship between lesion absorbed dose and the observed response as defined by percentage change in the 18F-fluoride SUVmean relative to baseline are shown in Figure 5. After 6 weeks there is no evident relationship between lesion dose and response (r2 = 0.07). However after 12 weeks there is an apparent log-linear relationship (r2 = 0.54) between the total absorbed dose from both therapies and the changes in the 18F-fluoride uptake. Based on the analysis of test-retest 18F-fluoride PET/CT imaging, Kurdziel et al [30] suggested a minimum percentage change of 21% should be used to identify real change in the SUVmean between two sequential scans of 18F-fluoride acquired 60 minutes post injection. For a change of > 21% in SUVmean of a lesion, a threshold of 5.0 Gy correctly identifies 94% of all responding lesions whilst no non-responding lesions are incorrectly identified as responding.
Figure 5.
Lesion response to 223Ra-dichloride treatment. (n=24 over 4 patients) A) Response to therapy 1 only assessed 6 weeks after baseline. B) Response to therapy 1 and 2 assessed 12 weeks after baseline.
18F-fluoride and 223Ra Comparison
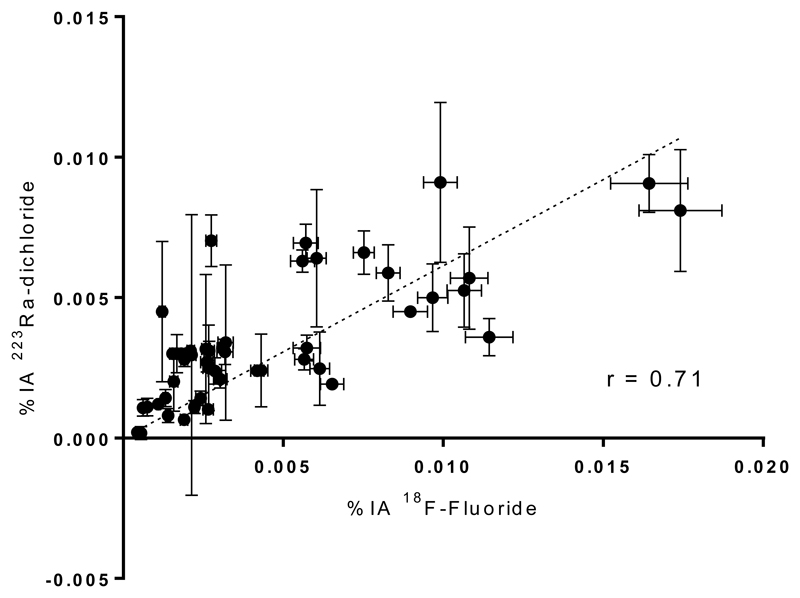
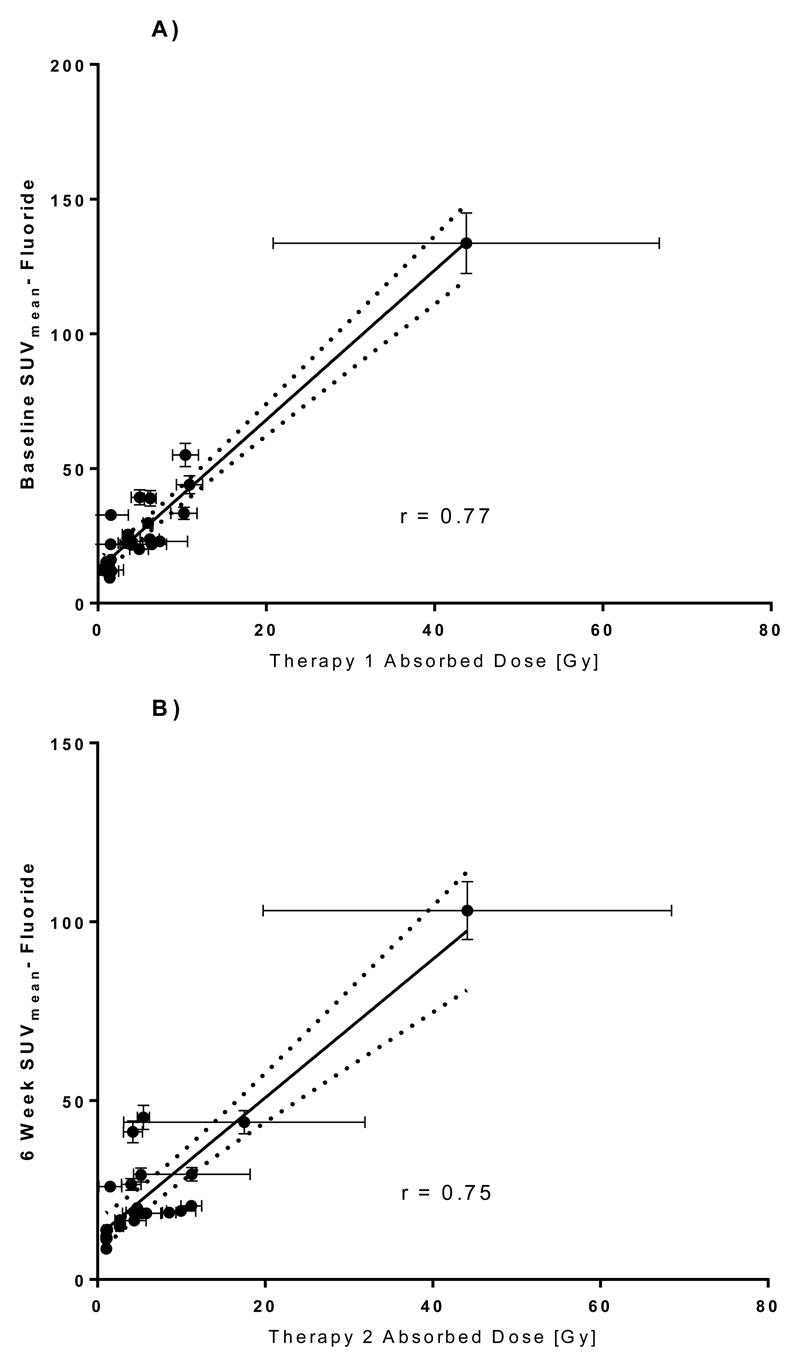
The relationship between %IA 223Ra and %IA 18F-fluoride is shown in Figure 6. A significant high correlation (r=0.71, p<0.0001) was observed. Furthermore, a high correlation is demonstrated between therapy 1 lesion absorbed dose and baseline 18F-fluoride SUVmean (r = 0.77, p<0.0001) as well as between therapy 2 lesion absorbed dose and 18F-fluoride SUVmean at 6 weeks (p<0.0001). (Figure 7.)
Figure 6.
Relationship between 223Ra uptake and 18F-Fluoride uptake. n = 58.
Figure 7.
Relationship between 223Ra absorbed dose to lesion and lesion 18F-fluoride SUVmean at the time of treatment. A) Dose from Therapy 1 vs Baseline PET. B) Absorbed dose from Therapy 2 vs 6 week PET.
18F-fluoride Image Analysis
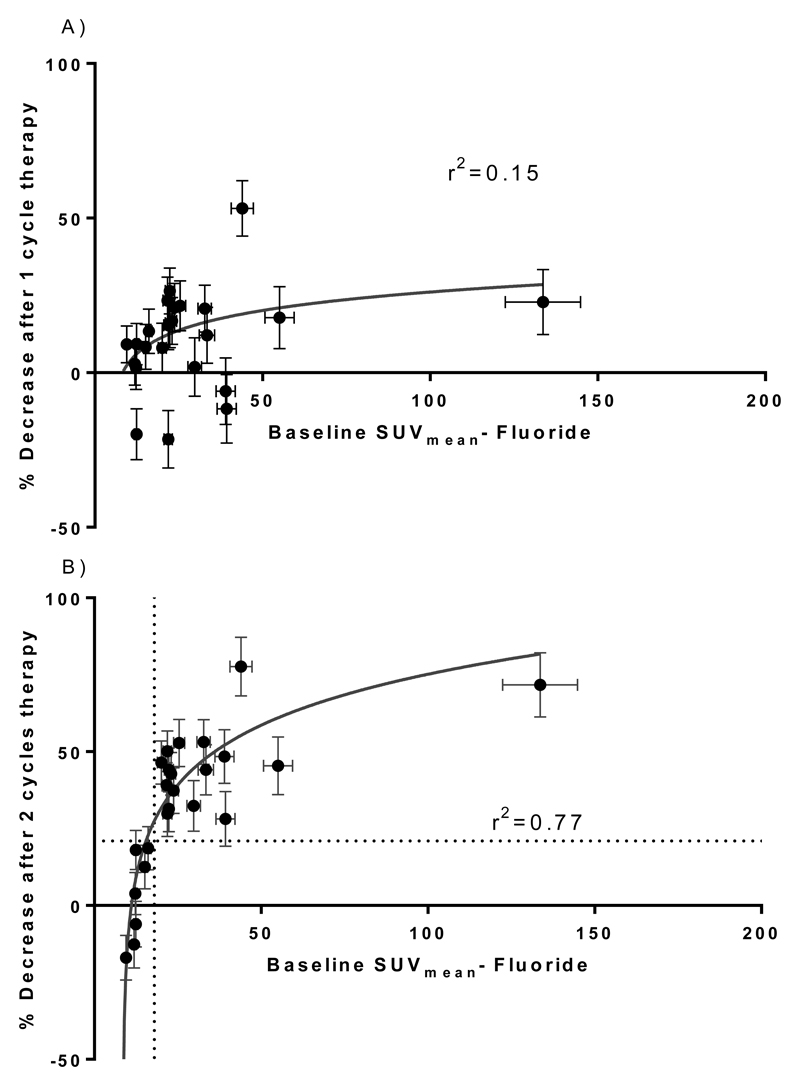
Figure 8 shows a log-linear dose response relationship between baseline SUVmean prior to treatment and the lesion responses as defined by percentage changes in SUVmean. In common with the 223Ra dose-response relationship, no significant relationship was observed 6 weeks after the first treatment. However at 12 weeks after the first treatment, a well-defined log linear relationship was observed (r2 = 0.77). If response is again defined as a change of >21% in the SUVmean of a lesion then a threshold between 17-20 partial volume corrected SUVmean correctly identifies 100% of all responding lesions whilst no non-responding lesions are incorrectly identified as responding.
Figure 8.
Relationship between baseline SUVmean and subsequent response to 223Ra dichloride. A) Changes following one cycle of 223Ra-dichloride. B) Changes following two cycles of 223Ra-dichloride. (n=24 over 4 patients)
Discussion
Absorbed doses in metastatic bone lesions ranged from 0.6 Gy to 44.1 Gy in this study. I.e. there is significant heterogeneity of absorbed dose across the patient population. For individual patients there was an average factor of 5.3 difference (range 2.5-11.0) between the maximum and minimum lesion dose. Emissions from the daughter isotopes of 223Ra contributed between 79% – 84% of this absorbed dose. The absorbed doses from 223Ra emissions only were 0.1 – 9.4 Gy which encompass the range observed in the study by Pacilio et al [11]. For deterministic cell killing effects using alpha emitters, a factor of 5 for relative biological effectiveness (RBE) has been suggested [31, 32]. However, this has not been validated in human studies and therefore no corrections for RBE were made to the dosimetry data presented in this study.
The limited count statistics associated with 223Ra whole-body imaging lead to uncertainties in the fitting of the time activity curves which in turn leads to the propagation of further uncertainty in the estimation of the absorbed dose. In this study the uncertainty associated with the absorbed dose estimates ranged from 0.03 Gy to 16.4 Gy. Nevertheless the median uncertainty in the lesion dose calculations (±1.0 Gy) was an order of magnitude less than the range of the calculated values and interobserver variation was not significant. No correlation was observed between uncertainty and lesion volume, nor between lesion absorbed dose and lesion volume. One way of reducing the uncertainty associated with these absorbed dose estimates could be to change the time points at which 223Ra images are acquired. In our study, the latest imaging time points were at 6 days after administration. Pacilio et al [11] imaged patients up to 24 days after administration, approximately 4 times the median effective half-life observed in our cohort and more in alignment with the MIRD recommendations for appropriate temporal sampling [22].
A further source of uncertainty associated with molecular radiotherapy dosimetry is the estimation of lesion volume. In this study, the FLAB segmentation technique was used to measure volume. This technique developed for radiotherapy outlining has been shown to be both accurate and reproducible [33]. The use of this particular algorithm reduces inter-operator variability in measurements of lesion volume to approximately 1% compared to ~15% for manual delineation [34]. In the example image shown in Figure 1, the PET derived outline of a rib lesion is seen to closely match the bony anatomy of the lesion demonstrated by the CT imaging. However, the use of a macroscopic volume is likely to give rise to a systematic error in the dose estimation since the true target volume, the endosteal layer, is an unknown fraction of the outlined volume.
Despite these uncertainties, a relationship between the absorbed dose to a lesion delivered over two cycles of therapy and the subsequent response as measured by 18F-fluoride imaging was demonstrated. The lack of an early response to 223Ra treatment has previously been observed and it was suggested that the flare phenomenon, whereby an increase in 18F-fluoride uptake is observed following treatment, may be partly responsible for this [35].
The hypothesised relationship between 18F-fluoride and 223Ra uptake was demonstrated (see Figure 6). The relationship is similar to the linear relationship between 223Ra and 99mTc-MDP uptake previously demonstrated by Pacilio et al [11]. A strong correlation between 18F-fluoride uptake and 223Ra absorbed dose was also observed, despite a wide range in the 223Ra lesion effective half-life.
The association between the 18F-fluoride uptake and 223Ra absorbed dose may therefore explain the apparent dose-response relationship between the SUVmean of a lesion measured prior to treatment with 223Ra-dichloride and the subsequent changes in SUVmean following two administrations of 223Ra-dichloride (Figure 8).
The potential for 18F-fluoride to act as a surrogate measure of 223Ra absorbed dose is appealing for a number of reasons. First, there is a higher coefficient of determination (r2 value) between the 18F-fluoride SUVmean and the lesion response (r2 = 0.77, Figure 8B) compared to that between the lesion absorbed dose and the lesion response (r2 = 0.52, Figure 5B). I.e. the 18F-fluoride uptake at baseline is a better indicator of the subsequent response than the absorbed dose estimate. This may reflect the greater uncertainty associated with the calculation of 223Ra absorbed dose from low count planar data. Second, the measurement of mean SUV within a lesion is an analysis technique more commonly used in routine clinical practice than the analysis of serial whole body images to calculate absorbed doses, although such dosimetry methodology is well established. Finally, the advantage of a surrogate measure for absorbed dose derived from a pre-treatment 18F-fluoride PET scan is that it represents prospective theragnostic information that could enable personalised treatment planning.
Earlier studies of 223Ra therapy have already demonstrated a relationship between the activity administered to a particular cohort of patients (e.g. 25 kBq/kg vs 50 kBq/kg) and the average change in pain index or PSA level across those cohorts [12, 13]. The wide range in the absorbed dose across multiple metastatic sites, along with the ability to image that dose distribution, either directly or through surrogate 18F-fluoride PET imaging, suggests that there is scope to further optimise the delivery of 223Ra-dichloride treatment at an individual level.
This study is limited by the number of patients recruited to the trial. In particular, the upper end of the dose-response curves could be better defined by additional data points. Nonetheless, if the relationship between initial 18F-fluoride and the subsequent response to therapy observed in this investigation can be replicated in a larger patient cohort, 18F-fluoride PET imaging can potentially provide a quantitative method for optimising the potential of 223Ra-dichloride treatment for individual patients.
Therefore future trials would need to address several issues. Whilst this study suggests that the individual lesion absorbed doses are predictive of subsequent changes observed with functional imaging, the relationship between absorbed dose and other clinically relevant endpoints including pain index, changes in ALP or PSA levels or prolonged survival should be investigated and quantified. This would further support the previous analysis of these patients by Cook et al [20] that described a qualitative association between changes in the average SUVmax of selected lesions and changes in ALP and PSA.
In this study a relationship between 18F-fluoride uptake, 223Ra absorbed dose and lesion response was demonstrated for two treatments of 110 kBq/kg. Further studies are required to investigate these results for alternative administration schedules including the current standard regimen of 6 x 55kBq/kg. The relationship between baseline uptake of 18F-fluoride and response to two treatments of 100 kBq/kg demonstrated that non-responding lesions could be identified prior to therapy. This leads to the hypothesis that a subset of patients who would benefit from increased activities of 223Ra-dichloride could be identified. Again, a suitably designed clinical trial would be required to test this hypothesis.
Conclusion
223Ra lesion dosimetry performed using planar WB and static images of 223Ra distribution show a wide range of absorbed doses across multiple sites of metastases. Evidence of a relationship between the 223Ra absorbed dose to a lesion and the subsequent response defined by serial 18F-Fluoride imaging was observed.
A relationship was also observed between the 18F-Fluoride uptake prior to 223Ra-dichloride treatment and the subsequent lesion response, indicating that 18F-fluoride SUVmean could potentially act as a predictor of lesion absorbed dose. This is likely due to the fact that uptake of 18F-fluoride in bone metastases is significantly correlated with both the corresponding uptake of 223Ra and the associated 223Ra absorbed dose. Larger trials are required to confirm these initial findings as well as to explore potential methodologies for optimising treatment.
Acknowledgements
NHS funding was provided to the NIHR Biomedical Research Centre at The Royal Marsden and the ICR. Data acquisition was funded by Bayer Healthcare Pharmaceuticals and Algeta ASA.
Funding: NHS funding was provided to the NIHR Biomedical Research Centre at The Royal Marsden and the ICR. Data acquisition was funded by Bayer Healthcare Pharmaceuticals and Algeta ASA.
Footnotes
Disclosure: Christopher C. Parker consults for Bayer Healthcare Pharmaceuticals and Algeta ASA.
Ethical approval: Informed consent was obtained from all individual participants in this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Christopher C. Parker, Department of Urology, Royal Marsden NHS Foundation Trust, Sutton, UK
Sue Chua, Department of Nuclear Medicine, Royal Marsden NHS Foundation Trust, Sutton, UK.
Glenn D. Flux, Joint Department of Physics, Royal Marsden NHS Foundation Trust, Sutton, UK & the Institute of Cancer Research, London, UK
References
- 1.Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nature reviews Clinical oncology. 2014;11:335–45. doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 2.Bodei L, Lam M, Chiesa C, Flux G, Brans B, Chiti A, et al. EANM procedure guideline for treatment of refractory metastatic bone pain. European journal of nuclear medicine and molecular imaging. 2008;35:1934–40. doi: 10.1007/s00259-008-0841-y. [DOI] [PubMed] [Google Scholar]
- 3.Florimonte L, Dellavedova L, Maffioli LS. Radium-223 dichloride in clinical practice: a review. European journal of nuclear medicine and molecular imaging. 2016;43:1896–909. doi: 10.1007/s00259-016-3386-5. [DOI] [PubMed] [Google Scholar]
- 4.Hobbs RF, Song H, Watchman CJ, Bolch WE, Aksnes AK, Ramdahl T, et al. A bone marrow toxicity model for Ra-223 alpha-emitter radiopharmaceutical therapy. Phys Med Biol. 2012;57:3207–22. doi: 10.1088/0031-9155/57/10/3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, et al. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clinical Cancer Research. 2005;11:4451–9. doi: 10.1158/1078-0432.ccr-04-2244. [DOI] [PubMed] [Google Scholar]
- 6.Hindorf C, Chittenden S, Aksnes AK, Parker C, Flux GD. Quantitative imaging of Ra-223-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nuclear medicine communications. 2012;33:726–32. doi: 10.1097/MNM.0b013e328353bb6e. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi A, Miwa K, Sasaki M, Baba S. A Monte Carlo study on (223)Ra imaging for unsealed radionuclide therapy. Medical physics. 2016;43:2965. doi: 10.1118/1.4948682. [DOI] [PubMed] [Google Scholar]
- 8.Chittenden SJ, Hindorf C, Parker CC, Lewington VJ, Pratt BE, Johnson B, et al. A Phase 1, Open-Label Study of the Biodistribution, Pharmacokinetics, and Dosimetry of 223Ra-Dichloride in Patients with Hormone-Refractory Prostate Cancer and Skeletal Metastases. J Nucl Med. 2015;56:1304–9. doi: 10.2967/jnumed.115.157123. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Kaneta T, Takano S, Sugiura M, Kawano T, Hino A, et al. Pharmacokinetics of single dose radium-223 dichloride (BAY 88-8223) in Japanese patients with castration-resistant prostate cancer and bone metastases. Ann Nucl Med. 2016;30:453–60. doi: 10.1007/s12149-016-1093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrasquillo JA, O'Donoghue JA, Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, et al. Phase I pharmacokinetic and biodistribution study with escalating doses of (2)(2)(3)Ra-dichloride in men with castration-resistant metastatic prostate cancer. European journal of nuclear medicine and molecular imaging. 2013;40:1384–93. doi: 10.1007/s00259-013-2427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacilio M, Ventroni G, De Vincentis G, Cassano B, Pellegrini R, Di Castro E, et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting Ra-dichloride. European journal of nuclear medicine and molecular imaging. 2015 doi: 10.1007/s00259-015-3150-2. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson S, Strang P, Aksnes AK, Franzen L, Olivier P, Pecking A, et al. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. European Journal of Cancer. 2012;48:678–86. doi: 10.1016/j.ejca.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Parker CC, Pascoe S, Chodacki A, O'Sullivan JM, Germa JR, O'Bryan-Tear CG, et al. A Randomized, Double-Blind, Dose-Finding, Multicenter, Phase 2 Study of Radium Chloride (Ra 223) in Patients with Bone Metastases and Castration-Resistant Prostate Cancer. Eur Urol. 2013;63:189–97. doi: 10.1016/j.eururo.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Segall GM. PET/CT with sodium 18F-fluoride for management of patients with prostate cancer. J Nucl Med. 2014;55:531–3. doi: 10.2967/jnumed.113.133546. [DOI] [PubMed] [Google Scholar]
- 15.Mick CG, James T, Hill JD, Williams P, Perry M. Molecular imaging in oncology: (18)F-sodium fluoride PET imaging of osseous metastatic disease. AJR American journal of roentgenology. 2014;203:263–71. doi: 10.2214/AJR.13.12158. [DOI] [PubMed] [Google Scholar]
- 16.Piert M, Zittel TT, Becker GA, Jahn M, Stahlschmidt A, Maier G, et al. Assessment of porcine bone metabolism by dynamic [F-18]fluoride ion PET: Correlation with bone histomorphometry. J Nucl Med. 2001;42:1091–100. [PubMed] [Google Scholar]
- 17.Neuman WF, Hursh JB, Boyd J, Hodge HC. ON THE MECHANISM OF SKELETAL FIXATION OF RADIUM. Annals of the New York Academy of Sciences. 1955;62:125–36. doi: 10.1111/j.1749-6632.1955.tb35369.x. [DOI] [Google Scholar]
- 18.Keightley J, Pearce A, Fenwick A, Collins S, Ferreira K, Johansson L. Standardisation of 223Ra by liquid scintillation counting techniques and comparison with secondary measurements. Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine. 2014;95C:114–21. doi: 10.1016/j.apradiso.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Zimmerman BE, Bergeron DE, Cessna JT, Fitzgerald R, Pibida L. Revision of the NIST Standard for (223)Ra: New Measurements and Review of 2008 Data. Journal of research of the National Institute of Standards and Technology. 2015;120:37–57. doi: 10.6028/jres.120.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook GJR, Parker C, Chua S, Johnson B, Aksnes AK, Lewington VJ. F-18-fluoride PET: changes in uptake as a method to assess response in bone metastases from castrate-resistant prostate cancer patients treated with Ra-223-chloride (Alpharadin) EJNMMI research. 2011;1 doi: 10.1186/2191-219X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buijs WCAM, Siegel JA, Boerman OC, Corstens FHM. Absolute organ activity estimated by five different methods of background correction. J Nucl Med. 1998;39:2167–72. [PubMed] [Google Scholar]
- 22.Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40:37s–61s. [PubMed] [Google Scholar]
- 23.Hatt M, Cheze le Rest C, Turzo A, Roux C, Visvikis D. A fuzzy locally adaptive Bayesian segmentation approach for volume determination in PET. IEEE Trans Med Imaging. 2009;28:881–93. doi: 10.1109/TMI.2008.2012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stabin MG, Siegel JA. Physical models and dose factors for use in internal dose assessment. Health Phys. 2003;85:294–310. doi: 10.1097/00004032-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Basic anatomical and physiological data: The skeleton. Annals of the ICRP. 1995;25:1–80. doi: 10.1016/S0146-6453(00)80004-4. [DOI] [PubMed] [Google Scholar]
- 26.Hindorf C. Dosimetry for radionuclide therapy with 223Ra-Cl2 with the biokinetics for the daughters measured separately. In preparation. [Google Scholar]
- 27.Kessler RM, Ellis JR, Eden M. Analysis of emission tomographic scan data - limitations imposed by resolution and background. journal of computer assisted tomography. 1984;8:514–22. doi: 10.1097/00004728-198406000-00028. [DOI] [PubMed] [Google Scholar]
- 28.BIPM, IEC, IFCC, ILAC, ISO, IUPAC et al. Evaluation of measurement data - Guide to the expression of uncertainty in measurement. Joint Committee for Guides in Metrology (JCMG) 2008;101 [Google Scholar]
- 29.Lin C, Bradshaw TJ, Perk TG, Harmon S, Eickhoff J, Jallow N, et al. Repeatability of Quantitative 18F-NaF PET: A Multicenter Study. J Nucl Med. 2016 doi: 10.2967/jnumed.116.177295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurdziel KA, Shih JH, Apolo AB, Lindenberg L, Mena E, McKinney YY, et al. The kinetics and reproducibility of 18F-sodium fluoride for oncology using current PET camera technology. J Nucl Med. 2012;53:1175–84. doi: 10.2967/jnumed.111.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sgouros G, Roeske JC, McDevitt MR, Palm S, Allen BJ, Fisher DR, et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med. 2010;51:311–28. doi: 10.2967/jnumed.108.058651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feinendegen LE, McClure JJ. Meeting report - Alpha-emitters for medical therapy - Workshop of the United States Department of Energy - Denver, Colorado, May 30-31, 1996. Radiation Research. 1997;148:195–201. doi: 10.2307/3579579. [DOI] [Google Scholar]
- 33.Hatt M, Le Rest CC, Albarghach N, Pradier O, Visvikis D. PET functional volume delineation: a robustness and repeatability study. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38:663–72. doi: 10.1007/s00259-010-1688-6. [DOI] [PubMed] [Google Scholar]
- 34.Hatt M, Rest CC-L, Aboagye EO, Kenny LM, Rosso L, Turkheimer FE, et al. Reproducibility of (18)F-FDG and 3 '-Deoxy-3 '-(18)F-Fluorothymidine PET Tumor Volume Measurements. Journal of Nuclear Medicine. 2010;51:1368–76. doi: 10.2967/jnumed.110.078501. [DOI] [PubMed] [Google Scholar]
- 35.Kairemo K, Joensuu T. Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer-Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics. 2015;5:413–27. doi: 10.3390/diagnostics5040413. [DOI] [PMC free article] [PubMed] [Google Scholar]