Abstract
Purpose
The Intergroup Exemestane Study (IES), an investigator-led study in 4724 postmenopausal patients with early breast cancer (ISRCTN11883920), has previously demonstrated that switching adjuvant endocrine therapy after 2-3 years tamoxifen to exemestane is associated with clinically relevant improvements in efficacy. Here we report the final efficacy analyses of this cohort.
Patients and methods
Patients who remained disease-free after 2-3 years of adjuvant tamoxifen were randomized to continue tamoxifen (T) or switch to exemestane (E) to complete a total of 5 years adjuvant endocrine therapy. Given the large number of non-breast cancer deaths now reported, breast-cancer-free survival (BCFS), censoring intercurrent deaths, is the primary survival endpoint of interest. Analyses focus on patients with ER positive (+) or unknown tumors (n=4599).
Results
At the time of data snapshot, median follow-up was 120 months. In the ER+/unknown population, 1111 BCFS events were observed, 508/2294 (22.1%) and 603/2305 (26.2%) in E and T groups respectively, corresponding to absolute difference (E-T) at 10 years of 4.0% (95%CI 1.2%-6.7%), with hazard ratio (HR) of 0.81 (95% CI 0.72-0.92) favoring E. This difference remained in multivariable analysis adjusting for nodal status, prior use of HRT and chemotherapy (HR=0.80 (95%CI 0.71-0.90); p<0.001).
A modest improvement in overall survival was seen with E, with absolute difference (E-T) at 10 years in the ER+/unknown population of 2.1% (95%CI -0.5%-4.6%), HR=0.89 (95%CI 0.78-1.01, p=0.08). For the ITT population, absolute difference was 1.6% (95%CI -0.9%-4.1%), HR=0.91 (95%CI 0.80-1.03, p=0.15).
Conclusions
The IES and contemporaneous studies have established that a strategy of switching to an aromatase inhibitor after 2-3 years of tamoxifen can lead to sustained benefits in terms of reduction of disease recurrence and breast cancer mortality.
Introduction
Despite improvements in adjuvant treatment, breast cancer remains the most frequent cause of cancer-related death in women, with approximately 508,000 deaths reported worldwide in 20111. For patients diagnosed with ER positive disease, risk of disease relapse remains for over 15 years after initial diagnosis, with recent research demonstrating the cumulative risk of relapse at 15 years to be comparable to that of ER negative patients in those receiving chemotherapy2.
Aromatase inhibitors reduce recurrence rates and 10-year breast cancer mortality rates compared with tamoxifen, but the optimal way to schedule aromatase inhibitors is still debated3.
The Intergroup Exemestane Study (IES) was an investigator-led, Pfizer sponsored trial assessing the impact on disease related outcome, adverse events, and quality of life of switching to exemestane after 2-3 years of tamoxifen compared with continuing to 5 years of tamoxifen4–11. The most recent update of efficacy analyses published in 2012 (data snapshot December 7, 2009) after a median follow-up of 91 months demonstrated that the highly statistically significant benefit of switching to exemestane on disease-free survival observed at initial publication was maintained, and this translated to a modest improvement in overall survival6.
IES was the first trial published to describe the benefits of switching from tamoxifen to an AI (exemestane) at 2-3 years, and was one of the pivotal generation of trials assessing the role of aromatase inhibitors in combination with or as a replacement for standard tamoxifen treatment3. Whether the strategy results in long-term sustained improvement in DFS or survival remains controversial, although our previous report suggested that his was the case6.
Recent analyses of the ATAC trial have sought to identify clinical and biological factors associated with disease relapse after completion of endocrine therapy12,13. Nodal involvement and tumor size are the most important clinical factors for predicting relapse both during and post treatment completion in ER positive breast cancer patients14,15. The other aim of this study, therefore, was to establish which prognostic features were important in the IES trial, which employed a ‘switching’ strategy, especially after the end of endocrine therapy.
Here we present the final efficacy analysis of the IES, along with exploratory analyses investigating clinical factors affecting the risk of distant relapse after completion of endocrine therapy.
Methods
Details of trial design, eligibility criteria and study procedures have been presented previously4–6. Briefly, eligible patients were post-menopausal women with ER+/unknown primary invasive breast cancer who remained disease free and on treatment after 2-3 years of tamoxifen. At randomisation women were allocated to continue tamoxifen (20 mg or [30 mg in Denmark] daily) or to switch to exemestane (25 mg daily) for the remainder of the 5 year endocrine therapy period. Timing of analyses was pre-planned, triggered by the last patient randomised reaching their 10 year follow-up. The current analysis includes all data received as of the 4th September 2013.
Efficacy analyses presented here have been performed on the main IES analysis population, which includes patients whose tumors were ER+ve (4052, 85.8%) plus those whose ER status remains unknown (547, 11.6%). Analyses exclude 125 patients (2.6%) with ER-negative disease, who would not have been eligible for the trial had their receptor status been known at trial entry. Intention to treat (ITT) analysis of OS is included for completeness.
Statistical analysis
The primary endpoint of the IES was DFS, defined as time from randomisation to local or distant breast cancer recurrence, new primary breast cancer or death without disease relapse (intercurrent death). As reported previously6, the proportion of patients experiencing intercurrent death has increased as the IES population ages, decreasing the sensitivity of DFS to detect differences between treatments in breast cancer outcome. Therefore we now regard breast cancer free survival (BCFS) in which intercurrent deaths are censored, as providing a more direct estimate of the treatment effect on breast cancer outcome in the long term. Other secondary endpoints presented include overall survival (OS, defined as time from randomisation to death from any cause), breast cancer specific survival (BCSS, defined as time from randomisation to breast cancer death (including death from unknown cause and other cause after recurrence), time to contralateral breast cancer (CLB, defined as time to contralateral breast cancer with patients censored at time of non-breast second primary cancer) and time to distant recurrence (TTDR, defined as time to distant recurrence or death from breast cancer or unknown cause without prior recurrence).
Kaplan-Meier plots, log-rank tests and Cox proportional hazards analyses were used to compare survival endpoints between randomised treatment groups. Multivariable analysis adjusting for known prognostic factors of nodal status, chemotherapy use and HRT use was also conducted.
For description of the sites of first distant recurrence the following groupings were used: visceral, bone or soft tissue/nodal, with patients assigned to multiple groups where relevant. Progression of metastatic disease subsequent to the initial distant recurrence was ignored. Events where site of recurrence was unknown were excluded from this part of the analysis.
The overall and age-related incidence of non-breast cancer second primary cancers was investigated to confirm the observation in our previous reports of a differential pattern according to randomised treatment5,6. For patients who reported more than one non-breast second primary cancer (n=6), the first reported event was included. Second primaries reported with no confirmed date of diagnosis were excluded (n=8).
Competing risks analyses were undertaken to assess the impact of randomised treatment on breast cancer events (local recurrence, distant recurrence, CLB, ipsilateral breast cancer (ILB), and BC death or death from unknown cause) whilst allowing for “competing risks” of intercurrent death and non-breast second primary cancer. Patients were included depending on which event occurred first; breast cancer event or competing risk event. Gray’s test was used to compare the two treatment groups with respect to breast cancer event in the presence of competing risks16.
Landmark analyses were performed to investigate the factors related to distant recurrence after the end of endocrine therapy. TTDR was the endpoint of interest, with survival time being partitioned at 2.5 years, representing the approximate end of endocrine therapy in IES. The impact of randomised treatment and a number of patient and tumor characteristics on TTDR after 2.5 years was assessed, both as single variables and together in a multivariable Cox proportional hazards model.
Full adverse event4–7,11 and quality of life9,10 data have been reported previously and are therefore not included in this manuscript, but we present here an updated estimate of post-treatment fracture incidence by treatment received. This includes all fractures occurring more than 6 months after treatment completion in patients who received at least 1 day of treatment, with events censored following relapse or new second primary cancer.
Analyses were performed using STATA version 13.2, (STATA Corp, College Station, TX). All statistical tests were two-sided with p<0.05 considered statistically significant.
Results
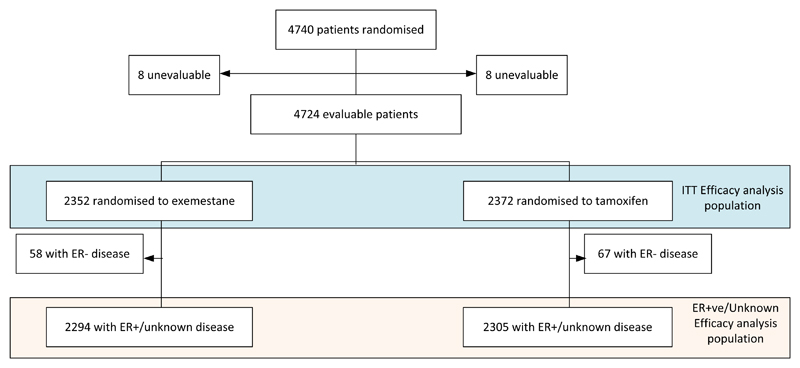
Between 1998 and 2003, 4724 evaluable patients were randomised from 366 sites in 37 countries. Of these, 4599 are known to be ER+ve or have unknown ER status (Figure 1).
Figure 1.
CONSORT diagram
Patient characteristics have previously been reported and were well balanced between treatment groups5,6. In summary, 2089/4724 (44.2%) patients were node positive and 1542/4724 (32.6%) patients had received adjuvant chemotherapy. Mean age at randomisation was 64.2 years (SD=8.2). At the time of data snapshot (04/09/2013) median follow-up in patients still known to be alive was 120.0 months (IQR: 114.8 to 122.0, range: 2.9 to 164.1), with current analysis based on over 39,000 women-years of follow-up. 74.7% of patients had at least 10 years follow-up or had previously died.
Efficacy
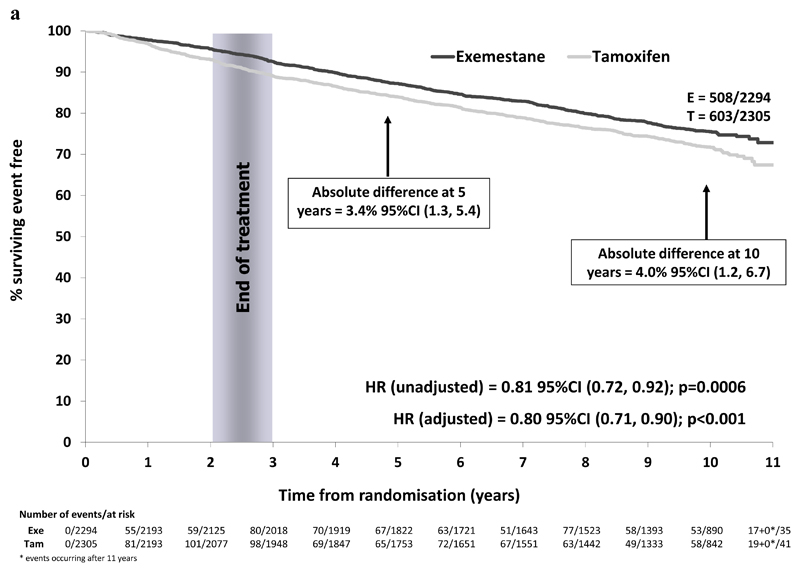
In the ER+ve/unknown population, 1111/4599 patients have experienced a BCFS event (exemestane 508/2294 (22.1%), tamoxifen 603/2305 (26.2%)). A reduction in risk of breast cancer related events was observed with an absolute difference at 10 years of 4.0% (95% CI 1.2% to 6.7%) and a hazard ratio of 0.81 (95% CI 0.72 to 0.92) in favor of switching to exemestane (Figure 2a).
Figure 2a.
Breast cancer free survival in the ER+/unknown population (N=4599)
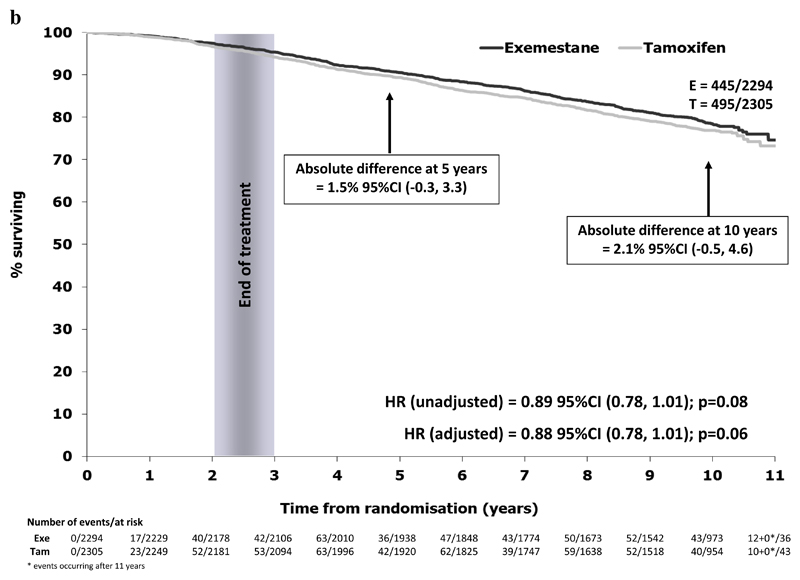
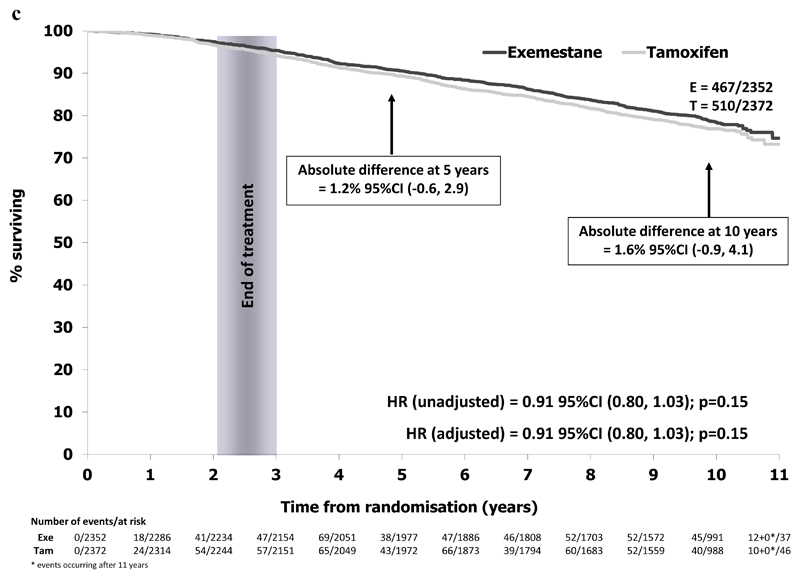
In the ER+ve/unknown population, 940/4599 patients have died (exemestane 445/2294 (19.4%), tamoxifen 495/2305 (21.5%)). A modest improvement in OS was seen with exemestane, with an absolute survival difference at 10 years of 2.1% (95% CI -0.5% to 4.6%) and a hazard ratio of 0.89 (95% CI 0.78 to 1.01) in favor of switching to exemestane, Figure 2b. The numerical difference in deaths was mainly seen in deaths due to breast cancer, with rates of intercurrent deaths similar between randomised treatment groups (Table 1). Results were similar when considering the ITT population (exemestane 467/2352 (19.9%), tamoxifen 510/2372 (21.5%), HR=0.91 (95% CI 0.80 to 1.03), Figure 2c.
Figure 2b.
Overall survival in the ER+/unknown population (N=4599)
Table 1. Efficacy events by treatment group in the ER+ve/unknown population (N=4599).
| Number of events contributing to endpoint of interest | ER+/unknown population |
||
|---|---|---|---|
| Exemestane (N=2294) | Tamoxifen (N=2305) | Total (N=4599) | |
| DFS First events | 650 (28.3%) | 742 (32.2%) | 1392 (30.3%) |
| Total BCFS events | 508 | 603 | 1111 |
| Distant recurrence | 369 | 420 | 789 |
| Local recurrence | 81 | 109 | 190 |
| Second primary breast cancer | 58 | 74 | 132 |
| Intercurrent death | 142 | 139 | 281 |
| All deaths | 445 (19.4%) | 495 (21.5%) | 940 (20.4%) |
| Breast cancer death | 263 | 310 | 573 |
| Death unknown cause | 40 | 46 | 86 |
| Death from other known cause | 142 | 139 | 281 |
| Other cancer | 40 | 60 | 100 |
| Vascular | 36 | 23 | 59 |
| Cardiac | 30 | 23 | 53 |
| Other | 36 | 33 | 69 |
| Distant recurrences | 403 (17.6%) | 469 (20.4%) | 872 (19.0%) |
| Distant recurrence to known site | 346 | 393 | 739 |
| Visceral only | 129 | 130 | 259 |
| Soft tissue/Nodal only | 29 | 25 | 54 |
| Visceral+Soft tissue/Nodal | 15 | 18 | 33 |
| Total sites not including bone | 173 | 173 | 346 |
| Bone only | 87 | 127 | 214 |
| Visceral+Bone | 60 | 63 | 123 |
| Visceral + Bone + Soft tissue/Nodal | 15 | 18 | 33 |
| Bone + Soft tissue/Nodal | 11 | 12 | 23 |
| Total sites including bone | 173 | 220 | 393 |
| BC death no previous recurrence | 17 | 28 | 45 |
| Death from unknown cause | 40 | 48 | 88 |
| Contralateral breast cancers | 56 (2.4%) | 75 (3.3%) | 131 (2.8%) |
| Non-breast second primary cancers | 143 (6.2%) | 191 (8.3%) | 334 (7.3%) |
| Uterus | 15 | 28 | 43 |
| GI-upper | 24 | 20 | 44 |
| GI-lower | 20 | 28 | 48 |
| Lung | 14 | 29 | 43 |
| Melanoma | 10 | 9 | 19 |
| Ovary | 10 | 8 | 18 |
| Hematological | 15 | 17 | 32 |
| Kidney | 5 | 8 | 13 |
| Other | 30 | 44 | 74 |
Figure 2c.
Overall survival in the ITT population (N=4724)
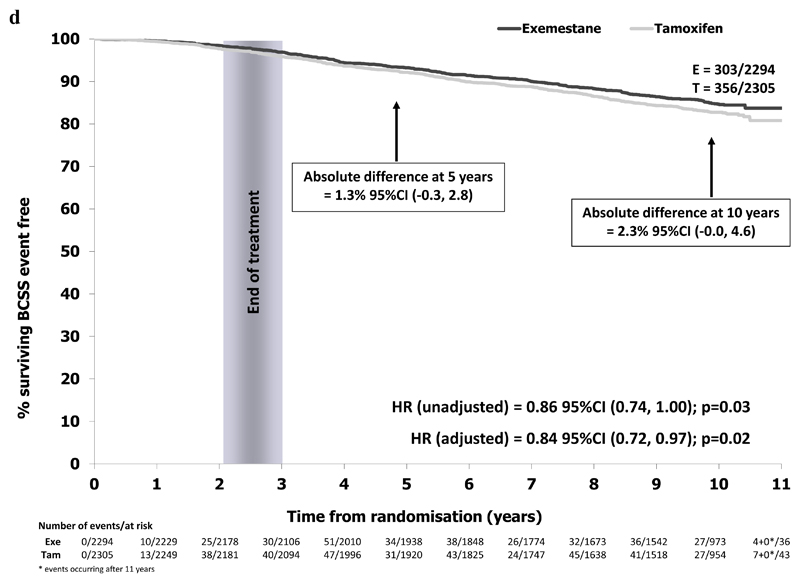
In the ER+ve/unknown population, 659/4599 BCSS events were reported (exemestane 303/2294 (13.2%), tamoxifen 356/2305 (15.4%)). Absolute BCSS difference at 10 years was 2.3% (95% CI -0.0% to 4.6%) with a hazard ratio of 0.84 (95% CI 0.72 to 0.98) in favor of switching to exemestane, Figure 2d.
Figure 2d.
Breast cancer specific survival in the ER+/unknown population (N=4599)
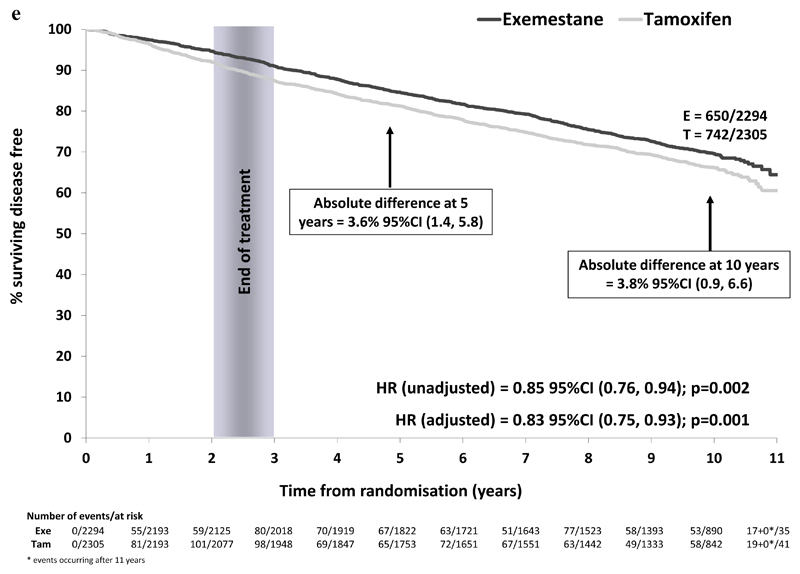
1392 DFS events have been reported in ER+/unknown patients only (exemestane 650/2294 (28.3%), tamoxifen 742/2305 (32.2%)). The highly significant improvement in DFS associated with switching to exemestane that we noted previously remains, with no convergence of survival curves seen (Figure 2e). This sustained benefit translated to an absolute difference in the proportion remaining alive and disease-free at 10 years of 3.8% (95%CI 0.9%, 6.6%). This difference remained in multivariable analyses adjusting for nodal status, prior HRT use and prior chemotherapy (HR favoring switch to exemestane of 0.83 (95%CI 0.75 to 0.93); p=0.001).
Figure 2e.
Disease free survival in the ER+/unknown population (N=4599)
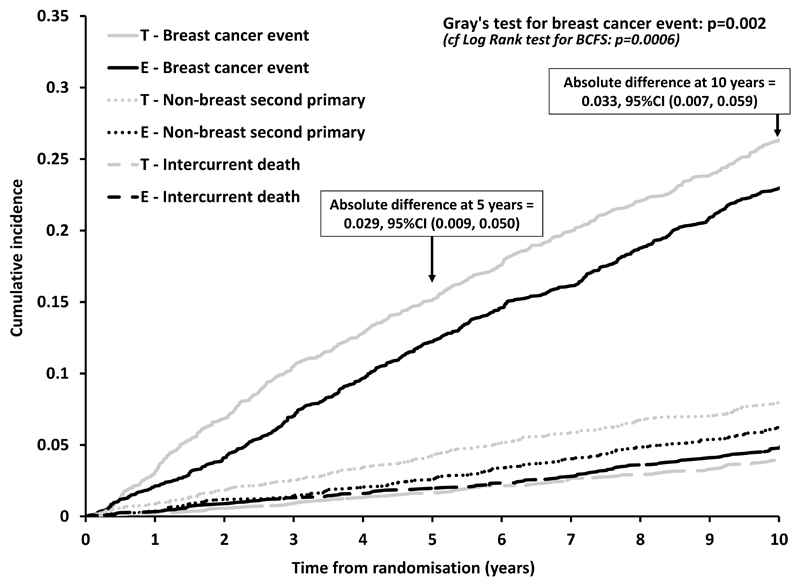
Using competing risks methodology, investigating all outcomes in a single analysis, the cumulative incidence of intercurrent deaths was seen to increase steadily throughout the follow-up period and was comparable between randomised treatment groups (Figure 3). Considering breast cancer events (after adjustment for competing risks), the early benefit from switching to exemestane was maintained throughout follow-up (Gray’s test p=0.002).
Figure 3.
Cumulative incidence of breast cancer event and of the competing risks intercurrent death and non-breast second cancer
No statistically significant difference was seen between randomised groups in the number of patients reporting a new primary CLBC (exemestane 56; tamoxifen 75; HR=0.73, 95% CI 0.52-1.03, Table 1) although the observed hazard ratio is consistent with other trials which have explored the additional preventative benefits of aromatase inhibitors compared with tamoxifen17. Numerically fewer non-breast second primary cancers were also reported with exemestane (143, compared to 191 with tamoxifen, Table 1). Analyses of incidence of distant recurrence and non-breast second primary cancer by age at randomisation reflect data presented previously, with a suggestion that second primary cancer incidence increases with age but no association was seen between age and distant recurrence incidence (trend tests p=0.08 and 0.22 respectively, Appendix 1).
Results of TTDR analyses across the entire follow-up period reflected other efficacy endpoints, with an absolute difference in the rate of distant recurrence or breast cancer death at 10 years of 2.6% (95% CI 0.2% to 5.1%) and a hazard ratio of 0.84 (95% CI 0.74 to 0.96) in favor of switching to exemestane. Analyses of TTDR after completion of endocrine therapy – equivalent to approximately 5 years after diagnosis - include 4147 patients (2091 exemestane, 2056 tamoxifen) known to be event free at 2.5 years post-randomisation (Table 2). No statistically significant difference in TTDR during this period was observed between randomised treatment groups (HR=0.94, 95% CI 0.80 to 1.10, p=0.41), reflecting the observation that the initial difference in disease outcome observed in the on-treatment period is maintained throughout the follow-up period. After inclusion in a multivariable Cox proportional hazards model, age at randomisation, nodal status, hormone receptor status, previous HRT use and tumor size, but not grade, had a significant effect on the risk of TTDR event after completion of endocrine therapy, i.e. of late relapse. Of note, risk of late distant recurrence in patients with tumor size greater than 5 cm at diagnosis was almost double that of patients with tumors of less than 2 cm (HR=1.92, 95% CI 1.28 to 1.90), and over six times higher in patients with 10 or more nodes involved compared to those who were node-negative at randomisation (HR=6.10, 95% CI 4.41 to 8.44), after adjustment for other factors.
Table 2. Factors affecting risk of TTDR event after 2.5 years (i.e. approximately 5 years after diagnosis).
| N | TTDR events | % | Unadjusted analysis |
Adjusted analysis (not including geographical region) |
Adjusted analysis (including geographical region) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value* | HR | 95% CI | p-value* | HR | 95% CI | p-value* | |||||||
| Randomised treatment | |||||||||||||||
| Tamoxifen | 2056 | 311 | 15.1 | 1 | - | 0.41 | 1 | - | 0.29 | 1 | - | 0.32 | |||
| Exemestane | 2091 | 300 | 14.3 | 0.94 | 0.80 - 1.10 | 0.92 | 0.78 - 1.08 | 0.92 | 0.79 - 1.08 | ||||||
| Age at randomisation | |||||||||||||||
| <60 | 1352 | 187 | 13.8 | 1 | - | 0.0003 | 1 | - | 0.008 | 1 | - | 0.004 | |||
| 60-69 | 1548 | 248 | 16.0 | 1.01 | 0.84 - 1.22 | 1.03 | 0.85 - 1.25 | 1.05 | 0.86 - 1.27 | ||||||
| >=70 | 999 | 176 | 17.6 | 1.43 | 1.17 - 1.76 | 1.36 | 1.09 - 1.70 | 1.41 | 1.13 - 1.76 | ||||||
| Nodal status | |||||||||||||||
| N- | 2227 | 183 | 8.2 | 1 | - | <0.0001 | 1 | - | <0.0001 | 1 | - | <0.0001 | |||
| 1-3N+ | 1256 | 220 | 17.5 | 2.22 | 1.83 - 2.70 | 2.11 | 1.72 - 2.59 | 2.09 | 1.70 - 2.56 | ||||||
| 4-9N+ | 386 | 131 | 33.9 | 4.8 | 3.84 - 6.01 | 4.41 | 3.45 - 5.63 | 4.28 | 3.35 - 5.46 | ||||||
| >=10N+ | 119 | 57 | 47.9 | 7.57 | 5.63 - 10.21 | 6.10 | 4.41 - 8.44 | 6.07 | 4.38 - 8.40 | ||||||
| Unknown | 159 | 20 | 12.6 | 1.59 | 1.01 - 2.53 | 1.63 | 1.02 - 2.60 | 1.82 | 1.14 - 2.92 | ||||||
| Previous chemotherapy use | |||||||||||||||
| Yes | 1305 | 248 | 19.0 | 1 | - | <0.0001 | 1 | - | 0.48 | 1 | - | 0.58 | |||
| No | 2842 | 363 | 12.8 | 0.66 | 0.56 - 0.77 | 1.07 | 0.89 - 1.30 | 1.06 | 0.87 - 1.28 | ||||||
| Hormone receptor status | |||||||||||||||
| ER+ and PgR+ | 2474 | 331 | 13.4 | 1 | - | 0.008 | 1 | - | 0.01 | 1 | - | 0.006 | |||
| ER+ and PgR-/unknown | 1204 | 206 | 17.1 | 1.3 | 1.09 - 1.55 | 1.29 | 1.08 - 1.54 | 1.33 | 1.11 - 1.60 | ||||||
| ER unknown and PgR unknown | 469 | 74 | 15.8 | 1.24 | 0.96 - 1.59 | 1.22 | 0.94 - 1.58 | 1.22 | 0.93 - 1.61 | ||||||
| Histological type | |||||||||||||||
| Ductal | 3157 | 450 | 14.3 | 1 | - | 0.02 | 1 | - | 0.30 | 1 | - | 0.29 | |||
| Lobular | 578 | 108 | 18.7 | 1.32 | 1.07 - 1.62 | 1.16 | 0.93 - 1.45 | 1.17 | 0.94 - 1.46 | ||||||
| Other/Unknown | 412 | 53 | 12.9 | 0.87 | 0.65 - 1.16 | 0.93 | 0.69 - 1.24 | 0.93 | 0.70 - 1.25 | ||||||
| Previous HRT use | |||||||||||||||
| Yes | 1021 | 115 | 11.3 | 1 | - | <0.0001 | 1 | - | 0.006 | 1 | - | 0.09 | |||
| No | 3035 | 473 | 15.6 | 1.47 | 1.20 - 1.80 | 1.35 | 1.10 - 1.67 | 1.20 | 0.97 - 1.49 | ||||||
| Unknown | 91 | 23 | 25.3 | 2.38 | 1.52 - 3.72 | 1.74 | 1.10 - 2.74 | 1.59 | 1.00 - 2.52 | ||||||
| Tumor size (cm) | |||||||||||||||
| <=2 | 2537 | 277 | 10.9 | 1 | - | <0.0001 | 1 | - | <0.0001 | 1 | - | <0.0001 | |||
| >2 & <=5 | 1431 | 292 | 20.4 | 2.03 | 1.72 - 2.39 | 1.51 | 1.28 - 1.80 | 1.51 | 1.27 - 1.79 | ||||||
| >5 | 94 | 26 | 27.7 | 3.05 | 2.04 - 4.56 | 1.92 | 1.28 - 1.90 | 1.96 | 1.30 - 2.96 | ||||||
| Unknown | 85 | 16 | 18.8 | 1.76 | 1.07 - 2.92 | 1.35 | 0.80 - 2.25 | 1.28 | 0.77 - 2.15 | ||||||
| Tumor grade | |||||||||||||||
| G1 | 737 | 73 | 9.9 | 1 | - | 0.0001 | 1 | - | 0.40 | 1 | - | 0.69 | |||
| G2 | 1785 | 263 | 14.7 | 1.52 | 1.17 - 1.97 | 1.16 | 0.89 - 1.51 | 1.14 | 0.88 - 1.49 | ||||||
| G3/Undifferentiated | 756 | 120 | 15.9 | 1.66 | 1.24 - 2.22 | 1.16 | 0.86 - 1.56 | 1.13 | 0.84 - 1.53 | ||||||
| Unknown | 869 | 155 | 17.8 | 1.91 | 1.45 - 2.52 | 1.29 | 0.96 - 1.72 | 1.20 | 0.89 - 1.61 | ||||||
| Region | |||||||||||||||
| USA | 325 | 26 | 8.0 | 0.53 | 0.35 - 0.79 | <0.0001 | 0.67 | 0.45 - 1.02 | 0.02 | ||||||
| UK | 512 | 54 | 10.5 | 0.63 | 0.47 - 0.84 | 0.69 | 0.51 - 0.94 | ||||||||
| Central & Eastern Europe | 754 | 134 | 17.8 | 1.20 | 0.98 - 1.46 | 1.15 | 0.93 - 1.44 | ||||||||
| Rest of Europe | 2351 | 367 | 15.6 | 1 | - | 1 | - | ||||||||
| Southern Hemisphere & Hong Kong | 205 | 30 | 14.6 | 1.01 | 0.70 - 1.47 | 0.96 | 0.66 - 1.40 | ||||||||
p-value for Likelihood ratio test. p-values are calculated with “unknown” categories included.
Fractures
No statistically significant difference was seen in the proportion of patients reporting at least one fracture event in the post-treatment period (exemestane 196/2105 (9.3%), tamoxifen 163/2036 (8.0%), p=0.14).
Discussion
This updated and final analysis of IES demonstrates that the benefit associated with switching to exemestane observed early in the follow-up period remains undiminished by further follow-up. As the IES population ages, incidence of non-breast cancer deaths and non-breast second primary cancers increase, leading to a dilution of OS results, however a modest benefit from switching to exemestane can still be seen, with absolute difference in OS at 10 years post-randomisation of 1.6%. As suggested previously, BCFS (which does not include non-breast cancer deaths) remains the most appropriate measure of treatment efficacy in this setting; an absolute benefit of 4.0% from switching to exemestane was observed at 10 years. Analyses taking into account competing events of intercurrent death and non-breast second primary cancer showed an absolute difference in breast cancer event at 10 years of 3%.
The IES trial compared treatments up to 5 years’ duration. Recent large randomised controlled trials18–20 have demonstrated an improvement in disease-related outcomes associated with continuing tamoxifen or aromatase inhibitor treatment past the standard five years of treatment. However long-term use of endocrine therapy is associated with many side-effects some of which have substantial impacts on patient well-being such as osteoporosis, vasomotor problems and musculo-skeletal conditions21. There remains great clinical need to identify patients who remain at high risk of disease relapse after completion of 5 years of endocrine therapy who may benefit from further treatment, and conversely patients who may be spared this due to low residual risk.
Results of analyses partitioned at 2.5 years post-randomisation support conclusions made previously that the difference in disease-related outcome observed at 10 years between treatment groups is due to maintenance of the initial on-treatment divergence between groups rather than any emerging post-treatment effect. Multivariable analyses of clinical factors affecting time to late distant recurrence identified age at randomisation, nodal involvement, hormone receptor status, previous HRT use and tumor size, although the relationship between HRT use and late distant recurrence is confounded by geographical region. The observation that tumor grade no longer retains prognostic significance in this setting after adjustment for other factors reflects previous analyses of retrospective case-series22 and comparable analyses of the ATAC trial12. The authors of this analysis also demonstrated the value of the PAM50-based risk of recurrence (ROR) score as an independent predictor of late distant recurrence; other molecular scores studied (IHC4, RS) did not add prognostic information when added to clinical data13.
In summary, the IES and other contemporaneous studies have established that a strategy of switching to an aromatase inhibitor after 2-3 years of tamoxifen can lead to sustained benefits in terms of reduction of disease recurrence and breast cancer mortality. Identifying patients who remain at higher risk of disease recurrence after the completion of 5 years of endocrine therapy (be it tamoxifen, aromatase inhibitor or a combination of the two) according to clinical factors such as nodal involvement and tumor size will aid decision making on the administration of further endocrine therapy or additional therapeutic agents.
Supplementary Material
Acknowledgements
We thank the women who took part in this study, the doctors, nurses, and support staff at local sites, and the monitors, data managers, trial coordinators, and study managers from the Argentine Breast Cancer Group, the Australian New Zealand Breast Cancer Trials Group, the Central and Eastern European Oncology Group, the Danish Breast Cancer Group, the Dutch Breast Cancer Research Group, the European Organisation for Research and Treatment of Cancer, the Grupo Espanol De Investigacion Del Cancer De Mama, the Gruppo Oncologico Nord Ovest, the Gruppo Oncologico Italiano di Ricerca Clinica, the International Breast Cancer Study Group, the International Collaborative Cancer Group, the Israeli Clinical Oncology Group, Italian Trials in Medical Oncology, the North West England Group, the Norwegian Breast Cancer Group, the Yorkshire Breast Group, the Federation Nationale Des Centres De Lutte Contre Le Cancer, the German Exemestane Adjuvant Group, the Wales Cancer Trials Network, US Oncology, the Swedish Breast Cancer Group and Pfizer. We also thank the Breast International Group for their support and the members of the study steering committee and the independent data monitoring committee. The trial coordinating units at Imperial College London and The Institute of Cancer Research also received funding from Cancer Research UK.
Research supported by Pfizer Inc.
References
- 1.WH. Breast cancer: prevention and control. 2013 [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative G. Dowsett M, Forbes JF, et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–52. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 4.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 5.Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet. 2007;369:559–70. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 6.Bliss JM, Kilburn LS, Coleman RE, et al. Disease-related outcomes with long-term follow-up: an updated analysis of the intergroup exemestane study. J Clin Oncol. 2012;30:709–17. doi: 10.1200/JCO.2010.33.7899. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–27. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 8.Bertelli G, Hall E, Ireland E, et al. Long-term endometrial effects in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES)--a randomised controlled trial of exemestane versus continued tamoxifen after 2-3 years tamoxifen. Ann Oncol. 2010;21:498–505. doi: 10.1093/annonc/mdp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallowfield LJ, Bliss JM, Porter LS, et al. Quality of life in the intergroup exemestane study: a randomized trial of exemestane versus continued tamoxifen after 2 to 3 years of tamoxifen in postmenopausal women with primary breast cancer. J Clin Oncol. 2006;24:910–7. doi: 10.1200/JCO.2005.03.3654. [DOI] [PubMed] [Google Scholar]
- 10.Fallowfield LJ, Kilburn LS, Langridge C, et al. Long-term assessment of quality of life in the Intergroup Exemestane Study: 5 years post-randomisation. Br J Cancer. 2012;106:1062–7. doi: 10.1038/bjc.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mieog JS, Morden JP, Bliss JM, et al. Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2-3 years of tamoxifen: a retrospective analysis of the Intergroup Exemestane Study. Lancet Oncol. 2012;13:420–32. doi: 10.1016/S1470-2045(11)70328-X. [DOI] [PubMed] [Google Scholar]
- 12.Sestak I, Dowsett M, Zabaglo L, et al. Factors predicting late recurrence for estrogen receptor-positive breast cancer. J Natl Cancer Inst. 2013;105:1504–11. doi: 10.1093/jnci/djt244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowsett M, Sestak I, Buus R, et al. Estrogen Receptor Expression in 21-Gene Recurrence Score Predicts Increased Late Recurrence for Estrogen-Positive/HER2-Negative Breast Cancer. Clin Cancer Res. 2015;21:2763–70. doi: 10.1158/1078-0432.CCR-14-2842. [DOI] [PubMed] [Google Scholar]
- 14.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 15.Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–71. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 16.Pintile M. Competing Risks: A Practical Perspective. Wiley; 2006. [Google Scholar]
- 17.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–8. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 18.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol (Meeting Abstracts) 2013;31:5. [Google Scholar]
- 20.Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. 2016;375:209–19. doi: 10.1056/NEJMoa1604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonning PE, Eikesdal HP. Aromatase inhibition 2013: clinical state of the art and questions that remain to be solved. Endocr Relat Cancer. 2013;20:R183–201. doi: 10.1530/ERC-13-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100:1179–83. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.