Abstract
Temperature influences the distribution, range and phenology of plants. The key transcriptional activators of the heat shock response in eukaryotes, the heat shock factors (HSFs), have undergone a large-scale gene amplification in plants. While HSFs are central in heat stress responses, their role in ambient temperature changes is less well understood. We show here that the warm ambient temperature transcriptome is dependent upon the HSFA1 clade of Arabidopsis HSFs, which cause a rapid and dynamic eviction of H2A.Z-nucleosomes at target genes. A transcriptional cascade results in the activation of multiple downstream stress responsive transcription factors, triggering large-scale changes to the transcriptome in response to elevated temperature. H2A.Z-nucleosomes are enriched at temperature responsive genes at non-inducible temperature, and thus likely confer inducibility of gene expression and higher responsive dynamics. We propose that the antagonistic effects of H2A.Z and HSF1 provide a mechanism to activate gene expression rapidly and precisely in response to temperature, while preventing leaky transcription in the absence of an activation signal.
Keywords: gene expression regulation, plant temperature sensing and signaling, transcriptomics, nucleosome dynamics, histone variant H2A.Z, plant epigenetics, heat shock transcription factors
Introduction
Warm temperature is an important cue for plants, which must be able to adapt to their environment (Wigge, 2013). In Arabidopsis, many of the growth and developmental responses to temperature are mediated by the bHLH transcription factor PHYTOCHROME INTERACTING FACTOR4 (Koini et al., 2009; Kumar et al., 2012), which is controlled by the thermosensor phyB (Jung et al., 2016; Legris et al., 2016). The phyB temperature perception mechanism relies on dark reversion, and consequently expression of the warm temperature transcriptome controlling development occurs at night (Jung et al., 2016). Plants are most likely to encounter higher temperatures in sunlight however, suggesting an additional mechanism to sense temperature during the day. Consistent with this, genes involved in response to heat stress are predominantly expressed in the light (Jung et al., 2016).
Thermal stress is a major threat to the cell, causing protein denaturation and compromising membrane integrity. Rising global temperature is estimated to reduce crop yields by 2.5 to 16% for every additional 1ºC of warming during hot summers (Battisti and Naylor, 2009). It is therefore important to understand the pathways and mechanisms by which warm temperature influences the cell. Work in yeast, Drosophila, plants and mammalian cells has led to a widely established model that genes encoding heat shock proteins (HSPs) are transcriptionally induced by heat shock factor (HSF)-class transcription factors (TFs) upon activation by heat stress (Jacob et al., 2017; Shivaswamy and Iyer, 2008; Zobeck et al., 2010). A genetic screen in plants revealed that in addition to HSFs (Miozzo et al., 2015), the chromatin state also influences the expression of warm temperature induced genes (Kumar and Wigge, 2010). Mutants deficient in the incorporation of H2A.Z-nucleosomes show a higher HSP70 expression and many phenotypes associated with warm temperature growth. Moreover it was shown that H2A.Z-nucleosome occupancy decreases in response to temperature at HSP70 and a few other genes (Kumar and Wigge, 2010), and heat stress results in a global increase in chromatin accessibility at responsive loci (Sullivan et al., 2014). It is unclear however, whether these dynamics of H2A.Z-nucleosomes reflect a passive process by which the stability of H2A.Z-nucleosomes responds directly to temperature, or result from increased transcriptional responsiveness of the loci, or a combination of these mechanisms.
The transcriptional activation of heat stress genes in plants is potentially complicated by the high degree of gene duplication. While yeast has a single HSF, Arabidopsis for example has 21 HSF family members (von Koskull-Dˆring et al., 2007). One clade in particular, the HSFA1 group, appears to be important for the early responses to heat stress (Yoshida et al., 2011). Activation of the HSF pathway in Arabidopsis is complex, with multiple downstream TFs being involved (Schramm et al., 2008). While the role of HSFA1 TFs in the response to heat stress is well established, it is still not clear if these factors are involved in the transcriptional response to warm temperature in the ambient range. While H2A.Z-nucleosomes have been implicated in regulating the warm temperature transcriptome, how they interact with TFs and other cis-acting factors is not clear.
In this study, we use genome-wide datasets to investigate the dynamics of both nucleosome and TF behavior to determine their contributions to the temperature transcriptome. We find that the day-time warm ambient temperature transcriptome is dependent on HSFA1 TFs and these are rapidly and robustly recruited to the promoters of responsive genes, activating their transcription. Moreover, we show that HSFA1a TFs are essential for H2A.Z eviction occurring in response to warm temperature at these genes. Activation of downstream TFs by the HSFA1-class results in a transcriptional cascade that can account for a large proportion of the day-time warm temperature transcriptome. Genes responding rapidly to warmer temperature display distinctive promoter architecture of heat shock elements (HSEs) and nucleosome positions. We propose that both HSFA1 class TFs and H2A.Z-nucleosomes enables a dynamic transcriptional response system to be activated upon passing a threshold temperature.
Results
A warm temperature transcriptome defined by the HSFA1 TFs
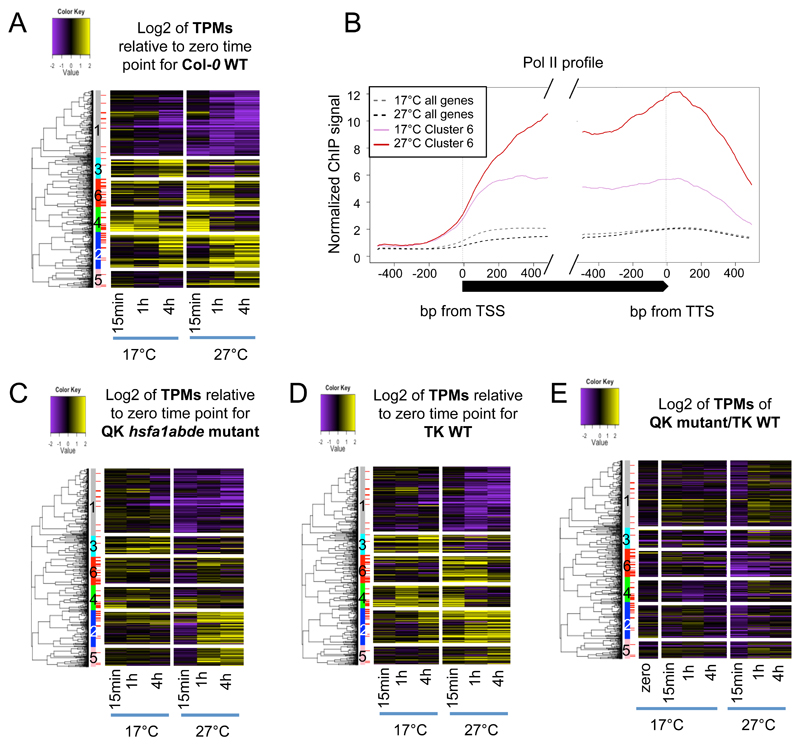
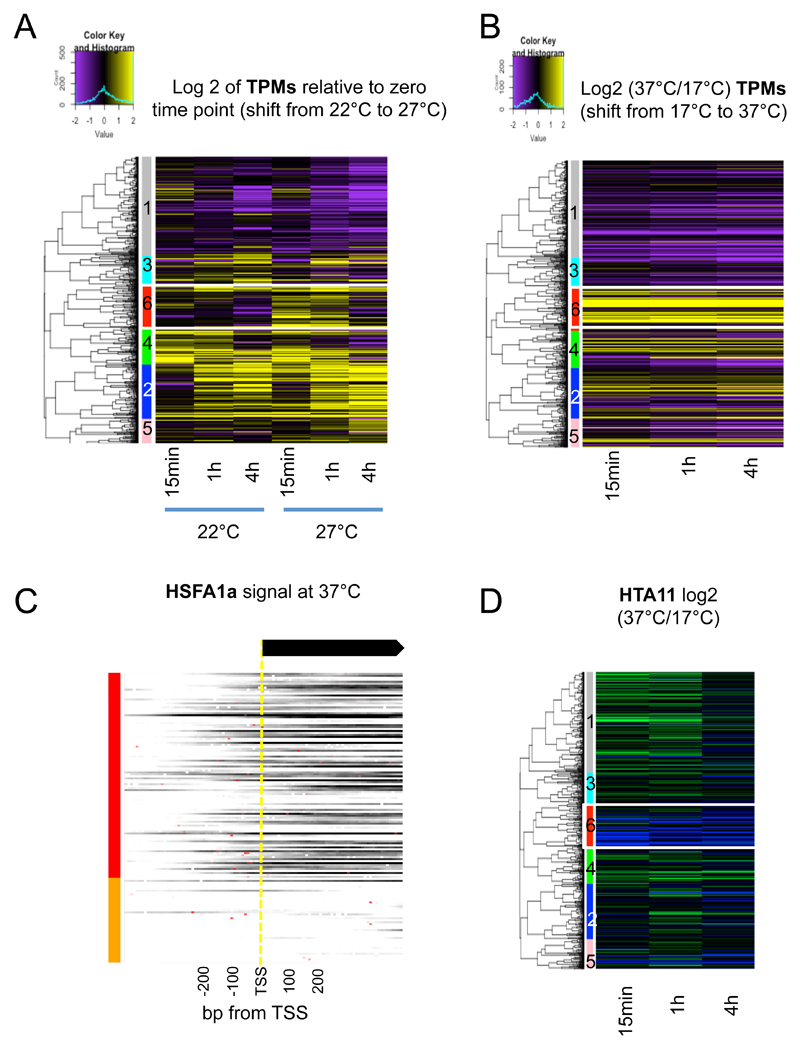
To analyze the day-time temperature response, we measured the temperature transcriptomes of plants shifted 1 hour after dawn from 17 to 27ºC for 0.25, 1 and 4 h. (Supplemental Figure 1A). A total of 1,035 transcripts show significant changes in these conditions (see Methods), and we refer to these as the “temperature responsive” transcripts (Supplemental Figure 1B). Hierarchical clustering of these temperature responsive genes reveals six major patterns of transcriptional response to warmer temperature (Figure 1A, Supplementary Figure 2A, Supplemental Dataset 1).
Figure 1. HSFA1 transcription factor family is regulating the transcriptional response to ambient temperature.
A Transcriptional patterns and dynamics of temperature-responsive genes (1,035 genes) in response to ambient temperature shift (17°C to 27°C) in Col-0 WT. The temperature-responsive genes were hierarchically clustered into six groups, based on the log2 ratio to the zero time point of transcript per million (TPM) values. Up-regulated genes are in yellow and down-regulated genes are in purple. The first sidebar to the left of the heatmap indicates the six clusters of temperature responsive genes. The second sidebar indicates target genes of HSFA1a at 27°C based on HSFA1a ChIP-seq performed on seedlings shifted for 15min from 17°C to 27°C.
B Average RNA Pol II occupancy profiles at TSS and TTS of cluster 6 genes (solid) and genome average (dotted) at 17°C (pink for cluster 6, grey for all genes) and after 15min of shift to 27°C (red for cluster 6, black for all genes).
C Transcriptional patterns in the QK hsfa1abde mutant. The genes and clusters are in the same order as in Figure 1A.
D Transcriptional patterns in the TK wild-type control, keeping the same order of genes and clusters as for Col-0 WT in Figure 1A. As the QK hsfa1abde mutant genome is a combination of the Ws and Col-0 backgrounds (Liu et al., 2011), we used the TK WT as a control as it was generated at the same time and thus serves as a suitable reference (Liu et al., 2011).
E Transcriptional changes between the QK hsfa1abde mutant and the TK wild-type control (log2(QK/TK) for each time point and temperature shift). The genes and clusters are in the same order as in Figure 1A.
Cluster 1 (350 genes, grey sidebar in Figure 1A) contains the genes that are predominantly repressed over time, and is enriched in genes involved in metabolic processes (based on a Gene Ontology, GO, enrichment analysis) (Eden et al., 2009), which are likely to be transcribed at a lower rate under stresses (more details on GO term analyses are in Supplemental Table 1). Cluster 2 (193 genes, blue) shows partial up-regulation after 1 and 4 h at 27ºC, but the patterns are similar to the 17ºC control samples at these time-points, suggesting the transcriptional dynamics may be endogenous to the experimental setup, and/or related to the circadian rhythm. The transcriptional activation of cluster 2 genes appears to be more rapid at 27ºC than 17ºC after 1 h (Supplemental Figure 2B). Cluster 3 (110 genes, cyan) demonstrates partial transcriptional repression but is not significantly enriched for any functional GO term. Cluster 4 (130 genes, green) demonstrates rapid and transient transcriptional activation at both 17°C and 27°C, followed by repression after 4 h, and is enriched in genes that are involved in stress and defence responses. Both transcriptional activation and repression seems to be faster at 27°C for the genes in this cluster (Supplemental Figure 2B). Cluster 5 (105 genes, pink) is the smallest cluster with a slight increase in transcriptional level at 27°C, but not enriched in a GO term. Cluster 6 (147 genes, red) genes are termed “rapidly temperature responsive”, as they show maximal expression within 15 min of 27ºC treatment. The peak of expression at 15 min is transient, as the expression of these genes returns to near basal levels after 4 h. These genes are highly enriched in biological process GO terms of heat and light responses (Supplemental Table 1), and include genes involved in the response to heat stress such as HSP70 and other HSPs, HSFA7A and DREB2A (Supplemental Dataset 1). The role of higher temperature in activating these genes is particularly clear when gene expression at 27ºC is normalized to that at 17°C (Supplementary Figure 2B). To confirm these results are a consequence of rapid transcriptional activation, we investigated RNA Pol II occupancy by ChIP-seq. We observe a corresponding increase in the relative amount of Pol II in the gene bodies of the cluster 6 genes in response to ambient temperature increase, which is absent in the control set of all genes (Figure 1B).
The Arabidopsis genome encodes 21 HSFs and members of the HSFA1 class are of particular importance in the early responses to heat since the hsfa1abde quadruple (QK) mutant is more sensitive to mild heat stress (Liu et al., 2011; Yoshida et al., 2011). We therefore investigated whether the QK mutant alters the warm temperature transcriptome. Consistent with a major role for this HSF clade, there is a global reduction in temperature responsiveness across all clusters in our experiment (Figure 1C and E, Supplemental Figure 2C), particularly clearly evident after 15 min of shifting to 27ºC. Clusters 2, 4 and 6 are most strongly perturbed, with many transcripts showing little or no temperature responsiveness. Interestingly, more than half the genes in cluster 6 (83/147 ~56%) lose temperature responsiveness (same criteria used to extract temperature responsive transcript, see Methods) after 15 min temperature shift in the QK mutant, as compared to its corresponding TK wild-type, which has the same background as QK but with HSFA1ABDE activity (Figure 1D). Lower proportions of genes in clusters 2 (59/193 ~31%) and 4 (21/130 ~16%) become unresponsive to temperature in QK.
HSFA1a binds to rapidly responsive genes and initiates a transcriptional cascade in response to warm temperature
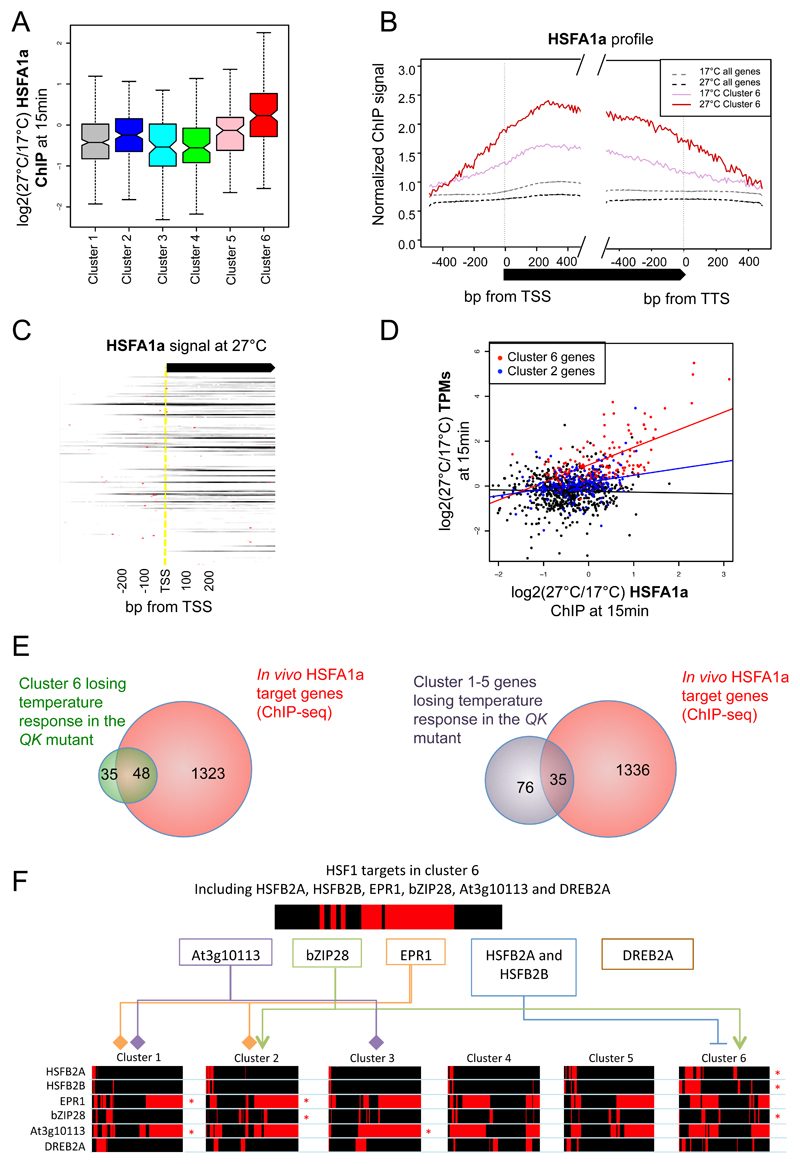
To determine if the effect of HSFA1a, a representative of the HSFA1 family TFs, on the warm ambient temperature transcriptome is direct, we performed ChIP-seq of HSFA1a on seedlings shifted from 17 to 27ºC for 15 min, and in controls kept at 17°C (Supplemental Figure 1A). We identified 1,371 genes that are directly bound by HSFA1a within 15 min of 27ºC (Supplemental Dataset 2). Specifically, ~46% of cluster 6 genes are directly bound by HSFA1a, and in clusters 1-5, 17% of genes are bound by HSFA1a, marked in red on the second sidebar of Figure 1A (Fisher’s exact P-value of the overlaps < 2.2e-16 for both groups) (Supplemental Figure 3A). By comparison, HSFA1a binds to only ~5% of the genes in Arabidopsis. Consistent with HSFA1a binding the promoters of temperature responsive genes, we observe strong enrichment for predicted heat shock elements (HSEs) in clusters 2 and 6 (Supplemental Table 2). For cluster 6 genes we found predicted HSEs within 44/84 (~52%) and 17/39 (~45%) of HSFA1a ChIP-seq peaks at 27 and 17ºC respectively.
While HSFA1a targets are significantly enriched in clusters 1, 2, 4 and 6 (Table 1), HSFA1a signal increases specifically for cluster 6 genes after 15 min of shift from 17ºC to 27ºC, (Figure 2A, Wilcoxon test P-value < 2.2e-16 when comparing cluster 6 genes to genes in clusters 1-5). For rapidly temperature responsive genes (cluster 6), there is significant signal for HSFA1a at the non-inductive temperature of 17ºC at HSEs and over the gene body, and this markedly increases at 27ºC (Figure 2B). Increase in HSFA1a occupancy upon temperature increase can be observed in most of cluster 6 genes (Figure 2C, red dots represent predicted HSEs). This increase in HSFA1a occupancy with temperature is not seen in other clusters, including cluster 2, which is also enriched for predicted HSEs (Supplemental Figure 3B-E).
Table 1. HSFA1a targets.
Enrichment of HSFA1a targets in the six clusters of temperature responsive genes. Number of HSFA1a targets in each cluster and Fisher’s exact test p-values are provided.
| Number of genes | Number of HSFA1a target genes | Fisher's exact test p-value (vs all genes) | |
|---|---|---|---|
| all genes | 27206 | 1325 | n.a. |
| cluster1 | 350 | 39 | 9.95E-06 |
| cluster2 | 193 | 44 | 7.30E-15 |
| cluster3 | 110 | 7 | 0.5035 |
| cluster4 | 130 | 36 | 1.32E-14 |
| cluster5 | 105 | 5 | 1 |
| cluster6 | 147 | 68 | < 2.2e-16 |
Figure 2. HSFA1a transcription factor binding is increasing at 27°C at the cluster 6 genes.
A Boxplot of changes for HSFA1a signal in the gene body between 17°C and 27°C after 15 min for genes in each temperature-responsive cluster. HSFA1a ChIP signal increases after temperature shift to 27°C specifically for cluster 6 genes (Wilcoxon test P-value < 2.2e-16 when comparing cluster 6 genes to genes in clusters 1-5). Non-overlapping notches indicate significant differences between populations’ medians.
B Genome-wide average HSFA1a binding profiles of HSFA1a show a higher HSFA1a occupancy at genes in cluster 6 (solid, pink) as compared to at 17°C (dotted, grey). The HSFA1a occupancy markedly increases after 15 min shift to 27°C (solid, red), as compared to 17°C (solid, pink),
C Gene-by-gene HSFA1a ChIP signal of cluster 6 genes at 27°C. Red dots indicate predicted HSEs from a known consensus motif.
D Correlation between changes in transcripts level (TPMs) and HSFA1a signal after 15min of shift from 17°C to 27°C. A strong positive correlation is observed between increase in transcripts level and HSFA1a signal for cluster 6 genes (red), as compared to an equal number of random genes from other temperature-responsive gene clusters (black) and cluster 2 genes (blue).
E Comparison of in vivo HSFA1a targets genes with genes losing temperature response in the QK hsfa1abde mutant that are either in the cluster 6 (left) or in clusters 1-5 (right). Of all the 147 temperature-rapid-response genes (cluster 6), 83 (~56%) become temperature-irresponsive in the QK mutant as compared to the TK wild-type. Temperature responsiveness is defined by log2(TPM 27°C 15 min/TPM 17°C 15 mins) >= 0.5, or the TPM 27°C at 15 mins is less than 2 (undetectable), while TPM 17°C at 15 mins is not. Of these 83 genes, 48 (~58%) are shown to be direct targets of HSFA1a (Fisher’s exact test P-value < 2.2e-16), signifying the crucial role of HSFA1a and other TFs in the HSFA families in transcriptional regulation temperature rapid responsive genes. For other temperature responsive genes (cluster 1-5), 35 out of 111 (~32%) of genes losing temperature-responsiveness are direct targets of HSFA1a. Note that there are 1,371 genes (~5%) predicted as targets of HSFA1a in the Arabidopsis genome.
F Representation of the transcriptional cascade in the clusters 1-6 genes (bottom) by the TFs that are in the cluster 6 and are HSFA1a targets (top). The direct targets of these TFs were determined based on the available DAP-seq dataset (O’Malley et al., 2016) and are represented in red stripes for each cluster (bottom). The linkers and asterisks indicates the temperature-responsive clusters whose members are significantly enriched in target genes of these temperature-responsive TFs. Known positive and negative regulatory relationships are indicated by arrows and blunt arrows, respectively. HSFB2A/B, EPR1, bZIP28, DREB2A and At3g10113 TFs in the cluster 6 are transcriptionally activated by HSFA1a, and they themselves regulate downstream temperature responsive targets.
Consistent with the central role of HSFA1a in shaping the rapid transcriptional responses to warm temperature, there is a positive correlation between changes in transcription and HSFA1a binding occupancy in response to shifting at 27ºC only for cluster 6 genes (P-value from linear model fitting = 1.5e-11, Figure 2D), in line with a previous study showing the major role of HSFA1a in regulating the response to heat stress (Liu et al., 2011). Within cluster 6, ~56% (83/147) of the genes become unresponsive to temperature in the QK mutant. Interestingly, we observe direct binding of HSFA1a to ~58% of these (48/83, Fisher’s exact test P-value < 2.2e-16). HSFA1a binding also occurs at ~32% (35/111, P-value =2.6e-15) of the other temperature-responsive genes that lose responsiveness in the hsfa1abde background in clusters 1-5 (Figure 2E). Despite HSEs being found in multiple clusters, the rapid increase in transcript abundance of the cluster 6 genes, accompanied by increased HSFA1a occupancy, is specific for this group of genes. Taken together, these results indicate that the presence of an HSE alone is insufficient to predict rapid gene induction by temperature. We note that the HSFA1a target genes identified here might be under-estimated, as the ChIP was performed using a HSFA1a-tagged line in a wild-type background, and thus might be subjected to interference by the native HSFA1a that could potentially decrease ChIP signal. We nonetheless observe a strong overlap of ~43% (83/194) between identified HSFA1a targets in all temperature-responsive genes (cluster 1-6), and the genes becoming unresponsive to temperature in the QK mutant.
While HSFA1a only binds directly to ~17% of the temperature responsive genes outside of cluster 6, we observe that the expression of up to 32% of these genes is perturbed in hsfa1abde (Figure 2E). This could be because some targets require HSFA1 b, d or e and cannot be bound by HSFA1a. We think this is unlikely however, since the HSFA1 clade has a highly conserved DNA binding domain and the HSFA1A knockout mutants are highly redundant. Furthermore, many of the temperature responsive genes outside of cluster 6 do not have clearly identifiable HSE in their promoters. As we show, several of the direct targets of HSFA1a are themselves TFs, it is likely that these are part of a transcriptional cascade that activates a broader range of transcriptional targets. We identified 9 TFs in cluster 6 that are directly bound by HSFA1a, and have previously been implicated in transcriptional responses, particularly to stresses. These HSFA1a targets are two members of the HFSB2 family (HSFB2A and HFSB2B), two members of the HSFA7 family (HSFA7A and HSFA7B), two closely related myb homeodomain TFs (EARLY PHYTOCHROME RESPONSE1 (EPR1) and At3g10113), and genes encoding the stress responsive TFs bZIP28, RAP2.4 and DREB2A. We were able to identify potential targets for 6 of these TFs in all temperature responsive clusters (HSFB2A, HFSB2B, EPR1, At3g10113, bZIP28 and DREB2A), using data generated by DNA affinity purification coupled with sequencing (DAP-seq) (O'Malley et al., 2016). Our analysis reveals a cascade by which these intermediate TFs are able to transmit the warm temperature signal to genes in other clusters. This cascade can therefore account for a large proportion of the temperature transcriptome, and likely contributes to the temporal variation in the responses we see (Figure 2F, Supplemental Table 3).
H2A.Z-nucleosomes signal transiently decreases at rapidly responsive genes at 27ºC
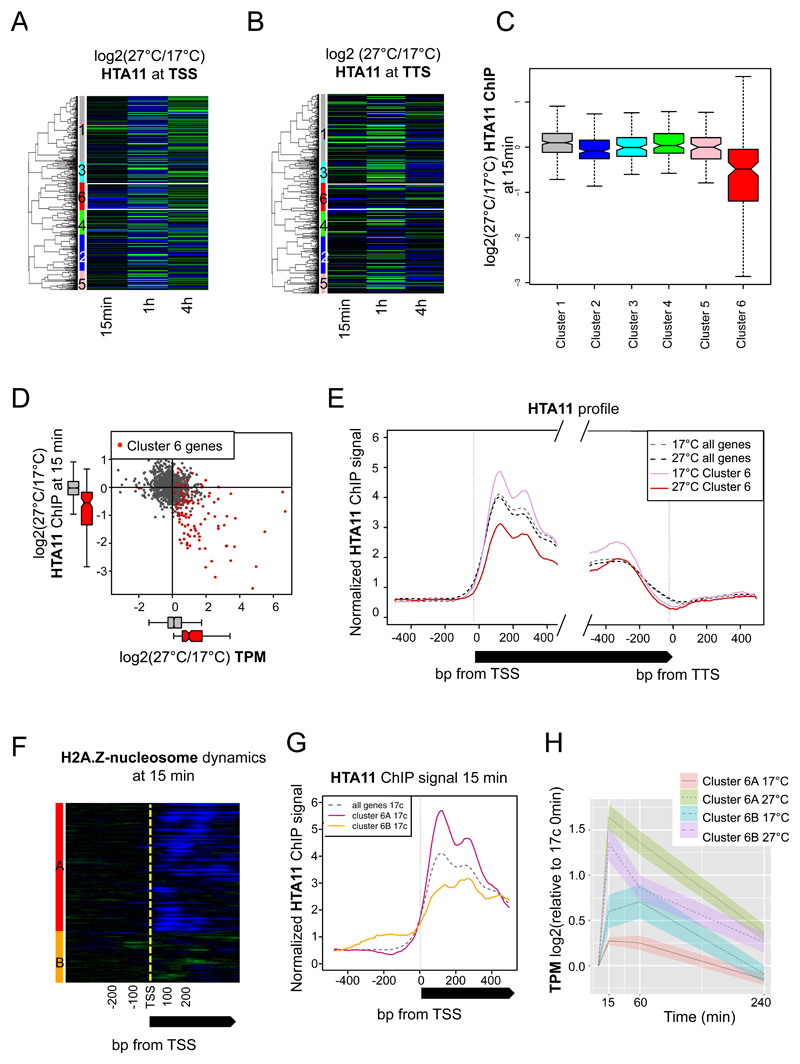
As H2A.Z-nucleosomes at the +1 nucleosome of HSP70 can be evicted in response to warm temperature (Kumar and Wigge, 2010) we used ChIP-seq of FLAG tagged HTA11, a broadly expressed H2A.Z gene, to determine if this is a global response of temperature responsive genes. We observe that cluster 6 genes show a rapid loss of HTA11 ChIP signal at the transcriptional start site (TSS) in response to 27ºC (Figure 3A). We were able to confirm this dynamic behavior for the occupancy of HTA9, a separate H2A.Z protein, using anti-HTA9 antibodies (Yelagandula et al., 2014), at HSP70 (Figure 6B and Supplemental Figure 8B), indicating that HTA11 ChIP signal is representative of H2A.Z-nucleosome occupancy for temperature responsive genes. The decrease of HTA11 ChIP signal on temperature responsive promoters is dynamic, since we observe signal returning after 1 h, and by 4 h the HTA11 ChIP signal is comparable to the starting levels at 17ºC. This depletion of H2A.Z-nucleosome occupancy is not confined to the +1 nucleosome, but also occurs in the gene body and the region surrounding the transcriptional termination site (TTS) (Figure 3B, Supplemental Figure 4A). We also sought to determine whether temperature responsiveness is a general property of H2A.Z-nucleosomes or whether this occurs only at specific loci. Interestingly, we see that H2A.Z-nucleosome eviction is only clearly apparent at cluster 6, and not at the other clusters (Figure 3C, Wilcoxon test P-value < 2.2e-16 when comparing cluster 6 genes to genes in clusters 1-5, and Supplemental Figure 4D). We also observed a strong negative correlation between transcription and HTA11 occupancy in response to shifting to 27ºC for 15 min (Figure 3D and Supplemental Figure 4B). This negative correlation is specific to cluster 6, as it is not observed elsewhere, even in clusters 2 and 4 (Supplemental Figure 5) where partial increases of transcription levels are observed, together with occurrence of predicted HSEs and HSFA1a binding.
Figure 3. HTA11 ChIP signal is transiently reduced at cluster 6 genes in response to the shift to 27°C.
A Dynamic profiles of +1 HTA11 ChIP (used to represent H2A.Z-nucleosomes) of temperature-responsive genes around TSS (same orders and clusters as in Figure 1A) reveal rapid loss of H2A.Z-nucleosome occupancy among the rapid temperature-responsive genes at 15 min (cluster 6, highlighted by white box). Green represents relative gain and blue represents relative loss of HTA11 ChIP signal between 17°C and 27°C.
B Dynamic profiles of HTA11 ChIP signal around the TTS of temperature-responsive genes (same orders as in Figure 1A). A rapid loss of H2A.Z-nucleosome occupancy is observed among the rapid temperature-responsive genes at 15 min (cluster 6, highlighted by white box).
C Changes for HTA11 signal at the TSS between 17°C and 27°C after 15 min for all genes in each temperature-responsive gene cluster. A strong reduction of HTA11 signal at 27°C is specifically observed in cluster 6 genes (Wilcoxon test P-value < 2.2e-16 when comparing cluster 6 genes to genes in clusters 1-5). Non-overlapping notches indicate significant differences between populations’ medians.
D Negative correlation between HTA11 occupancy at TSS and transcriptional changes (as TPMs) in response to 15 min shift from 17°C to 27°C for all temperature-responsive genes (black), and most prominently in cluster 6 genes (red).
E Average HTA11 occupancy profiles among the temperature-responsive genes in cluster 6 (solid), and genome-wide average (dotted). The loss of +1 H2A.Z-containing nucleosomes after 15 min shift to 27°C is observed only in cluster 6 genes (red compared to pink).
F Two subgroups of genes can be observed in the cluster 6 based on HTA11 dynamics in the plants shifted from 17°C to 27°C for 15min: 6A (red), which exhibit a predominant loss of H2A.Z occupancy at the +1 nucleosomes, as well as into the gene bodies, and 6B (orange) without any clear H2A.Z pattern.
G Average profiles of H2A.Z-containing nucleosomes of the genes in sub-class 6A (pink), is higher than for genes in sub-class 6B (orange), and the genome-wide average (dotted black).
H Average transcriptional changes of temperature-responsive genes (log2 ratio of TPMs normalized to the zero time point) for the sub-classes 6A (orange for 17°C and green for 27°C) and 6B (blue for 17°C and purple for 27°C) in Col-0 WT, which were previously identified by the re-clustering of cluster 6 genes based on HTA11 dynamics. The dynamic of transcriptional activation is stronger in genes of the sub-class 6A, notably because of a lower baseline transcriptional level compare to the sub-class 6B.
Figure 6. Interplay between HSFA1a and H2A.Z-containing nucleosome in the transcriptional response to temperature at cluster 6 genes.
A Changes of transcriptional levels (TPMs) among the cluster 6 genes are highly correlated with changes in HSF1 and HTA11 occupancies in response to 15min of shift from 17ºC to 27ºC. This is particularly clear in the sub-class 6A (red), and less so for 6B (orange).
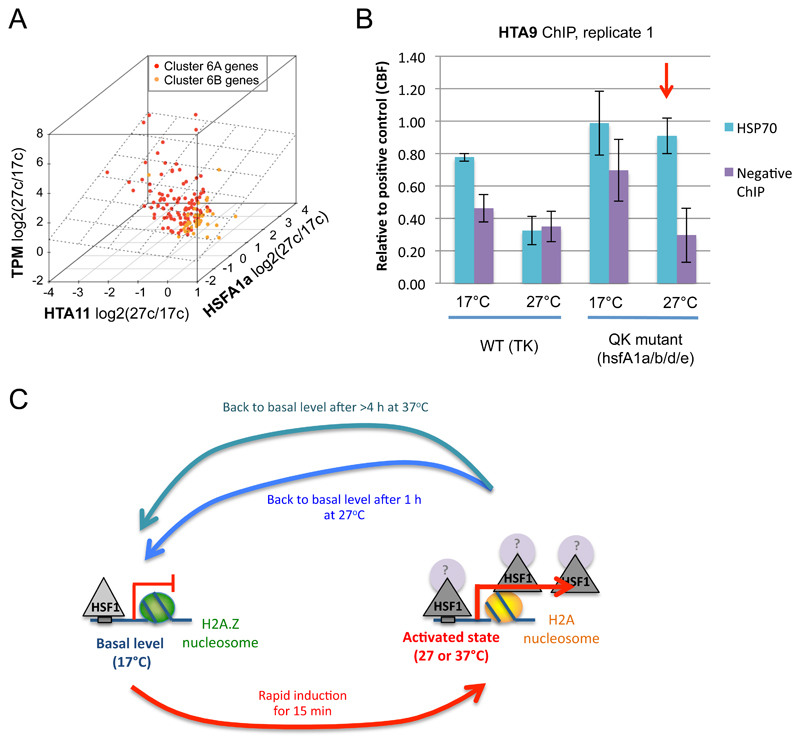
B ChIP-qPCR for HTA9 in the QK hsfA1a/b/d/e mutant and TK wild-type at HSP70 and AT3G12590, which is a negative control for H2A.Z. The ChIP results are first normalized by the INPUT and then by the positive control (CBF), so that they could be directly compared between samples. H2A.Z eviction at HSP70 in response to temperature shift is not anymore observed in the QK hsfA1a/b/d/e mutant at 27°C (red arrow). Error bars represent the standard errors of three technical replicates. The second biological replicate can be found in Figure S8B.
C) A model of transcriptional regulation of rapidly temperature responsive genes (cluster 6). At a low ambient growth temperature (17°C), HSFA1a is detectable at the promoters of the rapidly temperature responsive genes at a low level and at a small fraction of its target genes, insufficient to trigger transcriptional activation in Col-0 WT. Tightly bound H2A.Z-nucleosome, especially at the +1 position of these genes, may play a role to help restrict transcriptional activation at lower temperatures. At elevated ambient temperature (27°C), HSF1 is transiently further recruited to HSEs around the TSS as well as to the gene bodies of the targets, an event concomitant with depletion of H2A.Z from these regions and this is accompanied by robust transcriptional activation. The strong correlation between transcriptional activation, H2A.Z eviction, and HSF1 binding is also observed to a greater extent and for a longer period of time, when plants are subjected to heat stress (37°C).
H2A.Z loss at temperature-responsive genes is associated with increased chromatin accessibility
A key question is the extent to which H2A.Z-nucleosome eviction is required for transcriptional activation or a consequence of it. The occupancy of HTA11 at cluster 6 genes (solid lines, Figure 3E and Supplemental Figure 4C) is markedly higher than that of the genome-wide average at 17ºC (dotted lines), but within 15 min at 27ºC this drops below the average. Again, this high baseline HTA11 ChIP signal at 17ºC for the +1 nucleosome is not observed in clusters 2 and 4 (Supplemental Figure 5C-F). This trend is also apparent within cluster 6, as we can identify two subgroups based on HTA11 dynamics: 6A, which shows robust decreases in HTA11 ChIP signal, and 6B, where the change in HTA11 is less clear (Figure 3F). Interestingly, genes in 6A are also characterized by higher baseline HTA11 ChIP signal at 17ºC compared to both the genome average and subgroup 6B (Figure 3G). While transcripts in both 6A and 6B are activated within 15 min at 27ºC, 6A genes show a greater dynamic range with little or no expression at 17ºC compared to 6B genes that show moderate expression at this temperature (Figure 3H). These observations suggest that H2A.Z-nucleosomes may act to enhance the transcriptional dynamic range by keeping genes transcriptionally repressed under non-inductive conditions by restricting promoter and gene accessibility.
We also assessed the influence of H2A.Z-nucleosomes on the temperature transcriptome by comparing transcriptional responses of WT plants shifted to 27ºC for 15 min, with arp6-1, which is deficient in H2A.Z incorporation. Interestingly, transcriptional changes due to temperature shift and in arp6-1 appear to be more correlated in cluster 6, as compared to genome-wide (Pearson correlations, 0.24 for cluster 6 versus 0.12 genome-wide, Supplemental Figure 5G), and to any other cluster (Pearson correlations of 0.004, -0.01, -0.04, 0.05 and -0.06 for clusters 1 to 5 respectively). This further supports a model where H2A.Z-nucleosomes create a local chromatin environment refractory to transcription at 17ºC for genes in cluster 6, which is diminished when shifted to 27ºC or in arp6-1.
To measure chromatin accessibility, we investigated the accessibility of chromatin to micrococcal nuclease (MNase) genome-wide using sequencing (MNase-seq) on seedlings subjected to the same temperature shift regime as described above. Since nucleosomes protect DNA from MNase cleavage, this provides a robust assay of nucleosome accessibility. Genes within cluster 6 are enriched for MNase-protected sequences at 17ºC, but these become accessible after shifting to 27ºC (Supplemental Figure 6A). This higher protection of the +1 nucleosome at 17ºC is less distinct for genes in clusters 2 or 4 (Supplemental Figure 6B). Loss of HTA11 ChIP signal correlates with loss of MNase-seq signals at the +1 nucleosome position for cluster 6 genes (Supplemental Figure 6C, red dots), when shifted to 27ºC. This suggests that the genomic DNA at these genes becomes more accessible when H2A.Z is evicted compared with the other temperature responsive genes (Supplemental Figure 6C, black dots). To investigate whether the changes in DNA accessibility are specific to H2A.Z-containing nucleosomes, or whether they reflect broader changes in nucleosome dynamics, we investigated how other histones changed under the same temperature shift. H3 occupancy is indistinguishable between cluster 6 genes and the rest of the genome at 17ºC and 27ºC (Supplemental Figure 6D-E) or genes in clusters 2 and 4 (Supplemental Figure 6F). This suggests that the decrease in H2A.Z signal in response to heat does not reflect a general depletion of nucleosomes in response to greater transcription of these loci, but rather the specific exchange of H2A.Z-nucleosomes for H2A.
Promoter architecture of genes responding rapidly to temperature may facilitate transcriptional activation
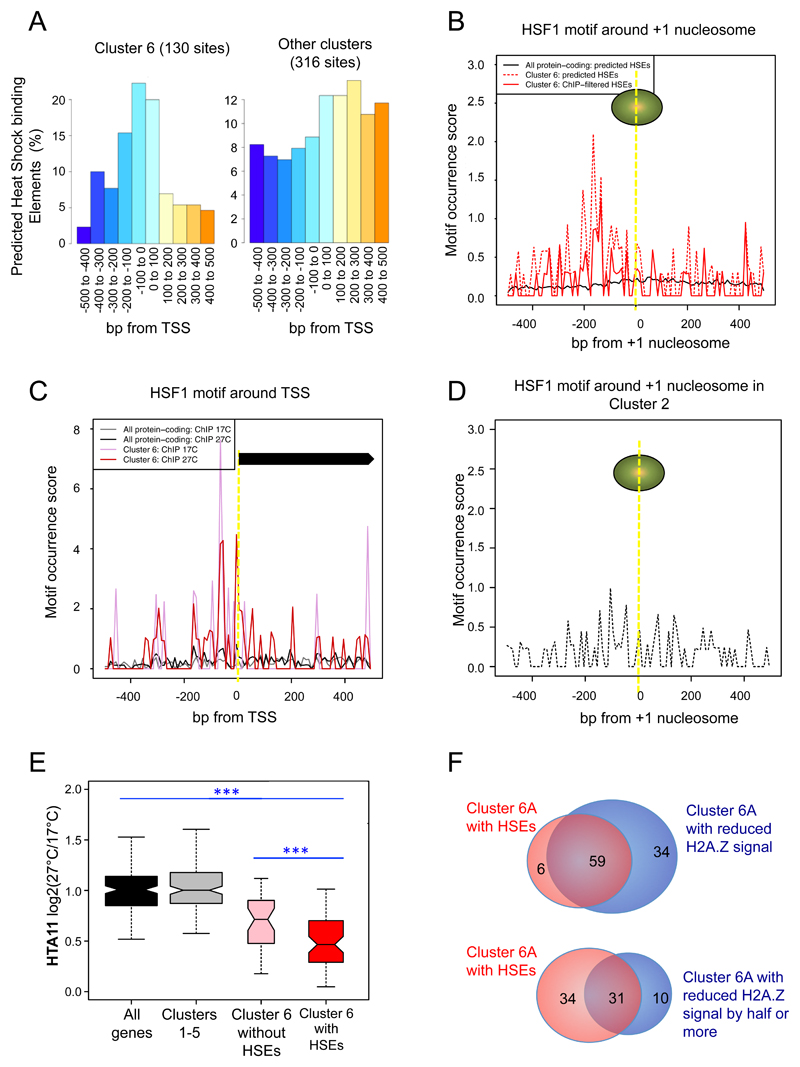
We have seen that HSFA1a binds to and activates transcription of a large proportion of the cluster 6 genes, and consistent with this we observe 130 instances of predicted HSEs among these genes, statistically greater than expected by chance (Figure 4A, Fisher’s exact test P-value < 2.2e-16). We do not observe this degree of enrichment for the other clusters, highlighting the importance of the HSF class of TF in mediating this response. Furthermore, in the promoters of cluster 6 genes, HSEs appear to be strongly positioned within 200 bp upstream of the TSS, adjacent to the +1 nucleosome that exhibits loss of HTA11 signal after the temperature shift (Figure 4B). This is also true for predicted HSEs in cluster 6 genes with bound HSFA1a (ChIP-filtered HSEs), based on our ChIP-seq experiments. Examining the target genes of HSFA1a, we also observed significant enrichment of HSE occurrences close to the TSS, as compared to other genes (Figure 4C). In contrast, the distribution of HSEs elsewhere in the genome, or even in cluster 2 where HSEs are also statistically enriched, do not show such a pattern in their distribution (Figure 4D). Consistent with the importance of HSF TFs in this response, we observe specific H2A.Z-nucleosome depletion upon shifting to 27ºC for cluster 6 genes with HSEs (red box), when compared to other genes (black) (Wilcoxon test P-values < 2.2e-16) (Figure 4E). This reduction of HTA11 signal is still greater than when compared to other temperature responsive gene clusters 1-5 (grey), or even cluster 6 genes without HSEs (pink). In addition, 59 of 65 of cluster 6 genes with HSEs show a reduced H2A.Z signal when shifted to 27ºC, and in 31 of these genes, the H2A.Z signal is reduced by more than half, providing further evidence that the HSEs at close proximity of H2A.Z-nucleosomes in cluster 6 genes are strongly associated with H2A.Z reduction (Figure 4F).
Figure 4. Promoter architecture of temperature rapid response genes may facilitate transcriptional activation.
A Predicted Heat Shock binding Elements (HSEs) are specifically enriched in temperature-responsive gene clusters 6, particularly within +/- 100bp upstream of TSS (Fisher’s exact test P-value < 2.2e-16). The same is not observed in other temperature-responsive genes.
B Predicted HSEs (dashed), and those confirmed and filtered by ChIP-seq evidence of HSFA1a from this study (solid), are specifically enriched in rapid temperature-responsive gene clusters 6 (red), just upstream of the +1 nucleosome. The same is not observed in other genes in the genome (black).
C ChIP-filtered HSEs are enriched in temperature-responsive gene clusters 6 around TSS. For the target genes of HSFA1a with ChIP-seq peaks (17°C ChIP in pink and 27°C ChIP in red) within 500bp from TSS, predicted HSEs are specifically enriched within +/-100bp upstream of TSS, as compared to HSEs found in other genes (17°C ChIP in grey and 27°C ChIP in black).
D Predicted HSEs (dashed) around +1 nucleosome for cluster 2 genes. HSEs are also enriched in this cluster, but without a clear positioning pattern in relation to the +1 nucleosome as in cluster 6 (Figure 4B)
E Changes for HTA11 signal at the TSS between 17°C and 27°C after 15 min for all genes, genes in clusters 1-5 and genes in cluster 6 with our without HSEs. A strong reduction of HTA11 signal at 27°C is specifically observed in cluster 6 genes with HSEs compared to genes in all other clusters (Wilcoxon test P-values < 2.2e-16), or even to cluster 6 genes without HSEs (P-value = 8.0e-4).
F In the sub-cluster 6A, comparison of genes with HSEs with genes showing a reduction of H2A.Z signal (top) or a reduction of H2A.Z signal by half or more (bottom). A strong enrichment in genes with HSEs is observed among genes with reduced H2A.Z signal in the sub-class 6A.
The transcriptional response of the warm temperature genes is triggered by absolute temperature
The transcriptional changes we observe could reflect a response to a relative change in temperature or be triggered by crossing an absolute temperature threshold. To distinguish between these possibilities, we generated transcriptomes, in conjunction with HTA11 and HSFA1a ChIP-seq datasets, for seedlings shifted from 17 to 22ºC, from 22 to 27ºC, as well as from 17 to 37ºC. While shifting seedlings from 22 to 27ºC elicits a robust induction of the warm temperature response (Figure 5A), comparable to the previous shift from 17 to 27ºC, only a very mild change in gene expression is seen for the 17 to 22ºC shift (Supplemental Figure 7A). This indicates that absolute temperature plays a pivotal role in determining the transcriptional response, since both these shifts were of the same magnitude (5ºC change), but had very different transcriptional outcomes. These results are supported by the HTA11 occupancy behavior, where shifting to 27ºC had a strong effect, but 22ºC did not (Supplemental Figure 7A-B). Furthermore, increasing the temperature to 37ºC causes a strong induction of the cluster 6 genes (Figure 5B and Supplementary Figure 7C), concomitant with a strong increase in HSFA1a signal at the responsive genes (Figure 5C), and a greater loss of HTA11 and MNase signals that are maintained over 4 h (Figure 5D and Supplemental Figure 7D-E). Interestingly, the transcriptional induction of HSP70 in plants shifted from 17ºC to 27ºC was observed only during the day but not in the dark, whereas HSP70 induction occurs in both light and dark when shifted to 37°C (Supplemental Figure 7F). Taken together, these results indicate that there is a key threshold temperature between 22ºC and 27ºC that must be passed for the induction of cluster 6 genes during the day, and once this is activated, further increasing temperature results in a quantitative increase in gene activation.
Figure 5. Transcriptional response to ambient warm temperature and to heat stress.
A Transcriptional patterns in Col-0 WT seedlings shifted from 22ºC to 27ºC. The clusters are in the same order as in Figure 1A. Transcriptional activation is also observed for genes in cluster 6 (highlighted with white box). Up-regulated genes are in yellow and down-regulated genes are in purple.
B Transcriptional patterns in Col-0 WT seedlings shifted from 12ºC to 37ºC. Transcriptional activation of the cluster 6 genes is stronger and persist longer when shifted to 37°C as compared to 27°C (highlighted with white box).
C Gene-by-gene HSFA1a ChIP signal of cluster 6 genes at 37°C. Further recruitment of HSF1 to the promoters and genes bodies of the cluster 6A genes resulted in elevated HSF1 occupancy at 37°C. Red dots indicate predicted HSEs from a known consensus motif.
D Dynamic profiles of HTA11 ChIP signal around the TTS of temperature-responsive genes Col-0 WT shifted from 12ºC to 37ºC. In plants shifted to 37°C, the H2A.Z eviction for cluster 6 genes (highlighted with white box) is stronger than shifting to 27°C and takes longer to be incorporated back into the nucleosomes (after 4 h). Green represents relative gain and blue represents relative loss of HTA11 ChIP signal between 17°C and 27°C.
HSFA1a binding at warm temperature is necessary to promote H2A.Z loss and transcriptional activation
To understand the relationship between HTA11, gene expression and HSFA1a more clearly, we asked if HTA11 and/or HSFA1a occupancy are good predictors of transcriptional level among the cluster 6 genes. We see a strong positive correlation between transcription and HSFA1a occupancy (P-value = 1.5e-11 for linear model fitting), and a negative relationship between transcription and HTA11 signal (Supplemental Table 4) (P-value = 1.1e-9), indicating that both HTA11 and HSFA1a can predict the transcriptional response to 27ºC to a certain extent. Interestingly, combining HSFA1a and HTA11 occupancies provides a prediction of gene expression upon shifting from 17ºC to 27ºC with higher confidence, based on a linear model fitted using transcriptional rate as a response (P-value = 8.5e-15, Figure 6A, Supplementary Table 4). Within cluster 6, the sub-class 6A genes (red dots) demonstrate a greater reduction of H2A.Z signal, gain of HSFA1a, and transcriptional induction, as compared to the sub-class 6B (orange). Such a correlation is not observed in randomly selected non-temperature-responsive genes (P-value = 0.38, Supplemental Figure 8A, Supplemental Table 4). These results show that both HSFA1 class TFs and H2A.Z-nucleosomes are necessary for rapid transcriptional responses to warmer temperatures, but how they influence each other is not clear.
To investigate whether H2A.Z-nucleosome eviction is mediated by temperature directly or via other factors, we performed in vitro ChIP on purified nucleosomes from Arabidopsis seedlings grown at 17ºC (see Supplemental Figure 8C and Methods). We observe that the H2A.Z-nucleosomes at +1 position near HSP70 are now not responsive to temperature in vitro (Supplemental Figure 8D). In addition, we also looked into the temperature responsiveness of reconstituted H2A.Z-nucleosomes in vitro using a fluorescence resonance energy transfer (FRET) assay. This allows us to directly assay the behavior of nucleosomes in response to temperature in the absence of other cellular components such as remodelers or TFs. We observe no significant change in DNA dynamics on the nucleosome at a wide range of ambient temperatures (Supplemental Figure 8E-F).
If H2A.Z-nucleosomes are not intrinsically temperature-responsive, could HSFA1a be one of the factors that influence DNA binding of H2A.Z-nucleosomes in response to temperature shift? To assess the role of HSFA1a on H2A.Z eviction in vivo, we performed ChIP-qPCR for HTA9 in the QK hsfa1abde mutant and TK WT, in response to temperature shift, at the HSP70 gene, at CBF, which is a positive control gene where H2A.Z signal remains after shift to 27ºC, and at AT3G12590, which is a negative control gene where no H2A.Z signal is observed. Interestingly, +1 H2A.Z-nucleosome at HSP70, as measured by ChIP, is no longer thermoresponsive in hsfa1abde (Figure 6B and Supplementary Figure 8B). This indicates that temperature dependent H2A.Z-nucleosome depletion at induced genes requires HSFA1a.
Discussion
A dynamic transcriptional cascade protects the cell from warm temperatures
We have constructed a comprehensive data set of temperature transcriptomes of WT and QK hsfa1abde mutant plants subjected to ambient temperature shift at three time points: 0.25, 1 and 4 h, with corresponding genome-wide binding profiles of the TF HSFA1a, Pol II, and histones H2A.Z and H3, in parallel with MNase nucleosome stability assays in consistent conditions (Supplemental Figure 1). We first observe a rapid induction of a core set of transcripts highly enriched for genes encoding cellular protective proteins such as chaperones within 15 min shift from 17 to 27°C. The activation of these genes is transient as they show a reduction of transcriptional levels after 1 h, and return to the baseline by 4 h. The warm temperature cellular protection pathway is therefore under tight transcriptional control and is rapidly attenuated when seedlings are maintained at warm temperatures, consistent with previous observations for heat stress (Ohama et al., 2016). Since chaperones function as ATPases, it may be important to control their expression levels for energy homeostasis within the cell. In Drosophila, maintaining flies at higher ambient temperature causes a depletion of body fat stores and a decline in fitness (Klepsatel et al., 2016). HSFA1a directly activates multiple activating TFs in cluster 6 genes, as well as the transcriptional repressors HSFB2A and HSFB2. These TFs may act in a negative feedback loop to fine tune the response to warm temperature, and genes up-regulated in hsfb1-1/hsfb2b (Ikeda et al., 2011) are highly enriched in cluster 6 (Fisher's exact test p-value < 2.2e-16, Supplemental Table 3). This indicates that HSFA1a and HSFB2A/B may act in an incoherent feed-forward loop, a common regulatory motif in biology (Milo et al., 2002).
In addition to HSFB2A and HSFB2B, we show that HSFA1a also directly regulates multiple key TFs involved in stress response signaling, in response to warm temperature: HSFA7A, DREB2A, RAP2.4, EPR1 and BZIP28 (Gao et al., 2008; Larkindale and Vierling, 2008; Lin et al., 2008; Ohama et al., 2017; Sakuma et al., 2006; Yoshida et al., 2011) (Figure 2F). This is well in line with earlier studies showing that the HSFA1 family are key regulators of genes involving in responses to heat as well as other abiotic stresses such as oxidative, osmotic and salt stresses (Liu et al., 2011). Strikingly, these TFs that are HSFA1 targets bind to the promoters of multiple genes that are differentially expressed upon the shift to 27ºC. It therefore appears that there is a transcriptional cascade from HSFA1a via these additional TFs, and this can partly account for differentially transcribed genes after shifting to 27ºC, as previously described for heat stress (Ohama et al., 2016). Consistent with this model, hsfa1abde shows a greatly attenuated transcriptional response to 27ºC, even for genes that are responsive to temperature but not bound by HSFA1a directly. This transcriptional cascade can help account for the diverse transcriptional responses to day-time warm temperature of genes in cluster 1-5 (Figure 1A) with both activation as well as repression of genes, occurring over different timescales. To this end, the direct control of a key set of TFs, including HSFB2A, HSFB2A, EPR1, At3g10113 and bZIP28 enables a complex transcriptional outcome in response to a single rapid stimulus. This role of HSFA1 TFs as master regulators of key TFs coordinating responses to heat stress and drought resembles the sequential waves of transcriptional activation observed in responding to the environment (Murray et al., 2004) and during cell differentiation (Boyd et al., 2014).
The heat protection response has an activation threshold and increases quantitatively above that
Temperature has diverse influences upon plant growth, development and survival. An open question is the extent to which these response pathways share common or separate initial temperature sensing events and downstream transcriptional cascades. In this study we have focused on the response to 27ºC during the day, typically not regarded as a heat stress (Balasubramanian et al., 2006). Interestingly however, much of the transcriptomic response appears to be conserved with higher temperature heat stress responses, suggesting that even 27ºC is sufficient to activate these pathways. The key regulators we observe (HSFA1, DREB2A, HSFA7, RAP2.4, BZIP28, MBF1C and HSFB2) have also previously been shown to be central in high temperature stress responses (Gao et al., 2008; Ikeda et al., 2011; Larkindale and Vierling, 2008; Lin et al., 2008; Liu et al., 2013; Schramm et al., 2008). The HSFA1 class TFs appear to activate both heat stress and warm ambient temperature pathways during the day, in agreement with the previous observation that hsfa1abde is hypersensitive to prolonged exposure to 27°C (Liu and Charng, 2013). In contrast to the heat stress pathway, growth and developmental responses to warm temperature, mediated via the evening complex (Box et al., 2015; Mizuno et al., 2014; Raschke et al., 2015) or thermosensory phytochromes (Jung et al., 2016; Legris et al., 2016), occur predominantly at night and control different target genes (Ezer et al., 2017; Jung et al., 2016).
Our results indicate that an absolute temperature, rather than relative temperature is important for cluster 6 genes activation and suggest the presence of a threshold between 22 and 27ºC for transcriptional induction of HSFA1a targets. Further experiments would be required to define the exact threshold temperature for cluster 6 genes activation, and this may vary from gene to gene depending on the promoter architecture. Above this activation threshold temperature, there is a quantitative increase in transcriptional induction with temperature, as seen in further activation of temperature responsive genes in plants shifted to 37ºC. Since HSFA1a is expressed constitutively in the plant, the temperature perception event in this pathway may occur at the level of the HSFA1 protein, consistent with the extensive post-translational modifications of HSFs in response to heat stress in plants (Ohama et al., 2016). HSFA1a binding signal is detectable by ChIP at a number of temperature rapid-response genes at 17°C (Figure 2B and Supplemental Figure 3D), suggesting that the TF may maintain genes in a poised state, ready for activation in response to warm temperature via the recruitment of additional HSFs and/or other components of transcriptional machinery complexes, a scenario which has been previously observed in yeast (Shivaswamy and Iyer, 2008; Zanton and Pugh, 2006).
A combinatorial role for H2A.Z and HSF1 to mediate temperature responsive gene expression
The day-time warm temperature transcriptome is important for cellular survival and is able to be rapidly activated and shutdown. While HSFA1 TFs are essential for this response, many genes with HSEs in their promoters are not rapidly activated by temperature, indicating additional factors might be required to confer rapid and robust temperature responsive gene expression. Nucleosome architecture at promoters can contribute to the transcriptional responsiveness of gene expression (Lam et al., 2008; Weber et al., 2014), and in this study we observe a strong tendency for temperature responsive promoters to have well-positioned H2A.Z-nucleosomes just downstream of HSEs (Figure 4B). Furthermore, we observe a specific temperature dependent depletion of H2A.Z-nucleosome signal at these loci at higher temperature. This drop is also associated with an increase in nucleosome accessibility as shown by a reduction of signal in the MNase-seq. However, this may not simply reflect a removal of nucleosomes by the transcriptional machinery upon the activation of transcription, because a concomitant drop in H3 is not observed. This is in agreement with a previous observation of an increase in nucleosome accessibility without reduction in nucleosome occupancy during rapid transcriptional activation (Mueller et al., 2017). This specific depletion of H2A.Z-nucleosomes in response to temperature requires HSFA1a activity, and it does not occur on chromatin in vitro or on reconstituted nucleosomes. Further experiments on H2A.Z occupancy changes in response to temperature using drugs blocking transcription, may reveal if H2A.Z eviction is indeed mediated by HSFA1a, or is a consequence of transcriptional activation. Here, we also observe strong H2A.Z eviction at genes transcriptionally activated in response to warm temperature, whereas a previous study suggested that H2A.Z eviction might be independent of expression changes (Kumar and Wigge, 2010). This discrepancy could potentially have arisen from differences in temperature shift regimes (shifting from 12°C to 27°C for 2 or 24 h, as opposed to 17°C to 27°C for 15 min in day-time), and a more restricted set of genes being analyzed by ChIP-qPCR (6 genes that showed a decreased or flat transcriptional response to temperature showed some level of H2A.Z decline by ChIP-qPCR).
Being refractory to transcription in vitro (Park et al., 2004; Thakar et al., 2010), H2A.Z-nucleosomes are well suited for creating inducible transcriptional switches (Figure 6C). At non-permissive conditions, loci can be maintained in a fully repressed state, but upon H2A.Z-nucleosome depletion, loci are fully accessible for rapid expression. In this way, the specific replacement of the H2A.Z-H2B dimer from nucleosomes may provide a particularly rapid and reversible transcriptional activation switch, and may also account for the paradoxical observation that H2A.Z-nucleosomes anti-correlate with H3-H4 turnover (Weber et al., 2014). This depletion of H2A.Z at transcriptionally activated genes in response to elevated temperature is in agreement with a recent study demonstrating depletion of H2A.Z at genes transcriptionally activated in response to drought stress and an overrepresentation of genes affected by drought stress among the genes mis-regulated in a H2A.Z-depleted mutant (Sura et al., 2017). This together with our results suggest a repressive role of H2A.Z at genes responding to different environmental stresses, possibly by creating a closed chromatin environment refractory to transcription.
The mechanism by which H2A.Z-nucleosomes are depleted in response to high temperature may involve additional chromatin-remodelling complexes. For example, in Drosophila, HSF1 cooperates with the FACT complex to maintain the chromatin in an open state for transcription (Saunders et al., 2003). We observe an increase of HSFA1a signal at the HSEs at warm temperature, just upstream of the +1 nucleosome (Figures 2C and 4B), and the signal appears to extend into the gene body. The presence of HSFA1a around the +1 H2A.Z-containing nucleosome suggests that HSFA1a may recruit RNA Pol II machinery and/or chromatin remodelers to facilitate transcription and H2A.Z eviction, though this cannot yet be confirmed in this study. Indeed, the recruitment of chromatin remodelers and modifiers to the gene promoters by specific TFs appears to be a common theme enabling the intricate regulation of transcriptional rate (Charoensawan et al., 2012; Lam et al., 2008; Stockinger et al., 2001; Teichmann et al., 2012; Vercruyssen et al., 2014; Wu et al., 2012; Zhang et al., 2014; Zhao et al., 2015). We observe a rapid eviction and re-insertion of H2A.Z upon temperature increase and it would be of interest to further investigate if the chromatin regulators that have been shown previously to deposit H2A.Z into chromatin are involved in this process. H2A.Z is incorporated into chromatin by the SWR1 chromatin remodeling complex, composed of PIE1, ARP6 and SWC6 in Arabidopsis (Choi et al., 2007; Deal et al., 2005; Lazaro et al., 2008; March-Diaz and Reyes, 2009; Noh and Amasino, 2003; Zilberman et al., 2008). The INO80 complex has also been shown to be involved in H2A.Z deposition in yeast (Papamichos-Chronakis et al., 2011). On the contrary, aside from the human chaperone protein ANP32E that was shown to remove H2A.Z from chromatin (Obri et al., 2014), less is known about H2A.Z eviction mechanism.
The broad pattern of activated HSFA1a we observe spreading across the gene body, beyond the predicted HSEs, is consistent with a role for HSFA1a in activating otherwise quiescent chromatin in a pioneer TF style of action (Fujimoto et al., 2012). It is also worth noting that HSF signal in gene bodies has already been observed for a few genes in mammals and drosophila (Gonsalves et al., 2011; Mahat et al., 2016) and that DAP-seq peaks for HSF TFs have been seen to be enriched in 5’UTR and CDS of genes (O'Malley et al., 2016). In Chlamydomonas, HSF1 plays a key role in preventing gene silencing and maintains nearby loci in a transcriptional responsive state (Strenkert et al., 2013). It will be of interest to determine the temperature dependent event activating HSFA1a that triggers this large-scale and rapid reprogramming of the transcriptome, as well as the mechanism by which H2A.Z-nucleosome can be rapidly removed and re-inserted into chromatin at temperature responsive loci.
Methods
Plant materials and growth conditions
We constructed FLAG tagged HTA11 (AT3G54560) and HSFA1a (AT4G17750) lines under the native promoters, which were used for H2A.Z and HSF1 ChIP experiments. The HTA11 and HSFA1a genomic clones were isolated from Col-0, tagged with a 3×FLAG epitopes directly upstream to the stop codons. The vectors were transformed into Col-0 WT plants. The QK hsfA1a/b/d/e mutant and its corresponding TK wild type, as well as a arp6-1 mutant were described previously (Deal et al., 2005; Liu et al., 2011). Seedlings were grown at constant 17°C in solid 1X Murashige and Skoog (MS) media in long days for H2A.Z, H3, H2B and H2A ChIP-seq as well as all the RNA-seq experiments, and in short days for the HSF1 ChIP-seq. The seedlings were grown through a nylon mesh and for temperature shift, transferred to liquid MS media pre-incubated at 17°C (control), 22°C, 27°C, 37°C at 1 h after dawn. The plant samples were collected at the shift (0 min) as well as 15 min, 1 h, and 4 h after the shift (Supplemental Figure 1A). For arp6-1 mutant, seedlings were harvested after 15 min “mock” shift from 17°C in solid media to 17°C in liquid media.
RNA-seq library preparation
Total RNA was isolated from 30 mg of ground seedlings. RNA quality and integrity were assessed on the Agilent 2200 TapeStation. Library preparation was performed using 1 µg of high integrity total RNA (RIN>8) using the TruSeq Stranded mRNA library preparation kit and TruSeq RNA Library Preparation Kit v2 (Illumina, RS-122-2101 and RS-122-2001). The libraries were sequenced on a HiSeq2000 using paired-end sequencing of 100 bp in length and NextSeq500 using paired-end sequencing of 75 bp in length.
RNA-seq mapping and differential expression analysis
The raw reads obtained from the sequencing facilities were analyzed using a combination of publicly available software and in-house scripts described in Supplemental Methods. No mis-match was allowed when mapping using Tophat, except for the QK hfsa1abde mutant and its corresponding TK WT for which data were analyzed for reads mapped with no mis-match as well with 4 mis-matches allowed, to account for potential discrepancies between the reference Col-0 genome, which was also used to map TK WT and QK mutant, which contain parts of Ws genome. The genes whose transcription affected by temperature (“temperature-responsive” genes) were identified using DESeq (Anders and Huber, 2010) through R Bioconductor. Further analyses on these genes were performed using their TPM values (Wagner et al., 2012), where different number of reads in libraries and transcript lengths were taken into account and normalized. The TPM values of different samples were normalized by those from the zero time point. Raw reads and process files were deposited on Gene Expression Omnibus (GSE79355). Hierarchical clustering of transcriptomic data was performed using the statistical programme R (R Development Core Team, 2011). Over-represented biological functions of temperature mis-regulated genes were assessed using GOrilla (Eden et al., 2009).
ChIP and ChIP-seq library preparation
ChIP experiment was performed as described (Gendrel et al., 2002) with minor modifications described in Supplemental Methods. In-house library preparation was done using the TruSeq ChIP sample preparation kit (Illumina, IP-202-1012) following the manufacturer’s instructions. The libraries were sequenced on HiSeq2000 and NextSeq500.
Analyses of ChIP-seq and nucleosome profiles
Sequenced ChIP-seq data were analyzed in house, following the same quality control and pre-processing as in RNA-seq. The read counts mapped to each bp in each sample were normalized by the sample’s genome-wide mappable reads coverage per bp, and used in the subsequent statistical analyses. The nucleosome and ChIP profiles were binned to generate “pile-up” ChIP profiles for different groups of genes/promoters using in-house R and Perl scripts. Nucleosome positioning and occupancy was determined using DANPOS (Chen et al., 2013). Plus one (+1) nucleosomes were defined as the first nucleosome found downstream from TSS, but not more than 250 bp from TSS. Peaks of HSFA1a ChIP-seq were called using MACS (Zhang et al., 2008).
In vitro Chromatin Immunoprecipitation (ChIP)
Seedlings expressing gHTA11::HTA11-3xFlag were grown in liquid 0.5X MS medium supplemented with 1% sucrose and vitamin B for 7 days at 22°C in long days and shifted to 17°C, 3 days prior to the material collection. The plant material was collected 1 h after dawn and immediately flash-frozen in liquid nitrogen. Nuclei were purified as previously described (Folta and Kaufman, 2006). The chromatin was digested with MNase to obtain mononucleosomes and an aliquot was taken as “Input before IP”. HTA11-3xFlag containing nucleosomes have been purified by immunoprecipitation and divided into 5 tubes: “Input after IP”, “17°C 15min”, “17°C 1h”, “27°C 15min”, “27°C 1h” (Supplemental Figure 8C). Input after IP sample was eluted with 100ng/µl of 3XFLAG in TE buffer. Other samples were incubated at 17°C or 27°C for 15 min or 1 h before washes. The nucleosomes were released with 100ng/µl of 3XFLAG in TE buffer.
In vitro nucleosome stability analysis by Single molecule FRET
A 155 bp DNA template containing a single 601 nucleosome positioning sequence with two fluorophores was generated by PCR. In all DNA constructs the donors and acceptors were separated by 76-81 bp (~24 nm), and positioned so that the FRET pair are close together in space in reconstituted nucleosomes. This way, the signal of the acceptor fluorophore is strong when the nucleosome is closed, and this signal is reduced when the nucleosome is destabilized (Supplemental Figure 8E). Two DNA constructs were generated: one with a FRET pair at the nucleosome extreme to measure DNA breathing (position Z), and the other at 27 bp from one nucleosome end to measure the nucleosome stability (Y). WT Arabidopsis thaliana H2A, H2A.Z, H2B, H3 and H4 histones were expressed in E. coli and purified as described previously (Robinson et al., 2008), and reconstituted by refolding an equimolar mixture of each of the four denatured histones by dialysis against a buffer containing 2 M NaCl. The intact histone octamers were fractionated from histone tetramers and hexamers by size-exclusion chromatography as described (Robinson et al., 2008). Two samples containing H2A and H2A.Z-Nucleosomes were mounted simultaneously on a 2-well culture insert (Ibidi) of two confined chambers. Samples and slides were equilibrated to reach desired temperature before mounting or after changing the temperature of the setup. Bursts of fluorescence were detected using the method described previously by Buning and co-workers (Buning et al., 2015).
Prediction of heat-shock elements (HSEs), regulatory motifs and target genes
Potential regulatory sequences of heat-shock proteins (HSEs) were predicted based on the consensus binding motifs using FIMO (Grant et al., 2011), as part of the MEME suite (Bailey et al., 2009). The HSE motifs and other plant TFs were analyzed using Protein Binding Microarray (PBM) by Franco-Zorrilla and co-workers (Franco-Zorrilla et al., 2014), whereas that of NF-Y was from the JASPAR database (Mathelier et al., 2014). Target genes were identified using PeakAnalyzer where their TSS are within 1 kb from the predicted TF binding sites or the peaks of ChIP-seq (Salmon-Divon et al., 2010).
Supplementary Material
Acknowledgements
S.C. was supported by an EMBO long-term fellowship. The Charoensawan lab is supported by the Thailand Research Fund (TRF) Grant for New Researcher [TRG5880067], Faculty of Science, Mahidol University, and the Crown Property Bureau Foundation. This work was supported by the Biotechnology and Biology Research Council [BB/I013350/1 to P.A.W. and D.R.]; P.A.W’s laboratory is supported by a Fellowship from the Gatsby Foundation [GAT3273/GLB]. We thank Pakkanan Chansongkrow for technical assistance on the analyses.
Footnotes
Conflict of interest
The authors have no conflicts of interest.
Accession numbers
Sequence data from this article can be found in the Gene Expression Omnibus under accession number (GSE79355).
Author contributions
S.C., V.C. and P.A.W conceived and designed the research. S.C., V.C., A.B., R.B., C.R., K.E.J. performed the experiments, under the supervisions of J.N., D.R. and P.A.W. S.C. and V.C. analyzed the data. S.C., V.C. and P.A.W wrote the manuscript, which was proofread and approved by all the authors.
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic acids research. 2009;37:W202–208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS genetics. 2006;2:26. doi: 10.1371/journal.pgen.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battisti DS, Naylor RL. Historical warnings of future food insecurity with unprecedented seasonal heat. Science. 2009;323:240–244. doi: 10.1126/science.1164363. [DOI] [PubMed] [Google Scholar]
- Box MS, Huang BE, Domijan M, Jaeger KE, Khattak AK, Yoo SJ, Sedivy EL, Jones DM, Hearn TJ, Webb AA, et al. ELF3 controls thermoresponsive growth in Arabidopsis. Curr Biol. 2015;25:194–199. doi: 10.1016/j.cub.2014.10.076. [DOI] [PubMed] [Google Scholar]
- Boyd M, Coskun M, Lilje B, Andersson R, Hoof I, Bornholdt J, Dahlgaard K, Olsen J, Vitezic M, Bjerrum JT, et al. Identification of TNF-alpha-responsive promoters and enhancers in the intestinal epithelial cell model Caco-2. DNA research : an international journal for rapid publication of reports on genes and genomes. 2014;21:569–583. doi: 10.1093/dnares/dsu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buning R, Kropff W, Martens K, van Noort J. spFRET reveals changes in nucleosome breathing by neighboring nucleosomes. Journal of physics. Condensed matter : an Institute of Physics journal. 2015;27:064103. doi: 10.1088/0953-8984/27/6/064103. [DOI] [PubMed] [Google Scholar]
- Charoensawan V, Janga SC, Bulyk ML, Babu MM, Teichmann SA. DNA sequence preferences of transcriptional activators correlate more strongly than repressors with nucleosomes. Molecular cell. 2012;47:183–192. doi: 10.1016/j.molcel.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xi Y, Pan X, Li Z, Kaestner K, Tyler J, Dent S, He X, Li W. DANPOS: dynamic analysis of nucleosome position and occupancy by sequencing. Genome research. 2013;23:341–351. doi: 10.1101/gr.142067.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Park C, Lee J, Oh M, Noh B, Lee I. Arabidopsis homologs of components of the SWR1 complex regulate flowering and plant development. Development (Cambridge, England) 2007;134:1931–1941. doi: 10.1242/dev.001891. [DOI] [PubMed] [Google Scholar]
- Deal RB, Kandasamy MK, McKinney EC, Meagher RB. The nuclear actin-related protein ARP6 is a pleiotropic developmental regulator required for the maintenance of FLOWERING LOCUS C expression and repression of flowering in Arabidopsis. The Plant cell. 2005;17:2633–2646. doi: 10.1105/tpc.105.035196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC bioinformatics. 2009;10:48. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezer D, Jung JH, Lan H, Biswas S, Gregoire L, Box MS, Charoensawan V, Cortijo S, Lai X, Stockle D, et al. The evening complex coordinates environmental and endogenous signals in Arabidopsis. Nature plants. 2017;3:17087. doi: 10.1038/nplants.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Kaufman LS. Isolation of Arabidopsis nuclei and measurement of gene transcription rates using nuclear run-on assays. Nature protocols. 2006;1:3094–3100. doi: 10.1038/nprot.2006.471. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Lopez-Vidriero I, Carrasco JL, Godoy M, Vera P, Solano R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2367–2372. doi: 10.1073/pnas.1316278111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Takaki E, Takii R, Tan K, Prakasam R, Hayashida N, Iemura S, Natsume T, Nakai A. RPA assists HSF1 access to nucleosomal DNA by recruiting histone chaperone FACT. Molecular cell. 2012;48:182–194. doi: 10.1016/j.molcel.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Gao H, Brandizzi F, Benning C, Larkin RM. A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16398–16403. doi: 10.1073/pnas.0808463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel AV, Lippman Z, Yordan C, Colot V, Martienssen RA. Dependence of heterochromatic histone H3 methylation patterns on the Arabidopsis gene DDM1. Science. 2002;297:1871–1873. doi: 10.1126/science.1074950. [DOI] [PubMed] [Google Scholar]
- Gonsalves SE, Moses AM, Razak Z, Robert F, Westwood JT. Whole-genome analysis reveals that active heat shock factor binding sites are mostly associated with non-heat shock genes in Drosophila melanogaster. PloS one. 2011;6:e15934. doi: 10.1371/journal.pone.0015934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant physiology. 2011;157:1243–1254. doi: 10.1104/pp.111.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Hirt H, Bendahmane A. The heat-shock protein/chaperone network and multiple stress resistance. Plant biotechnology journal. 2017;15:405–414. doi: 10.1111/pbi.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Domijan M, Klose C, Biswas S, Ezer D, Gao M, Khattak AK, Box MS, Charoensawan V, Cortijo S, et al. Phytochromes function as thermosensors in Arabidopsis. Science. 2016;354:886–889. doi: 10.1126/science.aaf6005. [DOI] [PubMed] [Google Scholar]
- Klepsatel P, Galikova M, Xu Y, Kuhnlein RP. Thermal stress depletes energy reserves in Drosophila. Scientific reports. 2016;6:33667. doi: 10.1038/srep33667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koini MA, Alvey L, Allen T, Tilley CA, Harberd NP, Whitelam GC, Franklin KA. High temperature-mediated adaptations in plant architecture require the bHLH transcription factor PIF4. Curr Biol. 2009;19:408–413. doi: 10.1016/j.cub.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alos E, Alvey E, Harberd NP, Wigge PA. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature. 2012;484:242–245. doi: 10.1038/nature10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Lam FH, Steger DJ, O'Shea EK. Chromatin decouples promoter threshold from dynamic range. Nature. 2008;453:246–250. doi: 10.1038/nature06867.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant physiology. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro A, Gomez-Zambrano A, Lopez-Gonzalez L, Pineiro M, Jarillo JA. Mutations in the Arabidopsis SWC6 gene, encoding a component of the SWR1 chromatin remodelling complex, accelerate flowering time and alter leaf and flower development. Journal of experimental botany. 2008;59:653–666. doi: 10.1093/jxb/erm332. [DOI] [PubMed] [Google Scholar]
- Legris M, Klose C, Burgie ES, Rojas CC, Neme M, Hiltbrunner A, Wigge PA, Schafer E, Vierstra RD, Casal JJ. Phytochrome B integrates light and temperature signals in Arabidopsis. Science. 2016;354:897–900. doi: 10.1126/science.aaf5656. [DOI] [PubMed] [Google Scholar]
- Lin RC, Park HJ, Wang HY. Role of Arabidopsis RAP2.4 in regulating light- and ethylene-mediated developmental processes and drought stress tolerance. Molecular plant. 2008;1:42–57. doi: 10.1093/mp/ssm004. [DOI] [PubMed] [Google Scholar]
- Liu HC, Charng YY. Common and distinct functions of Arabidopsis class A1 and A2 heat shock factors in diverse abiotic stress responses and development. Plant physiology. 2013;163:276–290. doi: 10.1104/pp.113.221168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HC, Liao HT, Charng YY. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant, cell & environment. 2011;34:738–751. doi: 10.1111/j.1365-3040.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang C, Chen J, Guo L, Li X, Li W, Yu Z, Deng J, Zhang P, Zhang K, et al. Arabidopsis heat shock factor HsfA1a directly senses heat stress, pH changes, and hydrogen peroxide via the engagement of redox state. Plant physiology and biochemistry: PPB / Societe francaise de physiologie vegetale. 2013;64:92–98. doi: 10.1016/j.plaphy.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Mahat DB, Salamanca HH, Duarte FM, Danko CG, Lis JT. Mammalian Heat Shock Response and Mechanisms Underlying Its Genome-wide Transcriptional Regulation. Molecular cell. 2016;62:63–78. doi: 10.1016/j.molcel.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March-Diaz R, Reyes JC. The beauty of being a variant: H2A.Z and the SWR1 complex in plants. Molecular plant. 2009;2:565–577. doi: 10.1093/mp/ssp019. [DOI] [PubMed] [Google Scholar]
- Mathelier A, Zhao X, Zhang AW, Parcy F, Worsley-Hunt R, Arenillas DJ, Buchman S, Chen CY, Chou A, Ienasescu H, et al. JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic acids research. 2014;42:D142–147. doi: 10.1093/nar/gkt997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milo R, Shen-Orr S, Itzkovitz S, Kashtan N, Chklovskii D, Alon U. Network motifs: simple building blocks of complex networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- Miozzo F, Saberan-Djoneidi D, Mezger V. HSFs, Stress Sensors and Sculptors of Transcription Compartments and Epigenetic Landscapes. Journal of molecular biology. 2015;427:3793–3816. doi: 10.1016/j.jmb.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Nomoto Y, Oka H, Kitayama M, Takeuchi A, Tsubouchi M, Yamashino T. Ambient temperature signal feeds into the circadian clock transcriptional circuitry through the EC night-time repressor in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:958–976. doi: 10.1093/pcp/pcu030. [DOI] [PubMed] [Google Scholar]
- Mueller B, Mieczkowski J, Kundu S, Wang P, Sadreyev R, Tolstorukov MY, Kingston RE. Widespread changes in nucleosome accessibility without changes in nucleosome occupancy during a rapid transcriptional induction. Genes & development. 2017;31:451–462. doi: 10.1101/gad.293118.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Molecular biology of the cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh YS, Amasino RM. PIE1, an ISWI family gene, is required for FLC activation and floral repression in Arabidopsis. The Plant cell. 2003;15:1671–1682. doi: 10.1105/tpc.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley RC, Huang SS, Song L, Lewsey MG, Bartlett A, Nery JR, Galli M, Gallavotti A, Ecker JR. Cistrome and Epicistrome Features Shape the Regulatory DNA Landscape. Cell. 2016;165:1280–1292. doi: 10.1016/j.cell.2016.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obri A, Ouararhni K, Papin C, Diebold ML, Padmanabhan K, Marek M, Stoll I, Roy L, Reilly PT, Mak TW, et al. ANP32E is a histone chaperone that removes H2A.Z from chromatin. Nature. 2014;505:648–653. doi: 10.1038/nature12922. [DOI] [PubMed] [Google Scholar]
- Ohama N, Kusakabe K, Mizoi J, Zhao H, Kidokoro S, Koizumi S, Takahashi F, Ishida T, Yanagisawa S, Shinozaki K, et al. The Transcriptional Cascade in the Heat Stress Response of Arabidopsis Is Strictly Regulated at the Level of Transcription Factor Expression. The Plant cell. 2016;28:181–201. doi: 10.1105/tpc.15.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends in plant science. 2017;22:53–65. doi: 10.1016/j.tplants.2016.08.015. [DOI] [PubMed] [Google Scholar]
- Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011;144:200–213. doi: 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Dyer PN, Tremethick DJ, Luger K. A new fluorescence resonance energy transfer approach demonstrates that the histone variant H2AZ stabilizes the histone octamer within the nucleosome. The Journal of biological chemistry. 2004;279:24274–24282. doi: 10.1074/jbc.M313152200. [DOI] [PubMed] [Google Scholar]
- Raschke A, Ibanez C, Ullrich KK, Anwer MU, Becker S, Glockner A, Trenner J, Denk K, Saal B, Sun X, et al. Natural variants of ELF3 affect thermomorphogenesis by transcriptionally modulating PIF4-dependent auxin response genes. BMC plant biology. 2015;15:197. doi: 10.1186/s12870-015-0566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, An W, Routh A, Martino F, Chapman L, Roeder RG, Rhodes D. 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. Journal of molecular biology. 2008;381:816–825. doi: 10.1016/j.jmb.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Qin F, Osakabe Y, Shinozaki K, Yamaguchi-Shinozaki K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heat-stress-responsive gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:18822–18827. doi: 10.1073/pnas.0605639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon-Divon M, Dvinge H, Tammoja K, Bertone P. PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC bioinformatics. 2010;11:415. doi: 10.1186/1471-2105-11-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Werner J, Andrulis ED, Nakayama T, Hirose S, Reinberg D, Lis JT. Tracking FACT and the RNA polymerase II elongation complex through chromatin in vivo. Science. 2003;301:1094–1096. doi: 10.1126/science.1085712. [DOI] [PubMed] [Google Scholar]
- Schramm F, Larkindale J, Kiehlmann E, Ganguli A, Englich G, Vierling E, von Koskull-Doring P. A cascade of transcription factor DREB2A and heat stress transcription factor HsfA3 regulates the heat stress response of Arabidopsis. The Plant journal : for cell and molecular biology. 2008;53:264–274. doi: 10.1111/j.1365-313X.2007.03334.x. [DOI] [PubMed] [Google Scholar]
- Shivaswamy S, Iyer VR. Stress-dependent dynamics of global chromatin remodeling in yeast: dual role for SWI/SNF in the heat shock stress response. Molecular and cellular biology. 2008;28:2221–2234. doi: 10.1128/MCB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Mao Y, Regier MK, Triezenberg SJ, Thomashow MF. Transcriptional adaptor and histone acetyltransferase proteins in Arabidopsis and their interactions with CBF1, a transcriptional activator involved in cold-regulated gene expression. Nucleic acids research. 2001;29:1524–1533. doi: 10.1093/nar/29.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenkert D, Schmollinger S, Schroda M. Heat shock factor 1 counteracts epigenetic silencing of nuclear transgenes in Chlamydomonas reinhardtii. Nucleic acids research. 2013;41:5273–5289. doi: 10.1093/nar/gkt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AM, Arsovski AA, Lempe J, Bubb KL, Weirauch MT, Sabo PJ, Sandstrom R, Thurman RE, Neph S, Reynolds AP, et al. Mapping and dynamics of regulatory DNA and transcription factor networks in A. thaliana. Cell Rep. 2014;8:2015–2030. doi: 10.1016/j.celrep.2014.08.019. [DOI] [PubMed] [Google Scholar]
- Sura W, Kabza M, Karlowski WM, Bieluszewski T, Kus-Slowinska M, Paweloszek L, Sadowski J, Ziolkowski PA. Dual Role of the Histone Variant H2A.Z in Transcriptional Regulation of Stress-Response Genes. The Plant cell. 2017;29:791–807. doi: 10.1105/tpc.16.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichmann SA, Wigge PA, Charoensawan V. Uncovering the interplay between DNA sequence preferences of transcription factors and nucleosomes. Cell Cycle. 2012;11:4487–4488. doi: 10.4161/cc.22666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar A, Gupta P, McAllister WT, Zlatanova J. Histone variant H2A.Z inhibits transcription in reconstituted nucleosomes. Biochemistry. 2010;49:4018–4026. doi: 10.1021/bi1001618. [DOI] [PubMed] [Google Scholar]
- Vercruyssen L, Verkest A, Gonzalez N, Heyndrickx KS, Eeckhout D, Han SK, Jegu T, Archacki R, Van Leene J, Andriankaja M, et al. ANGUSTIFOLIA3 binds to SWI/SNF chromatin remodeling complexes to regulate transcription during Arabidopsis leaf development. The Plant cell. 2014;26:210–229. doi: 10.1105/tpc.113.115907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Koskull-Dˆring P, Scharf K-D, Nover L. The diversity of plant heat stress transcription factors. Trends in plant science. 2007;12:452–457. doi: 10.1016/j.tplants.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Kin K, Lynch VJ. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory in biosciences = Theorie in den Biowissenschaften. 2012;131:281–285. doi: 10.1007/s12064-012-0162-3. [DOI] [PubMed] [Google Scholar]
- Weber CM, Ramachandran S, Henikoff S. Nucleosomes are context-specific, H2A.Z-modulated barriers to RNA polymerase. Molecular cell. 2014;53:819–830. doi: 10.1016/j.molcel.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Wigge PA. Ambient temperature signalling in plants. Current opinion in plant biology. 2013;16:661–666. doi: 10.1016/j.pbi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Wu MF, Sang Y, Bezhani S, Yamaguchi N, Han SK, Li Z, Su Y, Slewinski TL, Wagner D. SWI2/SNF2 chromatin remodeling ATPases overcome polycomb repression and control floral organ identity with the LEAFY and SEPALLATA3 transcription factors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:3576–3581. doi: 10.1073/pnas.1113409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelagandula R, Stroud H, Holec S, Zhou K, Feng S, Zhong X, Muthurajan UM, Nie X, Kawashima T, Groth M, et al. The histone variant H2A.W defines heterochromatin and promotes chromatin condensation in Arabidopsis. Cell. 2014;158:98–109. doi: 10.1016/j.cell.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim JM, Seki M, Todaka D, et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Molecular genetics and genomics : MGG. 2011;286:321–332. doi: 10.1007/s00438-011-0647-7. [DOI] [PubMed] [Google Scholar]
- Zanton SJ, Pugh BF. Full and partial genome-wide assembly and disassembly of the yeast transcription machinery in response to heat shock. Genes & development. 2006;20:2250–2265. doi: 10.1101/gad.1437506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Jing Y, Jiang Z, Lin R. The Chromatin-Remodeling Factor PICKLE Integrates Brassinosteroid and Gibberellin Signaling during Skotomorphogenic Growth in Arabidopsis. The Plant cell. 2014;26:2472–2485. doi: 10.1105/tpc.113.121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, et al. Model-based analysis of ChIP-Seq (MACS) Genome biology. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Yang S, Chen CY, Li C, Shan W, Lu W, Cui Y, Liu X, Wu K. Arabidopsis BREVIPEDICELLUS interacts with the SWI2/SNF2 chromatin remodeling ATPase BRAHMA to regulate KNAT2 and KNAT6 expression in control of inflorescence architecture. PLoS genetics. 2015;11:e1005125. doi: 10.1371/journal.pgen.1005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Coleman-Derr D, Ballinger T, Henikoff S. Histone H2A.Z and DNA methylation are mutually antagonistic chromatin marks. Nature. 2008;456:125–129. doi: 10.1038/nature07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobeck KL, Buckley MS, Zipfel WR, Lis JT. Recruitment timing and dynamics of transcription factors at the Hsp70 loci in living cells. Molecular cell. 2010;40:965–975. doi: 10.1016/j.molcel.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.