Abstract
Although much is now known about the mechanisms of radiation-induction of DNA double-strand breaks (DSB), there is less known about the conversion of DSB into chromosomal aberrations. In particular the induction and ‘rejoining’ of chromatid breaks has been a controversial topic for many years. However, its importance becomes clear in the light of the wide variation in the chromatid break response of human peripheral blood lymphocytes from different individuals when exposed to ionizing radiation, and the elevation of the frequency of radiation-induced chromatid breaks in stimulated peripheral blood lymphocytes of around 40% of breast cancer cases. A common assumption has been that chromatid breaks are merely expansions of initiating DSB, although the classic ‘breakage-first’ hypothesis (Sax, Ref. 44) was already challenged in the 50’s by Revell [30] who maintained that chromatid breaks were formed as a result of an incomplete exchange process initiated by two interacting lesions of an unspecified nature. Here we argue that both these models of chromatid break formation are flawed and we suggest an alternative hypothesis, namely that a radiation-induced DSB initiates an indirect mechanism leading to a chromatid break. This mechanism we suggest involves the nuclear enzyme topoisomerase IIα and we present evidence from topoisomerase IIα expression variant human cell lines and from siRNA treatment of human cells that supports this hypothesis.
Keywords: DNA double-strand break; Radiation; Chromatid break mechanisms; Topoisomerase IIα, siRNA; Human lymphocytes
1. Introduction
The chromosomal aberration response of cells from different individuals to a standard dose (0.5 Gy) of ionizing radiation shows a wide variation. This variation is strikingly demonstrated by the spread of frequencies of radiation-induced chromatid breaks observed in metaphase chromosomes of radiation-exposed phytohaemagglutinin-stimulated peripheral blood T-lymphocytes (PBL) from different normal individuals and sporadic breast and other cancer cases [1–11]. It has been proposed that the wide variation in ‘chromosomal radiosensitivity’ seen in PBL from different individuals, and elevation of radiosensitivity (with respect to controls) in approximately 80% of sporadic breast cancer cases (comprising some 40% showing radiosensitivity in the micronucleus test as well as about 40% showing radiosensitivity in the G2-assay of chromatid breaks) might result from the presence of mutations in low-penetrance genes that concomitantly confer both radiosensitivity and susceptibility to the disease [1–3]. Studies of monozygotic twins have also suggested that a high proportion of sporadic breast cancer cases are thought to occur in a susceptible sub-population as a result of mutations in such low-penetrance genes [12,13]. Carriers of the highly penetrant genes (e.g. BRCA1 and 2) however only account for 5 - 10% of cases. It has been suggested that mutations in low-penetrance susceptibility genes may also compromise DNA damage processing, lead to genome instability and confer chromosomal radiosensitivity [2,3,14]. Here we review and discuss evidence accumulating for a novel mechanism for chromatid breakage that could contribute to our understanding of chromosomal radiosensitivity.
For actively dividing lymphocytes, and a time interval of 1.5 h (in the presence of colcemid) between the radiation exposure and harvesting, the mitotic PBLs collected are primarily those that were in mid-late G2-phase of the cell-cycle at the time of exposure. The chromosomes of these mitotic cells show single chromatid breaks, defined as discontinuities in one chromatid arm that would potentially lead to terminal deletion of that segment of the chromatid. Such chromatid breaks seen at a fixed time after irradiation are induced linearly with radiation dose [15] indicating a ‘one-hit’ mechanism. We also know that DNA double-strand breaks (DSB) are the principal initiating lesions for chromatid breaks [16–18] and experiments using cells containing a single I-SceI site showed that only one DSB is required to initiate a chromatid break [19]. Other studies have since confirmed this (e.g. [20]). The question arises as to how a DSB leads to a chromatid break. Since the DSB is a sub-microscopic lesion, and chromatid breaks are, from their width, equivalent to the loss of many mega-bases of DNA, a mechanism involving more than just simple expansion of the DSB during chromatin condensation as a cell approaches mitosis seems probable.
2. Possible influence of cell-cycle perturbation on chromatid break frequency
Cell-cycle pertubation is a factor that could possibly influence observed frequencies of chromatid breaks, and in a study using human tumour cell lines Schwartz et al. [21] demonstrate an inverse relationship between radiation-induced mitotic inhibition and chromatid break frequency. However, studies of PBL from sporadic breast cancer patients and normal controls show only a very weak or insignificant correlation between cell-cycle delay and chromatid break frequency at the low radiation dose (0.5 Gy) used in the so-called ‘G2-assay’ of chromatid breaks [22,23]. Therefore, although cell-cycle checkpoint delay may have a small influence on the observed frequency of chromatid breaks in the G2 assay of actively dividing PBL, it is unlikely to be a major determinant of the chromatid break frequency.
3. Models of chromatid break formation
Although the disappearance of chromatid breaks with time following irradiation has been interpreted in terms of the classical ‘breakage-first’ model of Sax [44] by some authors, and has even been interpreted as a surrogate for DSB rejoining [24,25] we have previously noted a striking lack of correlation between the kinetics of the disappearance of chromatid breaks between irradiation and harvesting and kinetics of bulk DSB rejoining following radiation exposure. One of the most impressive examples is the lack of correspondence between ‘rejoining’ of chromatid breaks with time and DSB rejoining in the presence of the nucleoside analogue 9-β-D-arabinofuranosyladenine (araA). While rejoining of chromatid breaks was completely abrogated, rejoining of DSB was not affected [26]. Another example was provided by comparing the rejoining kinetics of chromatid breaks in wild-type CHO and Ku80 mutant xrs5 cells after irradiation. In the non-homologous DSB end-joining defective mutant chromatid break rejoining was found to be normal, again demonstrating a lack of correspondence between chromatid break and DSB rejoining [27,28]. With such data now available, the established classical view of radiation-induced chromatid breaks, that they are no more than expanded DSB [29], has to be questioned.
We have discussed Revell’s ‘exchange’ model [30] previously in more detail [26,31]. The exchange model differs from Sax’s breakage-first model [44] in that it proposes that a chromatid break results from an interaction between two initiating lesions. The model does not specify DSB (since these lesions were unknown at the time) as the initiating or interacting lesions, but one assumes that at some stage DNA must be broken for exchanges to take place, and involves mis-joining of two chromatin ends. Thus, a chromatid break would be caused if the exchange was incomplete at cell harvest. The exchange model postulates that two ‘hits’ would be required to form a chromatid break, and as a result a dose-squared relationship for chromatid breaks would be predicted. As mentioned above, chromatid breaks in human lymphocytes are formed as a linear function of dose, arguing against this two-hit model.
Also, Revell [30] predicted that the five different types of chromatid breaks would be equally likely, leading to a prediction that two out of the five types would be interchromatid exchanges. However, this prediction was not fulfilled since it was found that the frequency of interchromatid breaks is around 16% in both irradiated cells and cells treated with restriction endonucleases, as well as occurring spontaneously in unirradiated cells where the formation of two interacting lesions would be extremely unlikely (e.g. [32]), adding further support for a ‘one-hit’ model of chromatid breakage.
We previously proposed an alternative hypothesis that chromatid breaks are formed indirectly by the presence of a DSB within a chromatin loop domain and suggested that part of this process could result from the presence of a DSB during chromatid decatenation by topoisomerase IIα [15,26]. The ‘signal’ model [15] postulated that a ‘signalled’ DSB is the initiating lesion leading to a chromatid break via an intermediate step that could be the distortion of chromatin loop structure such that the process of decatenation of chromatids by topoisomerase IIα is disrupted, resulting in mis-joining of chromatin ends. In addition, topoisomerase IIα that is situated at the base of the chromatin loops can be poisoned by the simultaneous endogenous production of reactive oxygen species (ROS) in the same ionizing track that causes the initiating DSB (so maintaining a one-hit dose-response). Such poisoning of topoisomerase IIα by ROS has been demonstrated by treatment of human leukaemic cells with H2O2, which can activate topoisomerase IIα-mediated excision of chromatin loops [33]. Similarly, topoisomerase IIα can be poisoned by the presence of endogenous abasic sites in the DNA [34].
4. The role of topoisomerase IIα in the cell
Topoisomerase IIα is a DNA processing enzyme located in the cell nucleus at the base of looped chromatin domains comprising some 30-100 kbp, and unlike its sister isoform, topoisomerase IIβ, is expressed variably through the cell-cycle and at an increased level during G2, where chromatid decatenation takes place as cells progress towards mitosis [35–37]. In contrast, the β-isoform is expressed uniformly throughout the cell-cycle [38]. When poisoned, for example with etoposide, topoisomerase IIα will cause a protein-associated DSB [39]. Inhibition of topoisomerase IIα, for example by ICRF-193, prevents the dimer from opening, hence no DSB is induced [40].
5. Evidence for the role of topoisomerase IIα in chromatid break formation
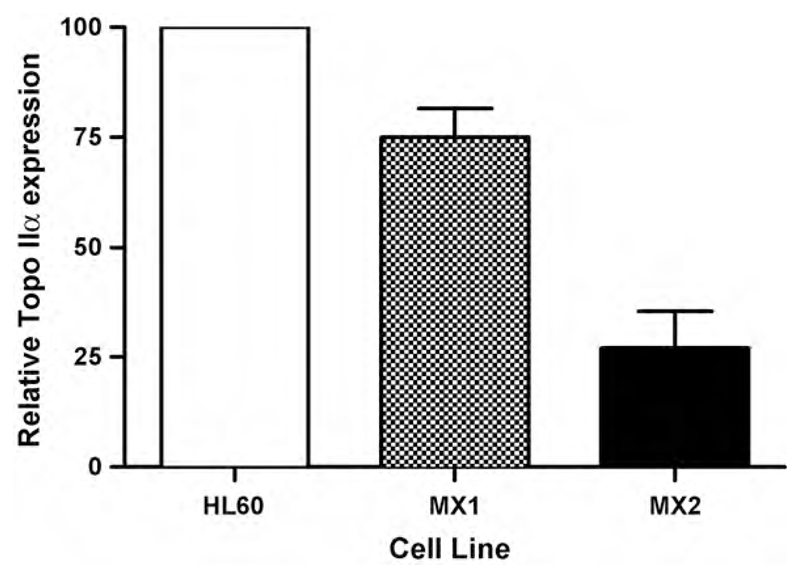
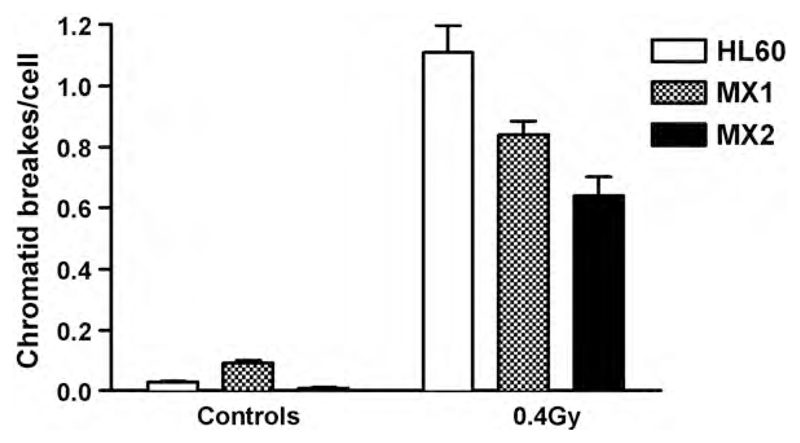
Recently [41] we have been able to test the involvement of topoisomerase IIα in the formation of chromatid breaks. Two variants of the human promyelocytic leukaemia (HL60) cell line have constitutive reduced expression of topoisomerase IIα. These variants (MX1 and MX2) were isolated many years ago after prolonged selection in the topoisomerase IIα poison mitoxantrone [42]. We confirmed the lowered expression of topoisomerase IIα using western blotting (Fig. 1) and showed that the chromosomal radiosensitivity, as measured by the frequency of chromatid breaks in the G2-assay, was correspondingly reduced with decreasing topoisomerase IIα expression (Fig. 2). Also, as a check on the phenotype of the variant lines, we also established that DSB rejoining was not different between the ‘normal’ HL60 and topoisomerase IIα variants.
Fig. 1.
Shows the relative expression level of topoisomerase IIα in promyelocytic leukaemic HL60 cells and two mitoxantrone-resistant expression variants (MX1 and MX2). Data was derived from Western blot analysis of cell extracts. Error bars represent standard errors of mean values from at least three experiments.
Fig. 2.
Shows the frequency of chromatid breaks in both irradiated (0.4 Gy) and unirradiated control samples of HL60, MX1 and MX2 cells measured in colcemid-blocked metaphases 1.5 h after irradiation. Error bars represent standard errors of mean values from 2 pooled experiments.
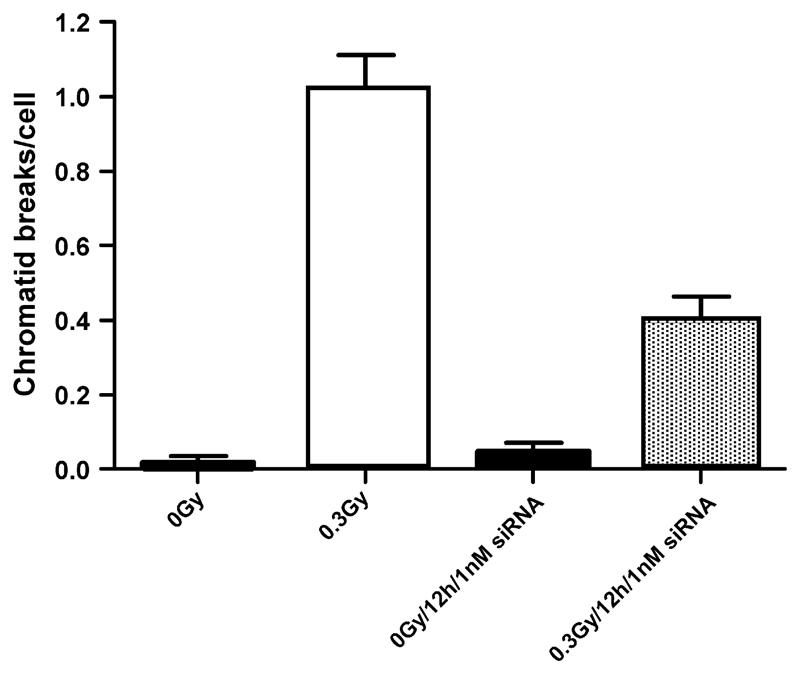
In another series of experiments [43] we used silencing RNA (siRNA) against topoisomerase IIα to transiently reduce its expression in human retinal pigment cells (hTERT-RPE) transformed with the hTERT gene (telomere reverse transcriptase). Following treatment for 12 h with siRNA, cells exposed to gamma-rays (0.3 Gy) were found to show significantly fewer chromatid breaks than untreated control cells (Fig. 3). The possibility that changes in cell-cycle progression were brought about by the siRNA could be eliminated by the fact that the mitotic index in siRNA-treated and irradiated cells was not significantly reduced [43].
Fig. 3.
Shows the frequency of chromatid breaks in irradiated and unirradiated control samples of hTERT-RPE cells with or without treatment with siRNA against topoisomerase IIα for 12 h. Error bars represent standard errors of mean values from at two experiments.
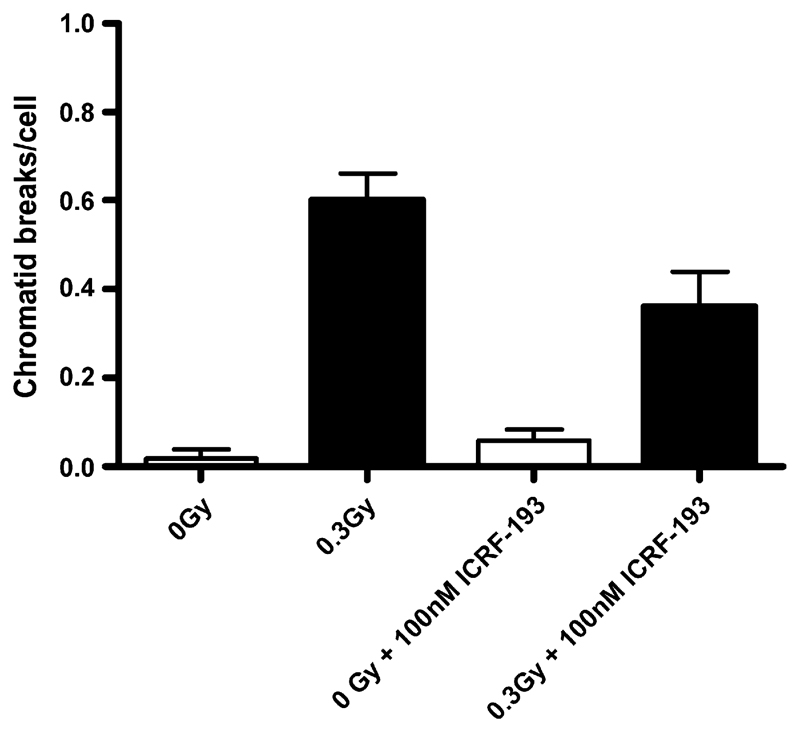
In a third strategy [43] we used the inhibitor ICRF-193 to reduce topoisomerase IIα function in hTERT-RPE cells. We found that reducing the function of topoisomerase IIα also led to a significant reduction in the frequency of radiation-induced chromatid breaks (Fig. 4).
Fig. 4.
Shows the frequency of chromatid breaks in irradiated and unirradiated hTERT-RPE cells treated with the topoisomerase inhibitor ICRF-193. Error bars represent standard errors of mean values from two experiments.
6. Discussion
Taken together, these experiments clearly suggest a significant role for topoisomerase IIα in formation of radiation-induced chromatid breaks and we interpret our findings as supportive of our proposed indirect mechanism for the formation of chromatid breaks, from initial DSB. We eliminated the possibility that reduced expression or functionality of topoisomerase IIα could affect DSB rejoining and also that cell-cycle kinetics were altered in such a way as to increase the time available for DSB rejoining to take place. Therefore we concluded that topoisomerase IIα is involved as an intermediate step in the formation of chromatid breaks. What could be the role for topoisomerase IIα in the progression from a DSB to a chromatid break?
One possibility is that during chromatid decatenation two (or more) topoisomerase IIα units, each comprising a dimer of the enzyme and located at the crossover points of chromatin loops, are poisoned by the presence of ROS sites in the vicinity. Coupled with the conformational changes in a chromatin loop brought about by the presence of a DSB, misjoining of the chromatin (30 nm fibre) ends produced by topoisomerase IIα scission of the DNA could occur, leading to a chromatid break as the cell progresses towards mitosis. A reduction in the number of topoisomerase IIα dimers at the loop crossover points (as in the experiments described here) would then reduce the probability of misjoining between chromatin ends and hence reduce the number of chromatid breaks resulting from exposure to a given dose of radiation.
It has been shown in many studies that when restriction endonucleases (producing DSB with 3′ hydroxyl and 5′ phosphoryl ends) are introduced into cells by membrane poration, they cause multiple chromosome and chromatid aberrations including chromatid breaks [17,18]. The DSB caused by such treatment would not be of the same type as those DSB with ‘dirty’ ends produced in cellular DNA by ionizing radiation. Moreover, there would be no additional ROS produced by this treatment. However, cells contain a background level of ROS that are produced during cell metabolism, and one could speculate that these ROS could poison topoisomerase IIα and, in interacting with a DSB, lead to a chromatid break. Such a model of chromatid breakage would, if applied to ionizing radiation, still yield a linear induction of chromatid breaks.
The variability in the chromosomal radiosensitivity of cells from different normal individuals, and different sporadic breast and other cancer sufferers, is thought to be due to mutations in low penetrance genes that have so far eluded identification. It is possible that variation in the expression of topoisomerase IIα in the stimulated PBL of these individuals could be partly responsible.
7. Conclusions
The classic breakage-first, and exchange models are not satisfactory explanations for the mechanism of radiation-induced chromatid breaks, and evidence (principally the lack of correlation between kinetics of rejoining of chromatid breaks and rejoining of DSB) suggests an indirect mechanism initiated by a single DSB, which is itself not directly involved.
We show that the mechanism underlying chromatid breaks involves topoisomerase IIα (dimers of which are present at the base of each chromatin loop) since reducing its expression or inhibiting the function of the enzyme significantly lowers radiation-induced chromatid break frequency. We postulate that DSB-initiated changes in chromatin loop structure during chromatid decatenation may cause topoisomerase IIα to become error-prone and lead to mis-joining of chromatid ends, which if incomplete are manifest as chromatid breaks.
Acknowledgements
The work was supported in part by the Breast Cancer Campaign and the Chief Scientist Office (Scottish Executive).
Footnotes
Conflict of interest
None.
References
- [1].Scott D, Spreadborough A, Levine E, Roberts SA. Genetic predisposition to breast cancer. Lancet. 1994;344:1444. doi: 10.1016/s0140-6736(94)90615-7. [DOI] [PubMed] [Google Scholar]
- [2].Scott D, Barber JBP, Spreadborough AR, Burrill W, Roberts SA. Increased radiosensitivity in breast cancer patients: a comparison of two assays. Int J Radiat Biol. 1999;75:1–10. doi: 10.1080/095530099140744. [DOI] [PubMed] [Google Scholar]
- [3].Roberts SA, Spreadborough AR, Bulman B, Barber JBP, Evans DRG, Scott D. Heritability of cellular radiosensitivity: A marker of low penetrance predisposition genes in breast cancer? Am J Human Genet. 1999;65:784–794. doi: 10.1086/302544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baria K, Warren C, Roberts SA, West CM, Scott D. Chromosomal radiosensitivity as a marker of predisposition to common cancers. Br J Cancer. 2001;84:892–896. doi: 10.1054/bjoc.2000.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baria K, Warren C, Eden OB, Roberts SA, West CM, Scott D. Chromosomal radiosensitivity in young cancer patients: possible evidence of genetic predisposition. Int J Radiat Biol. 2002;78:341–346. doi: 10.1080/09553000110117359. [DOI] [PubMed] [Google Scholar]
- [6].Buchholz TA, Wu X. Radiation-induced chromatid breaks as a predictor of breast cancer risk. Int J Radiat Oncol Biol Phys. 2001;49:533–537. doi: 10.1016/s0360-3016(00)01502-9. [DOI] [PubMed] [Google Scholar]
- [7].Riches AC, Bryant PE, Steel CM, Gleig A, Robertson AJ, Preece PE, Thompson AM. Chromosomal radiosensitivity in G2 phase lymphocytes identifies breast cancer patients with distinctive tumour characteristics. Br J Cancer. 2001;85:1157–11561. doi: 10.1054/bjoc.2001.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Papworth R, Slevin N, Roberts SA, Scott D. Sensitivity to radiation-induced chromosome damage may be a marker of genetic predisposition in young head and neck cancer patients. Br J Cancer. 2001;84:776–782. doi: 10.1054/bjoc.2000.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smart V, Curwen GB, Whitehouse CA, Edwards A, Tawn EJ. Chromosomal radiosensitivity: a study of the chromosomal G2 assay in human blood lymphocytes indicating significant inter-individual variability. Mutat Res. 2003;528:105–110. doi: 10.1016/s0027-5107(03)00076-9. [DOI] [PubMed] [Google Scholar]
- [10].Baeyens A, Thierens H, Claes K, Poppe B, Messiaen L, de Ridder L, Vral A. Chromosomal radiosensitivity in breast cancer patients with a known or putative genetic predisposition. Br J Cancer. 2002;87:1379–1385. doi: 10.1038/sj.bjc.6600628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baeyens A, Broecke RVD, Makar A, Thierens H, de Ridder L, Vral A. Chromosomal radiosensitivity in breast cancer patients: Influence of age of onset of the disease. Oncol Rep. 2005;13:347–353. [PubMed] [Google Scholar]
- [12].Peto J, Mack TM. High constant incidence in twins and other relatives of women with breast cancer. Nature Genet. 2000;26:411–414. doi: 10.1038/82533. [DOI] [PubMed] [Google Scholar]
- [13].Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer — Analyses of cohorts of twins from Sweden, Denmark, and Finland. New Eng, J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- [14].Peto J, Houlston RS. Genetics and the common cancers. Eur J Cancer. 2001;37:S88–S96. doi: 10.1016/s0959-8049(01)00255-6. [DOI] [PubMed] [Google Scholar]
- [15].Bryant PE. The signal model: a possible explanation for the conversion of DNA double-strand breaks into chromatid breaks. Int J Radiat Biol. 1998;73:243–251. doi: 10.1080/095530098142338. [DOI] [PubMed] [Google Scholar]
- [16].Natarajan AT, Obe G, van Zeeland AA, Palitti F, Meijers M, Verdegaal-Immerzell EAM. Molecular mechanisms involved in the production of chromosomal aberrations; II Utilization of Neurospora endonuclease for the study of aberration production by X-rays in G1 and G2 stages of the cell cycle. Mutat Res. 1980;69:293–305. doi: 10.1016/0027-5107(80)90094-9. [DOI] [PubMed] [Google Scholar]
- [17].Bryant PE. Enzymatic restriction of mammalian cell DNA using Pvu II and Bam H1: Evidence for double strand break origin of chromosome aberrations. Int J Radiat Biol. 1984;46:57–65. doi: 10.1080/09553008414551061. [DOI] [PubMed] [Google Scholar]
- [18].Natarajan AT, Obe G. Molecular mechanisms involved in the production of chromosomal aberrations III: Restriction endonucleases. Chromosoma. 1984;90:120–127. doi: 10.1007/BF00292448. [DOI] [PubMed] [Google Scholar]
- [19].Rogers-Bald M, Sargent RG, Bryant PE. Production of chromatid breaks by single DSB: evidence supporting the signal model. Int J Radiat Biol. 2000;76:23–29. doi: 10.1080/095530000138970. [DOI] [PubMed] [Google Scholar]
- [20].Bryant PE, Jones C, Armstrong G, Frankenberg-Schwager M, Frankenberg D. Induction of chromatid breaks by carbon-K ultra-soft X-rays. Radiat Res. 2003;159:247–250. doi: 10.1667/0033-7587(2003)159[0247:iocbbc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [21].Schwartz JL, Cowan J, Grdina DJ, Weichselbaum RR. Attenuation of G2-phase cell-cycle checkpoint control is associated with frequencies of unrejoined chromosome breaks in human tumor cells. Radiat Res. 1996;146:139–143. [PubMed] [Google Scholar]
- [22].Scott D, Spreadborough AR, Roberts SA. Less G2 arrest in irradiated cells of breast cancer patients than in female controls: a contribution to their enhanced chrmorosomal radiosensitivity? Int J Radiat Biol. 2003;79:405–411. doi: 10.1080/0955300031000150602. [DOI] [PubMed] [Google Scholar]
- [23].Pretazzoli V, Salone B, Bosi A, Olivieri G. Variability of G2 checkpoint sensitivity to low doses of X-rays (2 cGy): correlation with G2 chromatid aberrations but not with an adaptive response. Mutagenesis. 2000;15:531–535. doi: 10.1093/mutage/15.6.531. [DOI] [PubMed] [Google Scholar]
- [24].Sanford KK, Parshad R, Gantt R, Tarone RE, Jones GM, Price FM. Factors affecting the significance of G2 chromatin radiosensitivity in predisposition to cancer. Int J Radiat Biol. 1989;55:963–981. doi: 10.1080/09553008914551001. [DOI] [PubMed] [Google Scholar]
- [25].Parshad R, Price FM, Bohr VA, Cowan R, Zujewski JKA, Sanford KK. Deficient DNA repair capacity, a predisposing factor in breast cancer. Br J Cancer. 1996;74:1–5. doi: 10.1038/bjc.1996.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bryant PE, Gray LJ, Peresse N. Progress towards understanding the nature of chromatid breakage. Cytogenet Genome Res. 2004;104:65–71. doi: 10.1159/000077467. [DOI] [PubMed] [Google Scholar]
- [27].Macleod RAF, Bryant PE. Similar kinetics of chromatid aberrations in X-irradiated xrs 5 and wild-type Chinese hamster cells. Mutagenesis. 1990;5:407–410. doi: 10.1093/mutage/5.4.407. [DOI] [PubMed] [Google Scholar]
- [28].Bryant PE, Slijepcevic P. G2 chromatid aberrations: kinetics and possible mechanisms. Environ Mol Mutagen. 1993;22:250–256. doi: 10.1002/em.2850220412. [DOI] [PubMed] [Google Scholar]
- [29].Bender AM, Griggs HG, Bedford JS. Mechanisms of Chromosomal Aberration Production, III: Chemicals and Ionizing Radiation. Mut Res. 1974;23:197–212. doi: 10.1016/0027-5107(74)90140-7. [DOI] [PubMed] [Google Scholar]
- [30].Revell SH. The accurate estimation of chromatid breakage, and its relevance to a new interpretation of chromatid aberrations by ionizing radiations. Proc Royal Soc B. 1959;150:563–589. doi: 10.1098/rspb.1959.0043. [DOI] [PubMed] [Google Scholar]
- [31].Bryant PE. Origin of Chromosome Aberrations: Mechanisms. In: Obe G, Vijayalaxmi, editors. Chromosomal Alterations: Methods, Results and Importance in Human Health. Springer-Verlag; Berlin Heidelberg: 2007. pp. 177–199. [Google Scholar]
- [32].Harvey AN, Savage JRK. Investigating the nature of chromatid breaks produced by restriction endonucleases. Int J Radiat Biol. 1997;71:21–28. doi: 10.1080/095530097144373. [DOI] [PubMed] [Google Scholar]
- [33].Li T, Chen AY, Mao Y, Wang H, Liu LF. Activation of topoisomerase IImediated excision of chromosomal DNA loops during oxidative stress. Genes Develop. 1999;13:1553–1560. doi: 10.1101/gad.13.12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kingma PS, Corbett AH, Burcham PC, Marnett LJ, Osheroff N. Abasic sites stimulate double-stranded DNA cleavage mediated by topoisomerase II. J Biol Chem. 1995;270:21441–21444. doi: 10.1074/jbc.270.37.21441. [DOI] [PubMed] [Google Scholar]
- [35].Gasser SM, Laroche T, Falquet J, Boy de la Tour E, Laemmli UK. Metaphase chromosome structure: Involvement of topoisomerase II. J Mol Biol. 1986;188:613–629. doi: 10.1016/s0022-2836(86)80010-9. [DOI] [PubMed] [Google Scholar]
- [36].Drake FH, Zimmerman JP, McCabe FL, Bartus HF, Per SR, Sullivan DM, Ross WE, Mattern MR, Johnson RK, Crooke ST. Purification of topoisomerase II from amsacrine-resistant P388 leukemia cells. Evidence for two forms of the enzyme. J Biol Chem. 1987;262:16739–16747. [PubMed] [Google Scholar]
- [37].Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nature Reviews Mol Cell Biol. 2002;3:430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- [38].Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH. Proliferation and cell-cycle dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differen. 1991;2:209–214. [PubMed] [Google Scholar]
- [39].Davies SM, Robson CN, Davies SL, Hickson ID. Nuclear topoisomerase II levels correlate with the sensitivity of mammalian cells to intercalating agents and epipodophyllotoxins. J Biol Chem. 1988;263:17724–17729. [PubMed] [Google Scholar]
- [40].Tanabe K, Ikegami Y, Ishida R, Andoh T. Inhibition of topoisomerase II by antitumor agents bis(2,6-dioxopiperazine) derivatives. Cancer Res. 1991;51:4903–4908. [PubMed] [Google Scholar]
- [41].Terry SY, Riches AC, Bryant PE. A role for topoisomerase II alpha in the formation of radiation-induced chromatid breaks. Br J Cancer. 2008;99:670–674. doi: 10.1038/sj.bjc.6604514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Harker WG, Slade DL, Drake FH, Parr RL. Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II beta isoform. Biochem. 1991;30:9953–9961. doi: 10.1021/bi00105a020. [DOI] [PubMed] [Google Scholar]
- [43].Terry SY, Riches AC, Bryant PE. Suppression of topoisomerase IIα expression and function in human cells decreases chromosomal radiosensitivity. Mutat Res. 2009;663:40–45. doi: 10.1016/j.mrfmmm.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sax K. An analysis of X-ray induced chromosomal aberrations in Tradescantia. Genetics. 1940;25:41–68. doi: 10.1093/genetics/25.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]