Summary
Aim
Oxidative stress and endothelial dysfunction are two inter‐related conditions commonly seen in patients with cardiovascular risk factors. The enzyme, xanthine oxidase, is an important contributor to these phenomena but to a variable degree in different patient populations. This meta‐analysis will summarize the effect of allopurinol, an established xanthine oxidase inhibitor, on endothelial function among patients with different comorbidities.
Methods
Medline Complete, PubMed, ProQuest, ClinicalKey, Wiley Online Library, and Cochrane Central Register of Controlled Trials were searched till July 29, 2017. Meta‐analysis was planned for randomized controlled trials (RCTs) that investigated allopurinol effects on endothelial function. A random effect model was used to calculate the standardized mean difference (with 95% confidence intervals: CI) as an estimate of effect size. Heterogeneity was quantified by four types of information: Q statistics, I 2 statistic, Tau‐squared (T 2), and Tau (T).
Results
Thirty eligible studies were identified; 12 were included in the final analysis and subdivided among 3 patient’s groups: patients with chronic heart failure (CHF; 197 patients), patients with chronic kidney disease (CKD; 183 patients), and patients with type 2 diabetes mellitus (DM; 170 patients). Allopurinol was found to have a statistically significant benefit on endothelial function in patients with CHF and CKD but not in type 2 DM. The standardized mean differences and CI in the three patient’s groups were 0.776 (0.429, 1.122), 0.350 (0.009, 0.690), and 1.331 (−0.781, 3.444), respectively.
Conclusion
Allopurinol has an antioxidant property that might partially reverse endothelial dysfunction in patients with certain comorbidities. The importance of this property and the magnitude of the beneficial effect are likely to be related to the relative contribution of xanthine oxidase into the oxidative stress associated with different underlying pathologies.
Keywords: allopurinol, endothelial dysfunction, oxidative stress, xanthine oxidase, xanthine oxidase inhibitor
1. INTRODUCTION
Vascular oxidative stress is a state of imbalance between reactive oxygen species (ROS) and antioxidant enzymes of which the most important are superoxide dismutase (SOD), glutathione peroxidase, glutathione reductase, and catalase. A key source of ROS in the intravascular compartment is the xanthine oxidoreductase (XOR) system (the enzyme system better known for its involvement in uric acid production). This system exists in two forms, xanthine dehydrogenase (XDH) and xanthine oxidase (XO). The XDH form has NAD+ as the preferred redox partner and it is the predominant form in well‐oxygenated tissues, whereas XO, which is upregulated in hypoxic conditions,1 uses oxygen as an electron acceptor and produces ROS, mainly superoxide anion (O− 2) and hydrogen peroxide (H2O2),1, 2 which can damage all components of the cell (lipids, proteins, and DNA). Circulating XO has the ability to bind to the endothelial surface, where its ROS‐producing moiety can contribute to endothelial injury and endothelial dysfunction.3 Accordingly, XO inhibition has emerged as a novel therapeutic target among patients with cardiovascular (CV) risk factors, not only because XO has oxidative injury potential but also because serum uric acid concentrations per se have been found to correlate negatively with measures of endothelial function (eg, flow‐mediated dilatation—FMD). Such findings have been demonstrated in solely hyperuricemic individuals without any CV disease4 and in healthy individuals with uric acid concentrations within the physiologic range.5 Endothelial dysfunction, once it develops, is an important step in the progression of atherosclerosis,6, 7 and it has an important prognostic value for cardiovascular (CV) events in different populations.8, 9, 10 For these reasons, research is ongoing to find a therapeutic strategy which targets both these two phenomena—oxidative stress and endothelial dysfunction.
Allopurinol is a XO inhibitor whose effects on oxidative stress and endothelial dysfunction have been investigated in many clinical trials. The studies that have assessed the allopurinol effect on endothelial function have typically used two techniques: venous occlusion plethysmography (VOP) and flow‐mediated dilatation (FMD). VOP is the “gold standard” technique for investigating endothelial function via the infusion of different vasoactive substances locally into the brachial artery and the measurement of changes in forearm blood flow (FBF).11 While FMD is considered to be less invasive, it involves ultrasonic assessment of changes in brachial artery diameter in response to reactive hyperemia.12
In the published literature, there are a number of clinical trials that have evaluated the allopurinol effect on endothelial function (via these two techniques) with promising results in different patient populations. This review and meta‐analysis will summarize the results of these studies in different patient populations. However, because there is a wide range of XOR activity (more than 3‐fold variation in enzymatic activity among different individuals13) and also because the contribution of XO to oxidative stress may differ according to different patient comorbidities, the ability of allopurinol to produce beneficial effects on endothelial function might differ according to the baseline activity of XO and/or according to the underlying pathology in any given patient population.
2. METHODS
This review has been performed according to the PRISMA statement (preferred reporting items for systematic reviews and meta‐analysis)14 and has been registered on the PROSPERO register with registration number CRD42016046468 on August 24, 2016.
2.1. Search strategy
Medline Complete, PubMed, ProQuest Health & Medical Complete, ClinicalKey, Wiley Online Library Journals and Cochrane Central Register of Controlled Trials were searched using the search items in titles and abstracts, in combination with MESH terms: (allopurinol AND endothelial dysfunction OR endothelial function). The literature was searched till July 29, 2017. The articles included in the final analysis have been displayed in Figure 1.
Figure 1.
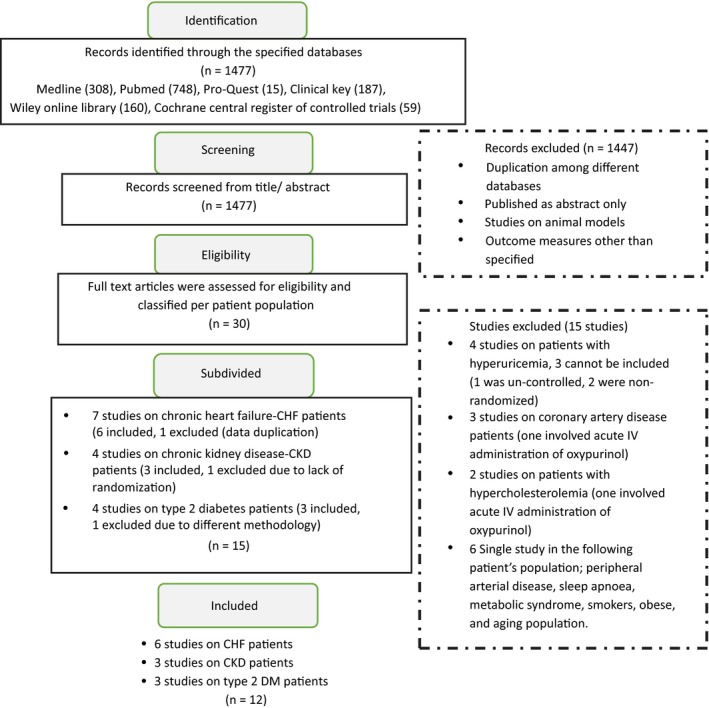
Flow diagram of the study selection procedure to assess the effect of allopurinol on endothelial function
2.2. Study selection
Original studies that met the following predetermined inclusion criteria were included in the study:
Published as full‐text article
Reported with either parallel or crossover design
Recruited human subjects randomized to allopurinol therapy or to control group (no treatment/or placebo)
Allopurinol administration for a minimum of 7 days
Assessed endothelial function as primary or secondary endpoints via VOP or FMD
Data reported as mean ± SD/SEM for each group after treatment or reported as % change from baseline.
Eligible studies were subdivided into different patient groups, such as chronic heart failure, chronic kidney disease, type 2 diabetes, hyperuricemia, and coronary artery disease. However, the final analysis requires a minimum of 3 studies per patient group.15 Nonhuman studies, review articles, duplicate publications and studies that involved acute administration of allopurinol for a period less than specified or involved acute administration of IV oxypurinol were excluded.
2.3. Data extraction and quality assessment
The following data including first author name, year of publication, study design, participant’s disease status, number of participants in the allopurinol group and control group, age, sex, and uric acid level in the study participants were extracted from eligible full‐text articles. Intervention strategies and outcomes included dose and duration of allopurinol therapy, forearm blood flow data (expressed as mL/min/100 mL), and percentage change in diameter of brachial artery from baseline in response to reactive hyperemia. The seven domains of the Cochrane risk of bias tool were used to evaluate the quality of the included studies (Table 1).
Table 1.
Assessment of risk of bias in the 12 included studies assessing the effect of allopurinol on endothelial function using Cochrane criteria
| Study | Ref | Random sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|---|
| Farquharson et al 2002 | 19 | L | U | L | L | L | L | L |
| Doehner et al 2002 | 20 | L | L | L | L | L | L | L |
| George et al 2006 | 21 | L | U | L | L | L | L | L |
| Tousoulis et al 2011 | 22 | L | U | L | L | L | L | L |
| Greig et al 2011 | 23 | L | L | L | L | L | L | L |
| Xiao et al 2016 | 24 | L | H | H | L | L | L | L |
| Kao et al 2011 | 26 | L | L | L | L | L | L | L |
| Bayram et al 2015 | 25 | L | H | L | L | L | L | L |
| Jalal et al 2016 | 27 | L | L | L | L | L | L | L |
| Butler et al 2000 | 28 | L | U | L | L | L | L | L |
| Dogan et al 2011 | 29 | L | U | U | L | L | L | L |
| Szwejkowski et al 2013 | 30 | L | L | L | L | L | L | L |
H, high risk of bias; L, low risk of bias; U, unclear risk of bias.
2.4. Quantitative data analysis
Meta‐analysis was conducted using Comprehensive Meta‐Analysis (CMA) V3 software (Biostat, Englewood, New Jersey, USA). Standardized mean difference (Hedges’s g) was used as an estimate of effect size rather than the raw mean difference. It is because the included studies have reported more than one scale/test to assess the outcome of endothelial function.16 Accordingly, the software divides the mean difference in each study by that study’s standard deviation to create the standardized mean difference that would be comparable across studies. Where some studies used SEM, SD was estimated using the following formula;
where,
n is the number of participants.
Subgroup analyses were carried out to check whether allopurinol had a tendency for different effect size based on baseline uric acid (if above or below 7 mg/dL) or the dose of allopurinol given.
2.5. Heterogeneity of the effect size
The observed effect size varies from one study to another, but a certain amount of variation is expected due to sampling error. The Q‐statistic provides a test of the null hypothesis that all studies included in the analysis share a common effect size. In such a case, it is expected that the value of Q would be equal to the degrees of freedom df (the number of studies minus 1). I 2 statistic tells us what proportion of the observed variance reflects differences in true effect sizes, rather than sampling error. Tau‐squared (T 2) is the estimate of the variance in true effect sizes (in log units). Tau (T) is the estimate of standard deviation of true effect sizes (in log units). All statistics are displayed in the footer section of forest plots.
2.6. Publication bias
One concern of publication bias is that some nonsignificant studies are missing from the analysis, and these studies if included, would nullify the observed effect. For that reason, classic fail‐safe N was calculated by CMA software. This statistic measure can be defined as the number of new, unpublished/retrieved studies with nonsignificant results that would be required to make the results of this meta‐analysis nonsignificant or that would bring P‐value ˃ alpha (.05).17, 18 Funnel plot was not carried out because of the small number of included studies in the analysis.
3. RESULTS
3.1. Search results
A total of 1477 records were identified through the database searched, and 1447 records were excluded based on title/abstract screening. The remaining 30 full‐text articles were assessed for eligibility. However, 15 studies were excluded as 4 of those studies were conducted on patients with hyperuricemia, but 3 did not meet the inclusion criteria; 3 more studies were conducted on patients with coronary artery disease; however, one of them involved the acute administration of oxypurinol; two studies were conducted on patients with hypercholesterolemia, and the remaining 6 studies were conducted on a different patient’s populations each (Figure 1). The remaining 15 studies were conducted on three patient populations: CHF, CKD, and type 2 DM. Moreover, 3 were excluded as one study (CHF) was a duplicate publication, one study (CKD) was non‐randomized, and the third study (type 2 DM) used a different methodology than specified. Thus, the final analysis included a total of 12 prospective studies that met the inclusion criteria, divided among 3 patient populations: CHF (6), CKD (3), and type 2 DM (3).
3.2. Study characteristics
3.2.1. Chronic heart failure
There are six RCTs19, 20, 21, 22, 23, 24 which assessed the allopurinol effect on endothelial function in CHF functional class II‐III patients. They were placebo‐controlled except one study where the control group did not receive placebo.24 They included a total of 197 patients with a mean age of 55‐69 years. Patients were predominantly males (74%‐100%), and serum uric acid varied from 5.4 to 9.4 mg/dL. Allopurinol was administered to all with a daily dose of 300 mg for a period of 1 week to 3 months and all studies, with one exception24 reported a significant drop in serum uric acid with allopurinol treatment that varied from −31% to −61%. No severe adverse events were reported.
3.2.2. Chronic kidney disease
There are 3 RCTs25, 26, 27 that assessed the allopurinol effect on endothelial function in CKD stage II‐IV patients. Two were placebo‐controlled.26, 27 They included a total of 183 patients with a mean age range 55‐74 years. Male gender constituted 47%‐87% of participants, and serum uric acid varied from 7.1 to 8.7 mg/dL. Allopurinol was administered to all with a daily dose of 300 mg for a period that varied from 3 months to 9 months. All studies reported a significant drop in serum uric acid with allopurinol therapy that varied from −19% to −41%. No severe adverse events were reported.
3.2.3. Diabetes mellitus (type 2)
Another 3 RCTs assessed the effect of allopurinol on endothelial function in patients with type 2 DM.28, 29, 30 All were placebo‐controlled and included a total of 170 patients with a mean age range 50‐65 year. Male gender constituted 51%‐91% of participants, and serum uric acid varied from 4.8 to 9.2 mg/dL in two studies. One study did not report baseline serum uric acid or its drop in response to allopurinol therapy.28 The administered allopurinol dose varied from 300 mg to 900 mg for periods of around 1 to 9 months. The reported significant drop in serum uric acid was −45% in one study and −54% in the other. No severe adverse events were reported.
3.3. Outcome results
3.3.1. CHF patients
Allopurinol improved endothelial function in 197 patients with mild‐moderate CHF. The effect size is the standardized mean difference (Hedges’s g), which was calculated as 0.776. Therefore, on average, CHF patients treated with allopurinol scored 0.776 a standard deviation higher than patients who received placebo. The 95% confidence interval of this estimate was (0.429, 1.122). Such effect size was statistically significant (P ˂ .001; Table 2). Q‐value is 8.746 with df = 5 and P = .120, indicating that all studies in the analysis share a common effect size. I 2 statistic states that 42.8% of the observed variance reflects differences in true effect size rather than sampling error. Tau‐squared (T 2) is the estimate of the variance in true effect sizes, which came to be 0.078. Tau (T) is the standard deviation of true effects that came to be 0.280. The relationship between allopurinol effect and baseline uric acid was not significant (P = .063). Publication bias assessment showed that classic fail‐safe N was 48.
Table 2.
Forest plot for allopurinol effect on endothelial function in chronic heart failure patients
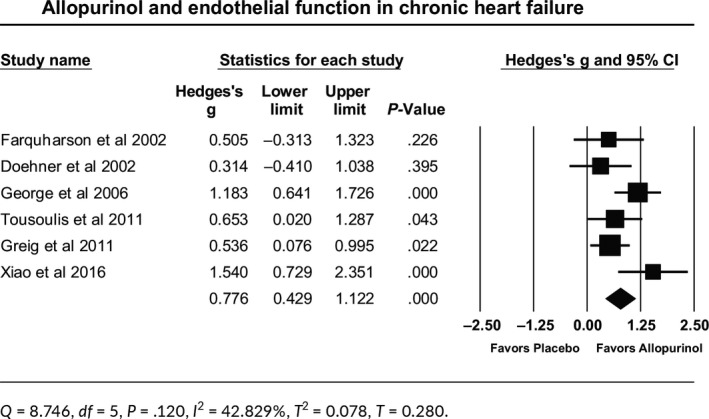
3.3.2. CKD patients
Allopurinol improved endothelial function in 183 patients with CKD stage II‐IV. The effect size is the standardized mean difference (Hedges’s g), which came to be 0.350. The 95% confidence interval of this estimate was (0.009, 0.690). Such effect size was statistically significant (P = .044; Table 3). Q‐value is 2.750 with df = 2 and P = .253, indicating that all studies in the analysis share a common effect size. I 2 statistic tells us that 27.3% of the observed variance reflects differences in true effect size rather than sampling error. Tau‐squared (T 2) came to be 0.025, and Tau (T) came to be 0.157. The relationship between allopurinol effect and baseline uric acid was not tested as baseline uric acid in the three studies was above 7 mg/dL.
Table 3.
Forest plot for allopurinol effect on endothelial function in patients with chronic kidney disease
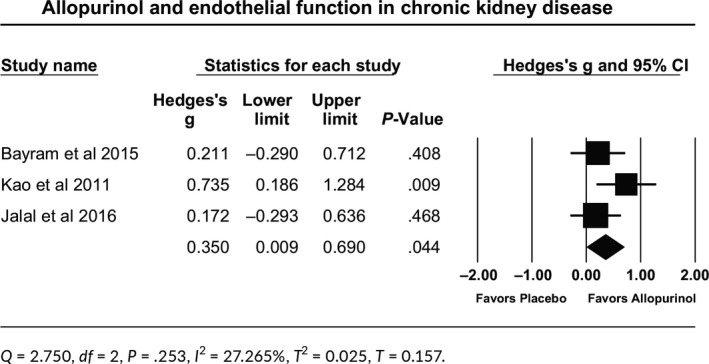
3.3.3. DM patients
Allopurinol had no significant effect on endothelial function in 170 patients with type 2 DM. The effect size was the standardized mean difference (Hedges’s g) came out to be 1.331. The 95% confidence interval of this estimate was (−0.781, 3.444; P = .217) (Table 4). Q‐value is 67.9 with df = 2 and P ˂ .001, indicating that effect size varies across studies. I 2 statistic states that 97% of the observed variance reflects differences in true effect size rather than sampling error. Tau‐squared (T 2) came to be 3.374, and Tau (T) came to be 1.837 (Table 4). The relationship between allopurinol effect and baseline uric acid was not tested as one of the studies did not report baseline or change in UA concentration.28 The three studies included in this meta‐analysis used different doses of allopurinol (300,28 600,30 and 900 mg daily29). However, the relationship between allopurinol effect and the dose administered was significant in favor of 900 mg dose as compared to 300 mg dose, with effect sizes of 3.308 vs 0.590, respectively (P ˂ .001). The effect size for 900 mg dose as compared to 600 mg dose was 3.308 vs 0.088, respectively (P ˂ .001).
Table 4.
Forest plot for allopurinol effect on endothelial function in patients with type 2 diabetes mellitus
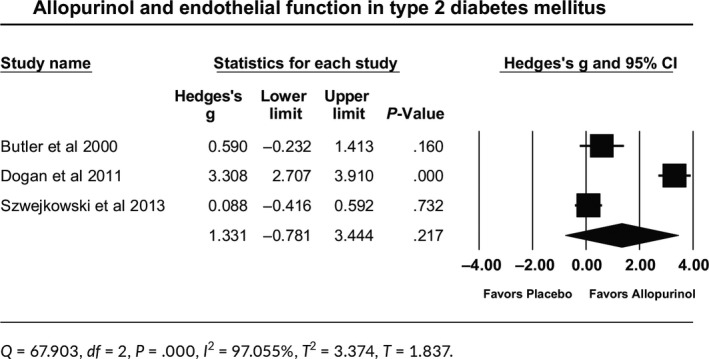
4. DISCUSSION
A number of studies in the published literature have assessed the effects of allopurinol on different parameters related to cardiovascular health in different patient populations. Few cohort studies have assessed its effect on cardiovascular events and mortality, and the results were inconsistent.31, 32, 33 On the other hand, its effect on endothelial dysfunction features prominently in some other studies and a few meta‐analyses have been published to summarize these effects in a small number of RCTs that included patients labeled as at risk of cardiovascular disease.34, 35, 36 All showed a statistically significant benefit of allopurinol therapy with mean increases in brachial artery flow‐mediated vasodilatation by respectively 2.50% (95% CI 0.15‐4.84),34 2.75% (95% CI 2.49‐3.01),35 and 1.67% (95% CI 0.83‐2.50).36 Two of these analyses also reported the effects on forearm blood flow in response to acetylcholine via VOP, with increases of respectively 68.80 (95% CI 18.70‐118.90)34 and 2.62 (95% CI 2.32‐2.91).35 These meta‐analyses included patients with different comorbidities and reached the conclusion that allopurinol has the potential to enhance endothelial function in general without specifying a specific patient population.
The primary purpose of this further meta‐analysis was to look more closely at the effect of allopurinol on endothelial function in different patient’s populations, as the contribution of xanthine oxidase activity to oxidative stress might differ from one patient population to another. The largest available number of well‐conducted studies was in patients with CHF. The six studies included patients with the same severity (functional class II‐III; mild‐moderate) and showed a statistically significant benefit of short‐term allopurinol therapy with the dose of 300 mg daily. This benefit was independent of the baseline uric acid level. Tests assessing heterogeneity of the effect size showed that all studies in the analysis shared a common effect size. The consistent benefit of allopurinol therapy in each individual study and in this meta‐analysis is in agreement with other observations in patients with CHF: for example, an increase in xanthine oxidase enzyme activity is reported in patients with CHF37 and alterations in other markers of oxidative stress such as decreased superoxide dismutase activity,37 elevated serum malondialdehyde (MDA),38 elevated serum Oxidised‐LDL,39 and elevated F2 isoprostane.40
For patients with CKD stage II‐IV, the results showed a statistically significant improvement although of a lesser magnitude to that obtained in patients with CHF, despite using the same dose of allopurinol (300 mg daily). Endothelial dysfunction and oxidative stress in patients with CKD are well documented41 and have been linked to reduce nitric oxide bioavailability,42 due to reduced endothelial and renal production,43 as well as increased NOS inhibitors, particularly including asymmetric dimethylarginine (ADMA).43, 44 Some studies have demonstrated rises in F2‐isoprostanes45, 46 while others have demonstrated a reduction in superoxide dismutase and glutathione peroxidase and a rise in plasma MDA and ADMA level.44 The contribution of xanthine oxidase into oxidative stress and endothelial dysfunction in CKD patients has mostly been investigated via the hyperuricemia that is commonly associated with CKD.47 However, uric acid which is a marker of upregulated XO activity has been identified as an independent predictor of endothelial dysfunction in patients with CKD,48 suggesting that lowering serum uric acid might be of therapeutic benefits. Direct measurement of XO activity has been shown in one study to be significantly higher in dialysis patients compared with uremic control patients and normal control subjects, despite the fact that investigators did not exclude patients on allopurinol therapy from the study.49 Such a finding suggests that XO enzyme activity is upregulated in patients with CKD, despite concomitant allopurinol therapy in some patients.
Another study used a more recent and highly sensitive assay of xanthine oxidoreductase activity to demonstrate the accelerated conversion of xanthine dehydrogenase (XDH) to xanthine oxidase (XO) with renal dysfunction.50 The results of these two studies are in agreement with the conclusion of this meta‐analysis. One study in the literature demonstrated that xanthine oxidoreductase (XOR) activity was lower among patients with renal dysfunction.51 However, it is worth mentioning that their assay measured both XDH and XO activity, not XO activity separately.
Finally, in patients with type 2 DM, the meta‐analysis failed to show a benefit of allopurinol therapy on endothelial function. This result may be explained by the fact that, although the number of patients included in the meta‐analysis is similar to that of the CHF and CKD groups, the included studies were heterogeneous in terms of dosage and duration of treatment, with the biggest effect relating to the 900 mg dose (Table 4). Oxidative stress‐induced alterations in major biomolecules in the cell and the status of plasma antioxidant potential in type 2 DM have been reported to involve increased lipid peroxidation, increased protein oxidation, reduced glutathione level, reduced catalase activity, and reduced superoxide dismutase activity.52, 53 The possible active role of xanthine oxidase in oxidative stress in type 2 DM has not received sufficient attention. A few studies have demonstrated increased XO activity in such patients and showed a positive correlation with HBA1C levels in the ranges of respectively 7.1%‐9.3% in one study54 and 7.6%‐8.8% in the other.55 The HBA1C range in the 170 patients included in this meta‐analysis was 6.1%‐7.25%.28, 29, 30 These results reflect that patients included in this meta‐analysis had better glycemic control and probably less upregulated XO. This may, at least in part, explain the finding that the patients showed no noticeable benefit in response to allopurinol therapy.
4.1. Limitations
This meta‐analysis was designed to explore the effect of allopurinol therapy on endothelial function on other patients’ populations in addition to the three groups analyzed. However, it was not feasible because of lack of adequate randomized clinical trials for other patients’ populations. The second limitation is that selection of papers, data extraction, quality assessment, and analysis was conducted by a single individual.
5. CONCLUSION
Oxidative stress and endothelial dysfunction are well recognized as two important phenomena in different patient populations with increased CV risk, and it is well understood that they are potentially important sources of morbidity and mortality. Accordingly, there is a clear mandate for therapeutic agents with antioxidant properties which might improve/reverse endothelial dysfunction. Allopurinol is a XO inhibitor with antioxidant properties that have shown promising benefits and improved endothelial function in patients with CHF and CKD (but not in type 2 DM in the small number of studies included). For that reason, further well‐designed, randomized controlled clinical trials with allopurinol as an antioxidant therapy are required in different patient populations to establish an evidence‐based recommendation for clinical practice. If allopurinol antioxidant properties were to be demonstrated on a large scale, the role of allopurinol as a valuable add‐on therapeutic approach for the preventative treatment of CV disease would depend on clinical trial evidence of improved mortality and morbidity in “at risk” patient populations.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENT
The author is very thankful to all the associated personnel in any reference that contributed in/for the purpose of this research.
Alem MM. Allopurinol and endothelial function: A systematic review with meta‐analysis of randomized controlled trials. Cardiovasc Ther. 2018;36:e12432 10.1111/1755-5922.12432
REFERENCES
- 1. Hassoun PM, Yu FS, Cote CG, et al. Upregulation of xanthine oxidase by lipopolysaccharide, interleukin‐1, and hypoxia. Role in acute lung injury. Am J Respir Crit Care Med. 1998;158:299‐305. [DOI] [PubMed] [Google Scholar]
- 2. Sarnesto A, Linder N, Raivio KO. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab Invest. 1996;74:48‐56. [PubMed] [Google Scholar]
- 3. Houston M, Estevez A, Chumley P, et al. Binding of xanthine oxidase to vascular endothelium. Kinetic characterization and oxidative impairment of nitric oxide‐dependent signaling. J Biol Chem. 1999;274:4985‐4994. [DOI] [PubMed] [Google Scholar]
- 4. Kato M, Hisatome I, Tomikura Y, et al. Status of endothelial dependent vasodilation in patients with hyperuricemia. Am J Cardiol. 2005;96:1576‐1578. [DOI] [PubMed] [Google Scholar]
- 5. Erdogan D, Gullu H, Caliskan M, et al. Relationship of serum uric acid to measures of endothelial function and atherosclerosis in healthy adults. Int J Clin Pract. 2005;59:1276‐1282. [DOI] [PubMed] [Google Scholar]
- 6. Celermajer DS, Sorensen KE, Gooch VM, et al. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111‐1115. [DOI] [PubMed] [Google Scholar]
- 7. Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833‐843. [DOI] [PubMed] [Google Scholar]
- 8. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673‐2678. [DOI] [PubMed] [Google Scholar]
- 9. Gokce N, Keaney JF Jr, Hunter LM, et al. Predictive value of noninvasively determined endothelial dysfunction for long‐term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769‐1775. [DOI] [PubMed] [Google Scholar]
- 10. Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191‐196. [DOI] [PubMed] [Google Scholar]
- 11. Wilkinson IB, Webb DJ. Venous occlusion plethysmography in cardiovascular research: methodology and clinical applications. Br J Clin Pharmacol. 2001;52:631‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Raitakari OT, Celermajer DS. Flow‐mediated dilatation. Br J Clin Pharmacol. 2000;50:397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerciolini R, Szumlanski C, Weinshilboum RM. Human liver xanthine oxidase: nature and extent of individual variation. Clin Pharmacol Ther. 1991;50:663‐672. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davey J, Turner RM, Clarke MJ, Higgins JP. Characteristics of meta‐analyses and their component studies in the Cochrane Database of Systematic Reviews: a cross‐sectional, descriptive analysis. BMC Med Res Methodol. 2011;11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Effect sizes based on means In: Borenstein M ed. Introduction to Meta‐Analysis. Hoboken, NY: John Wiley & Sons Ltd Publication; 2009:21‐32. [Google Scholar]
- 17. Borestein M, Hedges LV, Higgins JP, Rothstein HR. Publication bias In: Borenstein M ed. Introduction to Meta‐Analysis. 1st edn Hoboken, NJ: John Wiley & Sons. Ltd; 2009:277‐292. [Google Scholar]
- 18. Persaud R. Misleading meta‐analysis. “Fail safe N” is a useful mathematical measure of the stability of results. BMJ. 1996;312:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221‐226. [DOI] [PubMed] [Google Scholar]
- 20. Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo‐controlled studies. Circulation. 2002;105:2619‐2624. [DOI] [PubMed] [Google Scholar]
- 21. George J, Carr E, Davies J, Belch JJ, Struthers A. High‐dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation. 2006;114:2508‐2516. [DOI] [PubMed] [Google Scholar]
- 22. Tousoulis D, Andreou I, Tsiatas M, et al. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: the impact of inflammatory process and oxidative stress. Atherosclerosis. 2011;214:151‐157. [DOI] [PubMed] [Google Scholar]
- 23. Greig D, Alcaino H, Castro PF, et al. Xanthine‐oxidase inhibitors and statins in chronic heart failure: effects on vascular and functional parameters. J Heart Lung Transplant. 2011;30:408‐413. [DOI] [PubMed] [Google Scholar]
- 24. Xiao J, Deng SB, She Q, et al. Allopurinol ameliorates cardiac function in non‐hyperuricaemic patients with chronic heart failure. Eur Rev Med Pharmacol Sci. 2016;20:756‐761. [PubMed] [Google Scholar]
- 25. Bayram D, Tugrul SM, Inal S, Altuntas A, Kidir V, Orhan H. The effects of allopurinol on metabolic acidosis and endothelial functions in chronic kidney disease patients. Clin Exp Nephrol. 2015;19:443‐449. [DOI] [PubMed] [Google Scholar]
- 26. Kao MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol. 2011;22:1382‐1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jalal DI, Decker E, Perrenoud L, et al. Vascular function and uric acid‐lowering in stage 3 CKD. J Am Soc Nephrol. 2016;28:943‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746‐751. [DOI] [PubMed] [Google Scholar]
- 29. Dogan A, Yarlioglues M, Kaya MG, et al. Effect of long‐term and high‐dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011;20:182‐187. [DOI] [PubMed] [Google Scholar]
- 30. Szwejkowski BR, Gandy SJ, Rekhraj S, et al. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol. 2013;62:2284‐2293. [DOI] [PubMed] [Google Scholar]
- 31. Wei L, Mackenzie IS, Chen Y, Struthers AD, MacDonald TM. Impact of allopurinol use on urate concentration and cardiovascular outcome. Br J Clin Pharmacol. 2011;71:600‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010;5:1388‐1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kok VC, Horng JT, Chang WS, Hong YF, Chang TH. Allopurinol therapy in gout patients does not associate with beneficial cardiovascular outcomes: a population‐based matched‐cohort study. PLoS ONE. 2014;9:e99102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins P, Dawson J, Lees KR, McArthur K, Quinn TJ, Walters MR. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta‐analysis. Cardiovasc Ther. 2012;30:217‐226. [DOI] [PubMed] [Google Scholar]
- 35. Kanbay M, Siriopol D, Nistor I, et al. Effects of allopurinol on endothelial dysfunction: a meta‐analysis. Am J Nephrol. 2014;39:348‐356. [DOI] [PubMed] [Google Scholar]
- 36. Xin W, Mi S, Lin Z. Allopurinol therapy improves vascular endothelial function in subjects at risk for cardiovascular diseases: a meta‐analysis of randomized controlled trials. Cardiovasc Ther. 2016;34:441‐449. [DOI] [PubMed] [Google Scholar]
- 37. Landmesser U, Spiekermann S, Dikalov S, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine‐oxidase and extracellular superoxide dismutase. Circulation. 2002;106:3073‐3078. [DOI] [PubMed] [Google Scholar]
- 38. McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J. 1993;14:1493‐1498. [DOI] [PubMed] [Google Scholar]
- 39. Tsutsui T, Tsutamoto T, Wada A, et al. Plasma oxidized low‐density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;39:957‐962. [DOI] [PubMed] [Google Scholar]
- 40. Polidori MC, Pratico D, Savino K, Rokach J, Stahl W, Mecocci P. Increased F2 isoprostane plasma levels in patients with congestive heart failure are correlated with antioxidant status and disease severity. J Card Fail. 2004;10:334‐338. [DOI] [PubMed] [Google Scholar]
- 41. Ghiadoni L, Cupisti A, Huang Y, et al. Endothelial dysfunction and oxidative stress in chronic renal failure. J Nephrol. 2004;17:512‐519. [PubMed] [Google Scholar]
- 42. Schmidt RJ, Baylis C. Total nitric oxide production is low in patients with chronic renal disease. Kidney Int. 2000;58:1261‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baylis C. Nitric oxide deficiency in chronic kidney disease. Am J Physiol Renal Physiol. 2008;294:F1‐F9. [DOI] [PubMed] [Google Scholar]
- 44. Yilmaz MI, Saglam M, Caglar K, et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am J Kidney Dis. 2006;47:42‐50. [DOI] [PubMed] [Google Scholar]
- 45. Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009‐1016. [DOI] [PubMed] [Google Scholar]
- 46. Dounousi E, Papavasiliou E, Makedou A, et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am J Kidney Dis. 2006;48:752‐760. [DOI] [PubMed] [Google Scholar]
- 47. Jalal DI, Chonchol M, Chen W, Targher G. Uric acid as a target of therapy in CKD. Am J Kidney Dis. 2013;61:134‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanbay M, Yilmaz MI, Sonmez A, et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic kidney disease. Am J Nephrol. 2011;33:298‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Choi JY, Yoon YJ, Choi HJ, et al. Dialysis modality‐dependent changes in serum metabolites: accumulation of inosine and hypoxanthine in patients on haemodialysis. Nephrol Dial Transplant. 2011;26:1304‐1313. [DOI] [PubMed] [Google Scholar]
- 50. Murase T, Nampei M, Oka M, Miyachi A, Nakamura T. A highly sensitive assay of human plasma xanthine oxidoreductase activity using stable isotope‐labeled xanthine and LC/TQMS. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1039:51‐58. [DOI] [PubMed] [Google Scholar]
- 51. Fujimura Y, Yamauchi Y, Murase T, et al. Relationship between plasma xanthine oxidoreductase activity and left ventricular ejection fraction and hypertrophy among cardiac patients. PLoS ONE. 2017;12:e0182699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tiwari BK, Pandey KB, Abidi AB, Rizvi SI. Markers of oxidative stress during diabetes mellitus. J Biomark. 2013;2013:378790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress‐A concise review. Saudi Pharm J. 2016;24:547‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kuppusamy UR, Indran M, Rokiah P. Glycaemic control in relation to xanthine oxidase and antioxidant indices in Malaysian Type 2 diabetes patients. Diabet Med. 2005;22:1343‐1346. [DOI] [PubMed] [Google Scholar]
- 55. Miric DJ, Kisic BM, Filipovic‐Danic S, et al. Xanthine oxidase activity in type 2 diabetes mellitus patients with and without diabetic peripheral neuropathy. J Diabetes Res. 2016;2016:4370490. [DOI] [PMC free article] [PubMed] [Google Scholar]


