Abstract
In randomized controlled trials (RCTs), sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors have been shown to confer glycaemic and extra‐glycaemic benefits. The DARWIN‐T2D (DApagliflozin Real World evIdeNce in Type 2 Diabetes) study was a multicentre retrospective study designed to evaluate the baseline characteristics of patients receiving dapagliflozin vs those receiving selected comparators (dipeptidyl peptidase‐4 inhibitors, gliclazide, or glucagon‐like peptide‐1 receptor agonists), and drug effectiveness in routine clinical practice. From a population of 281 217, the analysis included 17 285 patients initiating dapagliflozin or comparator glucose‐lowering medications (GLMs), 6751 of whom had a follow‐up examination. At baseline, participants starting dapagliflozin were younger, had a longer disease duration, higher glycated haemoglobin (HbA1c) concentration, and a more complex history of previous GLM use, but the clinical profile of patients receiving dapagliflozin changed during the study period. Dapagliflozin reduced HbA1c by 0.7%, body weight by 2.7 kg, and systolic blood pressure by 3.0 mm Hg. Effects of comparator GLMs were also within the expected range, based on RCTs. This real‐world study shows an initial channelling of dapagliflozin to difficult‐to‐treat patients. Nonetheless, dapagliflozin provided significant benefits with regard to glucose control, body weight and blood pressure that were in line with findings from RCTs.
Keywords: cohort study, dapagliflozin, glycaemic control, observational study, type 2 diabetes, weight control
1. INTRODUCTION
Sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors exert a variety of favourable glycaemic and extra‐glycaemic effects1; in phase III randomized controlled trials (RCTs), SGLT2 inhibitors lowered glycated haemoglobin (HbA1c) concentration by ~0.5% to 0.7%, body weight by 2 to 3 kg, and blood pressure by 3 to 4 mm Hg in people with type 2 diabetes (T2D).2, 3 Although RCTs deliver the best evidence for clinical decisions, they have limited external transferability.4 Real‐world retrospective studies using routinely accumulated clinical data are regaining interest for their ability to collect large representative datasets in a relatively short time. These studies are subject to channelling bias (or confounding by indication),5 especially for newly marketed drugs.6 This can be addressed using strategies of propensity score matching (PSM).7 When a sufficient overlap between patient groups exists,8 PSM can generate quasi‐experimental comparisons that make retrospective studies closer to RCTs. For instance, 2 RCTs indicate that SGLT2 inhibitors improve cardiovascular outcomes in patients with T2D and high risk for or established cardiovascular disease9, 10 and 2 retrospective studies on administrative databases have confirmed these findings in broader T2D patient populations.11, 12
The DARWIN‐T2D study was a multicentre Italian retrospective study, designed to evaluate the use and effectiveness of dapagliflozin, the first‐in‐class SGLT2 inhibitor in Italy, compared with other glucose‐lowering medications (GLMs) in routine clinical practice.13 In the present study, we report data on baseline clinical characteristics with their temporal trends, along with an initial assessment of effectiveness.
2. MATERIALS AND METHODS
We have previously published a detailed description of the design of the present study13 and an expanded Methods section can be found in the Supporting Information. Briefly, the DARWIN‐T2D study was a multicentre nationwide retrospective study, designed to evaluate the baseline clinical characteristics and the change in glycaemic and extra‐glycaemic effectiveness variables in patients initiated on dapagliflozin vs patients initiated on dipeptidyl peptidase‐4 (DPP‐4) inhibitors, gliclazide, or glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), in Italian diabetes outpatient clinics. The study flowchart is shown in Figure S1 in Appendix S1. Automated software retrospectively extracted data from the same electronic chart system at all centres.
2.1. Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are expressed as percentages. Normality was checked using the Kolmogorov–Smirnov test and non‐normal variables were log‐transformed. Comparison between the 2 groups was performed using the two‐tailed unpaired Student's t test or χ2 test. Adjusting for multiple testing was performed using the Benjamini–Hochberg procedure. The two‐tailed paired Student's t test was used to compare data collected at follow‐up with those collected at baseline. P values <.05 were taken to indicate statistical significance.
3. RESULTS
3.1. Baseline clinical characteristics
The study collected data from 46 Italian diabetes specialist outpatient clinics, for a total background population of 281 217 patients with T2D. For the purpose of the present analysis, we extracted detailed information on 17 285 patients who initiated dapagliflozin (n = 2484), a DPP‐4 inhibitor (n = 6594: sitagliptin 57.2%; alogliptin 19.7%; vildagliptin 19.6%; saxagliptin 3.5%), gliclazide (n = 5960), or a long‐acting GLP‐1RA (n = 2247: liraglutide 73.3%; exenatide extended release 26.7%) between March 15, 2015 and December 31, 2016.
The baseline clinical characteristics of patients in the 4 groups are shown in Table S1 in Appendix S1. Significant differences among groups were detected for most variables: patients initiated on dapagliflozin were younger, had longer diabetes duration and higher fasting plasma glucose level, HbA1c level and blood pressure than patients in the other 3 groups and were more obese than patients initiated on DPP‐4 inhibitors or gliclazide, but less obese than patients initiated on a GLP‐1RA. The baseline lipid profile was slightly but significantly worse in the dapagliflozin vs the DPP‐4 inhibitor and gliclazide groups, but not when compared with the GLP‐1RA group. The frequency of microangiopathy at baseline was higher in patients initiated on dapagliflozin than in the other 3 groups, whereas the frequency of macroangiopathy was lower in the dapagliflozin than in the DPP‐4 inhibitor and gliclazide groups.
Metformin and insulin use were more common in the dapagliflozin group than in the other 3 groups, at the time of prescription of the new drugs and in the previous prescription. More than half of patients receiving dapagliflozin were insulin‐treated, about half of whom were on basal‐bolus insulin. The historical therapeutic regimen was also more complex in the dapagliflozin than in the other groups. All concomitant cardiovascular medications were less frequent among dapagliflozin users than in the control groups.
Heterogeneity arising from the centre effect, geographical location, and temporal trends are described in the Supporting Information (Table S2, S3, Figures S2, S3 in Appendix S1).
3.2. Analyses of effectiveness
Changes in glycaemic and extra‐glycaemic effectiveness variables were calculated for patients having a follow‐up visit 3 to 12 months after baseline and still being on drug. Of the 17 285 patients evaluated at baseline, n = 6751 (39.1%) had a follow‐up visit: n = 830 (33.4%) for dapagliflozin, n = 2999 (45.5%) for DPP‐4 inhibitors, n = 2111 (35.4%) for gliclazide, and n = 811 (36.1%) for GLP‐1RAs (Table 1, Figure 1).
Table 1.
Change in glycaemic and extra‐glycaemic effectiveness variables in the 4 groups
| Dapagliflozin | DPP‐4 inhibitors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Follow‐up | Delta | P | n | Baseline | Follow‐up | Delta | P | |
| Age, years | 830 | 60.2 ± 9.3 | 2999 | 67.2 ± 9.1 | ||||||
| Sex male % | 830 | 61.2 | 2999 | 59.3 | ||||||
| BMI, kg/mq | 748 | 33.1 ± 6.0 | 2644 | 29.1 ± 4.9 | ||||||
| Duration, years | 830 | 12.4 ± 8.2 | 2999 | 11.0 ± 7.7 | ||||||
| Follow‐up, days | 830 | 168.1 ± 55.5 | 2999 | 185.7 ± 49.0 | ||||||
| Weight, kg | 735 | 92.8 ± 19.0 | 90.1 ± 18.8 | −2.7 ± 3.5 | <.001 | 2420 | 79.7 ± 15.2 | 79.2 ± 15.0 | −0.5 ± 3.1 | <.001 |
| SBP, mm Hg | 513 | 138.6 ± 18.0 | 135.7 ± 17.1 | −3.0 ± 17.7 | <.001 | 1702 | 136.3 ± 18.0 | 135.6 ± 17.7 | −0.7 ± 19.6 | .122 |
| DBP, mm Hg | 512 | 80.4 ± 10.3 | 79.1 ± 9.2 | −1.3 ± 9.9 | .004 | 1701 | 78.3 ± 9.1 | 78.0 ± 9.0 | −0.3 ± 10.3 | .293 |
| FPG, mg/dL | 616 | 174.7 ± 53.3 | 146.6 ± 45.0 | −28.2 ± 54.5 | <.001 | 2004 | 153.4 ± 37.1 | 141.9 ± 34.1 | −11.5 ± 39.9 | <.001 |
| HbA1c, % | 751 | 8.6 ± 1.4 | 7.9 ± 1.3 | −0.7 ± 1.2 | <.001 | 2531 | 7.8 ± 0.9 | 7.1 ± 0.9 | −0.6 ± 1.0 | <.001 |
| Total cholesterol, mg/dL | 426 | 175.1 ± 40.6 | 171.6 ± 40.6 | −3.5 ± 34.7 | .039 | 1361 | 171.5 ± 35.7 | 166.1 ± 34.8 | −5.4 ± 29.8 | <.001 |
| HDL cholesterol, mg/dL | 409 | 45.9 ± 13.8 | 47.6 ± 13.2 | 1.6 ± 8.1 | <.001 | 1293 | 48.9 ± 13.9 | 49.2 ± 13.6 | 0.3 ± 7.9 | .235 |
| TG, mg/dL | 420 | 171.0 ± 134.1 | 155.0 ± 113.4 | −15.9 ± 135.4 | .016 | 1325 | 142.2 ± 84.3 | 134.1 ± 72.3 | −8.1 ± 70.3 | <.001 |
| LDL cholesterol, mg/dL | 387 | 95.3 ± 32.8 | 93.3 ± 33.4 | −2.0 ± 27.1 | .147 | 1238 | 94.4 ± 31.0 | 90.2 ± 30.3 | −4.2 ± 26.7 | <.001 |
| Gliclazide | GLP‐1RA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | Follow‐up | Delta | P | n | Baseline | Follow‐up | Delta | P | |
| Age, years | 2111 | 67.5 ± 9.2 | 811 | 61.7 ± 9.1 | ||||||
| Sex male % | 2111 | 59.0 | 811 | 54.1 | ||||||
| BMI, kg/mq | 1851 | 29.8 ± 5.1 | 736 | 35.2 ± 5.7 | ||||||
| Duration, years | 2111 | 11.7 ± 7.5 | 811 | 10.0 ± 7.1 | ||||||
| Follow‐up, days | 2111 | 185.4 ± 59.4 | 811 | 168.8 ± 52.3 | ||||||
| Weight, kg | 1741 | 82.1 ± 16.0 | 82.1 ± 15.9 | −0.1 ± 3.2 | .393 | 685 | 97.9 ± 17.9 | 95.5 ± 18.0 | −2.4 ± 4.0 | <.001 |
| SBP, mm Hg | 1390 | 139.5 ± 19.6 | 139.6 ± 19.2 | 0.1 ± 19.2 | .894 | 386 | 140.2 ± 18.7 | 138.8 ± 16.8 | −1.4 ± 18.6 | .138 |
| DBP, mm Hg | 1389 | 78.7 ± 9.6 | 78.3 ± 9.1 | −0.3 ± 10.0 | .206 | 386 | 80.7 ± 9.6 | 81.0 ± 9.7 | 0.3 ± 10.7 | .556 |
| FPG, mg/dL | 1439 | 166.5 ± 44.8 | 151.7 ± 39.6 | −14.8 ± 49.6 | <.001 | 473 | 156.4 ± 35.6 | 139.2 ± 36.3 | −1‐7.2 ± 40.9 | <.001 |
| HbA1c, % | 1710 | 8.1 ± 1.2 | 7.5 ± 1.1 | −0.6 ± 1.3 | <.001 | 688 | 7.8 ± 0.8 | 7.1 ± 0.9 | −0.6 ± 1.0 | <.001 |
| Total cholesterol, mg/dL | 868 | 173.6 ± 39.8 | 165.6 ± 37.0 | −8.0 ± 31.3 | <.001 | 332 | 175.1 ± 43.1 | 163.5 ± 39.6 | −11.6 ± 35.8 | <.001 |
| HDL cholesterol, mg/dL | 826 | 47.4 ± 13.3 | 47.4 ± 12.9 | 0.0 ± 7.6 | 1.000 | 318 | 45.2 ± 11.4 | 45.6 ± 11.7 | 0.4 ± 7.0 | .255 |
| TG, mg/dL | 843 | 155.1 ± 104.7 | 142.2 ± 78.3 | −12.9 ± 83.2 | <.001 | 336 | 174.0 ± 112.5 | 165.0 ± 102.8 | −8.9 ± 105.4 | .121 |
| LDL cholesterol, mg/dL | 785 | 95.7 ± 33.6 | 90.2 ± 31.8 | −5.5 ± 26.4 | <.001 | 308 | 95.1 ± 35.2 | 85.0 ± 35.4 | −10.1 ± 32.3 | <.001 |
Abbreviations: DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; SBP, systolic blood pressure; TG, triglycerides.
For each treatment group and for each variable, the number of patients with available data in both the baseline and follow‐up visit are reported. Values are mean ± SD.
Figure 1.
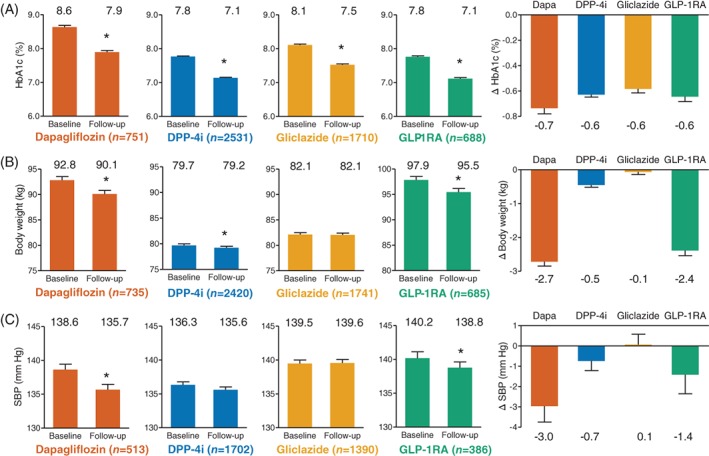
Within‐group analysis of effectiveness. For A, glycated haemoglogin (HbA1c), B, body weight and C, systolic blood pressure, panels show baseline and follow‐up values, along with changes from baseline (right panels). Numbers on top of columns indicate the corresponding values. Numbers between brackets indicates the number of available measures for each outcome. *P < .05 vs baseline. DPP‐4i, dipeptidyl peptidase‐4 inhibitor; Dapa, dapagliflozin; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; SBP, systolic blood pressure
Patients initiated on dapagliflozin, after an average of 168 days (5.5 months), showed significant improvements in fasting plasma glucose (−28.2 mg/dL), HbA1c (−0.7%), body weight (−2.7 kg), systolic blood pressure (−3.0 mm Hg), diastolic blood pressure (−1.3 mm Hg), total cholesterol (−3.5 mg/dL), HDL cholesterol (+1.6 mg/dL) and triglycerides (−15.9 mg/dL), with no change in LDL cholesterol. For patients initiating dapagliflozin on a background of insulin therapy, HbA1c significantly declined by 0.8% (from 9.1% to 8.3%) and body weight significantly declined by 2.2 kg (from 93.2 to 91.0 kg).
Patients initiated on a DPP‐4 inhibitor, after an average 185 days (6.1 months), showed significant improvements in fasting plasma glucose (−11.5 mg/dL), HbA1c (−0.6%), body weight (−0.5 kg), total cholesterol (−5.4 mg/dL), triglycerides (−8.1 mg/dL) and LDL cholesterol (−4.2 mg/dL).
Patients initiated on gliclazide, after an average of 185 days (6.1 months), showed significant improvements in fasting plasma glucose (−14.8 mg/dL), HbA1c (−0.6%), total cholesterol (−8.0 mg/dL), triglyceride (−12.9 mg/dL) and LDL cholesterol (−5.5 mg/dL).
Patients initiated on a GLP‐1RA, after an average of 169 days (5.6 months), showed significant improvements in fasting plasma glucose (−17.2 mg/dL), HbA1c (−0.6%), body weight (−2.4 kg), total cholesterol (−11.6 mg/dL) and LDL cholesterol (−10.1 mg/dL).
The percentage of patients meeting the composite endpoint of simultaneous HbA1c and body weight reduction was 56.9% for dapagliflozin, 40.5% for DPP‐4 inhibitors, 28.7% for gliclazide, and 56.2% for GLP‐1RAs (Figure S4 in Appendix S1). The corresponding percentages for the combined endpoint of simultaneous HbA1c, body weight and SBP reductions were 31.5%, 18.2%, 12.3% and 29.7%, respectively.
For each group, we estimated the expected improvement in cardiovascular risk using the UK Prospective Diabetes Study risk engine.14 Patients initiated on dapagliflozin showed the most consistent and significant reduction in the estimated 10‐year risk of coronary heart disease, fatal coronary heart disease, stroke and fatal stroke (Table S4 in Appendix S1).
Results of PSM are described in the Supporting Information. Common support between the dapagliflozin and comparator groups was very low (Figure S5 in Appendix S1) and the conditions for comparing matched cohorts were precarious (Figure S6 in Appendix S1); therefore, outcome analysis after PSM was not performed.
4. DISCUSSION
The DARWIN‐T2D study was a large retrospective study using clinical data routinely accumulated in electronic charts, involving 17 285 patients, of whom 6751 underwent a follow‐up examination. The comparison of baseline characteristics showed statistically significant and clinically meaningful differences in most variables between patients who started dapagliflozin and those who started a comparator. This was expected from an individualized therapy approach, but the HbA1c divide among groups was striking. A baseline HbA1c value of 8.7% reflects that dapagliflozin was being preferentially used in patients with moderately to severely uncontrolled diabetes. The reason for this channelling is probably 2‐fold. First, incretin‐based therapies were reimbursed by the Italian Healthcare System only if initiated in patients with an HbA1c between 7.5% and 9.0%, whereas such a limitation was not imposed on SGLT2 inhibitors. Dapagliflozin was reimbursed only in association with metformin and/or insulin (including the basal‐bolus regimen), whereas incretin‐based therapies could be reimbursed with multiple combinations of GLMs but not with basal‐bolus insulin, and gliclazide had no reimbursement limitations. These different criteria were responsible for enriching insulin therapy among patients initiating dapagliflozin. The high baseline HbA1c and the more frequent use of previous GLMs suggests that many patients initiating dapagliflozin were poor responders to other GLM classes. Second, newly marketed drugs are initially tested on the most difficult‐to‐treat patients, whereas the peculiar characteristics of patients initiating a new drug are expected to mitigate over time when the new drug becomes more established. A time‐trend analysis showed that the overall differences in clinical characteristics at the time patients initiated dapagliflozin vs other treatments were tapering over time, indicating that dapagliflozin was being progressively used in less severe cases.
The analysis of effectiveness was based on 6751 patients still on drug at follow‐up, about 6 months after baseline. The within‐group analysis in the total cohort showed improvements in HbA1c (−0.7%), FPG (−28.2 mg/dL), body weight (−2.7 kg) and systolic blood pressure (−3.0 mm Hg) after initiation of dapagliflozin that were consistent with findings from phase III RCTs.2, 3 A benefit for lipid profile was also observed, confirming the increase in HDL cholesterol and reduction of triglycerides seen in RCTs,15 but with no significant increase in LDL cholesterol. Among patients initiating dapagliflozin on a background of insulin therapy, HbA1c and body weight declined more than in the corresponding RCTs.16 Effectiveness in patients initiated on DPP‐4 inhibitors, gliclazide, or a GLP‐1RA was also well within the range expected from RCTs.17 Cardiovascular risk estimated using the UK Prospective Diabetes Study risk engine,14 improved with all treatments, but only dapagliflozin therapy was associated with a significant reduction in the projected risk of all the 4 endpoints, because of simultaneous improvements in glucose, body weight, blood pressure and lipids.
Between‐group comparisons of effectiveness were hampered by massive differences in baseline clinical characteristics that could not be overcome by PSM. Further analyses will be performed on the DARWIN‐T2D database and its follow‐up extension to provide more information on comparative effectiveness.
The present study has all the limitations inherent to its retrospective nature, including patient heterogeneity, risk of inverse causality, and concerns with regard to data quality and missingness. Importantly, the DARWIN‐T2D database is not yet linked with administrative registries and data on hard cardiovascular endpoints are not available. Major strengths of the DARWIN‐T2D study include the large sample size with nationwide distribution, the extensive patient characterization and, above all, the automatic data extraction from the same electronic chart, which granted uniform data coding and limited reporting errors.
In conclusion, during the first 21 months after marketing authorization approval in Italian routine clinical practice, dapagliflozin was prescribed to the most difficult‐to‐treat patients, many of whom were poor responders to previous GLMs and > 50% were on insulin. A “launch effect” and differential reimbursement criteria were responsible for this massive channelling, but the scenario is progressively changing. Nonetheless, even in difficult‐to‐treat patients, the effectiveness of dapagliflozin with regard to glycaemic and extra‐glycaemic endpoints was similar to results obtained in RCTs and > 50% of patients experienced a simultaneous reduction in HbA1c and body weight.
COMPOSITION OF THE DARWIN‐T2D DATABASE
Agostino Consoli and Gloria Formoso (Dipartimento di Medicina e Scienze dell'Invecchiamento ‐ Università Degli studi G. D'Annunzio di Chieti‐Pescara); Giovanni Grossi (Ospedale San Francesco di Paola ‐ Azienda Sanitaria Provinciale di Cosenza); Achiropita Pucci (Azienda Sanitaria Provinciale di Cosenza); Giorgio Sesti and Francesco Andreozzi (Azienda Ospedaliero Universitaria di Catanzaro); Giuseppe Capobianco (Azienda Sanitaria Locale Napoli 2 Nord); Adriano Gatti (Ospedale San Gennaro dei Poveri ‐ Azienda Sanitaria Locale Napoli 1 Centro); Riccardo Bonadonna, Ivana Zavaroni and Alessandra Dei Cas (Azienda Ospedaliero Universitaria di Parma); Giuseppe Felace (Ospedale di Spilimbergo ‐ Azienda per l'Assistenza Sanitaria n.5 Friuli Occidentale); Patrizia Li Volsi (Ospedale di Pordenone ‐ Azienda per l'Assistenza Sanitaria n.5 Friuli Occidentale); Raffaella Buzzetti and Gaetano Leto (Ospedale Santa Maria Goretti ‐ Azienda Sanitaria Locale di Latina); Gian Pio Sorice (Fondazione Policlinico Universitario A. Gemelli, Roma); Paola D'Angelo (Ospedale Sandro Pertini ‐ Azienda Sanitaria Locale Roma 2); Susanna Morano (Azienda Ospedaliera Universitaria Policlinico Umberto I, Roma); Antonio Carlo Bossi (Ospedale di Treviglio ‐ Azienda Socio Sanitaria Territoriale Bergamo Ovest); Edoardo Duratorre (Ospedale Luini Confalonieri di Luino Azienda Socio Sanitaria Territoriale Sette Laghi).
Ivano Franzetti (Ospedale Sant'Antonio Abate di Gallarate Azienda Socio Sanitaria Territoriale Valle Olona); Paola Silvia Morpurgo (Ospedale Fatebenefratelli ‐ Azienda Socio Sanitaria Territoriale Fatebenefratelli Sacco); Emanuela Orsi (Fondazione IRCCS Ca’ Granda ‐ Ospedale Maggiore Policlinico di Milano); Fabrizio Querci (Ospedale Pesenti Fenaroli di Alzano Lombardo ‐ Azienda Socio Sanitaria Territoriale Bergamo Est); Massimo Boemi† and Federica D'Angelo (Presidio Ospedaliero di Ricerca INRCA‐IRCCS di Ancona); Massimiliano Petrelli (Azienda Ospedaliero Universitaria Ospedali Riuniti di Ancona); Gianluca Aimaretti and Ioannis Karamouzis (Azienda Ospedaliero Universitaria Maggiore della Carità di Novara); Franco Cavalot (Azienda Ospedaliero Universitaria San Luigi Gonzaga, Orbassano); Giuseppe Saglietti† (Ospedale Madonna del Popolo di Omegna ‐ Azienda Sanitaria Locale Verbano Cusio Ossola); Giuliana Cazzetta (Casa della Salute, Ugento ‐ Distretto Socio Sanitario Gagliano del Capo ‐ Azienda Sanitaria Locale di Lecce); Silvestre Cervone (Presidio ospedaliero San Marco in Lamis Distretto Socio Sanitario San Marco in Lamis ‐ Azienda Sanitaria Locale di Foggia); Eleonora Devangelio (Distretto Socio Sanitario di Massafra ‐ Azienda Sanitaria Locale di Taranto); Olga Lamacchia (Azienda Ospedaliero Universitaria Ospedali Riuniti di Foggia); Salvatore Arena (Ospedale Umberto I – Azienda Sanitaria Provinciale di Siracusa); Antonino Di Benedetto (Azienda Ospedaliera Universitaria Policlinico G. Martino di Messina); Lucia Frittitta (Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione Garibaldi di Catania); Carla Giordano (Azienda Universitaria Policlinico Paolo Giaccone di Palermo); Salvatore Piro (Azienda Ospedaliera di Rilievo Nazionale e di Alta Specializzazione Garibaldi di Catania); Manfredi Rizzo, Roberta Chianetta and Carlo Mannina (Azienda Universitaria Policlinico Paolo Giaccone di Palermo); Roberto Anichini (Ospedale San Jacopo di Pistoia – Azienda USL Toscana Centro); Giuseppe Penno (Azienda Ospedaliero Universitaria Pisana); Anna Solini (Azienda Ospedaliera Universitaria Pisana); Bruno Fattor (Comprensorio Sanitario di Bolzano ‐ Azienda Sanitaria della Provincia Autonoma di Bolzano); Enzo Bonora and Massimo Cigolini (Azienda Ospedaliero Universitaria Integrata di Verona); Annunziata Lapolla and Nino Cristiano Chilelli (Complesso Socio Sanitario Ai Colli ‐ Azienda ULSS n.6 Euganea); Maurizio Poli (Ospedale Girolamo Fracastoro di San Bonifacio ‐ Azienda ULSS n.9 Scaligera); Natalino Simioni and Vera Frison (Ospedale di Cittadella ‐ Azienda ULSS n.6 Euganea); Carmela Vinci (Azienda ULSS n.4 Veneto Orientale).
Supporting information
Appendix S1. Online Appendix.
ACKNOWLEDGMENTS
We are grateful for the technical support of Alessia Russo, Italian Diabetes Society.
Conflict of interest
G.P.F. has received grant support, lecture or advisory board fees from AstraZeneca, Boehringer‐Ingelheim, Eli Lilly, NovoNordisk, Sanofi, Genzyme, Abbott, Novartis and Merck Sharp & Dohme.
A.C. has received consultancy or speaker fees from Abbott, AstraZeneca, Boehringer‐Ingelheim, Bruno Farmaceutici, Janssen, Eli‐Lilly, Merck Sharp & Dohme, Novartis, NovoNordisk, Roche, Sanofi‐Aventis, Servier and Takeda. He has also received research grants from Eli‐Lilly and NovoNordisk. A.G. has received consultancy or speaker fees from AstraZeneca, Boehringer‐Ingelheim, Eli‐Lilly, Merck Sharp & Dohme, Sanofi‐Aventis and Takeda. He has also received a research grant from AstraZeneca. G.S. has received fees for advisory work or lectures from Servier, Intarcia Therapeutics Inc, NovoNordisk, Janssen, Boehringer‐Ingelheim, Eli Lilly, AstraZeneca, Merck Sharp & Dohme Italy, Lilly, Sanofi, Novartis, Abbott and Takeda. A.A. has received research grants, lecture or advisory board fees from Merck Sharp & Dome, AstraZeneca, Novartis, Boeringher‐Ingelheim, Sanofi, Mediolanum, Janssen, NovoNordisk, Lilly, Servier and Takeda. G.Z., D.B. and I.B. declare no conflict of interest.
Author contributions
Author contributions were as follows: design, data collection and analysis: G.P.F., G.Z., D.B., I.B., A.C., G.S. and A.A.; manuscript writing: G.P.F. and A.A.; and manuscript revising for intellectual content: G.Z., A.G., A.C. and G.S. All authors approved the final version of the manuscript.
Fadini GP, Zatti G, Baldi I, et al. Use and effectiveness of dapagliflozin in routine clinical practice: An Italian multicentre retrospective study. Diabetes Obes Meta. 2018;20:1781–1786. 10.1111/dom.13280
Funding information The DARWIN‐T2D study was funded by the Italian Diabetes Society, with support from AstraZeneca. The external funding source had no role in study design and conduction, nor in the writing of the manuscript and decision to publish.
REFERENCES
- 1. Abdul‐Ghani MA, Norton L, Defronzo RA. Role of sodium‐glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515‐531. [DOI] [PubMed] [Google Scholar]
- 2. Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium‐glucose co‐transporter 2 inhibitors on blood pressure: a systematic review and meta‐analysis. J Am Soc Hypertens. 2014;8:262‐275, e269. [DOI] [PubMed] [Google Scholar]
- 3. Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co‐transport‐2 inhibitors in type 2 diabetes: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457‐466. [DOI] [PubMed] [Google Scholar]
- 4. Frieden TR. Evidence for health decision making ‐ beyond randomized, controlled trials. N Engl J Med. 2017;377:465‐475. [DOI] [PubMed] [Google Scholar]
- 5. Patel A, Billot L. Reality and truth. Balancing the hope and the hype of real‐world evidence. Circulation. 2017;136:260‐262. [DOI] [PubMed] [Google Scholar]
- 6. Gagne JJ, Bykov K, Willke RJ, Kahler KH, Subedi P, Schneeweiss S. Treatment dynamics of newly marketed drugs and implications for comparative effectiveness research. Value Health. 2013;16:1054‐1062. [DOI] [PubMed] [Google Scholar]
- 7. Austin PC, Laupacis A. A tutorial on methods to estimating clinically and policy‐meaningful measures of treatment effects in prospective observational studies: a review. Int J Biostat. 2011;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014;49:1701‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 11. Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular outcomes and risks after initiation of a sodium glucose co‐transporter 2 inhibitor: results from the EASEL population‐based cohort study. Circulation. 2017. 10.1161/CIRCULATIONAHA.117.031227 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose Cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose Cotransporter‐2 inhibitors). Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fadini GP, Zatti G, Consoli A, Bonora E, Sesti G, Avogaro A. Rationale and design of the DARWIN‐T2D (DApagliflozin real world evIdeNce in type 2 diabetes): a multicenter retrospective nationwide Italian study and crowdsourcing opportunity. Nutr Metab Cardiovasc Dis. 2017;27:1089‐1097. [DOI] [PubMed] [Google Scholar]
- 14. Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101:671‐679. [PubMed] [Google Scholar]
- 15. Matthaei S, Bowering K, Rohwedder K, Sugg J, Parikh S, Johnsson E. Durability and tolerability of dapagliflozin over 52 weeks as add‐on to metformin and sulphonylurea in type 2 diabetes. Diabetes Obes Metab. 2015;17:1075‐1084. [DOI] [PubMed] [Google Scholar]
- 16. Wilding JP, Woo V, Soler NG, et al. Long‐term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405‐415. [DOI] [PubMed] [Google Scholar]
- 17. Phung OJ, Scholle JM, Talwar M, Coleman CI. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 diabetes. JAMA. 2010;303:1410‐1418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Online Appendix.


