Abstract
This study evaluated the patterns of care and health care resource use (HCRU) in patients with metastatic squamous cell carcinoma of the head and neck (SCCHN) who received ≥3 lines of systemic therapy in the United Kingdom (UK). Oncologists (n = 40) abstracted medical records for patients with metastatic SCCHN who initiated third‐line systemic therapy during 1 January 2011–30 August 2014 (n = 220). Patient characteristics, treatment patterns and SCCHN‐related HCRU were summarised descriptively for the metastatic period; exploratory multivariable regression analyses were conducted on select HCRU outcomes. At metastatic diagnosis, most patients had an Eastern Cooperative Oncology Group performance status (PS) of 0/1 (95%). For patients with PS 0/1, the most common first‐line treatment was cisplatin+5‐fluorouracil (5‐FU); docetaxel was the most common second‐ and third‐line treatment. For patients with PS ≥ 2, the most common first‐, second‐, and third‐line treatments were carboplatin+5‐FU, cetuximab, and methotrexate, respectively. Most patients received supportive care during (85%) and after (89%) therapy. This study provides useful information, prior to the availability of immunotherapy, on patient characteristics, treatment patterns, HCRU, and survival in a real‐world UK population with metastatic SCCHN receiving ≥3 lines of systemic therapy. Patterns of care and HCRU varied among patients with metastatic SCCHN; specific systemic therapies varied by patient PS.
Keywords: health care resource use, squamous cell carcinoma of the head and neck, treatment patterns
1. INTRODUCTION
Approximately 139,000 incident cases of head and neck cancer occur each year in Europe (Grégoire, Lefebvre, Licitra, & Felip, 2010). This type of cancer includes a wide range of malignant tumours originating in the upper aerodigestive tract, including the larynx, oral cavity, oropharynx, piriform sinus, and hypopharynx (Cancer Research UK, 2016a,2016b; Jones, 2014). Approximately 90% of head and neck cancers are classified as squamous cell carcinomas of the head and neck (SCCHN) (Grégoire et al., 2010). Patients with squamous cell carcinomas can experience pain, cough, bleeding, vocal cord paralysis or swallowing dysfunction, as well as masses or ulcerations in the neck, oral cavity, or oropharynx (Argiris, Karamouzis, Raben, & Ferris, 2008). In the United Kingdom (UK), approximately, 8,500–9,000 cases of SCCHN are diagnosed annually (Jones, 2014), and the incidence has been increasing since 1995 (Gillison, Chaturvedi, Anderson, & Fakhry, 2015).
Tobacco use and alcohol consumption are risk factors for SCCHN (National Cancer Institute, 2013), and an association between human papillomavirus (HPV) and SCCHN, in particular for oropharyngeal tumours, has been observed (Gillison et al., 2015; Pfister & Fury, 2014; Syrjänen, 2010). Approximately one‐half to two‐thirds of patients with SCCHN initially present with locally or regionally advanced disease, and 10% of patients with SCCHN have metastatic disease at diagnosis (Gold, Lee, & Kim, 2009; Won et al., 2011). Roughly 65% of patients with locally or regionally advanced SCCHN will relapse after primary therapy and require additional treatment (Won et al., 2011). Median overall survival for patients with metastatic SCCHN is approximately 6–10 months (van der Linden et al., 2016; Malhotra et al., 2014; Vermorken et al., 2013; Won et al., 2011), with 1‐year survival rates ranging between 38% and 43% (Adamo et al., 2004; Raguse, Gath, Oettle, & Bier, 2006; Won et al., 2011).
Treatment options for metastatic SCCHN are limited, and there is no consensus on a treatment pathway for metastatic disease among European and UK‐specific treatment guidelines. Guidelines published more than 5 years ago from the European Society for Medical Oncology (ESMO) note that palliative chemotherapy is the recommended treatment for most patients with advanced, recurrent or metastatic SCCHN who are not candidates for surgery or radiation therapy (Grégoire et al., 2010). In contrast with the ESMO guidelines, multidisciplinary guidelines published in 2011 for the treatment of head and neck cancer issued by ENT‐UK suggest that there is little evidence supporting the use of palliative chemotherapy but that there may be an emerging role for biological targeted therapies (e.g., cetuximab) in recurrent/metastatic disease (Roland & Paleri, 2011). Finally, the National Institute for Health and Care Excellence (NICE) clinical guidelines describe general treatment strategies (surgery, radiotherapy, or both, with or without concomitant chemotherapy) for advanced upper aerodigestive cancers but do not recommend a specific regimen for metastatic SCCHN (NICE, 2016).
Treatment patterns and the standard of care are evolving, as the immuno‐oncologic agent nivolumab was recently approved by the European Medicines Agency (EMA) for the treatment of patients with advanced or metastatic SCCHN with disease progression on or after platinum‐based chemotherapy. Nivolumab is available in the UK via the Cancer Drugs Fund for patients meeting select criteria (NICE, 2017). Both nivolumab and pembrolizumab have been approved by the United States Food and Drug Administration for patients with advanced or metastatic SCCHN with disease progression on or after platinum‐based chemotherapy, and studies are on‐going in earlier lines of treatment. Data are also emerging to suggest that other immuno‐oncologic therapies (e.g., ipilimumab) may be effective for SCCHN (Burtness, 2015). With continuing improvements in treatment for advanced cancer, patients with recurrent/metastatic SCCHN increasingly may be candidates for later lines of therapy following disease progression. However, background information against which to assess the utility of emerging therapies in routine practice—specifically, real‐world data characterising treatment patterns among patients with metastatic SCCHN in the European Union and the UK—is quite limited. Current treatments for the metastatic population, the sequence of successive treatments, and the impact of performance status (PS) are uncharacterised in the literature. van der Linden et al. (2016) evaluated treatments and costs associated with metastatic SCCHN in the Netherlands, but this study was not focused on patients who had progressed beyond the second line of therapy in the metastatic setting. To our knowledge, no previous studies have evaluated treatment patterns by PS or supportive care use among patients with metastatic SCCHN. The objectives of our study were to characterise and report the real‐world treatment patterns and health care resource use (HCRU), both overall and by PS, for patients with a diagnosis of metastatic SCCHN who have progressed to later lines of systemic therapy in the UK.
2. METHODS
2.1. Study design and data source
We conducted a retrospective cohort study of patients with metastatic SCCHN (either stage IVC SCCHN at initial presentation or recurrent SCCHN with distant metastasis) who had received at least three lines of systemic therapy for metastatic disease.
Medical record data were abstracted retrospectively by oncology specialists who treat patients with metastatic SCCHN in the UK. The participating oncology specialists abstracted patient data using a secure, web‐based data collection form that was developed by the authors based on study objectives. Physicians were instructed to select medical records using a quasi‐random selection approach (i.e., selecting medical records for patients whose surname began with a randomly generated letter between A and Z). Abstracted data were de‐identified and anonymous to the authors. This study was submitted to RTI International's Institutional Review Board and was deemed exempt from full review. Based on local ethical review board requirements in the UK, this study was similarly exempt from a full ethics committee review by the UK's National Research Ethics Service.
2.2. Physician and patient selection criteria
Physicians were medical oncologists, clinical oncologists, or haematologists/oncologists who had been in practice for 5–30 years since their date of oncology board certification or end of residency. They had a case load of at least three patients with metastatic SCCHN treated with at least third‐line therapy for metastatic disease in the last 12 months. Data were extracted for patients who were aged 18 years or older on the date of metastatic SCCHN diagnosis and who started third‐line systemic therapy for metastatic SCCHN between 1 January 2011 and 30 August 2014. Data were extracted between 6 March 2015 and 29 April 2015. Patients were excluded if they were enrolled in treatment or interventional studies related to SCCHN at any time from the time of diagnosis of metastatic disease until the end of available medical record data. Patients could be either dead or alive at the time of medical record abstraction.
2.3. Study measures
Physician self‐reported characteristics included patient case load (i.e., the number of patients with metastatic SCCHN treated with third‐line therapy in the last 12 months), number of years in practice, medical specialty, and the geographic region in the UK where they practised.
Patient characteristics abstracted from the medical records included year of birth, sex, race/ethnicity, availability of supplemental private insurance (in addition to the national health plan), smoking status, HPV status, and presence of comorbidities or medical history at the time of diagnosis of metastatic disease. Patients’ age at diagnosis of metastatic disease and age at start of third‐line therapy for metastatic disease were calculated from patients’ year of birth. Stage of disease at initial diagnosis and primary site of SCCHN were collected. For patients with initial diagnoses of stage I through stage IVB disease, the types of cancer‐directed treatments and supportive care services patients received before diagnosis of metastatic disease were collected and analysed. Eastern Cooperative Oncology Group (ECOG) or Karnofsky Performance Scale PS at metastatic SCCHN diagnosis but prior to the first line of systemic therapy (“at metastatic diagnosis”), as well as at start of each subsequent therapy line, was documented. Karnofsky Performance Scale PS scores were converted to ECOG scores to facilitate comparison of PS across all patients (ESMO, 2008; Oken et al., 1982).
Systemic therapy treatment patterns from the time of metastatic SCCHN diagnosis until the time of last medical record or death were evaluated. Treatment guidelines and published literature informed which treatments were listed on the data collection form (Grégoire et al., 2010; National Comprehensive Cancer Network, 2013), although physicians were able to enter other treatments that were not listed. Survival data were analysed using Kaplan–Meier methods to estimate survival from initiation of third‐line systemic therapy for metastatic SCCHN, as well as from the time of metastatic diagnosis. For patients who were still alive or had unknown survival status, observation was censored at the date of the last entry in their medical record.
The number and percentage of patients receiving specific supportive care services after metastatic diagnosis and prior to discontinuation of last systemic therapy, as well as after discontinuation of last systemic therapy until death or last medical record were documented. Supportive care services were also documented before metastatic diagnosis among the subset of patients initially presenting with stage I‐IVB disease. Information on metastatic SCCHN‐related HCRU (i.e., outpatient visits and hospitalisations) was collected and reported separately for the periods during systemic therapy and after the end of last systemic therapy. In addition to the number of SCCHN‐related outpatient visits and hospitalisations, length of stay for hospitalisation, and time on wards (e.g., general ward, oncology, intensive care unit) were collected. Hospitalisations with discharge dates occurring on the same day as admission dates were assumed to have a 1‐day length of stay (i.e., hospitalisation length of stay was calculated per visit by subtracting the admission date index from the discharge date index and adding 1).
2.4. Statistical analyses
All study measures were summarised descriptively through the tabular display of values characterising mean and standard deviations (SDs) for continuous variables of interest and frequency distributions for categorical variables. Time‐related outcomes (e.g., survival) were estimated using the Kaplan–Meier method.
Exploratory multivariable logistic regression analyses were conducted to formally assess the risk of covariate‐adjusted HCRU outcomes of interest (i.e., whether patients had any SCCHN‐related hospitalisations, emergency department visits, office visits/consults, outpatient visits in hospital clinics, and outpatient palliative care visits). Covariates that were expected to be associated with the HCRU outcomes of interest were included in the models (e.g., age at metastatic diagnosis, sex, PS at metastatic diagnosis, comorbidity burden). Odds ratios from the logistic regression models are reported.
All analyses were conducted using SAS statistical software, versions 9.3 and 9.4 (Cary, NC: SAS Institute, Inc.; 2011, 2013).
3. RESULTS
3.1. Physician characteristics
A total of 40 physicians were recruited from across the UK for study participation. Participating physicians were specialists in the fields of clinical oncology (52.5%) and medical oncology (47.5%). Mean (SD) years in practice since oncology board certification or end of residency was 10.9 (5.4), with the majority of participating physicians in practice for 5–10 years (52.5%). The number of patients with metastatic SCCHN treated with at least third‐line therapy in the last 12 months by participating physicians ranged from 3 to 60 patients, with a mean (SD) of 25.9 (18.2) patients. In all, 19 (47.5%) of the participating physicians were from the Greater London and South East region of the UK, with the remaining physicians from the Midlands and East (22.5%), North (20.0%) and South West (10.0%).
3.2. Patient characteristics
A total of 220 patients from the UK were included in the study (Table 1). Mean (SD) age at metastatic SCCHN diagnosis was 59.0 (8.0) years, and mean (SD) age at start of third‐line systemic therapy for metastatic SCCHN was 60.7 (8.0) years. The study cohort was disproportionately male (73.6%), of white/Caucasian ethnicity (90.5%), and without supplemental private insurance at the time of metastatic SCCHN diagnosis (92.7%). Most patients were former (58.2%) or current smokers (26.8%) at the time of metastatic diagnosis. The majority of patients (51.8%) had initial diagnoses of stage IVC disease, 47.7% had initial diagnoses of stage I through stage IVB disease, and only 1 patient (0.5%) reported having an unknown stage at initial diagnosis. The oropharynx was the most common primary anatomic site reported (36.8%).
Table 1.
Patient characteristics
| N | % | |
|---|---|---|
| Number of patients (%) | 220 | 100.0 |
| Age at start of third‐line systemic therapy (years) | ||
| Mean (SD) | 60.7 (8.0) | |
| 18–44 | 9 | 4.1 |
| 45–54 | 38 | 17.3 |
| 55–64 | 107 | 48.6 |
| 65–74 | 60 | 27.3 |
| 75+ | 6 | 2.7 |
| Sex | ||
| Male | 162 | 73.6 |
| Female | 58 | 26.4 |
| Race/ethnicity | ||
| White/Caucasian | 199 | 90.5 |
| African/black | 3 | 1.4 |
| Asian or Pacific Islander | 9 | 4.1 |
| Middle Eastern | 4 | 1.8 |
| Indian subcontinent (Indian, Pakistani, Bangladeshi) | 5 | 2.3 |
| Supplemental private insurance | ||
| Yes | 4 | 1.8 |
| No | 204 | 92.7 |
| Not reported | 12 | 5.5 |
| Smoking status | ||
| Current smoker | 59 | 26.8 |
| Former smoker | 128 | 58.2 |
| Non‐smoker | 27 | 12.3 |
| Not reported | 6 | 2.7 |
| Initial clinical stage at SCCHN diagnosis | ||
| I | 4 | 1.8 |
| II | 16 | 7.3 |
| III | 37 | 16.8 |
| IVA | 28 | 12.7 |
| IVB | 20 | 9.1 |
| IVC | 114 | 51.8 |
| Not reported | 1 | 0.5 |
| Primary site | ||
| Lip/oral cavity | 23 | 10.5 |
| Nasopharynx | 41 | 18.6 |
| Oropharynx | 81 | 36.8 |
| Hypopharynx | 28 | 12.7 |
| Larynx (supraglottis, glottis or subglottis) | 36 | 16.4 |
| Nasal cavity/paranasal sinuses (maxillary sinus, nasal cavity or ethmoid sinus) | 9 | 4.1 |
| Not reported | 2 | 0.9 |
| Cancer‐directed treatments before metastatic SCCHN diagnosisa,b | ||
| N (patients initially diagnosed with stages I‐IVB) | 105 | 47.7 |
| Any surgery | 68 | 64.8c |
| Any radiotherapy | 88 | 83.8c |
| Any systemic chemotherapy | 63 | 60.0c |
| Not reported | 3 | 2.9c |
SCCHN: squamous cell carcinoma of the head and neck; SD: standard deviation.
Characteristics were assessed at metastatic SCCHN diagnosis unless otherwise specified.
Totals may sum to more than 100% because respondents were able to provide multiple answers.
Supporting Information Table S1 provides additional details on the cancer‐directed treatments that were received before metastatic SCCHN diagnosis.
Percentage of the 105 patients initially diagnosed with stage I‐IVB SCCHN.
Among patients with initial diagnoses of stage I through stage IVB disease, 83.8% received any radiotherapy, 64.8% received any surgery, and 60.0% received any systemic chemotherapy prior to diagnosis with metastatic disease (Table 1). Most patients received a combination of these cancer‐directed treatments (Supporting Information Table S1). Only 4.8% of patients received radiotherapy alone, 13.3% of patients received surgery alone; there were no patients who received systemic chemotherapy alone.
Most patients (71.8%) were reported to have at least one comorbidity at the time of metastatic SCCHN diagnosis; 23.6% of patients were reported to have no comorbidity; and 4.5% of patients had an unknown comorbidity status. Comorbidities reported in more than 10% of the population included hypertension (42.7%), chronic obstructive pulmonary disease or asthma (26.4%), alcohol abuse or alcoholism (10.9%), diabetes with end organ involvement (10.5%), and diabetes without end organ involvement (10.5%). Only two patients were reported to be HPV positive.
At metastatic diagnosis, 15.9% of patients had an ECOG PS score of 0, 78.6% had an ECOG PS score of 1, and 4.1% had an ECOG PS score ≥ 2 (Figure 1). There was a downward trend in PS as patients progressed to later lines of therapy.
Figure 1.
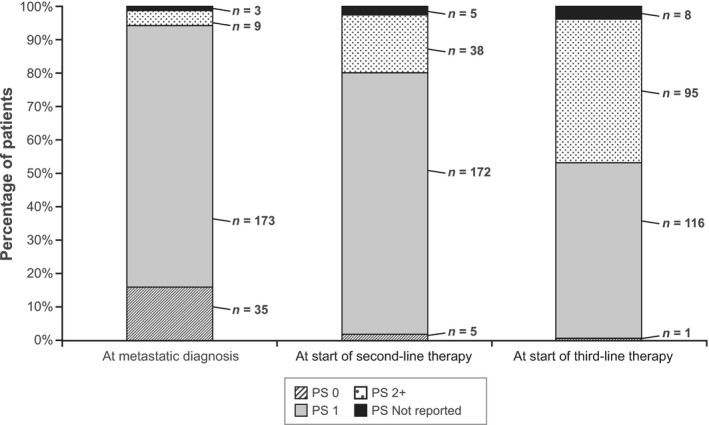
ECOG performance status scores stratified by line of therapy. ECOG: Eastern Cooperative Oncology Group; PS: performance status. Notes: Distributions are shown at each time for the full sample of 220 patients. Of the 4 patients who received fourth‐line therapy, 2 patients had PS 1, 1 patient had PS ≥ 2 and 1 patient had unknown PS (not shown). Karnofsky Performance Scale scores for 20 patients at metastatic diagnosis (prior to receipt of first‐line therapy), 19 patients at start of second‐line therapy and 15 patients at start of third‐line therapy were converted to ECOG/World Health Organization scores using the conversion guide found at: http://oncologypro.esmo.org/Guidelines-Practice/Practice-Tools/Performance-Scales
3.3. Treatment patterns
3.3.1. Anticancer treatment use
Of the 220 study participants (all of whom received at least three lines of systemic therapy for metastatic SCCHN), only 4 patients (1.8%) received four lines of therapy; no patients received five or more therapy lines. A total of 36 distinct chemotherapy regimens were reported across all lines of therapy (Table 2); the majority of reported regimens were single‐agent therapies (55.1%), followed by combination regimens consisting of two (36.9%) or three (8.0%) agents. Among all 36 distinct drug regimens, the most commonly reported were single‐agent docetaxel (21.1%), cisplatin + 5‐fluorouracil (5‐FU) (16.4%), and single‐agent cetuximab (9.3%).
Table 2.
Systemic treatment regimens used across lines of therapy
| N | % | |
|---|---|---|
| Number (%) of regimens reporteda | 664 | 100.0 |
| Single agents | 366 | 55.1 |
| Docetaxel | 140 | 21.1 |
| Cetuximab | 62 | 9.3 |
| Paclitaxel | 44 | 6.6 |
| Methotrexate | 44 | 6.6 |
| Gemcitabine | 34 | 5.1 |
| Cisplatin | 14 | 2.1 |
| Carboplatin | 14 | 2.1 |
| Capecitabine | 7 | 1.1 |
| Vinorelbine | 6 | 0.9 |
| Other (1 regimen)b | 1 | 0.2 |
| Two‐agent regimens | 245 | 36.9 |
| Cisplatin + 5‐FU | 109 | 16.4 |
| Carboplatin + paclitaxel | 33 | 5.0 |
| Carboplatin + 5‐FU | 30 | 4.5 |
| Gemcitabine + vinorelbine | 15 | 2.3 |
| Carboplatin + gemcitabine | 15 | 2.3 |
| Capecitabine + cisplatin | 11 | 1.7 |
| Cisplatin + docetaxel | 7 | 1.1 |
| Other (12 regimens)b | 25 | 3.8 |
| Three‐agent regimens | 53 | 8.0 |
| Cetuximab + cisplatin + 5‐FU | 36 | 5.4 |
| Carboplatin + cetuximab + 5‐FU | 8 | 1.2 |
| Other (5 regimens)b | 9 | 1.4 |
5‐FU: 5‐fluorouracil.
“Paclitaxel” refers to the non‐protein‐bound form of the drug. Systemic treatments by therapy line are presented in Supporting Information Table S2.
Regimens were reported among all 220 patients.
Other regimens were reported in ≤5 patients each.
The most frequently used regimens varied by line of therapy and by PS measured at the start of each line of therapy (Table 3, with additional details on all systemic treatments used in each line of therapy presented in Supporting Information Table S2). In the first line, cisplatin + 5‐FU was the most commonly reported regimen (46.4%) overall, followed by cetuximab + cisplatin + 5‐FU (16.4%), and carboplatin + 5‐FU (11.8%). Cisplatin + 5‐FU was the most common first‐line treatment among patients with PS 0 or 1, and carboplatin + 5‐FU was the most common first‐line treatment among patients with PS ≥ 2. In the second line, the most common regimens overall were single‐agent docetaxel (45.0%) and single‐agent paclitaxel (12.7%), with single‐agent cetuximab (8.6%) and carboplatin + paclitaxel (8.6%) tied for the third most commonly reported regimen. Docetaxel was the most common second‐line treatment among patients with PS 0 or 1, and cetuximab was the most common second‐line treatment among patients with PS ≥ 2. In the third line, single‐agent cetuximab (19.6%) and single‐agent methotrexate (19.6%) were the most commonly reported regimens overall, followed by single‐agent docetaxel (18.2%). Docetaxel was the most common third‐line treatment among patients with PS 0 or 1, and methotrexate was the most common third‐line treatment among patients with PS ≥ 2. Overall, the mean number of cycles varied across regimens and lines of therapy, ranging from 4.1 cycles for third‐line docetaxel to 6.6 cycles for third‐line cetuximab. Among the four patients overall who received fourth‐line therapy, no patient received the same fourth‐line therapy (Supporting Information Table S2). Overall, the most frequently used regimen sequence was cisplatin + 5‐FU as first‐line therapy, single‐agent docetaxel as second‐line therapy, and single‐agent cetuximab as third‐line therapy. This sequence of regimens was received by 8.6% of patients (n = 19).
Table 3.
Patients receiving the most commonly used systemic treatments in each line of therapy, overall and by ECOG PS
| First line | Overall | PS 0 | PS 1 | PS 2+ | |
|---|---|---|---|---|---|
| N = 220 | n = 35 (15.9%) | n = 173 (78.6%) | n = 9 (4.1%) | ||
| n (%) | Mean (SD) No. of cycles | n (%) | n (%) | n (%) | |
| Cisplatin + 5‐FU | 102 (46.4) | 5.7 (0.9) | 20 (57.1) | 78 (45.1) | 3 (33.3) |
| Cisplatin + cetuximab + 5‐FU | 36 (16.4) | 6.3 (1.1) | 5 (14.3) | 31 (17.9) | 0a |
| Carboplatin + 5‐FU | 26 (11.8) | 5.7 (0.8) | 3 (8.6) | 16 (9.3) | 5 (55.6) |
| Cisplatin + capecitabine | 10 (4.5)a | N/Ab | 3 (8.6) | 7 (4.1)a | 0a |
| Cisplatin + 5‐FU + paclitaxel | 2 (0.9)a | N/Aa | 0a | 1 (0.6)a | 1 (11.1) |
| Second line | Overall | PS 0 | PS 1 | PS 2+ | |
|---|---|---|---|---|---|
| N = 220 | n = 5 (2.3%) | n = 172 (78.2%) | n = 38 (17.3%) | ||
| n (%) | Mean (SD) No. of cycles | n (%) | n (%) | n (%) | |
| Docetaxel | 99 (45.0) | 5.3 (1.3) | 1 (20.0) | 84 (48.8) | 10 (26.3) |
| Paclitaxel | 28 (12.7) | 5.6 (2.1) | 2 (40.0) | 18 (10.5) | 8 (21.1) |
| Carboplatin + paclitaxel | 19 (8.6) | 5.8 (0.7) | 0a | 16 (9.3) | 3 (7.9)a |
| Cetuximab | 19 (8.6) | 6.0 (1.9) | 0a | 7 (4.1)a | 12 (31.6) |
| Carboplatin + gemcitabine | 2 (0.9)a | N/Aa | 1 (20.0) | 0a | 1 (2.6)a |
| Cetuximab + 5‐FU | 1 (0.5)a | N/Aa | 1 (20.0) | 0a | 0a |
| Third line | Overall | PS 0 | PS 1 | PS 2+ | |
|---|---|---|---|---|---|
| N = 220 | n = 1 (<1.0%) | n = 116 (52.7%) | n = 95 (43.2%) | ||
| n (%) | Mean (SD) No. of cycles | n (%) | n (%) | n (%) | |
| Docetaxel | 40 (18.2) | 4.1 (1.6) | 1 (100.0) | 29 (25.0) | 10 (10.5)a |
| Cetuximab | 43 (19.6) | 6.6 (2.5) | 0a | 27 (23.3) | 15 (15.8) |
| Methotrexate | 43 (19.6) | 4.2 (1.7) | 0a | 22 (19.0) | 21 (22.1) |
| Gemcitabine | 27 (12.3)a | N/Ab | 0a | 6 (5.2)a | 16 (16.8) |
5‐FU: 5‐fluorouracil; ECOG: Eastern Cooperative Oncology Group; N/A: not available; PS: performance status; SD: standard deviation.
PS was requested before each line of therapy but was not reported for all patients; therefore, the total number of patients by PS in each line of therapy is <220, and the sum varies among lines of therapy. For each line of therapy, the three most commonly used treatments, overall and by ECOG PS, are presented in bold. Where two or three regimens were reported with equal frequency within a stratum (line of therapy and PS), more than three treatments are presented in bold for that stratum.
This regimen was not one of the three most commonly used in this stratum.
Mean (SD) number of cycles are reported only for the top regimens overall.
3.3.2. Supportive care use
Among the 105 patients initially diagnosed with stage I‐IVB SCCHN, 91.4% received some amount of supportive care prior to diagnosis of metastatic disease (Table 4). Only one patient (1.0%) did not receive any supportive care during this period, and the remaining 7.6% of patients had unknown supportive care use. Patients most commonly received nutritional support (73.3%), dental care for radiotherapy effects (61.9%), pain and symptom management (60.0%), support for smoking and alcohol cessation (47.6%), and speech and swallowing therapy (44.8%).
Table 4.
Patients receiving supportive care measures by phase of treatment
| Before metastatic diagnosis in patients presenting with earlier stage disease (n = 105) (47.7%) | After metastatic diagnosis and before discontinuation of systemic therapy (N = 220) (100%) | After discontinuation of systemic therapy (n = 164) (74.5%)a | |
|---|---|---|---|
| Number (%) of patients | |||
| Audiology | 27 (25.7) | 3 (1.4) | 1 (0.6) |
| Dental care for radiotherapy effects | 65 (61.9) | 49 (22.3) | 16 (9.8) |
| Depression assessment and management | 13 (12.4) | 23 (10.5) | 15 (9.1) |
| Nutritional support | 77 (73.3) | 129 (58.6) | 81 (49.4) |
| Pain and symptom management | 63 (60.0) | 117 (53.2) | 95 (57.9) |
| Support for smoking and alcohol cessation | 50 (47.6) | 16 (7.3) | 5 (3.0) |
| Speech and swallowing therapy | 47 (44.8) | 49 (22.3) | 15 (9.2) |
| Tracheotomy care | 10 (9.5) | 3 (1.4) | 1 (0.6) |
| Wound management | 18 (17.1) | 8 (3.6) | 3 (1.8) |
| Xerostomia management | 28 (26.7) | 53 (24.1) | 23 (14.0) |
| Antiemetics | NC | 131 (59.6) | 65 (39.6) |
| Management of oral and gastrointestinal mucositis | NC | 65 (29.6) | 27 (16.5) |
| Haematological growth factor/transfusions | NC | 49 (22.3) | 19 (11.6) |
| Any supportive care | 96 (91.4) | 186 (84.5) | 146 (89.0) |
| None | 1 (1.0) | 6 (2.7) | 5 (3.0) |
| Not reported | 8 (7.6) | 28 (12.7) | 13 (7.9) |
NC: not collected.
Patients could have received multiple types of supportive care.
56 patients were still receiving systemic therapy at the time of data collection.
Among all 220 patients, 84.5% received some amount of supportive care following metastatic SCCHN diagnosis and prior to discontinuation of last systemic therapy. More than 50% of patients received antiemetics (59.6%), nutritional support (58.6%), and pain and symptom management (53.2%) during this period. In all, 56 patients were still receiving systemic therapy for metastatic SCCHN at the time of data collection. Among the 164 patients who discontinued last systemic therapy during the study, 89.0% received some amount of supportive care during the period following discontinuation. Although the overall percentage of patients receiving some amount of supportive care remained similar during and after discontinuation of systemic therapy, the proportions of patients using particular supportive care services varied: for example, use of antiemetics decreased (from 59.6% to 39.6%), use of nutritional support decreased (from 58.6% to 49.4%), and use of pain and symptom management increased (from 53.2% to 57.9%) after discontinuation of systemic therapy.
3.4. Health care resource use
During systemic therapy, the HCRU categories most commonly reported for patients were outpatient office visits (70.0% with any visit) and palliative care visits (44.6% with any visit) (Table 5). Among patients with any visit in either setting, a mean (SD) of 24.8 (13.7) outpatient office visits and 3.8 (3.0) palliative care visits were reported. Fewer patients had outpatient visits in a hospital clinic or cancer centre, emergency department visits on an outpatient basis, or hospitalisations (overnight or day admissions, excluding emergency department visits).
Table 5.
Health care resource use related to metastatic SCCHN, overall
| During systemic therapy, N = 220 (100.0%) | For supportive care after end of last systemic therapy, n = 164 (74.5%) | |
|---|---|---|
| Number (%) of patients | ||
| Outpatient office visitsa | 154 (70.0) | 83 (50.6) |
| Outpatient hospital clinic or cancer centre visits | 77 (35.0) | 60 (36.6) |
| ED visits | 45 (20.5) | 23 (14.0) |
| Palliative care visits in outpatientb, community health service, or home settings | 98 (44.6) | 59 (36.0) |
| Hospitalisations (overnight or day admissions excluding ED visits) | 59 (26.8) | 14 (8.5) |
| Hospice, long‐term care, or other end‐of‐life/palliative care services | 18 (8.2) | 31 (18.9) |
| Mean (SD) number of visits (among patients with any visits) | ||
| Outpatient office visitsa | 24.8 (13.7) | 3.4 (2.4) |
| Outpatient hospital clinic or cancer centre visits | 7.9 (6.1) | 3.3 (2.5) |
| ED visits | 1.8 (1.2) | 1.4 (0.8) |
| Palliative care visits in outpatientb, community health service, or home settings | 3.8 (3.0) | 3.1 (2.0) |
| Hospitalisations (overnight or day admissions excluding ED visits) | 2.9 (2.8) | 1.1 (0.3) |
| Hospice, long‐term care, or other end‐of‐life/palliative care services | 1.2 (0.4) | 1.6 (0.6) |
ED: emergency department; SCCHN: squamous cell carcinoma of the head and neck; SD: standard deviation.
Outpatient office visits included visits/consults at the responding physician's office, with a general practitioner, or at a free‐standing oncology clinic.
Outpatient palliative care visit settings excluded those settings already captured (i.e., visits to the responding physician's office, with a general practitioner, or at a free‐standing oncology clinic, hospital clinic, or cancer centre).
Among all 220 patients, 38.6% of patients had no hospitalisations, 11.4% had one hospitalisation, 6.8% had two hospitalisations, 8.6% had more than two hospitalisations, and 34.6% had an unknown number of stays. Among the 59 patients with any hospitalisations during this period, a mean (SD) of 2.9 (2.8) visits was reported overall. Among the 171 hospitalisations that were reported, admissions were primarily to oncology wards (83.6%) and general wards (14.0%). Among the 90 hospitalisations with known admission and discharge dates, the mean (SD) length of stay was 5.2 (3.4) days.
Among the 164 patients who discontinued last systemic therapy during the study, HCRU declined after the end of last systemic therapy across all categories of care, with two exceptions (outpatient hospital clinic or cancer centre visits; hospice, long‐term care, or other end‐of‐life care services) (Table 5). The percentage of patients with at least one office visit decreased during this period (from 70.0% to 50.6%), as did the percentage of patients with at least one palliative care visit (from 44.6% to 36.0%). The proportion of patients with at least one emergency department visit on an outpatient basis or at least one hospitalisation also decreased during this period (from 20.5% to 14.0% and from 26.8% to 8.5%, respectively). In addition, among patients with any visit across settings, the mean number of visits declined after the end of last systemic therapy, with the exception of hospice, long‐term care, or other end‐of‐life care services. After the end of last systemic therapy, 48.2% of patients had no hospitalisations, 7.9% had one hospitalisation, 0.6% had two hospitalisations, and 43.3% had an unknown number of stays; no patients had more than two hospitalisations during this period. Among the 15 hospitalisations that were reported, admissions were primarily to oncology wards (66.7%) and general wards (26.7%). Among the 10 hospitalisations with known admission and discharge dates, the mean (SD) length of stay was 6.9 (3.5) days.
Table 6 presents the multivariable regression results describing the risk of specific health care visits following metastatic disease diagnosis. The odds of having had an SCCHN‐related outpatient office visit/consult at the responding physician's office or with a general practitioner or at a free‐standing oncology clinic were lower for patients with an ECOG score of 1 at metastatic diagnosis (odds ratio [OR] = 0.23 [95% confidence interval (CI), 0.07–0.76] vs. those patients with an ECOG score of 0 at metastatic diagnosis); ORs were higher as the number of comorbidities increased (OR = 1.92 [95% CI, 1.37–2.69]). The odds of having had an SCCHN‐related outpatient visit in a hospital clinic or cancer centre were lower for patients with an ECOG score of 2+ at metastatic diagnosis (OR = 0.08 [95% CI, 0.01–0.84] vs. those patients with an ECOG score of 0 at metastatic diagnosis). The odds of having an SCCHN‐related emergency department visit were higher as the number of comorbidities increased (OR = 1.34 [95% CI, 1.06–1.68]). The odds of having had an SCCHN‐related outpatient palliative care visit were lower for patients with an ECOG score of 2+ at metastatic diagnosis (OR = 0.07 [95% CI, 0.01–0.80] vs. those patients with an ECOG score of 0 at metastatic diagnosis); ORs were higher as the number of comorbidities increased (OR = 1.87 [95% CI, 1.45–2.41]). In addition, the receipt of surgery or systemic therapy for non‐metastatic disease and the receipt of radiotherapy for metastatic disease were associated with select categories of HCRU.
Table 6.
Multivariable analysis of risk of health care resource use following metastatic SCCHN diagnosis
| Covariates | OR (95% CI) | ||||
|---|---|---|---|---|---|
| SCCHN‐related hospitalisation visit | SCCHN‐related ED visit | SCCHN‐related office visit | SCCHN‐related outpatient hospital clinic or cancer centre visit | SCCHN‐related outpatient palliative care visit | |
| Age at metastatic diagnosis | 1.01 (0.97–1.05) | 0.97 (0.93–1.02) | 0.99 (0.94–1.03) | 0.98 (0.95–1.02) | 0.96 (0.92–1.00) |
| Female sex (referent group: males) | 0.64 (0.30–1.40) | 0.97 (0.45–2.12) | 0.74 (0.34–1.59) | 0.82 (0.42–1.60) | 1.22 (0.60–2.46) |
| Comorbidity burdena | 1.47 (1.17–1.85) | 1.34 (1.06–1.68) | 1.92 (1.37–2.69) | 0.99 (0.80–1.21) | 1.87 (1.45–2.41) |
| Non‐stage IVC at initial diagnosis of SCCHN (referent group: stage IVC) | 1.92 (0.27–13.73) | 1.32 (0.18–9.82) | 0.30 (0.04–2.25) | 0.42 (0.07–2.73) | 2.32 (0.38–14.13) |
| Received any supports and services for treating complications associated with SCCHN before metastatic diagnosisb | 2.56 (0.43–15.40) | 1.60 (0.27–9.34) | 0.64 (0.10–4.01) | 0.35 (0.07–1.79) | 1.62 (0.33–8.05) |
| Received surgery for non‐metastatic diseaseb | 0.81 (0.30–2.15) | 1.35 (0.49–3.72) | 5.08 (1.66–15.59) | 4.80 (1.79–12.88) | 0.69 (0.27–1.80) |
| Received radiotherapy for non‐metastatic diseaseb | 0.86 (0.23–3.22) | 0.70 (0.17–2.85) | 1.19 (0.27–5.16) | 1.00 (0.25–4.04) | 0.40 (0.10–1.64) |
| Received systemic therapy for non‐metastatic diseaseb | 0.34 (0.11–1.03) | 0.78 (0.25–2.50) | 4.31 (1.21–15.29) | 3.65 (1.15–11.54) | 0.68 (0.23–2.04) |
| Performance status at metastatic diagnosis using converted ECOG scores (referent group: ECOG score = 0) | |||||
| ECOG 1 | 0.52 (0.22–1.21) | 0.62 (0.26–1.46) | 0.23 (0.07–0.76) | 0.71 (0.32–1.60) | 0.82 (0.35–1.90) |
| ECOG 2+ | 0.46 (0.08–2.65) | 0.13 (0.01–1.54) | 0.23 (0.03–1.52) | 0.08 (0.01–0.84) | 0.07 (0.01–0.80) |
| Received radiotherapy for metastatic diseaseb | 1.56 (0.40–6.15) | 4.83 (1.31–17.85) | 3.83 (0.45–32.30) | 7.39 (1.25–43.62) | 1.05 (0.28–3.98) |
CI: confidence interval; ECOG: Eastern Cooperative Oncology Group; ED: emergency department; OR: odds ratio; SCCHN: squamous cell carcinoma of the head and neck.
The comorbidity burden control variable is a count of the number of comorbid conditions recorded, ranging from 0 to 28.
For these binary variables, the referent groups were patients who were not reported to have received the respective services.
3.5. Survival
The estimated median survival from start of third‐line treatment was 8.8 months (95% CI, 8.0–10.4 months). Estimated median survival from time of first diagnosis of metastatic SCCHN was 31.2 months (95% CI, 29.9–34.1 months) (results not shown); however, since patients in this study were selected for having received a third‐line therapy (and therefore for having survived long enough to receive such), this likely overestimates the expected survival of the general population of patients with metastatic SCCHN.
4. DISCUSSION
Limited data are available for countries outside of the United States that describe treatment patterns in advanced SCCHN, characterise information by PS and provide information on supportive care use. To our knowledge, only one previous study has explored utilisation of systemic treatments associated with metastatic SCCHN in Europe (van der Linden et al., 2016). The purpose of the present study was to describe the real‐world treatment patterns in the UK, including cancer‐directed and supportive care therapy, as well as HCRU, in patients with metastatic SCCHN who have received multiple lines of therapy. This information will provide a useful historical context for the evaluation of new systemic treatments developed for metastatic disease.
Patients’ age >50 years and smoking have both been identified as risk factors for head and neck cancer in the UK (Macmillan Cancer Support, 2012). This study included a total of 220 patients, with a median age of 60 years; more than 90% of the study population was Caucasian, and 85% were either current or former smokers. While a significant amount of research has emphasised the relevance of HPV as a prognostic risk factor in this population, as well as the recommendation to include it as part of a patient's risk assessment (Gillison et al., 2000; Grégoire et al., 2010; Pfister & Fury, 2014; Syrjänen, 2010), data on HPV status were not reported for the majority of this cohort.
Approximately, half of patients (51.8%) in the present study had initial diagnoses of stage IVC disease, which is much higher than what has been previously reported (~10%) (Gold et al., 2009). The majority of patients had relatively good PS at diagnosis and prior to receipt of first‐line therapy. Overall, there was substantial heterogeneity in the types of systemic therapy received by patients, both overall and by PS. A total of 36 distinct chemotherapy regimens were reported across all lines of therapy, which was likely a function of the few effective therapeutic options available for these patients at the time of this study. For most patients with local, regional, and metastatic recurrences of SCCHN, the ESMO guidelines recommend palliative chemotherapy as the standard treatment, with a first‐line option of cetuximab plus a platinum agent and 5‐FU noted for “fit patients” (Grégoire et al., 2010). In the current study, 17.7% of patients received this triplet regimen in the first‐line setting. Most patients, however, were reported to have received a platinum agent and 5‐FU without cetuximab as first‐line therapy, and the choice of platinum agent varied by PS. Not surprisingly, for patients with PS score of 0 or 1, the most common first‐line treatment was cisplatin + 5‐FU; for patients with PS ≥ 2, the most common first‐line treatment was carboplatin + 5‐FU. Cisplatin tends to be more poorly tolerated as compared with carboplatin, which likely explains its less‐frequent use for patients with poorer PS. Docetaxel was the most common second‐ and third‐line treatments for patients with a PS score of 0 or 1 (vs. cetuximab and methotrexate, respectively, for patients with PS ≥ 2).
Among the 105 patients with initial diagnoses of stage I through stage IVB disease, most received surgery, radiotherapy, or systemic therapy, alone or in combination, prior to diagnosis with metastatic disease. These findings were consistent with ESMO guidelines, which note that specific combinations of these treatment options for early stage (I–II) and locally advanced stage (III–IV) disease depend on the primary tumour location and extension, as well as expected patient outcomes and prognoses (Grégoire et al., 2010).
Although supportive care has been acknowledged by ESMO as a critical aspect of care in oncology, the use of supportive care measures is not well characterised in the literature specific to SCCHN in any European country. ESMO has issued supportive care guidelines spanning a variety of issues, including management of pain, febrile neutropenia, oral and gastrointestinal mucositis, bone health, antiemetic prophylaxis, and palliative care (Cherny, 2014; Cherny, Catane, & Kosmidis, 2003; Coleman, Body, Aapro, Hadji, & Herrstedt, 2014; Crawford, Caserta, & Roila, 2010; de Naurois et al., 2010; Peterson, Boers‐ Doets, Bensadoun, & Herrstedt, 2015; Ripamonti, Santini, Maranzano, Berti, & Roila, 2012; Roila et al., 2010; Schrijvers & Cherny, 2014). In keeping with these guidelines, supportive care use was reported for the majority of patients with metastatic SCCHN throughout the course of their disease, both before and after discontinuation of systemic therapy for metastatic disease. Although pain control, nutritional support, and antiemetics were consistently reported as top supportive care services for metastatic disease, their use (as well as the use of other types of supportive care) varied in the periods during and after systemic therapy.
Health care resource use findings indicated that the proportion of patients using specific health care resources generally decreased in the period after discontinuation of systemic therapy (with the exception of the following two categories: outpatient visits in a hospital clinic or cancer centre; hospice, long‐term care, or other end‐of‐life care services). Furthermore, after the end of last systemic therapy, the mean number of visits among patients with any use of a health care resource category declined, with the exception of hospice, long‐term care, or other end‐of‐life care services. These findings likely reflect changes in treatment strategies following withdrawal from cancer‐directed therapy (e.g., in which goals may transition to providing supportive services and maximising time at home or with family). Most patients had outpatient visits to the responding physician's office, with a general practitioner, or at a free‐standing oncology clinic both during systemic therapy (70.0%) and after end of last systemic therapy (50.6%). In comparison, during the same periods, lower proportions of patients were reported to have received outpatient palliative care (44.6% and 36.0%, respectively), outpatient visits in a hospital clinic or cancer centre (35.0% and 36.6%, respectively), emergency department visits (20.5% and 14.0%, respectively), and hospitalisations (26.8% and 8.5%, respectively).
Exploratory logistic regression results found that patients with PS ≥ 2 (n = 9) at metastatic diagnosis had a lower likelihood of having certain categories of HCRU (i.e., outpatient visits in a hospital clinic or cancer centre, outpatient palliative care visits). These results are counterintuitive and should be considered “exploratory” given the nature of the analysis and the potential for immortal time bias (discussed later as a potential limitation). In addition to PS at metastatic diagnosis, other factors significantly associated with select measures of HCRU included comorbidity burden, receipt of surgery or receipt of systemic therapy for non‐metastatic disease, and receipt of radiotherapy for metastatic disease.
This study is characterised by a number of strengths. The selection of patients from routine practice provides real‐world evidence regarding the demographic and clinical characteristics of patients with metastatic SCCHN and treatment patterns among this population. The data collection form was designed by the study team to capture relevant data from medical records. In addition, because the investigators were not informed of any particular research hypothesis, there is no reason to believe that the information is biased.
However, limitations of this study also must be considered when interpreting the results. Most notably, the eligibility criterion requiring patients to have received at least three lines of systemic therapy for SCCHN introduced the potential for survival (immortal time) bias as compared with an unselected population with metastatic SCCHN. This is evident in evaluating overall survival times in the present study versus those reported for less stringently selected patients with metastatic SCCHN in published literature. For example, in the current study, median estimated survivals from time of metastatic diagnosis (31.2 months) and from start of third‐line therapy (8.8 months) were both longer than the 6.0‐month median survival from the start of systemic therapy observed by van der Linden et al. (2016). Thus, survival findings from this study apply to our study population only and are not estimates of survival for all patients in the general population diagnosed with metastatic SCCHN.
In addition, the stringent selection of patients also likely influenced the findings related to patient characteristics, including PS, treatment patterns, and HCRU. In particular, patients with PS ≥ 2 (n = 9) at the first diagnosis of metastatic disease received three lines of therapy; these patients were likely “more fit” and/or perhaps had better outcomes from treatments received than what is typical for the general population of patients with newly diagnosed metastatic SCCHN with poor PS. It is also important to take into account the subjectivity of a clinician's assessment when assigning a PS rating; some patients may have been mischaracterised as having PS ≥ 2 when they perhaps had a PS of 1.
Furthermore, because the goal of the study was to evaluate treatment patterns and outcomes among patients treated in routine clinical practice, the results are limited to those treatments that were available at the time of the study. Specifically, patients participating in any interventional clinical trial, including those of PD‐1 inhibitors, at the time of our study (January 2011–August 2014) were not included. Patients selected for study inclusion represent a convenience sample, in that the records were obtained from physicians who were willing to participate in the study. Our findings may therefore not be generalisable to the overall metastatic SCCHN population in the UK and must be considered within the context of an evolving treatment landscape, given the recent availability of nivolumab, as well as the potential future availability of other treatments that are under clinical investigation in this setting.
The importance of HPV status and prognosis in SCCHN is appreciated, and although treatment guidelines recommend testing for HPV DNA in oropharyngeal tumours (Grégoire et al., 2010; NICE, 2016; Roland & Paleri, 2011), limited data were reported regarding HPV status for this population in this study. The data collected in this study were potentially subject to data‐entry errors as they were directly entered by physicians; the authors did not review patients’ medical record data to confirm the accuracy of information collected. Moreover, physicians reported data based on patients’ medical records to which they had access. HCRU information pertinent to the patient's SCCHN condition and study objectives but not captured in the patient's chart to which the participating physician had access was not included in our analysis and may be underreported. Data on hospitalisations in patients’ charts also lacked dates of service for a substantial proportion of the study's sample, leading to less robust estimates of hospitalisation length of stay data. Finally, the data collection form was designed to prioritise collection of key information in support of study objectives while balancing physician time burden. There may be additional measures or analyses that would be useful in understanding variations in treatment and outcomes. For example, differential patient follow‐up time was not accounted for in the exploratory regression analyses and more advanced models that account for differential follow‐up (e.g., Cox proportional hazards models) were not feasible with the data collected (i.e., lack of dates of service for many HCRU categories).
5. CONCLUSION
This study provides useful information on patient characteristics, treatment patterns, HCRU, and survival in a real‐world population of patients with metastatic SCCHN who received at least three lines of systemic therapy in the UK in the pre‐immunotherapy era. Patterns of care and HCRU varied among patients with metastatic SCCHN, and specific systemic therapies varied by patient PS. Given the lack of existing data on this particular patient population and on‐going development efforts to identify new treatments for metastatic SCCHN, findings from the current study help to fill critical gaps in the literature. With the EMA approval of nivolumab for advanced SCCHN, as well as several other studies of immunotherapy on‐going, future real‐world research should explore how treatment patterns have evolved and the extent to which these treatments provide meaningful improvement in outcomes relative to the pre‐immunotherapy era.
CONFLICT OF INTEREST
This study was performed under a research contract between Eli Lilly and Company and RTI Health Solutions and was funded by Eli Lilly and Company. Elizabeth M. La, Sandra E. Talbird and James A. Kaye are employees of RTI Health Solutions. Emily Nash Smyth, Li Li, Aimee Bence Lin and Lee Bowman are employees of Eli Lilly and Company.
Supporting information
ACKNOWLEDGEMENTS
This research and preparation of this publication were sponsored by Eli Lilly and Company. The authors acknowledge the role of A+A Research in recruiting oncology specialists to participate in this study and in supporting data collection. Kate Lothman of RTI Health Solutions provided medical writing services that were funded by Eli Lilly and Company.
La EM, Smyth EN, Talbird SE, et al. Treatment patterns and health care resource use in patients receiving multiple lines of therapy for metastatic squamous cell carcinoma of the head and neck in the United Kingdom. Eur J Cancer Care. 2018;27:e12862 10.1111/ecc.12862
Funding information
This study was funded by Eli Lilly and Company.
REFERENCES
- Adamo, V. , Ferraro, G. , Pergolizzi, S. , Sergi, C. , Laudani, A. , Settineri, N. , … Spitaleri, G. (2004). Paclitaxel and cisplatin in patients with recurrent and metastatic head and neck squamous cell carcinoma. Oral Oncology, 40, 525–531. [DOI] [PubMed] [Google Scholar]
- Argiris, A. , Karamouzis, M. V. , Raben, D. , & Ferris, R. L. (2008). Head and neck cancer. Lancet, 371, 1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtness, B. (2015). Moving forward in the management of squamous cell carcinoma of the head and neck: Promising immuno‐oncology approaches. American Journal of Hematology/Oncology, 11, 28–31. [Google Scholar]
- Cancer Research UK (2016a). About laryngeal cancer data. Retrieved from http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/laryngeal-cancer#heading-Six [last accessed 8 December 2016].
- Cancer Research UK (2016b). About Oral Cancer Data. Retrieved from http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oral-cancer#heading-Five [last accessed 8 December 2016].
- Cherny, N. I. , & ESMO Guidelines Working Group . (2014). ESMO Clinical Practice Guidelines for the management of refractory symptoms at the end of life and the use of palliative sedation. Annals of Oncology, 25(Suppl. 3), iii143–iii152. [DOI] [PubMed] [Google Scholar]
- Cherny, N. I. , Catane, R. , Kosmidis, P. , & ESMO Taskforce on Supportive and Palliative Care . (2003). ESMO takes a stand on supportive and palliative care. Annals of Oncology, 14, 1335–1337. [DOI] [PubMed] [Google Scholar]
- Coleman, R. , Body, J. J. , Aapro, M. , Hadji, P. , Herrstedt, J. , & ESMO Guidelines Working Group . (2014). Bone health in cancer patients: ESMO Clinical Practice Guidelines. Annals of Oncology, 25(Suppl. 3), iii124–iii 137. [DOI] [PubMed] [Google Scholar]
- Crawford, J. , Caserta, C. , Roila, F. , & ESMO Guidelines Working Group . (2010). Hematopoietic growth factors: ESMO Clinical Practice Guidelines for the applications. Annals of Oncology, 21(Suppl. 5), v248–v2451. [DOI] [PubMed] [Google Scholar]
- de Naurois, J. , Novitzky‐Basso, I. , Gill, M. J. , Marti, F. M. , Cullen, M. H. , Roila, F. , & ESMO Guidelines Working Group . (2010). Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Annals of Oncology, 21(Suppl. 5), v252–v256. [DOI] [PubMed] [Google Scholar]
- European Society for Medical Oncology (ESMO) (2008). Performance scales: Karnofsky and ECOG scores. Retrieved from http://oncologypro.esmo.org/Guidelines-Practice/Practice-Tools/Performance-Scales [last accessed 22 February 2016].
- Gillison, M. L. , Chaturvedi, A. K. , Anderson, W. F. , & Fakhry, C. (2015). Epidemiology of human papillomavirus‐positive head and neck squamous cell carcinoma. Journal of Clinical Oncology, 33, 3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillison, M. L. , Koch, W. M. , Capone, R. B. , Spafford, M. , Westra, W. H. , Wu, L. , … Sidransky, D. (2000). Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute, 92, 709–720. [DOI] [PubMed] [Google Scholar]
- Gold, K. A. , Lee, H. Y. , & Kim, E. S. (2009). Targeted therapies in squamous cell carcinoma of the head and neck. Cancer, 115, 922–935. [DOI] [PubMed] [Google Scholar]
- Grégoire, V. , Lefebvre, J. L. , Licitra, L. , & Felip, E. (2010). Squamous cell carcinoma of the head and neck: EHNS‐ESMO‐ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 21(Suppl. 5), v184–v186. [DOI] [PubMed] [Google Scholar]
- Jones, T. M. (2014). Tumour‐infiltrating lymphocytes in the risk stratification of squamous cell carcinoma of the head and neck. British Journal of Cancer, 110, 269–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan Cancer Support (2012). Risk factors and causes of head and neck cancer. Retrieved from http://www.macmillan.org.uk/cancerinformation/cancertypes/headneck/aboutheadneckcancers/causes.aspx [last accessed 22 August 2016].
- Malhotra, B. , Moon, J. , Kucuk, O. , Clark, J. I. , Urba, S. G. , Wolf, G. T. , & Worden, F. P. (2014). Phase II trial of biweekly gemcitabine and paclitaxel with recurrent or metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group study S0329. Head and Neck, 36, 1712–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute at the National Institutes of Health (2013). Cancer topics, national cancer institute fact sheet, head and neck cancers. Retrieved from https://www.cancer.gov/types/head-and-neck/head-neck-fact-sheet [last accessed 8 December 2016].
- National Comprehensive Cancer Network (2013). NCCN clinical practice guidelines in oncology (NCCN Guidelines): Head and neck cancers, Version 2.2013. Retrieved from http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site [last accessed 14 March 2014].
- National Institute for Health and Care Excellence (NICE) (2016). Cancer of the upper aerodigestive tract: Assessment and management in people aged 16 and over. NG36. Retrieved from https://www.nice.org.uk/guidance/ng36 [last accessed 13 May 2016]. [PubMed]
- National Institute for Health and Care Excellence (NICE) (2017). Nivolumab for treating squamous cell carcinoma of the head and neck after platinum‐based chemotherapy. TA490. Retrieved from https://www.nice.org.uk/guidance/ta490 [last accessed 5 February 2018].
- Oken, M. M. , Creech, R. H. , Tormey, D. C. , Horton, J. , Davis, T. E. , McFadden, E. T. , & Carbone, P. P. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology, 5, 649–655. [PubMed] [Google Scholar]
- Peterson, D. E. , Boers‐ Doets, C. B. , Bensadoun, R. J. , Herrstedt, J. , & ESMO Guidelines Committee . (2015). Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow‐up. Annals of Oncology, 26(Suppl. 5), v139–v151. [DOI] [PubMed] [Google Scholar]
- Pfister, D. G. , & Fury, M. G. (2014). New chapter in our understanding of human papillomavirus‐related head and neck cancer. Journal of Clinical Oncology, 32, 3349–3352. [DOI] [PubMed] [Google Scholar]
- Raguse, J. D. , Gath, H. J. , Oettle, H. , & Bier, J. (2006). Oxaliplatin, folinic acid and 5‐fluorouracil (OFF) in patients with recurrent advanced head and neck cancer: A phase II feasibility study. Oral Oncology, 42, 614–618. [DOI] [PubMed] [Google Scholar]
- Ripamonti, C. I. , Santini, D. , Maranzano, E. , Berti, M. , Roila, F. , & Guidelines Working ESMO Group . (2012). Management of cancer pain: ESMO Clinical Practice Guidelines. Annals of Oncology, 23(Suppl. 7), vii139–vii 154. [DOI] [PubMed] [Google Scholar]
- Roila, F. , Herrstedt, J. , Aapro, M. , Gralla, R. J. , Einhorn, L.H. , Ballatori, E. , … ESMO/MASCC Guidelines Working Group (2010). Guideline update for MASCC and ESMO in the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting: Results of the Perugia consensus conference. Annals of Oncology, 21(Suppl. 5), v232–v243. [DOI] [PubMed] [Google Scholar]
- Roland N. J., & Paleri V. (Eds.) (2011). Head and neck cancer: Multidisciplinary management guidelines (4th edn). London: ENT UK. [Google Scholar]
- Schrijvers, D. , Cherny, N. I. , & ESMO Guidelines Working Group . (2014). ESMO Clinical Practice Guidelines on palliative care: Advanced care planning. Annals of Oncology, 25(Suppl. 3), iii138–iii 142. [DOI] [PubMed] [Google Scholar]
- Syrjänen, S. (2010). The role of human papillomavirus infection in head and neck cancers. Annals of Oncology, 21(Suppl. 7), vii243–vii 245. [DOI] [PubMed] [Google Scholar]
- van der Linden, N. , Buter, J. , Pescott, C. P. , Lalisang, R. I. , de Boer, J. P. , de Graeff, A. , … Uyl‐de Groot, C. A. (2016). Treatments and costs for recurrent and/or metastatic squamous cell carcinoma of the head and neck in the Netherlands. European Archives of Oto‐rhino‐laryngology, 273, 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermorken, J. B. , Licitra, L. , Stöhlmacher‐Williams, J. , Dietz, A. , Lopez‐ Picazo, J. M. , Hamid, O. , … Gauler, T. C. (2013). Phase II study of pemetrexed in combination with cisplatin and cetuximab in recurrent or metastatic squamous cell carcinoma of the head and neck. European Journal of Cancer, 49, 2877–2883. [DOI] [PubMed] [Google Scholar]
- Won, Y. W. , Park, Y. H. , Ahn, M. J. , Do, I. G. , Ko, Y. H. , & Park, K. (2011). A phase II study of combination chemotherapy with capecitabine and cisplatin in patients with metastatic or recurrent squamous cell carcinoma of the head and neck. Annals of Oncology, 22, 417–423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


