Abstract
Previous global studies of guselkumab have demonstrated clinical benefits in patients with psoriasis. The aim of this 52‐week, phase 3 study was to evaluate efficacy and safety of guselkumab in Japanese patients with moderate to severe plaque‐type psoriasis. Patients randomly received guselkumab 50 mg or 100 mg at weeks 0, 4 and every 8 weeks, or placebo with cross‐over to guselkumab 50 mg or 100 mg at week 16. Co‐primary end‐points were the proportion of patients achieving Investigator's Global Assessment (IGA) cleared/minimal (0/1) and 90% or more improvement in Psoriasis Area and Severity Index (PASI‐90) at week 16. Overall, 192 patients were randomized to placebo, guselkumab 50 mg or 100 mg. At week 16, patients in the placebo group were crossed over to guselkumab 50 mg or 100 mg. At week 16, a significantly (P < 0.001) higher proportion of patients receiving guselkumab 50 mg and 100 mg versus placebo achieved IGA 0/1 (92.3% and 88.9% vs 7.8%) and PASI‐90 (70.8% and 69.8% vs 0%). Patients in guselkumab 50 mg and 100 mg groups achieved significant improvement versus placebo in PASI‐75 (89.2% and 84.1% vs 6.3%, P < 0.001) at week 16; improvement was maintained through week 52. Incidences of treatment‐emergent adverse events were comparable among the groups through week 16; the most commonly reported was nasopharyngitis. No new safety concerns were observed until week 52. In conclusion, guselkumab treatment demonstrated superior efficacy over placebo and was well tolerated in Japanese patients with moderate to severe plaque‐type psoriasis.
Keywords: guselkumab, interleukin‐23, Japan, long term, plaque psoriasis
Introduction
Psoriasis is a chronic immune‐mediated inflammatory skin disease that affects nearly 1–2% of the population worldwide and 0.3% in Japan.1, 2, 3 Because moderate to severe plaque‐type psoriasis has a significant impact on patients’ well‐being,4 treatment goals should aim to achieve rapid control of disease, followed by maintenance of long‐term remission to improve overall quality of life (QoL).
The recent development of biologic therapies targeting tumor necrosis factor‐α (TNF‐α),5, 6, 7 interleukin (IL)‐17,8 IL‐12 and IL‐23,9, 10 and IL‐23 alone11, 12 for the treatment of psoriasis have contributed to an increased understanding of the pathogenesis. IL‐23 plays a key role in T‐helper (Th)17 cell differentiation and survival as well as upstream regulation of IL‐17A, a central pro‐inflammatory effector cytokine involved in psoriasis pathogenesis.13, 14, 15 Recent evidence has demonstrated that selective inhibition of IL‐23 results in further improvement in signs and symptoms of psoriasis.16
Guselkumab is a fully human immunoglobulin (Ig)G1 lambda monoclonal antibody that blocks the downstream signaling of IL‐23 by specifically binding to the p19 subunit of IL‐23. The efficacy of guselkumab treatment has been demonstrated in previous global studies, including head‐to‐head comparison studies of psoriasis (guselkumab vs adalimumab),16, 17, 18 and in plaque psoriasis and palmoplantar pustulosis studies in Japanese patients.19, 20 The phase 3 study reported here was conducted to evaluate the efficacy and safety of guselkumab versus placebo in Japanese patients with moderate to severe plaque‐type psoriasis.
Methods
Patients
Patients (aged ≥20 years) were eligible if they had moderate to severe plaque‐type psoriasis for 6 months or more with a Psoriasis Area and Severity Index (PASI) score of 12 or more, an Investigator's Global Assessment (IGA) score of 3 or more, and an involved body surface area (BSA) of 10% or more at baseline; and were candidates for phototherapy or systemic treatment for psoriasis. Patients diagnosed with psoriatic arthritis (PsA), using Classification Criteria for Psoriatic Arthritis (CASPAR) and with active PsA (defined as ≥3 swollen joints and ≥3 tender joints at baseline and C‐reactive protein of 0.3 mg/dL or more at baseline) were also included. Patients were excluded if they had non‐plaque‐type psoriasis, drug‐induced psoriasis, latent or active tuberculosis, chronic or recurrent infectious disease, malignancy within 5 years (except non‐melanoma skin cancer or cervical carcinoma that had been treated, and with no evidence of recurrence within 3 months), anaphylactic reactions, or history or current signs or symptoms of any severe, progressive or uncontrolled medical disorders. Patients who had received prior treatment with guselkumab, anti‐TNF‐α agents within 3 months or five half‐lives, whichever was longer, biological therapy targeting IL‐12, IL‐17 or IL‐23 within 6 months, systemic immunosuppressants (e.g. methotrexate, cyclosporin) within 4 weeks, or phototherapy within 4 weeks of enrolment were also excluded.
Study design
This was a phase 3, randomized, double‐blind, placebo‐controlled study conducted in Japan (35 sites) from 15 January 2015 to 11 November 2016. The study comprised a placebo‐controlled period (weeks 0–16), a placebo cross‐over and active treatment period (weeks 16–52) and a long‐term extension phase (Fig. 1). Eligible patients were randomized (1:1:1) to guselkumab 50 mg, 100 mg or placebo, with s.c. injections at weeks 0, 4, and every 8 weeks (q8w) thereafter. Patients receiving placebo were crossed over to receive (1:1) guselkumab 50 mg or 100 mg at weeks 16 and 20 and every q8w thereafter (Fig. 1). Randomization was performed centrally using a computer‐generated randomization scheme, balanced using randomly permuted blocks and stratified by presence of PsA. Study site personnel, investigators and patients were blinded to treatment allocation until week 52 database lock. The study was approved by the institutional review board and was conducted according to the Declaration of Helsinki and Good Clinical Practice standards. Patients provided written informed consent before study participation. The study's Clinical Trial Registration number was NCT02325219.
Figure 1.
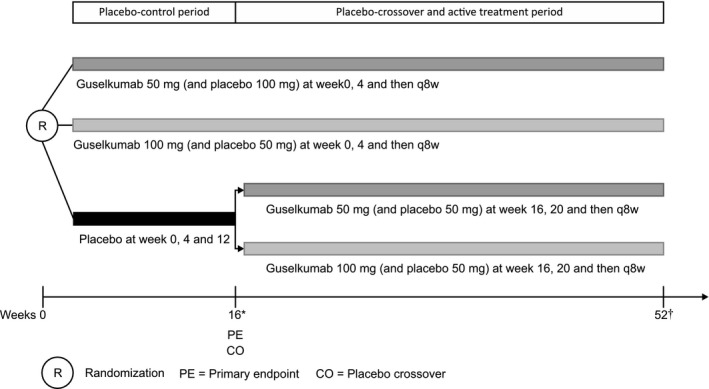
Study design. *During the cross‐over at week 16, patients in guselkumab groups received placebo. †The last study drug administration in the double‐blind treatment phase occurred at week 44. Patients received treatment at week 52 only if they agreed to continue participation in the long‐term extension phase of the study.
Study end‐points
The co‐primary end‐points were the proportion of patients achieving an IGA cleared/minimal (0/1) and PASI‐90 response (≥90% improvement in PASI from baseline) at week 16. The key secondary end‐points included the proportion of patients who achieved a PASI‐75 response (≥75% improvement in PASI from baseline) and change from baseline in the Dermatology Life Quality Index (DLQI) score at week 16.
Other secondary end‐points evaluated at week 16 and through week 52 include proportion of patients with IGA 0, IGA 0/1, PASI‐50, PASI‐75, PASI‐90 (≥90% improvement) and PASI‐100 (100% improvement); change and percentage improvement in the Nail Psoriasis Area and Severity Index (NAPSI) score from baseline; proportion of patients with a scalp‐specific Investigator's Global Assessment (ss‐IGA) score of 0 (absence of disease) or ss‐IGA 0/1 (very mild disease) who had a baseline score of 2 or more; proportion of patients who achieved a DLQI score of 0 or 1 (among patients with a baseline DLQI score of >1); and proportion of patients who achieved a reduction of 5 or more in the DLQI score from baseline. Changes from baseline in the EuroQOL 5 dimensions questionnaire (EQ‐5D) and physical and mental component summary (PCS and MCS) scores of the 36‐Item Short form Health Assessment Questionnaire (SF‐36) were assessed through week 48. In the subset of patients with active PsA, efficacy end‐points through week 52 included proportion of patients achieving American College of Rheumatology (ACR)‐20, ACR‐50 and ACR‐70 (≥20%, ≥50% and ≥70% improvement from baseline, respectively), improvements from baseline in the tender and swollen joint counts, improvements from baseline in the patient's assessment of pain (visual analog scale [VAS]) and patient's and physician's global assessment of disease activity, and proportion of responders on the Health Assessment Questionnaire – Disability Index.
Safety assessments included treatment‐emergent adverse events (TEAE) and laboratory evaluations through week 52. Antibodies to guselkumab in serum were detected using a validated, specific, sensitive and drug‐tolerant electrochemiluminescence immunoassay method using the Meso Scale Discovery platform (Gaithersburg, MD, USA) that incorporates an acid dissociation step to improve detection of antibodies to guselkumab in the presence of excess guselkumab.
Statistical analysis
The randomized analysis set included all randomized patients for efficacy analyses, and data were analyzed by treatment groups. The co‐primary end‐points were assessed in a multiplicity‐adjusted, fixed‐sequence testing procedure. Except for PsA, binary efficacy end‐points at week 16 (e.g. co‐primary end‐points) were analyzed using Fisher's exact test; continuous efficacy end‐points at week 16 were evaluated using ancova with treatment as a factor and baseline as a covariate; categorical efficacy end‐points at week 16 with two or more levels were analyzed using the Cochran–Mantel–Haenszel test. Based on the previous phase 2 study,16 a sample size of 180 patients (n = 60 per group) was estimated to provide 98% or more power to detect significant differences between guselkumab and placebo treatments in the co‐primary end‐points. All statistical tests were two‐sided (α = 0.05).
Patients who discontinued the study drug because of lack of efficacy, a TEAE of worsening psoriasis or starting a protocol‐prohibited psoriasis treatment were considered non‐responders for binary end‐points or had baseline values carried forward for continuous end‐points. Last observation was carried forward for other patients with missing data. Safety analyses included all patients receiving one or more study drug administration and were summarized by actual treatment received.
Results
Out of 220 patients screened, 192 were randomized to placebo (n = 64), guselkumab 50 mg (n = 65) or 100 mg (n = 63). At week 16, patients in the placebo group were re‐randomized to guselkumab 50 mg or 100 mg (n = 26 each). Overall, 173 of the 192 (90.1%) patients completed the study till week 52. A total of 19 patients discontinued the study agent through week 52; 15 patients prior to week 16 (placebo, n = 12; guselkumab 50 mg, n = 2; guselkumab 100 mg, n = 1) and four patients from weeks 16–52 (guselkumab 50 mg, n = 3; placebo to 100 mg, n = 1; Fig. 2). Demographics and baseline characteristics were generally well‐balanced across groups, except for the lower mean bodyweight in the guselkumab 50 mg group (67.76 kg) versus the guselkumab 100 mg (74.27 kg) and placebo group (71.56 kg) (Table 1).
Figure 2.
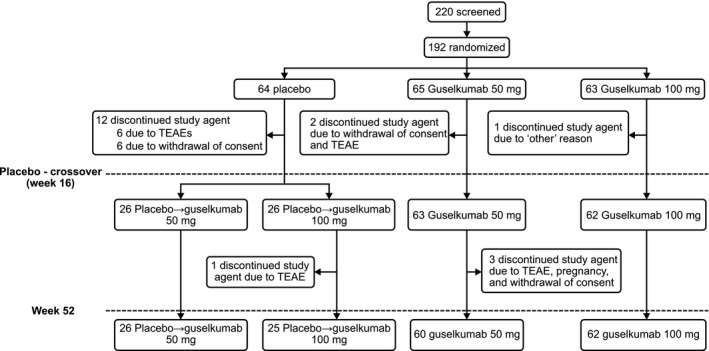
Patient disposition. TEAE, treatment emergent adverse event. The “other” reason was patient did not meet the tuberculosis screening inclusion criterion.
Table 1.
Demographics and baseline characteristics
| Characteristics | Placebo (n = 64) | Guselkumab | |
|---|---|---|---|
| 50 mg (n = 65) | 100 mg (n = 63) | ||
| Age, mean (SD), years | 48.3 (10.56) | 50.1 (12.66) | 47.8 (11.07) |
| Men, n (%) | 54 (84.4) | 44 (67.7) | 47 (74.6) |
| Bodyweight, mean (SD), kg | 71.56 (14.01)† | 67.76 (15.02) | 74.27 (16.04) |
| BMI, kg/m2, mean (SD) | 25.42 (4.791)† | 24.61 (4.514) | 26.33 (5.032) |
| Duration of psoriasis, mean (SD), years | 13.66 (10.291) | 15.25 (9.170) | 14.39 (9.227) |
| BSA of lesion (%), mean (SD) | 33.6 (18.39) | 38.0 (19.94) | 37.9 (21.48) |
| PASI score (0–72), mean (SD) | 25.92 (12.341) | 25.60 (11.680) | 26.73 (12.196) |
| IGA score (0–4), n (%) | |||
| Cleared (0) | 0 | 0 | 0 |
| Minimal (1) | 0 | 0 | 0 |
| Mild (2) | 0 | 1 (1.5) | 0 |
| Moderate (3) | 52 (81.3) | 50 (76.9) | 51 (81.0) |
| Severe (4) | 12 (18.8) | 14 (21.5) | 12 (19.0) |
| DLQI score (0–30), mean (SD) | 10.6 (7.74) | 10.4 (6.25) | 10.3 (7.27) |
| EQ‐5D score (0–100), mean (SD) | 61.6 (23.69) | 60.3 (23.34) | 59.8 (24.05) |
| SF‐36 PCS, mean (SD) | 46.0 (12.40) | 44.8 (16.47) | 45.7 (15.18) |
| SF‐36 MCS, mean (SD) | 44.6 (9.26) | 47.5 (9.18) | 46.3 (9.85) |
| NAPSI score (0–8), n ‡ | 42 | 44 | 40 |
| Mean (SD) | 3.6 (2.25) | 3.8 (1.96) | 3.7 (2.22) |
| Diagnosis of PsA§, n (%) | 10 (15.6) | 11 (16.9) | 10 (15.9) |
| Diagnosis of active PsA, n (%) | 4 (6.3) | 3 (4.6) | 3 (4.8) |
| ACR components, mean (SD) | |||
| Tender joint count | 2.6 (2.32) | 10.0 (15.13) | 5.1 (5.86) |
| Swollen joint count | 5.9 (7.14) | 4.1 (3.59) | 3.7 (4.76) |
| Patient's assessment of pain (VAS) | 5.90 (2.778) | 4.43 (3.028) | 3.01 (3.634) |
| Patient's global assessment of disease activity (VAS) | 6.48 (2.507) | 4.74 (2.876) | 3.51 (3.674) |
| Physician's global assessment of disease activity (VAS) | 4.73 (3.044) | 5.46 (2.979) | 2.90 (2.402) |
| Prior treatments, n (%) | |||
| Topical agents | 64 (100.0) | 65 (100.0) | 62 (98.4) |
| Phototherapy | 27 (42.2) | 39 (60.0) | 30 (47.6) |
| PUVA | 6 (9.4) | 15 (23.1) | 10 (15.9) |
| UV‐B | 24 (37.5) | 33 (50.8) | 22 (34.9) |
| Conventional systemic | 38 (59.4) | 40 (61.5) | 37 (58.7) |
| Apremilast | 2 (3.1) | 3 (4.6) | 0 |
| Cyclosporin | 34 (53.1) | 33 (50.8) | 28 (44.4) |
| Methotrexate | 7 (10.9) | 7 (10.8) | 6 (9.5) |
| Tofacitinib | 2 (3.1) | 0 | 0 |
| Biologic agents | 10 (15.6) | 14 (21.5) | 11 (17.5) |
| Adalimumab | 2 (3.1) | 7 (10.8) | 4 (6.3) |
| Brodalumab | 1 (1.6) | 0 | 2 (3.2) |
| Infliximab | 6 (9.4) | 5 (7.7) | 4 (6.3) |
| Ixekizumab | 0 | 1 (1.5) | 2 (3.2) |
| Secukinumab | 2 (3.1) | 2 (3.1) | 0 |
| Ustekinumab | 2 (3.1) | 7 (10.8) | 2 (3.2) |
† n = 63. ‡Patients diagnosed with nail psoriasis. §Diagnosis based on Classification Criteria for Psoriatic Arthritis (CASPAR). ACR, American College of Rheumatology; BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; EQ‐5D, EuroQol 5 dimensions questionnaire; IGA, Investigator's Global Assessment; IQ, interquartile range; PASI, Psoriasis Area and Severity Index; PCS, Physical Component Score; PSSD, Psoriasis Symptoms and Signs Diary; SF‐36, Medical Outcomes Study 36‐Item; PsA, psoriatic arthritis; PUVA, psoralen plus ultraviolet A light therapy; UV‐B, narrowband ultraviolet B therapy; VAS, visual analog scale.
[Correction added on 23 July 2018, after first online publication: The data in the following rows have been corrected; Apremilast, Cyclosporin, Methotrexate, Tofacitinib, Adalimumab, Brodalumab, Infliximab, Ixekizumab, Ustekinumab. Also, a new row for Secukinumab has been added under ‘Biologic agents’.]
Efficacy through week 16
At week 16, significantly greater proportions of patients in the guselkumab groups (50 mg and 100 mg) versus placebo achieved IGA 0/1 (92.3% and 88.9% vs 7.8%, respectively; both P < 0.001) and PASI‐90 responses (70.8% and 69.8% vs 0%, respectively; both P < 0.001) (Table 2).
Table 2.
Efficacy outcomes at week 16 and through week 52 (All randomized patients)
| Week 16 | Through week 52† | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | Guselkumab 50 mg | Guselkumab 100 mg | Placebo → guselkumab 50 mg | Placebo → guselkumab 100 mg | Guselkumab 50 mg | Guselkumab 100 mg | |
| Physician‐reported outcomes | 64 | 65 | 63 | 26 | 26 | 65 | 63 |
| IGA 0 | 0 (0) | 29 (44.6) | 28 (44.4) | 14 (53.8) | 13 (50.0) | 35 (53.8) | 37 (58.7) |
| IGA 0/1 | 5 (7.8) | 60 (92.3)* | 56 (88.9)* | 26 (100) | 23 (88.5) | 57 (87.7) | 57 (90.5) |
| PASI‐50 | 9 (14.1) | 61 (93.8)* | 60 (95.2)* | 26 (100) | 25 (96.2) | 64 (98.5) | 62 (98.4) |
| PASI‐75 | 4 (6.3) | 58 (89.2)* | 53 (84.1)* | 26 (100) | 24 (92.3) | 60 (92.3) | 57 (90.5) |
| PASI‐90 | 0 | 46 (70.8)* | 44 (69.8)* | 24 (92.3) | 19 (73.1) | 49 (75.4) | 49 (77.8) |
| PASI‐100 | 0 | 21 (32.3)* | 17 (27.0)* | 10 (38.5) | 11 (42.3) | 25 (38.5) | 30 (47.6) |
| PASI, % improvement from baseline, mean (SD) | 0.2 (45.53) | 88.9 (17.34) | 88.7 (17.77) | 96.7 (4.45) | 91.4 (14.02) | 91.6 (17.12) | 92.5 (15.39) |
| NAPSI, n | 42 | 44 | 40 | 15 | 18 | 44 | 40 |
| Change in NAPSI, mean (SD) | −0.2 (1.13) | −1.2 (1.61)** | −1.5 (1.78)* | −3.3 (2.34) | −1.4 (1.54) | −2.8 (1.94) | −2.7 (2.20) |
| % improvement from baseline, mean (SD) | 1.0 (59.38) | 31.6 (43.56) | 39.1 (48.93) | 79.2 (25.62) | 44.9 (53.56) | 74.4 (35.11) | 75.3 (41.32) |
| ss‐IGA responders, n | 57 | 58 | 58 | 21 | 24 | 58 | 58 |
| ss‐IGA 0 | 2 (3.5) | 28 (48.3)* | 37 (63.8)* | 14 (66.7) | 18 (75.0) | 39 (67.2) | 45 (77.6) |
| ss‐IGA 0/1 | 6 (10.5) | 43 (74.1)* | 48 (82.8)* | 18 (85.7) | 23 (95.8) | 49 (84.5) | 50 (86.2) |
| Patient‐reported outcomes | |||||||
| DLQI, n | 64 | 65 | 63 | 26 | 26 | 65 | 63 |
| Change in DLQI score, mean (SD) | −0.8 (5.40) | −8.3 (5.87)* | −8.5 (6.95)* | −10.1 (7.79) | −6.5 (5.05) | −9.2 (6.39) | −9.0 (7.28) |
| DLQI score >1 at baseline, n | 61 | 61 | 60 | 24 | 25 | 64 | 60 |
| DLQI 0/1 | 4 (6.6) | 41 (64.1) | 41 (68.3) | 18 (75.0) | 20 (80.0) | 47 (73.4) | 46 (76.7) |
| EQ‐5D, n † | 64 | 65 | 63 | 26 | 26 | 65 | 63 |
| Change in EQ‐5D VAS, mean (SD) | 2.45 (22.44) | 21.20 (23.54)* | 18.43 (26.21)* | 20.38 (22.09) | 7.00 (29.49) | 20.88 (29.65) | 21.70 (26.58) |
| Change in EQ‐5D index score, mean (SD) | 0.05 (0.14) | 0.20 (0.20) | 0.18 (0.21) | 0.28 (0.15) | 0.15 (0.14) | 0.20 (0.20) | 0.21 (0.23) |
| SF‐36† | 64 | 65 | 63 | 26 | 26 | 65 | 63 |
| Change in PCS, mean (SD) | 0.3 (9.90) | 7.4 (15.65)* | 7.3 (14.40)* | 8.8 (12.13) | 4.4 (7.60) | 8.2 (14.22) | 8.4 (15.16) |
| Change in MCS, mean (SD) | 1.3 (8.21) | 4.0 (7.22)* | 5.3 (9.63)* | 4.5 (9.92) | 5.0 (11.00) | 5.7 (9.04) | 5.6 (9.32) |
*P < 0.001 versus placebo. **P = 0.002 versus placebo. †EQ‐5D and SF‐36 (PCS and MCS) outcomes were assessed through week 48. Values are presented as n (%) unless otherwise specified. DLQI, Dermatology Life Quality Index; EQ‐5D, EuroQol‐5 dimensions questionnaire; IGA, Investigator Global Assessment; MCS, Mental Component Score; NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; PCS, Physical Component Score; PSSD, Psoriasis Symptoms and Signs Diary; SD, standard deviation; SF‐36, 36‐Item Short form Health Assessment Questionnaire; ss‐IGA, scalp‐specific Investigator Global Assessment.
Significantly greater improvements were observed in the guselkumab groups in achieving IGA 0, PASI‐50, PASI‐75 and PASI‐100 responses versus placebo at week 16 (all P < 0.001; Table 2). ss‐IGA 0 or 0/1 responses were significantly higher among guselkumab 50 mg and 100 mg groups versus placebo (both P < 0.001) and higher mean improvements in NAPSI were also observed (50 mg, P = 0.002; 100 mg, P < 0.001) (Table 2). Mean change from baseline DLQI score at week 16 was significantly greater among patients in the guselkumab groups (50 mg and 100 mg) versus placebo (−8.3 and −8.5 vs −0.8, respectively; both P < 0.001) (Table 2). Improvements in EQ‐5D and PCS and MCS scores of SF‐36 at week 16 were also significantly greater in both guselkumab groups versus placebo (both P < 0.001; Table 2).
Efficacy from week 16 through week 52
Improvement in IGA 0/1 responses and PASI‐90 responses was maintained from week 16 through week 52 in both guselkumab 50 mg (87.7% and 75.4%, respectively) and 100 mg groups (90.5% and 77.8%, respectively; Table 2, Fig. 3). From weeks 32–52, a consistent tendency for higher PASI‐100 response was observed in the guselkumab 100 mg group. Through week 52, both guselkumab groups showed sustained improvements in NAPSI and ss‐IGA (0 or 0/1) responses. Placebo cross‐over patients showed improvement similar to those who initially received guselkumab for all efficacy measures (Table 2). Improvements in the EQ‐5D VAS and index scores, and SF‐36 scores observed in the guselkumab groups at week 16 were generally maintained through week 48 (Table 2).
Figure 3.
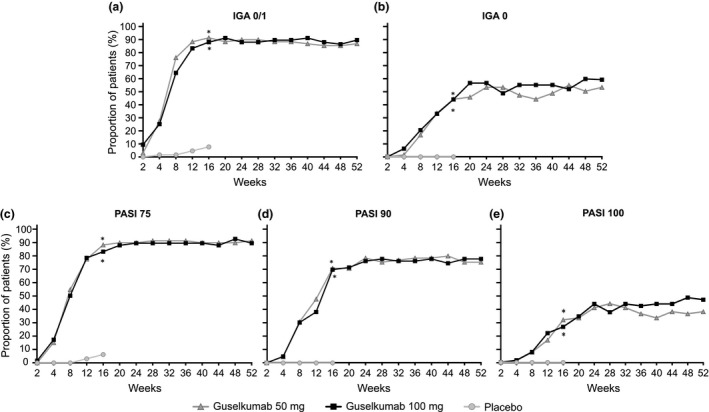
Efficacy outcomes through week 52. (a) Proportion of patients achieving IGA 0/1. (b) Proportion of patients achieving IGA 0. (c) Proportion of patients achieving PASI‐75. (d) Proportion of patients achieving PASI‐90. (e) Proportion of patients achieving PASI‐100. *P < 0.001 for guselkumab versus placebo. IGA 0/1, Investigator Global Assessment score of cleared (0) or minimal (1); IGA 0, Investigator Global Assessment score of 0 (cleared); PASI‐75, 75% or more improvement in Psoriasis Area and Severity Index score from baseline; PASI‐90, 90% or more improvement in Psoriasis Area and Severity Index score from baseline; PASI‐100, 100% improvement in Psoriasis Area and Severity Index score from baseline.
Previous exposure to biological therapy
A total of 35 out of 192 (18%) patients had prior treatment with biologics (placebo, n = 10; guselkumab 50 mg, n = 14; 100 mg, n = 11) and 157 (82%) patients (placebo, n = 54; guselkumab 50 mg, n = 51; 100 mg, n = 52) were naive to biologic therapy. At week 16, both guselkumab groups demonstrated greater IGA 0/1 (50 mg, 98.0%; 100 mg, 88.5%) and PASI‐90 (50 mg, 72.5%; 100 mg, 73.1%) responses versus placebo (9.3% for IGA 0/1 and 0% for PASI‐90) among biologic‐naive patients. Similarly, both guselkumab groups showed better IGA 0/1 (50 mg, 71.4%; 100 mg, 90.9%) and PASI‐90 (50 mg, 64.3%; 100 mg, 54.5%) versus placebo (0% for both IGA 0/1 and PASI‐90 responses) among biologic‐experienced patients, although most response rates were slightly lower than those for biologic‐naive patients.
Efficacy in patients with PsA
A total of 31 of the 192 patients (placebo, n = 10; guselkumab 50 mg, n = 10; guselkumab 100 mg, n = 11) were diagnosed with PsA based on CASPAR, and 10 were diagnosed with active PsA. At week 16, three of the three patients with active PsA in each of the guselkumab groups achieved an ACR‐20 response, compared with none of the four patients in the placebo group. Individual ACR components such as tender and swollen joint counts and the patient's assessment of pain also showed improvements that were consistent with the overall ACR response (Table 3). Improvements were generally maintained through week 52 for both guselkumab treatment groups. Similarly, improvements were also observed among placebo cross‐over patients after initiating guselkumab treatment at week 16.
Table 3.
Efficacy end‐points related to patients with active psoriatic arthritis
| Efficacy outcomes | Week 16 | Through week 52 | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 4) | Guselkumab 50 mg (n = 3) | Guselkumab 100 mg (n = 3) | Placebo → guselkumab 50 mg (n = 1) | Placebo → guselkumab 100 mg (n = 1) | Guselkumab 50 mg (n = 3) | Guselkumab 100 mg (n = 3) | |
| ACR response, n (%) | |||||||
| ACR‐20 | 0 | 3 (100.0) | 3 (100.0) | 1 (100.0) | 1 (100.0) | 3 (100.0) | 1 (33.3) |
| ACR‐50 | 0 | 2 (66.7) | 3 (100.0) | 1 (100.0) | 1 (100.0) | 2 (66.7) | 1 (33.3) |
| ACR‐70 | 0 | 1 (33.3) | 3 (100.0) | 1 (100.0) | 0 | 2 (66.7) | 1 (33.3) |
| Change in tender joint count, mean (SD) | 1.8 (2.36) | −8.3 (10.12) | −9.3 (4.93) | −3.0 (–) | 4.0 (–) | −10.3 (9.45) | −10.7 (7.23) |
| Change in swollen joint count, mean (SD) | 0.5 (2.52) | −3.3 (2.52) | −9.0 (3.00) | −10.0 (–) | −3.0 (–) | −4.3 (1.53) | −3.0 (8.19) |
| Change in patient's assessment of pain (VAS), mean (SD) | 0.80 (1.47) | −5.07 (2.20) | −5.43 (0.64) | −5.60 (–) | −4.80 (–) | −4.60 (4.00) | −3.63 (3.07) |
| Change in patient's global assessment of disease activity (VAS), mean (SD) | 0.55 (1.75) | −5.60 (1.71) | −6.00 (1.11) | −6.60 (–) | −4.80 (–) | −5.07 (3.33) | −3.63 (2.45) |
| Change in physician's global assessment of disease activity (VAS), mean (SD) | 1.63 (3.25) | −5.70 (3.12) | −3.27 (1.08) | −10.00 (–) | −2.10 (–) | −6.17 (1.52) | −2.20 (2.42) |
| HAQ‐DI responders, n (%) | 0 | 2 (66.7) | 2 (66.7) | 1 (100.0) | 0 | 2 (66.7) | 1 (33.3) |
ACR, American College of Rheumatology; HAQ‐DI, Health Assessment Questionnaire – Disability Index; PsA, psoriatic arthritis; SD, standard deviation; VAS, visual analog scale.
Safety through week 16
The proportions of patients experiencing one or more TEAE were comparable across groups (50 mg, 46.2% [30/65]; 100 mg, 46.0% [29/63]) and placebo group (56.3% [36/64]) (Table 4). The majority of the TEAE were mild in severity. Serious TEAE were reported in four patients: two patients in the placebo group (acute cholecystitis, and pemphigoid and exacerbation of psoriasis), and one patient each in the guselkumab 50 mg (colon adenoma and rectal adenocarcinoma) and 100 mg groups (bacterial prostatitis). Treatment discontinuation occurred in one patient in the guselkumab 50 mg group due to colon adenoma and rectal adenocarcinoma and six patients in the placebo group, of which four were due to exacerbation of psoriasis. Rectal adenocarcinoma was the only malignancy observed during the placebo‐controlled period, reported in a 57‐year old man on day 54. Injection site reactions were reported in two patients (placebo and guselkumab 50 mg group, n = 1 each).
Table 4.
Safety at week 16 and through week 52 (safety analysis set)
| n (%) | Week 16 | Through week 52 | |||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 64) | Guselkumab 50 mg (n = 65) | Guselkumab 100 mg (n = 63) | Placebo → guselkumab 50 mg (n = 26) | Placebo → guselkumab 100 mg (n = 26) | Guselkumab 50 mg (n = 65) | Guselkumab 100 mg (n = 63) | |
| Patients with one or more TEAE | 36 (56.3) | 30 (46.2) | 29 (46.0) | 19 (73.1) | 23 (88.5) | 57 (87.7) | 54 (85.7) |
| Common TEAE † | |||||||
| Nasopharyngitis | 7 (10.9) | 14 (21.5) | 8 (12.7) | 7 (26.9) | 7 (26.9) | 28 (43.1) | 24 (38.1) |
| TEAE of infections | 14 (21.9) | 18 (27.7) | 15 (23.8) | 12 (46.2) | 13 (50.0) | 37 (56.9) | 38 (60.3) |
| Patients with one or more serious TEAE | 2 (3.1) | 1 (1.5) | 1 (1.6) | 1 (3.8) | 3 (11.5) | 4 (6.2) | 2 (3.2) |
| Acute cholecystitis ‡ | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Pemphigoid | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Psoriasis | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Colon adenoma | 0 | 1 (1.5) | 0 | 0 | 0 | 1 (1.5) | 0 |
| Rectal adenocarcinoma | 0 | 1 (1.5) | 0 | 0 | 0 | 1 (1.5) | 0 |
| Bacterial prostatitis ‡ | 0 | 0 | 1 (1.6) | 0 | 0 | 0 | 1 (1.6) |
| Atrial fibrillation | 0 | 0 | 0 | 1 (3.8) | 0 | 0 | 0 |
| Congestive cardiac failure | 0 | 0 | 0 | 1 (3.8) | 0 | 0 | 0 |
| Cataract | 0 | 0 | 0 | 0 | 1 (3.8) | 0 | 0 |
| Diabetic retinopathy | 0 | 0 | 0 | 0 | 1 (3.8) | 0 | 0 |
| Macular hole | 0 | 0 | 0 | 0 | 1 (3.8) | 0 | 0 |
| Wrist fracture | 0 | 0 | 0 | 0 | 1 (3.8) | 0 | 0 |
| Retinal detachment | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 |
| Angina pectoris | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 |
| Cerebral infarction | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 |
| Loss of consciousness | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 |
| Varicose vein | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1.6) |
| TEAE leading to discontinuation of study agent | 6 (9.4) | 1 (1.5) | 0 | 0 | 1 (3.8) | 3 (4.6) | 0 |
| Psoriasis | 4 (6.3) | 0 | 0 | 0 | 1 (3.8) | 0 | 0 |
| Pemphigoid | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Cholecystitis acute | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Psoriatic arthropathy | 1 (1.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Colon adenoma | 0 | 1 (1.5) | 0 | 0 | 0 | 1 (1.5) | 0 |
| Rectal adenocarcinoma | 0 | 1 (1.5) | 0 | 0 | 0 | 1 (1.5) | 0 |
| Hepatitis B DNA assay positive § | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 |
| Pregnancy | 0 | 0 | 0 | 0 | 0 | 1 (1.5) | 0 |
| TEAE of injection site reaction | 1 (1.6) | 1 (1.5) | 0 | 0 | 2 (7.7) | 6 (9.2) | 4 (6.3) |
†Common TEAE occurred in more than 10 patients in the guselkumab groups. ‡Serious infection. §The patient who initially tested positive for hepatitis B DNA had a negative retest; therefore, hepatitis B reactivation was not confirmed in this patient. TEAE, treatment emergent adverse event.
Safety through week 52
The proportions of patients experiencing one or more TEAE through week 52 remained comparable between the guselkumab 50 mg and 100 mg groups (Table 4). Among patients who crossed over to guselkumab, 73.1% (19/26) receiving 50 mg and 88.5% (23/26) receiving 100 mg experienced one or more TEAE. The most commonly reported TEAE across all groups was nasopharyngitis and the majority of TEAE were mild in severity through week 52. A total of eight additional patients reported serious TEAE from week 16 through week 52: one patient in the placebo to 50 mg group (congestive cardiac failure and atrial fibrillation), three patients in the placebo to 100 mg group (diabetic retinopathy, wrist fracture; cataract and macular hole, n = 1 each), three patients in guselkumab 50 mg (cerebral infarction in right thalamus and angina pectoris; retinal detachment; and loss of consciousness, n = 1 each) and one patient in guselkumab 50 mg (varicose vein). Three additional TEAE led to treatment discontinuation from week 16 through week 52: pregnancy and hepatitis B DNA assay positive (n = 1 each in the guselkumab 50 mg group) and psoriasis flare (n = 1 in the placebo to 100 mg group). The patient who initially tested positive for hepatitis B DNA had a negative retest; therefore, hepatitis B reactivation was not confirmed in this patient. (Table 4). One patient in the guselkumab 50 mg group experienced a major adverse cardiovascular event (MACE) of cerebral infarction involving the right thalamus on day 289. This patient, a 60‐year‐old man, had a history of stroke, hypertension and hyperlipidemia, and a 25‐year history of smoking. No action was taken with the study agent and the event was resolved after 12 days from onset. Injection site reactions were reported in 9.2% (6/65) of patients in the guselkumab 50 mg group, 6.3% (4/63) in the 100 mg, and 0% and 7.7% (2/26) in the placebo to 50 mg and placebo to 100 mg, respectively. None discontinued treatment due to injection site; there were no new occurrences of malignancy, no deaths, anaphylactic reactions, possible serum sickness‐like reactions or active tuberculosis reported through week 52. No clinically important laboratory abnormalities were observed among all guselkumab‐treated patients, including placebo cross‐over patients.
Immunogenicity
Antibodies to guselkumab were detected in 13 of 180 (7.2%) patients through week 52 and more than half of these patients (eight of 13) had low titer levels (≤1:80). One of these 13 patients was positive for neutralizing antibodies to guselkumab, but still achieved a PASI‐100 response at week 52. Also, no definitive differences in efficacy (PASI‐90 and IGA 0/1 response rates) and pharmacokinetics (serum guselkumab concentrations) were observed between patients who were positive or negative for antibodies to guselkumab (data not shown). Of the 13 patients who were positive for antibodies to guselkumab, five experienced injection site reactions, which were mild in severity and did not lead to treatment discontinuation.
Discussion
This phase 3 study demonstrated the superiority of guselkumab than placebo in treating Japanese patients with moderate to severe plaque‐type psoriasis based on significant improvements in co‐primary (IGA 0/1 and PASI‐90) and secondary efficacy end‐points at week 16. The safety profile of guselkumab was comparable with that of placebo during the 16‐week placebo‐controlled period. The 50 mg and 100 mg guselkumab groups showed no notable differences in safety outcomes through week 52.
Patients treated with guselkumab showed significantly greater improvements in PASI‐75, PASI‐90, PASI‐100, IGA 0/1 and IGA 0 responses at week 16 versus placebo and improvement was maintained long‐term through week 52. The onset of action of guselkumab was rapid, with significant response observed as early as week 2. Improvements were also observed among placebo cross‐over patients after switching to guselkumab at week 16 through week 52, and levels of improvements were similar to those observed for patients originally randomized to the guselkumab 50 mg or 100 mg groups. Treatment responses in regional forms of psoriasis, such as nail and scalp disease, were also observed based on the improvements in NAPSI scores and ss‐IGA scores. Guselkumab treatment was efficacious regardless of the previous use of biologics, as significant treatment success was observed among both biologic‐naive and biologic‐experienced patients.
Patient‐reported outcome measures such as DLQI, EQ‐5D, and SF‐36 PCS and MCS scores, improved throughout the study, indicating improvement in signs and symptoms of psoriasis and overall QoL with guselkumab treatment. Additionally, in a small number of patients with active PsA, guselkumab treatment resulted in improvements in joint signs and symptoms at week 16 that were maintained through week 52. Findings from a global phase 2 study of guselkumab also demonstrated improvements in the signs and symptoms associated with PsA, further establishing the efficacy of guselkumab for PsA.21
The study was limited by a small sample size and a placebo‐control period of 16 weeks. Although this study was not designed to statistically compare efficacy responses between the guselkumab 50 mg and 100 mg doses, a dose response was generally not observed for most efficacy measures. However, over time, a tendency for higher PASI‐100 and IGA 0 response, the most stringent efficacy criteria indicating complete clearance of psoriasis, was observed in the guselkumab 100 mg group. In patients with scalp psoriasis, which is often resistant to treatment, guselkumab 100 mg dose consistently showed higher ss‐IGA 0 response rates. This is a particularly important consideration given that new biologics have recently been developed to address the growing demand for treatments that can provide higher levels of improvement and overall QOL of psoriatic patients while maintaining a favorable safety profile.
Overall, guselkumab was generally well‐tolerated and the safety profile was consistent with previous studies in Japanese patients as well as in global populations.16, 17, 18, 19, 20 The incidence of TEAE and serious TEAE were comparable across guselkumab groups and placebo, and indicate no dose effect between guselkumab 50 mg and 100 mg. One MACE (cerebral infarction) occurred in the guselkumab 50 mg group in this study. The incidence rate of MACE with guselkumab was low in the current study and was similar to that of the global studies, wherein one MACE (myocardial infarction) was reported with guselkumab in VOYAGE 1 and 2 studies.17, 18 No impact of antibodies to guselkumab on pharmacokinetics, efficacy and safety were observed, although a small number of patients who were positive for antibodies to guselkumab precludes drawing any definitive conclusions.
These findings further support that guselkumab by targeting the IL‐23 pathway can be a valuable therapeutic option in management of psoriasis. IL‐23, besides its influence on promoting proliferation and survival of IL‐17A‐producing Th17 cells, is found to be associated with stimulation of additional Th17 cytokines (e.g. IL‐22), which may also be produced by other cell types (e.g. Th22 cells, innate lymphoid type 3 cells and γδ T cells).22, 23, 24 Therefore, selective blockade of IL‐23 may result in inhibition of pathogenic Th17 cells and preclude downstream production of multiple effector cytokines such as IL‐17A, IL‐22 and TNF‐α,25 and may also enhance accumulation of regulatory T cells.26 Because many cells other than Th17 produce IL‐17A and/or IL‐22 in response to IL‐23, inhibition of IL‐23 may impact the regulation of these pathogenic cells by IL‐23 and their cytokine production.22, 23, 24 This may possibly explain the sustained effects of guselkumab observed in treating psoriasis and why longer dosing intervals are possible compared with other biologics such as anti‐TNF‐α and anti‐IL‐17 agents.17
In conclusion, guselkumab demonstrated robust efficacy and a favorable safety profile in Japanese patients with moderate to severe plaque‐type psoriasis. Improvement in efficacy measures was maintained with a q8w dosing regimen through week 52. Overall, these findings suggest that guselkumab has potential as a novel therapeutic option in Japanese patients with psoriasis. The long‐term efficacy and safety of guselkumab treatment beyond 52 weeks will be further evaluated in the ongoing extension phase of this study.
Acknowledgments
The authors thank the study participants without whom this study would not have been accomplished and also thank the investigators for their participation in the study. Writing assistance was provided by Ramji Narayanan, ISMPP CMPP™ (SIRO Clinpharm, Thane, India) funded by Janssen Pharmaceutical, Japan. Madhavi Patil, Ph.D. (SIRO Clinpharm) provided additional editorial support. Publication support was provided by Kenichiro Tsutsumi (Janssen Pharmaceutical). Funding: This study was funded by Janssen Pharmaceutical, Tokyo, Japan.
Conflict of Interest
M. O. has received honoraria and/or research grants as a consultant and/or advisory board member and/or paid speaker and/or investigator from Abbvie, Boehringer‐Ingelheim, Celgene, Eisai, Janssen, Kyowa‐Kirin, LEO Pharma, Eli Lilly, Maruho, Novartis, Pfizer, Tanabe‐Mitsubishi, Nichiiko, Torri, Bayer, Pola Pharma, Taiho, Bristol‐Myers Squibb, Astellas, Otsuka, Mochida, Nippon Zoki, Actelion, Sanofi, Kaken Pharmaceuticals, Teijin Pharma, Nippon Kayaku, Shionogi, Ono and Galderma. H. N. has received honoraria and/or research grants as an advisory board member and/or speaker from ABC Pharma, Kyowa Hakko Kirin, Abbvie, Mitsubishi‐Tanabe Pharma, LEO Pharma, Maruho, Eli Lilly Japan, Janssen. H. K., H. M., R. G. and R. Z. are employees of Janssen Pharmaceutical.
References
- 1. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5(1): e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lebwohl M. Psoriasis. Lancet 2003; 361(9364): 1197–1204. [DOI] [PubMed] [Google Scholar]
- 3. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370(9583): 263–271. [DOI] [PubMed] [Google Scholar]
- 4. Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population‐based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol 2016; 17(1): 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Papp KA, Tyring S, Lahfa M et al A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152(6): 1304–1312. [DOI] [PubMed] [Google Scholar]
- 6. Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366(9494): 1367–1374. [DOI] [PubMed] [Google Scholar]
- 7. Menter A, Tyring SK, Gordon K et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58(1): 106–115. [DOI] [PubMed] [Google Scholar]
- 8. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371(4): 326–338. [DOI] [PubMed] [Google Scholar]
- 9. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371(9625): 1675–1684. [DOI] [PubMed] [Google Scholar]
- 10. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371(9625): 1665–1674. [DOI] [PubMed] [Google Scholar]
- 11. Sofen H, Smith S, Matheson RT et al Guselkumab (an IL‐23‐specific mAb) demonstrates clinical and molecular response in patients with moderate‐to‐severe psoriasis. J Allergy Clin Immunol 2014; 133(4): 1032–1040. [DOI] [PubMed] [Google Scholar]
- 12. Kopp T, Riedl E, Bangert C et al Clinical improvement in psoriasis with specific targeting of interleukin‐23. Nature 2015; 521(7551): 222–226. [DOI] [PubMed] [Google Scholar]
- 13. Ness‐Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL‐17A‐ and IL‐22‐producing human Vgamma2Vdelta2 T cells. J Immunol 2010; 184(12): 7268–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin‐23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin‐17. J Biol Chem 2003; 278(3): 1910–1914. [DOI] [PubMed] [Google Scholar]
- 15. Wilson NJ, Boniface K, Chan JR et al Development, cytokine profile and function of human interleukin 17‐producing helper T cells. Nat Immunol 2007; 8(9): 950–957. [DOI] [PubMed] [Google Scholar]
- 16. Gordon KB, Duffin KC, Bissonnette R et al A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015; 373(2): 136–144. [DOI] [PubMed] [Google Scholar]
- 17. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76(3): 418–431. [DOI] [PubMed] [Google Scholar]
- 18. Blauvelt A, Papp KA, Griffiths CE et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76(3): 405–417. [DOI] [PubMed] [Google Scholar]
- 19. Nemoto O, Hirose K, Shibata S, Li K, Kubo H. Safety and efficacy of guselkumab in Japanese patients with moderate‐to‐severe plaque psoriasis: a randomised, placebo‐controlled, ascending dose study. Br J Dermatol 2017; 178: 689–696. [DOI] [PubMed] [Google Scholar]
- 20. Terui T, Kobayashi S, Okubo Y, Murakami M, Hirose K, Kubo H. Guselkumab, an anti‐IL‐23 monoclonal antibody in palmoplantar pustulosis: a randomized, double‐blind, placebo‐controlled phase 2 study assessing efficacy and safety in Japanese patients. JAMA Dermatol 2018; 154: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gottlieb AB, Deodhar AA, Boehncke W‐H, Dong B. Efficacy and safety of guselkumab, an anti‐IL23 Monoclonal antibody, in patients with active psoriatic arthritis: a phase 2a, randomized, double‐blind, placebo‐controlled study. J Am Acad Dermatol 2017; 76(6): AB111. [Google Scholar]
- 22. Villanova F, Flutter B, Tosi I et al Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44 + ILC3 in psoriasis. J Invest Dermatol 2014; 134(4): 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ward NL, Umetsu DT. A new player on the psoriasis block: IL‐17A‐ and IL‐22‐producing innate lymphoid cells. J Invest Dermatol 2014; 134(9): 2305–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Teunissen MB, Munneke JM, Bernink JH et al Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol 2014; 134(9): 2351–2360. [DOI] [PubMed] [Google Scholar]
- 25. Blauvelt A. New concepts in the pathogenesis and treatment of psoriasis: key roles for IL‐23, IL‐17A and TGF‐β1. Expert Rev Dermatol 2007; 2(1): 69–78. [Google Scholar]
- 26. Maxwell JR, Zhang Y, Brown WA et al Differential roles for interleukin‐23 and interleukin‐17 in intestinal immunoregulation. Immunity 2015; 43(4): 739–750. [DOI] [PubMed] [Google Scholar]


