Abstract
A 63‐year‐old man with cirrhosis, hepatocellular carcinoma, and coagulopathy was diagnosed with a sinus venosus atrial septal defect (ASD) and partial anomalous pulmonary venous return (PAPVR) of the right upper pulmonary vein (RUPV). Transcatheter repair by positioning a stent graft in the superior vena cava was planned. Based on three‐dimensional (3D) reconstruction of gated cardiac CTA, a 28 mm × 7 cm Endurant II® aortic extension stent graft (Medtronic, MN) was chosen. A 3D model printed from the CTA was used to simulate device deployment, demonstrating successful exclusion of the sinus venosus ASD with return of the RUPV to the left atrium (LA). Post simulation, the 3D model was used for informed consent. The patient was then taken to the hybrid operating room. On‐table cone beam CT was performed and registered with the CTA images. This enabled overlay of 3D regions of interest to live 2D fluoroscopy. The stent graft was then deployed using 3D regions of interest for guidance. Hemodynamics and angiography demonstrated successful exclusion of the sinus venosus ASD and unobstructed return of RUPV to the LA. This is the first report of comprehensive use of contemporary imaging for planning, simulation, patient consent, and procedural guidance for patient‐centered complex structural intervention in repair of sinus venosus ASD with PAPVR. We propose this as a process model for continued innovation in structural interventions.
Keywords: cardiac imaging, computed tomography, cone beam, congenital, heart defects, techniques
1. INTRODUCTION
Advances in cardiovascular imaging now provide high resolution three‐dimensional (3D) data. These may be used as templates for 3D printing and can also be imported into the catheterization lab for live guidance during complex interventions. These innovations enable patient‐centered novel structural interventions. We report the first 3D image‐fusion‐guided transcatheter repair of a sinus venosus atrial septal defect (ASD) in a poor surgical candidate after pre‐procedural simulation in a 3D printed model. We describe this process as a template for development of future patient‐centered structural interventions (Figure 1, Supporting Information Video 1).
Figure 1.
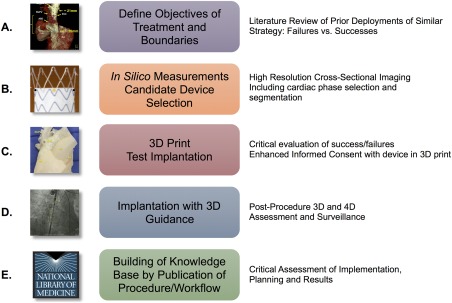
Process map for development of patient‐centered structural heart interventions. A, Problem, objectives, and boundaries defined with candidate solutions and pitfalls identified from literature review. B, Software based measurements of 3D anatomic structures based on high‐resolution cross‐sectional imaging. Candidate devices selected based upon these measurements. C, After 3D segmentation of CT, 3D model is printed and candidate device is deployed to simulate procedure and identify any potential sources of failure. D, Procedure performed in patient using 3D guidance from CT data imported into fluoroscopy system. Post‐procedure imaging using high‐resolution CT to critically evaluate deployment and procedure. E, Complete process reported publicly including candid critique of process and procedure. [Color figure can be viewed at http://wileyonlinelibrary.com]
2. CASE REPORT
A 63‐year‐old man with cirrhosis, hepatocellular carcinoma, thrombocytopenia, and coagulopathy under consideration for liver transplantation presented with shortness of breath and edema. Transthoracic echocardiography (TTE) showed right ventricular (RV) enlargement, hypokinesis, and left to right shunt through a congenital heart lesion. Cardiac MRI revealed a shunt from the left atrium (LA) to the right atrium (RA) via a superior sinus venosus ASD (length 3.5 cm, area 4.4 cm2). The right upper pulmonary vein (RUPV) connected to the LA as expected but it also abnormally drained into the superior vena cava (SVC) leading to partial anomalous pulmonary venous return (PAPVR). The estimated Qp:Qs was 1.9:1. Due to liver failure, surgical repair was a poor option, and a transcatheter approach was developed (Figure 1).
2.1. Pre‐procedural 3D planning
Our objective was to exclude the SVC from the LA and the RUPV (by covering the sinus venosus ASD) (Figure 2A). Our boundaries were to avoid obstructing neighboring venous structures (e.g., azygos vein) or extending into the RA (could induce ectopy). We required the diameter and external radial strength of the stent graft to ensure apposition to the proximal SVC. This was expected to prevent residual shunt from the LA, or leak. An important concern was to avoid obstructing the RUPV which could induce segmental pulmonary edema and pulmonary hypertension (Figure 1A,B).
Figure 2.
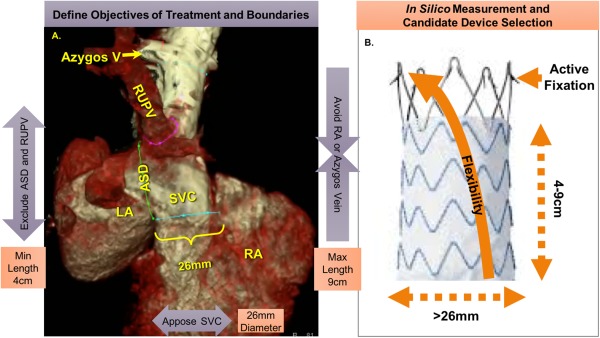
Objectives for transcatheter repair of sinus venosus ASD with PAPVR and measures required for candidate device. A, 3D reconstruction of gated cardiac CTA with regions of interest outlined. Objectives were to exclude the ASD and anomalous RUPV (>4 cm) while avoiding extending into the RA or covering the azygos vein (<9 cm) with a device at least the diameter of the SVC near the RA (>26 mm). B, One candidate device demonstrating measures identified in (A); additional important features such as active fixation barbs and stent flexibility to conform to the SVC. [Color figure can be viewed at http://wileyonlinelibrary.com]
Based on these objectives and measurements of the gated cardiac CTA (Figure 2A), we required a device >4 cm in length to exclude both the RUPV and sinus venosus ASD, but <9 cm to avoid covering the azygos vein or extending into the RA. A diameter of at least 26 mm was required to match the proximal SVC at the floor of the sinus venosus ASD and prevent shunt “endograft leak.” To optimize safety, the device would be flexible and conform to the curvature of the SVC. It would use active fixation barbs to prevent embolization (unlike arterial targets, the SVC had no calcifications to ensure stable positioning of the device, and many coverings such as PTFE can be slippery). After reviewing stent grafts from multiple vendors, one device met these conditions: the self‐expanding Endurant II® 28 mm × 7 cm aortic extension (Medtronic, MN) (Figure 2B).
2.2. 3D print test implantation
A 3D model was printed using tissue‐mimicking rubber‐like material (TangoPlus, 3D Printers Canada and Objet Geometries Ltd.) after segmentation of CTA images (segmentation time: <5 min) (Figure 3A,B). A cone‐beam CT of the 3D model was acquired and co‐registered with the pre‐procedural CTA to validate the model (Supporting Information Video 1). The stent graft was then implanted in the SVC of the model under fluoroscopy (Figure 3C). Post‐simulation cone‐beam CT of the model demonstrated successful exclusion of the sinus venosus ASD and return of the RUPV to the LA (Figure 3D). The 3D model was shown to the patient for informed consent (Figure 1C).
Figure 3.
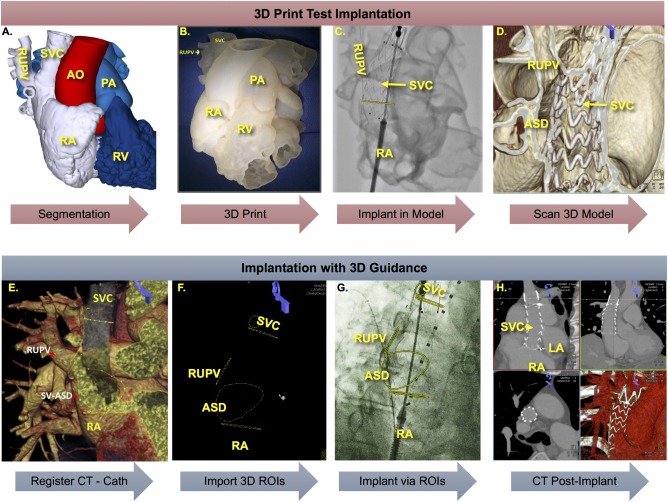
Simulation of device implantation in 3D printed model and implantation in patient using 3D image guidance. 3D segmentation of gated cardiac CTA in MIMICS (A) followed by 3D print of segmented structures of interest (B). Implantation of candidate stent graft in 3D printed model under fluoroscopy (C) and cone‐beam CT of results (D) demonstrated successful exclusion of ASD and RUPV. 3D guidance of implant in patient after importing gated cardiac CTA and registration with cone‐beam CT of patient on table (E) followed by overlay of ROIs onto fluoroscopy (F). G, Implant of stent graft guided by 3D ROIs demarcating the ASD and RUPV with proximal and distal ends of SVC outlined in orthogonal planes. H, Post‐procedure gated CTA demonstrated successful exclusion of ASD and RUPV without obstruction. [Color figure can be viewed at http://wileyonlinelibrary.com]
2.3. Implantation with 3D guidance
3D regions of interests (ROIs) were electronically marked on the CTA (ASD, RUPV, azygos vein, proximal and distal ends of the SVC) using software tools (syngo iGuide toolbox®, Siemens Healthcare GmbH, Germany) (Figure 3E). The patient was taken to the hybrid operating room equipped with a robotic angiographic imaging system (Artis zeego ®, Siemens). On‐table cone‐beam CT (5 sec protocol) was performed during simultaneous contrast injection into the SVC and RUPV (Supporting Information Video 2). Cone‐beam CT images were co‐registered with pre‐procedural CTA (8 min from start to finish) using commercially available tools (syngo Inspace 3D3D fusion®, Siemens) (Figure 3E,F). Co‐registration enabled real‐time overlay of 3D ROIs onto live 2D fluoroscopy (Figure 3G). It also allowed optimization of C‐arm angles for orthogonal view of the SVC at the floor of the ASD and the ostium of the azygos vein as well as en‐face view of the ASD.
The stent graft delivery system was advanced into position using the 3D ROIs as guidance (Figure 1D) and carefully deployed in the SVC with the uncovered portion at the level of the azygos vein and the covered portion beginning above the RUPV and extending below the floor of the sinus venosus ASD, just above the SVC‐RA junction (Figure 3G, Supporting Information Video 3 and Methods).
Post deployment, C‐arm angles were optimized for en‐face and side‐on view of the ASD based on the co‐registered CTA/cone‐beam CT images. SVC and RUPV venograms demonstrated successful exclusion of the RUPV with unobstructed flow from RUPV into the LA and no residual shunt across the ASD (Supporting Information Video 3). Simultaneous measurement of LA and RUPV pressures demonstrated no gradient. Repeat oximetry did not reveal a residual shunt.
2.4. Post‐procedure imaging and follow‐up
Post‐procedure day 1 CTA confirmed that the device remained in appropriate position without evidence of RUPV stenosis (Figure 3H). The risk of thrombosis in a freshly implanted stent was considered higher than the bleeding risk; therefore, the patient was discharged on dual anti‐platelet therapy (aspirin and clopidogrel). As our patient lives in another country, he was unable to follow‐up with us; on telephonic follow‐up, he reported improvement in symptoms at 6 months. TTE in his home country showed normalization of RV volume followed by successful liver transplant shortly after.
3. DISCUSSION
We report the first comprehensive use of contemporary imaging for planning, 3D prototyping and simulation, patient consent, and procedural guidance for a patient‐centered complex structural intervention in the transcatheter repair of a sinus venosus ASD with PAPVR. We propose this as a process model for innovation in structural heart interventions (Figure 1).
First, we defined our objectives and boundaries to prevent complications. We reviewed literature for successes and failures: Garg et al. have reported sinus venosus ASD exclusion using a balloon‐mounted covered stent (Advanta V12, Maquet Medical, Rastatt, Germany) in the SVC under trans‐esophageal echocardiography (TEE)/angiography guidance 1. Crystal et al. used three self‐expanding stent grafts (Zenith Flex AAA extension cuffs, Cook Medical, Bloomington, IN) of progressively increasing sizes in the SVC under TEE guidance 2. There were no reported complications, and this strategy appeared feasible and low complexity. Next, we obtained high‐resolution cross‐sectional imaging and made measurements based on our objectives to identify candidate devices.
Third, we 3D printed the cardiac CTA to identify potential problems with device implantation. We then validated the 3D model followed by procedure simulation in the validated 3D model. Post‐simulation, the 3D model was used for informed consent.
Finally, we performed stent graft implantation under 3D image fusion guidance as conventional 2D fluoroscopy and angiography provided limited information, and we were unfamiliar with the TEE appearance of the stent graft and delivery system. The projected 3D ROI markers automatically tracked with changes in C‐arm angulations, table positions, and image zoom. This enabled visualization of the anatomic target for device deployment in a quasi‐3D environment. We and other authors feel that this may allow safer and more accurate device placement and decrease the need for repeated angiography 3. Importing the CT data also allowed optimization of C‐arm angles to minimize parallax during device deployment and angiography. Post‐procedure CT confirmed optimal positioning of the device and appeared remarkably similar to the position of the stent graft within the 3D model.
While the procedure was successful with no significant complications, we report the following concerns. First, is there a risk of thrombus formation on the stent graft material? Thrombus on the endoluminal side may be acceptable but on the LA side, it could be a source for systemic embolization. Second, what is the effect of the active fixation barbs on the SVC? Active fixation is safe in the aorta with a thick vascular wall, but is there risk of perforation in the SVC? Third, patient age at treatment: application of this technique to children who are still subject to somatic growth may present a problem. While the stent graft may be oversized to allow for future re‐dilation, venous fluid dynamics may be compromised with unknown consequences. Some children may not have the venous anatomy to accommodate an 18‐Fr delivery system. Fourth, does the 3D print truly mimic target tissue properties such that it serves as a reliable simulation to identify potential complications? Recent work from our institution examined the tensile properties of mitral tissue in comparison to different 3D print materials to best simulate mitral interventions 4; studies will continue to optimize the fidelity of 3D printed models for ex vivo testing. Additionally, there remains no pathway to reimbursement for 3D printing which can be extremely expensive; our case was supported by institutional research funding. Finally, although the ROIs from CTA are automatically tracked in real time with changes in C‐arm angulations, table positions and image zoom, they do not track with cardiac or respiratory motion. Introduction of stiff devices may distort vasculature and make projected ROIs less accurate; however, angiography based 2D re‐registration tools and software based 3D vascular deformation correction algorithms are available to improve image fusion accuracy further. In our case, these limitations did not affect the procedure as we were deploying a linear device in a linear target with fairly straightforward anatomy during mechanical breath holds.
Despite these limitations, meticulous planning can mitigate the risks of these innovative procedures. Currently, however, we cannot recommend this process for low complexity patients: sinus venosus ASDs with PAPVR are surgically corrected with excellent results, and patients able to tolerate conventional therapies should be treated as such. However, routine use of this technique may be possible in the future as outcomes from more cases are reported.
In conclusion, our process may be optimal to identify innovative but straightforward procedures for high complexity patients with co‐morbidities that preclude conventional therapies.
CONFLICT OF INTEREST
Ponraj Chinnadurai is a full‐time senior staff scientist at Advanced Therapies Division, Siemens Medical Solutions USA Inc.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Methods
Supporting Information Video 1
Supporting Information Video 2
Supporting Information Video 3
ACKNOWLEDGMENTS
Authors would like to thank Avni Patel: Houston Methodist Hospital, Houston, TX; Stephen H. Little, MD: Houston Methodist DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX; and Alan Lumsden, MD: Houston Methodist DeBakey Heart and Vascular Center, Houston Methodist Hospital, Houston, TX.
Thakkar AN, Chinnadurai P, Breinholt JP, Lin CH. Transcatheter closure of a sinus venosus atrial septal defect using 3D printing and image fusion guidance. Catheter Cardiovasc Interv. 2018;92:353–357. 10.1002/ccd.27645
REFERENCES
- 1. Garg G, Tyagi H, Radha AS. Transcatheter closure of sinus venosus atrial septal defect with anomalous drainage of right upper pulmonary vein into superior vena cava—An innovative technique. Catheter Cardiovasc Interv 2014;84:473–477. [DOI] [PubMed] [Google Scholar]
- 2. Crystal MA, Vincent JA, Gray WA. The wedding cake solution: A percutaneous correction of a form fruste superior sinus venosus atrial septal defect. Catheter Cardiovasc Interv 2015;86:1204–1210. [DOI] [PubMed] [Google Scholar]
- 3. Krishnaswamy A, Tuzcu EM, Kapadia SR. Integration of MDCT and fluoroscopy using c‐arm computed tomography to guide structural cardiac interventions in the cardiac catheterization laboratory. Catheter Cardiovasc Interv 2015;85:139–147. [DOI] [PubMed] [Google Scholar]
- 4. Vukicevic M, Puperi DS, Jane Grande‐Allen K, Little SH. 3D Printed Modeling of the Mitral Valve for Catheter‐Based Structural Interventions. Ann Biomed Eng 2017;45:508–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Methods
Supporting Information Video 1
Supporting Information Video 2
Supporting Information Video 3


