Abstract
Objectives
Genitourinary syndrome of menopause (GSM) combines the conditions of vulvovaginal atrophy (VVA) and urinary tract dysfunction, which is a result of urethral atrophy. There are several treatment methods available for the management of vulvovaginal symptoms of GSM, whereas urinary tract dysfunction often remains overlooked and undertreated. The objective of this pilot study was to assess the safety and efficacy of intraurethral Er:YAG laser treatment of urinary symptoms of GSM.
Patients and Methods
Patients with diagnosed GSM, having less than 5% of vaginal superficial cells in the cytology, vaginal pH higher than 5, with urinary symptoms of GSM (dysuria, frequency, urgency) and impaired continence due to urethral atrophy, received two sessions of intraurethral Er:YAG laser with a 3‐week interval in‐between the sessions. Laser energy was delivered in non‐ablative way using Erbium SMOOTH™ mode technology and a 4‐mm thick cannula. Therapeutic efficacy was determined using ICIQ‐SF, the 1‐hour pad test and VAS scores. Occurrence of adverse effects was followed at every visit. Follow ups (FU) were at 3 and 6 months.
Results
29 female patients fulfilling the inclusion criteria were included in this pilot study and received two sessions of the intraurethral non‐ablative Erbium SMOOTH™ laser therapy. Significant improvement was observed in all measured parameters at both FU. ICIQ‐SF improved by an average of 64% at 3 months FU and by 40% at 6 months. The 1‐hour pad test showed a reduction of the quantity of leaked urine by 59% at 3 months FU and by 42% at 6 months FU. All urinary symptoms of GSM improved. Dysuria dropped to 13% and 31% of baseline values at three and 6 months respectively, urinary urgency dropped to 23% and 47% and frequency dropped to 22% and 43% after 3 and 6 months, respectively. Adverse effects were mild and transient.
Conclusions
Our findings suggest that intraurethral Er:YAG laser is an efficacious and safe modality for treatment of urinary symptoms of GSM, however, prospective, randomized, and controlled trials with larger number of patients are needed to better assess the long‐term effect of this novel procedure. Lasers Surg. Med. 50:802–807, 2018. © 2018 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: Erbium SMOOTH™ laser, genitourinary syndrome of menopause, impaired continence, intraurethral, urethral atrophy
INTRODUCTION
Genitourinary syndrome of menopause (GSM) combines the conditions of vulvovaginal atrophy (VVA) and urinary tract dysfunction that are associated with estrogen deficiency. The syndrome may include but is not limited to genital symptoms of dryness, burning, and irritation; sexual symptoms of lack of lubrication, discomfort or pain, and impaired function; and urinary symptoms 1 of urgency, dysuria, frequency, and recurrent tract infections 2. All these symptoms may interfere with sexual function and overall quality of life. GSM symptoms usually occur after the onset of menopause, with a prevalence ranging from 65% to 84%, depending on the number of years since menopause 3.
Several therapeutic options are available to alleviate GSM symptoms, including hormonal and non‐hormonal products. Moisturizers and lubricants tend to provide only temporal relief of mild symptoms, whereas local vaginal estrogen administration offers long‐term relief and is usually the treatment of choice 4. This treatment nevertheless needs to be individualized, offering the most appropriate dose, regimen, duration of use, and route of administration, in order to maximize the benefit and minimize the risk 5. Some women are reluctant to commit to long‐term administration of hormone replacement therapy (HRT) or have a special health condition, for example, breast cancer or are breast cancer survivors. In such patients, non‐hormonal approaches are the first‐line choices for managing urogenital symptoms or atrophy‐related urinary symptoms 6.
Vaginal laser therapy, such as fractional CO2 and non‐ablative Erbium SMOOTH™ laser therapy have been established as promising treatment modalities for management of vulvovaginal symptoms of GSM 7, 8, 9, 10, 11, with Erbium SMOOTH™ having also show good results in treatment of stress urinary incontinence (SUI) 12, 13, 14, 15, vaginal relaxation syndrome (VRS) 16, 17 and mild prolapses 18. The safety and efficacy of the intraurethral Erbium SMOOTH™ procedure for the treatment of type III SUI have been demonstrated in our recent study 19. Nevertheless urinary tract dysfunction as a result of GSM remains an overlooked issue; therefore, the aim of this pilot study was to establish the safety and efficacy of intraurethral application of non‐ablative Erbium SMOOTH™ laser for the treatment of urinary symptoms of GSM. The premise of our aim is the fact that the thickness of mucosa and the rich vascularization of the submucosa confer its sealing properties, since they are the main contributory factors of the reduction of both the caliber of the urethral opening and the radius of the urethral cylinder 20.
MATERIALS AND METHODS
Patients
A total of 29 postmenopausal women (age range 56–77 years, mean age 66) with a diagnosis of GSM were prospectively enrolled at the Urogynecology Department of the Uroclinic in Mendoza, Argentina, between January and March, 2016. Study was approved by the Ethics Review Board of the Uroclinica and all participants gave written informed consent. The procedures followed were in accordance with the Helsinki Declaration. The inclusion criteria were clinical urinary symptoms of GSM (dysuria, frequency, and urgency), cytological proof of less than 5% of vaginal superficial cells and vaginal pH higher than 5. A urodynamic study was performed with each patient for a diagnosis of overactive detrusor and the measurement of Valsalva leak point pressure (VLPP). VLPP lower than 60 cm of H2O was considered as type III SUI 20, 21. Patients with genital prolapse of Pelvic Organ Prolapse Quantification (POP‐Q) stage more than I and Body Mass Index (BMI) higher than 35 kg m−2 were excluded from the study. None of the patients had received local or systemic HRT for the last 6 months prior the initiation of this study.
Procedure
All patients were treated with 2,940 nm Er:YAG laser (SP Spectro, Fotona, Slovenia). The laser energy was delivered using a 4 mm thick cannula. Immediately before the laser treatment, urine was evacuated form the bladder and the cannula was inserted into the urethra. Laser beam delivery was initiated at the proximal end of the urethra and continued step‐wise toward the urethra orifice. Non‐ablative laser energy was deposited using Erbium SMOOTH™ technology, with four SMOOTH™ pulses delivered at each location with a frequency of 1.4 Hz. Spot size was 4 mm and fluence was set to 1.5 J/cm2. After a train of four SMOOTH™ pulses was delivered, the cannula was pulled outwards by 2.5 mm until the urethra orifice had been reached. One treatment session consisted of four passes. Geometry of the beam that is expanding into conical shape with the angle of 22°, enabled the treatment of the whole urethral wall (360°).
Parameters used during the intraurethral laser procedure are derived from the RenovaLase® protocol (developed by Fotona d.o.o.), which has been shown to improve the vulvovaginal symptoms of GSM 7, 8, 9, 10, 11 and to promote neoangiogenesis 22, a highly desirable effect that improves the trophism of the mucosal tissue.
Patients were treated in an outpatient clinical setting and received no anesthesia. Pretreatment medication consisted of 1 g of oral ciprofloxacin one hour prior the procedure. Post‐treatment care consisted of oral administration of 1 g of vitamin C and 25,000 Units of vitamin A per day for 60 days. Patients had two laser treatment sessions 3 weeks apart. FU evaluations were scheduled 3 and 6 months after the second laser treatment. Nutrient supplementation has been prescribed as a part of general practice in our clinic, since it is known that high percentage of aging population nowadays has a certain degree of nutrient deficiency 23. Vitamin C has an important function as a cofactor for the ferrous (Fe[II]) and 2‐oxoglutarate dependent dioxygenases in collagen synthesis 24 and vitamin A is an important cofactor needed at the level of the epithelium 25.
Patient discomfort and potential adverse events were monitored during treatment and at every FU appointment.
The primary clinical outcome measures were subjective assessment of the severity of urinary symptoms of GSM; dysuria, frequency, and urgency were assessed on a VAS scale (0–100; 0 = total absence of symptoms and 100 = worst symptom). The severity of patient's incontinence was assessed using ICIQ‐UI SF and the 1‐hour pad test, which was done without retrograde filling. Recommendations of International Continence Society (ICS) have been followed.
The improvement rate in each of the outcome measures was calculated as a ratio between the values determined at each FU and the baseline values. Improvement rates higher than 50% of the baseline value were considered a clinically meaningful improvement.
A Friedman test (related‐samples Friedman's two‐way analysis of variance by ranks) with post‐hoc pairwise comparison using Bonferroni correction for multiple comparison (SPSS version 23, IBM SPSS Statistics, IBM Corp., NY) was run to determine if there were differences in endpoint measures during the course of our pilot study.
RESULTS
Our pilot study included twenty‐nine patients, aged between 56 and 77 years, with mean (SD) = 66 (5.6). They were all diagnosed with GSM, having less than 5% of vaginal superficial cells in the cytology, vaginal pH higher than 5, with urinary symptoms of GSM (dysuria, frequency, urgency) and impaired continence due to urethral atrophy.
The average baseline VAS values for dysuria, urgency, and frequency were 66, 58, and 49, respectively (Table 1 and Fig. 1). At the 3‐month FU, VAS values were 8.3, 13, and 11, respectively and at the 6‐month FU these values were 20, 28, and 21, respectively. There is a statistically significant difference (P < 0.0005) between values at 3‐month FU as well as at 6‐month FU appointment compared to baseline values (Table 1).
Table 1.
Effect of Intraurethral Laser Treatment During the Course of the Study
| Baseline (B)a | 3‐month FUb | 6‐month FUc | P * | post hoc test # | |
|---|---|---|---|---|---|
| Dysuria (VAS 0‐100) | 66 (21) | 8.3 (7.6) | 20 (11) | <0.0005 | a vs. b, c <0.0005 |
| Urgency (VAS 0‐100) | 58 (28) | 13 (12) | 28 (15) | <0.0005 | a vs. b, c <0.001 |
| Frequency (VAS 0‐100) | 49 (20) | 11 (8.4) | 21 (8.8) | <0.0005 | a vs. b, c <0.0005 |
| ICIQ‐UI | 13 (4.1) | 5.2 (3.6) | 8.1 (4.8) | <0.0005 | a vs. b, c <0.0005 |
| 1‐h pad test (g) | 42 (17) | 16 (5.2) | 23 (10) | <0.0005 | a vs. b, c <0.0005 |
Results are presented as mean (SD).
Friedman test (related samples Friedman's two‐way analysis of variance by ranks). Significance level is set to 0.05.
Post‐hoc multiple comparison with a Bonferroni correction for multiple comparisons.
a‐cTime points of the study.
Figure 1.
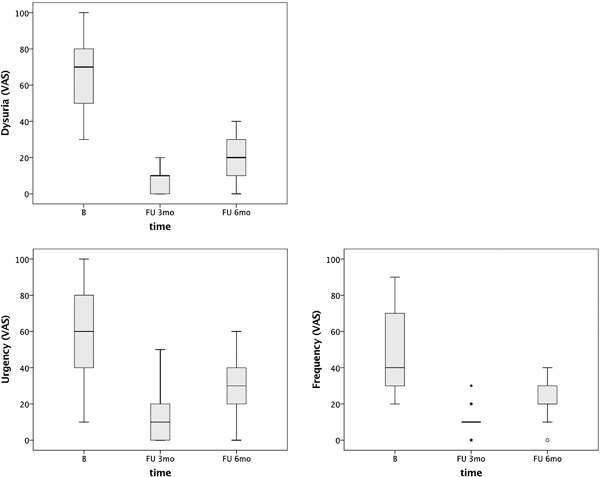
Effect of intraurethral laser treatment on three urinary symptoms of GSM: dysuria (a), urgency (b), and frequency (c). The horizontal line within the box indicates a median, boundaries of the box indicate the 1st and 3rd quartile, and whiskers indicate the minimum and maximum values. Empty circles indicate outliers with values 1.5 × IQR above and below the marked quartiles (Q1 and Q3) and asterisks indicate outliers with values 3 × IQR above and below the Q1 and Q3.
According to ICIQ‐UI, 48% (14/29) of patients suffered from moderate incontinence, 38% (11/29) from severe, and 14% (4/29) from very severe incontinence. The ICIQ‐UI range at baseline was 6–21. According to the 1‐hour pad test, 1–10 g h−1 of urine leakage was considered a mild incontinence, 11–50 g h−1 was considered moderate and >50 g h−1 was considered severe incontinence 26. Based on pad test results at baseline none of the women suffered from mild incontinence, 93% (27/29) suffered from moderate incontinence and 7% (2/29) from severe urinary incontinence. Average ICIQ‐UI and 1‐hour pad test values at baseline were 13 and 42, respectively. Values at the 3‐month FU were 5.2 and 16, respectively and at the 6‐month FU they were 8.1 and 23 (Table 1 and Fig. 2). ICIQ‐UI scores and 1‐hour pad weight at the 3‐ and 6‐month FU were statistically significantly different from baseline values (P < 0.0005) (Table 1).
Figure 2.
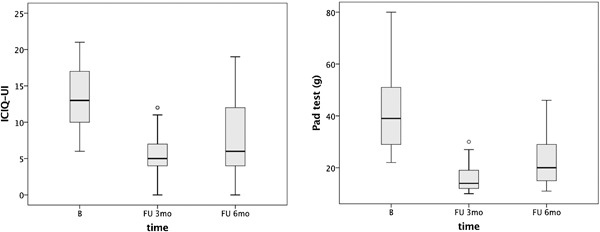
Effect of intraurethral laser treatment on UI, determined by ICIQ‐UI SF (a) and 1‐hour pad test (b). The horizontal line within the box indicates a median, boundaries of the box indicate the 1st and 3rd quartile, and whiskers indicate the minimum and maximum values. Empty circles indicate outliers with values 1.5 × IQR above and below the marked quartiles (Q1 and Q3) and asterisks indicate outliers with values 3 × IQR above and below the Q1 and Q3.
After 3 months, dysuria improved in all of the patients, urgency improved in 93% of patients and frequency improved in 97% of patients (Table 2). Average improvement rates in dysuria, frequency, and urgency at the 3‐month FU were 87%, 79%, and 77%, respectively (Table 3). At the 6‐month FU average values were 64%, 44%, and 52%, respectively.
Table 2.
Improvement in Outcome Measures
| Outcome measure | Dysuria | Urgency | Frequency | ICIQ‐UI SF | 1‐h pad test |
|---|---|---|---|---|---|
| 3‐month FU | |||||
| Improved a (%) | 100 (n = 29) | 93 (n = 27) | 97 (n = 27) | 79 (n = 23) | 90 (n = 26) |
| Not improved b (%) | 0 | 7 (n = 2) | 3 (n = 1) | 21 (n = 6) | 10 (n = 3) |
| Worse c (%) | 0 | 0 | 0 | 0 | 0 |
| 6‐month FU | |||||
| Improved a (%) | 76 (n = 22) | 66 (n = 19) | 62 (n = 18) | 45 (n = 13) | 38 (n = 11) |
| Not improved b (%) | 24 (n = 7) | 31 (n = 9) | 38 (n = 11) | 48 (n = 14) | 59 (n = 17) |
| Worse c (%) | 0 | 3 (n = 1) | 0 | 7 (n = 2) | 3 (n = 1) |
Data is presented as a percentage of patients with corresponding improvement rate, which was calculated as ratio between values at FU and baseline values (n = absolute number of patients).
If improvement was ≥50%.
If improvement was <50%.
If improvement was negative (relative to baseline).
Table 3.
Average Improvement Rates (%) from Baseline Values
| 3‐month FU | 6‐month FU | |
|---|---|---|
| Dysuria | 87 (12) | 64 (25) |
| Urgency | 79 (18) | 44 (35) |
| Frequency | 77 (18) | 52 (23) |
| ICIQ‐UI | 64 (25) | 40 (31) |
| 1‐h pad test (g) | 59 (13) | 42 (20) |
Results are presented as mean (SD).
The ICIQ‐UI scores and 1‐hour pad test revealed a clinically meaningful improvement in 79% and 90% of patients at 3‐month FU, respectively. After 6 months clinically meaningful improvement was observed in 45% and 38% of patients (Table 2).
Observed side effects were mild and transient and mostly resolved without intervention in less than 24 hours. Dysuria and minimal hematuria were observed in four patients. One patient suffered from urinary infection, which was treated with antibiotics.
DISCUSSION
The results of our pilot study suggest that intraurethral laser treatment using Erbium SMOOTH™ technology is a safe and effective treatment for the management of urinary symptoms of GSM. To our knowledge, this is the first study using laser to specifically target urinary mucosa for relief of GSM symptoms. Urinary symptoms are usually managed indirectly with the use of vaginal estrogens, which have a locoregional effect, resulting in improved trophism of the vaginal mucosa as well as urethral mucosa 27.
According to a recent observational study by Palma et al. 3 there appears to be significant confusion about proper therapy for GSM. Treatment of GSM is heterogeneous, there seems to be no clear indication to differentiate between hormonal and non‐hormonal therapies and physicians do not follow a clear indication for the treatment of GSM 3. Their study showed that almost 40% of women spontaneously discontinued the therapy, with inadequate symptom relief being the main reason for discontinuation besides disruption and interference with sexual spontaneity, safety concerns, side effects, vaginal placement, and messiness.
The mechanism of continence has not been fully clarified yet. But it is known that the thickness of the urethral mucosa and the rich vascularization of the submucosa confer its sealing properties, since they are the main contributory factors of the reduction of both the caliber of the urethral opening and the radius of the urethral cylinder 21. The idea behind our intraurethral procedure is to improve continence by improving the urethral trophism. The non‐ablative Erbium SMOOTH™ technology and its thermal effect on the mucosal tissue has been well established 28. Reorganization of existing collagen fibers 22, formation of new ones (neocollagenesis) 22, and neoangiogenesis 22 have been successfully applied for the treatment of various non‐surgical gynecological indications, including GSM, vaginal relaxation syndrome (VRS) and stress urinary incontinence (SUI). We hypothesized that by creating a superficial warming process we would promote an initial vasodilation effect improving the performance of the intrinsic mechanism of continence by decreasing the radius of the urethra due to the improvement in the submucosal vascular plexus and increased thickness of the epithelium 22.
Addressing the trophism of the vaginal canal as well as the urethral mucosa improves not only the symptoms of GSM but also symptoms of urinary incontinence (UI) 19, which opens an important future research area, that should focus on synergistic effect of the combined vaginal and urethral application of Erbium SMOOTH™ laser. Based on the severity of the GSM and impaired continence the appropriate sequence of the Erbium SMOOTH™ protocols should also be taken into consideration.
Erbium SMOOTH™ technology was shown to be more efficient and have a longer lasting effect than topical estriol treatment, which starts to diminish as soon as the hormone therapy has ceased 12.
The intraurethral laser procedure performed in this study successfully reduced the symptoms of dysuria, urgency, and frequency in our patients. A statistically significant long‐term effect was observed, and the positive effects appear to last up to 6 months following laser treatment. The results on impaired continence are comparable to results of our previous study 19, where type III SUI was treated using intraurethral Erbium SMOOTH™ technology. In this study a 1‐hour pad test revealed 82% and 50% of women achieving a clinical improvement after 3 and 6 months, respectively. ICIQ‐UI scores showed that a clinically significant improvement was seen in 82% of women after 3 months and in 79% of women after 6 months.
GSM is a chronic condition that has a very high impact on quality of life and requires the constant management of symptoms, most commonly in the form of daily to weekly application of topical or intravaginal medicine. Such treatment has a relatively low patient compliance, leaving patients improperly treated and dissatisfied 3. A non‐ablative Erbium SMOOTH™ therapy proves to be a valid and reliable alternative to such treatment, as its effects last up to 6 months, and based on the modality, both VVA 10, 12 and urinary symptoms can be addressed. Most importantly, Erbium SMOOTH™ Er:YAG laser treatment can be used in patient populations, for which other treatment methods are not recommended and should be used with care 9. Our pilot study was performed in a relatively small number of patients, but our promising results indicate the need for larger controlled studies, with a more sound assessment of safety and efficacy, especially for longer periods of time during which the need for maintenance treatments could be assessed. One important issue that needs to be addressed in the future is also the synergistic effect of intraurethral and vaginal Erbium SMOOTH™ laser treatment for improving the symptoms of GSM as well as impaired continence.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form of Disclosure of Potential Conflicts of Interest and have disclosed the following: Zdenko Vižintin and Neža Koron are employees of Fotona d.o.o., the manufacturer of a medical device used in the study. Other authors have no conflicts of interest to declare.
REFERENCES
- 1. Gandhi J, Chen A, Dagur G, et al. Genitourinary syndrome of menopause: An overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am J Obstet Gynecol 2016; 215(6):704–711. [DOI] [PubMed] [Google Scholar]
- 2. Neves‐E‐Castro M, Birkhauser M, Samsioe G, et al. EMAS position statement: the ten point guide to the integral management of menopausal health. Maturitas 2015; 81(1):88–92. [DOI] [PubMed] [Google Scholar]
- 3. Palma F, Xholli A, Cagnacci A. Management of vaginal atrophy: A real mess. Results from the AGATA study. Gynecol Endocrinol 2017; 33(9):702–707. [DOI] [PubMed] [Google Scholar]
- 4.NAMS. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause J North Am Menopause Soc 2013; 20(9):888–902. [DOI] [PubMed] [Google Scholar]
- 5.NAMS. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause J North Am Menopause Soc 2017; 24(7):728–753. [DOI] [PubMed] [Google Scholar]
- 6.ACOG. The Use of Vaginal Estrogen in Women With a History of Estrogen‐Dependent Breast Cancer. Committee Opinion No. 659. Obs Gynecol 2016; 127:e93‐6. [DOI] [PubMed] [Google Scholar]
- 7. Tadir Y, Gaspar A, Lev‐Sagie A, et al. Light and energy based therapeutics for genitourinary syndrome of menopause: Consensus and controversies. Lasers Surg Med 2017; 49(2):137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pitsouni E, Grigoriadis T, Falagas M, Tsiveleka A, Salvatore S, Athanasiou S. Microablative fractional CO2laser for the genitourinary syndrome of menopause: Power of 30 or 40W? Lasers Med Sci 2017; 32(8):1865–1872. [DOI] [PubMed] [Google Scholar]
- 9. Gambacciani M, Levancini M b, Cervigni M. Vaginal erbium laser: the second‐generation thermotherapy for the genitourinary syndrome of menopause. Climacteric 2015; 18(5):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gambacciani M, Levancini M. Short‐term effect of vaginal erbium laser on the genitourinary syndrome of menopause. Minerva Ginecol 2015; 67(2):97–102. [PubMed] [Google Scholar]
- 11. Levancini AMbc, Gambacciani M. Vaginal erbium laser as a treatment for genitourinary syndrome of menopause: Preliminary results [Láser erbium vaginal como tratamiento del síndrome genitourinario de la menopausia: resultados preliminares]. Rev Chil Obstet Ginecol 2015; 80(2):145–150. [Google Scholar]
- 12. Gaspar A, Brandi H, Gomez V, Luque D. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers Surg Med 2017; 49(2):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fistonić N, Fistonić I, Lukanovič A, Findri Guštek Š, Sorta Bilajac Turina I, Franić D. First assessment of short‐term efficacy of Er:YAG laser treatment on stress urinary incontinence in women: prospective cohort study. Climacteric 2015; 18(sup1):37–42. [DOI] [PubMed] [Google Scholar]
- 14. Ogrinc UB, Senčar S, Lenasi H. Novel minimally invasive laser treatment of urinary incontinence in women. Lasers Surg Med 2015; 47(9):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lapii GA, Yakovleva AY, Neimark AI. Structural reorganization of the vaginal mucosa in stress urinary incontinence under conditions of Er:YAG laser treatment. Bull Exp Biol Med 2017; 162(4):510–514. [DOI] [PubMed] [Google Scholar]
- 16. Gaviria J, Lanz J. Laser Vaginal Tightening (LVT)—evaluation of a novel noninvasive laser treatment for vaginal relaxation syndromele. J Laser Heal Acad 2012; 2012(1):59–66. [Google Scholar]
- 17. Gaviria PJE, Korosec B, Fernandez J, Montero G. Up to 3‐year follow‐up of patients with vaginal relaxation syndrome participating in laser vaginal tightening. J Laser Heal Acad 2016; 2016:1–6. [Google Scholar]
- 18. Ogrinc UB, Sencar S. Non‐ablative vaginal erbium YAG laser for the treatment of cystocele. Ital J Gynaecol Obstet 2017; 29(1):19–25. [Google Scholar]
- 19. Gaspar A, Brandi H. Non‐ablative erbium YAG laser for the treatment of type III stress urinary incontinence (intrinsic sphincter deficiency). Lasers Med Sci 2017; 32(3):685–691. [DOI] [PubMed] [Google Scholar]
- 20. Bump RC, Coates KW, Cundiff GW, Harris RL, Weidner AC, Brame RG. Diagnosing intrinsic sphincteric deficiency: comparing urethral closure pressure, urethral axis, and valsalva leak point pressures. Am J Obstet Gynecol 1997; 177(2):303–310. [DOI] [PubMed] [Google Scholar]
- 21. McGuire EJ, Fitzpatrick CC, Wan J, et al. Clinical assessment of urethral sphincter function. J Urol 1993; 150(5 Pt 1):1452–1454. [DOI] [PubMed] [Google Scholar]
- 22. Lapii GA, Yakovleva AY, Neimark AI. Structural reorganization of the vaginal mucosa in stress urinary incontinence under conditions of Er:YAG laser treatment. Bull Exp Biol Med 2017; 162(4):510–514. [DOI] [PubMed] [Google Scholar]
- 23.WHO. Keep fit for life: meeting the nutritional needs of older persons. WHO. 2002; 2(2):156210–115. http://apps.who.int/iris/bitstream/handle/10665/42515/9241562102.pdf;jsessionid=A595ABC44D5AC7AB4A68463E0A5F0932?sequence=1. Accessed March 23, 2018. [Google Scholar]
- 24. Lykkesfeldt J, Michels AJ, Frei B, Vitamin C. Adv Nutr 2014; 5(1):16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ghosh A, Pallavi S, Nagpal B, Hegde U, Archana S, Nagpal J. Nutrition and oral health: A review. Indian J Appl Res 2015; 5(11):546–549. [Google Scholar]
- 26. Moore KH. Urogynecology: Evidence‐Based Clinical Practice. 2nd edition London: Springer‐Verlag; 2013. [Google Scholar]
- 27. Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause. Obstet Gynecol 2014; 124(6):1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fistonić N, Fistonić I, ŠF Guštek, et al. Minimally invasive, non‐ablative Er:YAG laser treatment of stress urinary incontinence in women—A pilot study. Lasers Med Sci 2016; 31(4):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]


