Summary
Chronic helminth infection with Schistosoma (S.) mansoni protects against allergic airway inflammation (AAI) in mice and is associated with reduced Th2 responses to inhaled allergens in humans, despite the presence of schistosome‐specific Th2 immunity. Schistosome eggs strongly induce type 2 immunity and allow to study the dynamics of Th2 versus regulatory responses in the absence of worms. Treatment with isolated S. mansoni eggs by i.p. injection prior to induction of AAI to ovalbumin (OVA)/alum led to significantly reduced AAI as assessed by less BAL and lung eosinophilia, less cellular influx into lung tissue, less OVA‐specific Th2 cytokines in lungs and lung‐draining mediastinal lymph nodes and less circulating allergen‐specific IgG1 and IgE antibodies. While OVA‐specific Th2 responses were inhibited, treatment induced a strong systemic Th2 response to the eggs. The protective effect of S. mansoni eggs was unaltered in μMT mice lacking mature (B2) B cells and unaffected by Treg cell depletion using anti‐CD25 blocking antibodies during egg treatment and allergic sensitization. Notably, prophylactic egg treatment resulted in a reduced influx of pro‐inflammatory, monocyte‐derived dendritic cells into lung tissue of allergic mice following challenge. Altogether, S. mansoni eggs can protect against the development of AAI, despite strong egg‐specific Th2 responses.
Keywords: allergy and immunology, antigen‐presenting cells, asthma, B lymphocytes, helminths, Schistosoma mansoni, Th2 cells
1. INTRODUCTION
The prevalence of allergies and asthma has dramatically increased in developed countries over the last decades, and the incidence rates continue to increase especially in low‐ and middle‐income countries.1 It has been suggested that environmental factors, such as an increased exposure to air pollutants and tobacco smoke,2 but also an overly sanitary lifestyle, with decreased exposure to parasites, may play an important role in the increased prevalence of asthma.
The protective effect of parasitic infections against allergic asthma has been introduced as one of many elements in the so‐called “old friends hypothesis”.3, 4, 5, 6 The relationship between helminths and asthma is complex, with factors such as worm species, timing, intensity and chronicity of infection, as well as host genetics at interplay,7 and a causal link in humans has yet to be demonstrated. Acute or light helminth infections seem to promote allergic sensitization and allergic symptoms, while chronic helminth infections are more often associated with protection.7, 8 This may also explain why deworming at population level has been shown to result in enhanced skin‐prick test positivity or rates of eczema in some cases, while having no effect in others.8 A large body of epidemiological and experimental studies has shown that, despite heterogeneity in the results, especially hookworm infections have been consistently found to reduce allergic sensitization.9, 10 Schistosoma ssp. has also been reported to be protective against allergic sensitization in humans.9, 11
Schistosoma ssp. infections consist of an acute phase dominated by a strong Th2 response to the eggs and a chronic phase with a diminished Th2 response and increased activity of regulatory immune cells.12 To distinguish between egg‐induced and worm‐induced protection from AAI, experimental infections with mixed sex or male Schistosoma worms were performed.13, 14, 15, 16, 17, 18, 19 However, these reports revealed conflicting results, as some indicated a reduction in AAI in the presence of egg‐producing infections,14, 16, 18, 19 whereas others showed a reduction in the absence of eggs.13, 17 In addition, some studies show protection from AAI during the acute (5‐11 weeks),17, 19 and others during the chronic (12‐16 weeks)14, 18 phase of infection, which elicit characteristically different immune responses.
From an immunological perspective, the conundrum that Th2‐inducing helminth infections can dampen symptoms linked to allergic Th2 responses as observed in humans and mouse models7, 8 is still subject to discussion. Often, the immunomodulatory activity of helminths is associated with the induction of a regulatory network. In mouse models, the rodent nematodes Heligmosomoides polygyrus and Nippostrongylus brasiliensis revealed important insights into the role of regulatory T (Treg)20 and B (Breg)21 cells as well as the regulatory cytokine IL‐1022, 23 in protection against AAI. Treg and Breg cells as well as IL‐10 have also been described to mediate protection induced by S. mansoni infections.14, 16, 17, 18, 19 However, data showing that the acute phase S. mansoni infections and/or the presence of eggs are important for protection suggest that the induction of a regulatory network is not the sole determinant of immunomodulation.
To further explore the dynamics and interplay between Th2 responses and regulatory responses in the protective effect of S. mansoni infections against AAI, we used isolated eggs instead of a full natural infection. We show that eggs are equally protective as a natural S. mansoni infection in a prophylactic setting, despite the induction of a strong egg‐specific Th2 response. Egg treatment did not lead to Treg cell expansion or enhanced activity markers following allergen challenge, and the observed protection was independent of both Treg cells and B cells. Instead, S. mansoni egg‐induced protection was associated with a reduced pulmonary influx of pro‐inflammatory monocyte‐derived dendritic cells (moDCs). This study shows that, although inducing egg‐specific Th2 responses, S. mansoni eggs can protect from AAI, closely resembling the human situation.
2. MATERIAL AND METHODS
2.1. Mice
Female C57BL/6 mice (Harlan) were housed under SPF conditions in the animal facility of the Leiden University Medical Center (Leiden, The Netherlands) and used for experiments at 6‐12 weeks of age. All animal studies were performed in accordance with the Animal Experiments Ethical Committee of the Leiden University Medical Center. The Dutch Experiments on Animals Act is established under European Guidelines (EU directive no. 86/609/EEC regarding the Protection of Animals used for Experimental and Other Scientific Purposes). B6.129S2‐Ighmtm1Cgn/J (μMT) mice (C57BL/6 background) were kindly provided by B. Lambrecht, Ghent University (Belgium), and originally purchased from Jackson Laboratory (Bar Harbor, USA).
2.2. Preparation of Schistosoma mansoni eggs
Eggs were isolated from trypsinized livers of hamsters infected for 50 days with a Puerto Rican strain of S. mansoni, washed with RPMI medium containing 300U/mL penicillin, 300 μg/mL streptomycin (both Sigma‐Aldrich, St. Louis, MO, USA) and 500 μg/mL amphotericin B (Thermo Fisher Scientific, Waltham, MA, USA) and frozen at −80°C until use. To investigate whether freeze‐thawed eggs still release a comparable protein content to that of freshly cultured eggs, excretory‐secretory product (eggES) of freeze‐thawed and fresh eggs was compared by silver staining after 48 hours of egg culture.
2.3. Allergic airway inflammation model, egg treatment and Treg cell depletion
Mice were sensitized by i.p. injection of OVA (10 μg/mL; Invivogen, San Diego, CA, USA) emulsified in alum adjuvant (2 mg/mL; Thermo Fisher Scientific) on day 0 and 7. Seven to 10 days after the last injection, mice were challenged for 3 consecutive days by either exposure to OVA aerosols (10 mg/mL in PBS, 30minutes) or by intranasal (i.n.) administration of 50 μg OVA/50 μL PBS. Mice were sacrificed 24 hours after the last challenge. Animals in the treatment group received two i.p. injections of 5000 S. mansoni eggs diluted in sterile PBS in on day 11 and day 4 prior to allergic sensitization. To deplete Treg cells, mice were treated i.p. with anti‐CD25‐depleting (clone PC61) or control (anti‐β‐galactosidase, clone GL113) antibody (500 μg/mouse) 6 days prior to the first egg injection and again 6 days prior to the first allergic sensitization (2 days before second egg injection).24
2.4. Tissue preparation
BAL fluid was collected by flushing the lungs with 1 mL PBS/2 mmol/L EDTA (Invitrogen), followed by additional two lavages to collect remaining cells. The 1st BAL flush was kept separate for cytokine analysis in cell‐free supernatant, and the cells from all flushes were pooled for flow cytometry. Perfused lungs were cut into small pieces and digested using collagenase III (100 U/mL; Worthington, Lakewood, NJ, USA) and DNase (2000 U/mL; Sigma‐Aldrich) for 1 hour at 37°C. Digested lungs were homogenized through 70 μm cell strainers (BD Biosciences, Franklin Lakes, NJ, USA) and remaining red blood cells lysed. In some cases, one side of the lung was tied off with surgical suture and removed, and the other side inflated with and collected into 3.9% PFA/PBS. Mediastinal lymph nodes and spleens were homogenized through 70‐μm filters, and spleens were subjected to red blood cell lysis. Blood for assessment of Treg cells was collected from the tail vein 6 days after the first injection of anti‐CD25 or control antibody and red blood cells were lysed. For serum collection, blood was collected by heart puncture, spun down and the serum stored at −20°C until further analysis.
2.5. Flow cytometry
The cellular composition of BAL fluid was determined by staining with fluorescently labelled antibodies against B220 (RA3‐6B2), CD3 (17A2), CD11b (M1/70), CD11c (HL3), Gr‐1 (RB6‐8C5), MHCII (M5/114.15.2) and SiglecF (E50‐2440) directly ex vivo. Treg cells in BAL fluid, medLNs and blood were identified by staining with live/dead fixable aqua dead cell stain kit (Thermo Scientific) and fluorescently labelled antibodies against CD3 (17A2), CD4 (GK1.5), CD25 (PC61.5), CTLA‐4 (UC10‐4B9), Foxp3 (FJK‐16s) and GITR (YGITR 765). DCs in lung tissue were identified by staining with live/dead fixable aqua dead cell stain kit (Thermo Scientific) and fluorescently labelled antibodies against CD3 (17A2), CD11b (M1/70), CD11c (HL3), CD19 (MB19‐1), CD64 (X54‐5/7.1), CD103 (2E7), Gr‐1 (RB6‐8C5), MHCII (A5/114.15.2), Nk1.1 (PK136) and SiglecF (E50‐2440). For all stainings, FcγR‐binding inhibitor (2.4G2, kind gift of L. Boon, Bioceros) was added. Flow cytometry was performed using a FACS Canto II and FACSDiva software (BD Biosciences) followed by data analysis using FlowJo.
2.6. Histology
Lungs were collected into 3.9% PFA/PBS and the tissue transferred into 70% ethanol after 1‐2 days. Lungs were then embedded in paraffin, sliced and stained for inflammatory cell infiltration using haematoxylin and eosin (H&E; both Klinipath). Stained slices were analysed under a Olympus BX41 light microscope (Olympus). Peribronchial inflammation as assessed by H&E staining was scored on a scale 0‐4 by two blinded, independent investigators.
2.7. ELISA and CBA
OVA‐ and SEA‐specific IgG1 and IgE antibodies were measured in serum. Ninety‐six‐well Nunc Maxisorp plates (Thermo Fisher Scientific) were coated with 25 μg/mL of the respective antigen diluted in buffer (1M sodium carbonate) at 4°C overnight and subsequently incubated with serial dilutions of sera, biotinylated detection antibodies against IgG1 and IgE (BD Biosciences) and horseradish peroxidase‐conjugated streptavidin (BD Biosciences). Optical densities were measured after addition of TMB peroxidase substrate (KPL). The concentration of the cytokines, IL‐5, IL‐10, IL‐13 and IFN‐γ, was detected in cell‐free supernatants of BAL fluid and cell cultures using either ELISA kits or BD cytometric bead array (CBA) Flex‐set kits (BD Biosciences) followed by flow cytometry measurement on a FACS Canto II (BD Biosciences). The chemokine CCL2 was also measured using a CBA Flex‐set kit.
2.8. Statistical analysis
Statistical analysis was performed with GraphPad Prism (version 7.02) using unpaired t test for comparison of 2 groups, one‐way ANOVA for comparison of more than two groups and two‐way ANOVA for comparison of more than two groups while correcting for a batch effect between different experiments. All data are presented as mean ± SE of the mean (SEM). P‐values < 0.05 were considered statistically significant.
3. RESULTS
3.1. Schistosoma mansoni eggs protect against OVA/alum‐induced AAI
We and others have previously reported that chronic, but not acute, mixed infection with S. mansoni, which most closely resembles the natural situation in humans, protects mice from allergic airway inflammation.14, 16, 18, 19 A plausible explanation for the differential effect of acute and chronic infection might be the changing balance between Th2 and regulatory responses. To further explore the dynamics and interplay between Th2 and regulatory responses in helminth infections, we first tested whether S. mansoni liver‐derived eggs can confer protection from AAI in the absence of worms. These eggs were isolated from livers of infected hamsters and frozen prior to use. We have confirmed that the excretory‐secretory product (eggES) of these freeze‐thawed eggs is similar to that of freshly isolated, mature liver eggs (Figure S1A). Mice were treated twice with 5 x 103 eggs by i.p. injection prior to allergic sensitization with OVA emulsified in alum adjuvant (Figure 1A). This treatment resulted in a profound suppression of overall cellularity and eosinophilia both in the bronchoalveolar lavage (BAL) fluid (Figure 1 B) and in lung tissue (Figure S1B), accompanied by a reduction in various other leucocyte populations (Figure 1C). The reduction in AAI in treated mice was also reflected by the reduction in cellular infiltration around the airways (Figure 1D) as assessed by histology. Assessment of serum immunoglobulins revealed that egg treatment ablates the OVA‐specific IgG1 and IgE response (Figure 1E). We also assessed local cytokine production in BAL fluid, as well as local recall responses to the allergen by restimulation of medLN with OVA. In both BAL fluid and OVA‐restimulated medLN cell cultures, the production of allergic Th2 cytokines IL‐5 and IL‐13 was greatly increased in allergic mice, but blocked upon egg treatment (Figure 1F,G). IL‐10, while not detectable in BAL fluid, followed the same pattern as IL‐5 and IL‐13 in OVA‐restimulated medLN cell cultures (Figure 1G). Additionally, IFN‐γ could hardly be detected in both BAL fluid and OVA‐restimulated medLN cell cultures (Figure 1F,G). Collectively, these data show that S. mansoni egg administration prior to allergic sensitization inhibits the development of OVA‐induced AAI.
Figure 1.
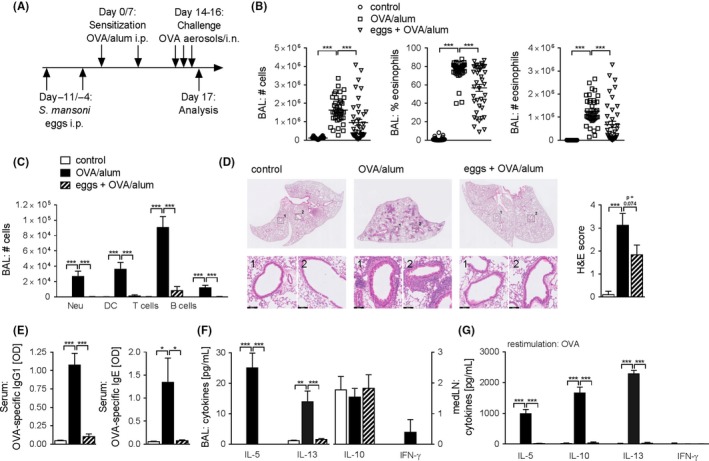
Schistosoma mansoni eggs protect against OVA/alum‐induced AAI. A, Schematic representation of the experimental model. B, Total number of cells, percentage of eosinophils and total number of eosinophils in BAL fluid as assessed by FACS. Summary of multiple experiments. C, Total number of neutrophils (Neu), dendritic cells (DC), T cells and B cells in BAL fluid as assessed by FACS. Representative of multiple experiments, n = 4‐5. D, Representative images of haematoxylin and eosin (H&E) histology staining from PFA‐fixed sections (scale bar = 100 μm). Scores for severity of cellular infiltration around the airways on a scale of 0‐4 were assessed by two blinded observers. The average of both scores is displayed. Significant difference was determined by unpaired t test, *P < 0.05. E, OVA‐specific IgG1 and OVA‐specific IgE antibodies in serum measured by ELISA. Representative of multiple experiments, n = 4‐5. F, Cytokine concentration in BAL fluid measured by CBA. Representative of multiple experiments, n = 4‐5. G, Cytokine concentration in medLN cell supernatants after 4 d restimulation with OVA (10 μg/mL) measured by CBA. Representative of multiple experiments, n = 4‐5. Significant differences were determined by two‐way ANOVA following Tukey's multiple comparison test (B) or one‐way ANOVA following Dunnett's multiple comparisons test (C‐F) and are indicated with *P < 0.05, **P < 0.01, ***P < 0.001
3.2. Protection occurs despite the induction of an egg‐specific Th2 response
Human and animal hosts are known to mount a strong type 2 immune responses to egg deposition in live infections. To determine whether egg treatment induced a systemic, antigen‐specific cytokine response in our model, we restimulated spleen cell cultures with soluble egg antigens (SEA). SEA restimulation profoundly increased the production of IL‐5, IL‐10 and IL‐13, but not IFN‐γ, in mice that had received isolated eggs compared to naïve or allergic, untreated mice (Figure 2A). In the medLN, similar cytokine profiles were observed following SEA restimulation (Figure S2). Furthermore, OVA‐restimulated spleen cell cultures induced a strong Th2 cytokine production in the allergic group (Figure 2B). Strikingly, these data show a systemic inhibition of OVA‐specific type 2 immunity, in addition to the local inhibition observed in Figure 1. Additionally, high levels of IgG1 were observed; however, the SEA‐specific IgE response was found to be weak (Figure 2C). These data show that egg treatment induces a fully developed Th2 response to egg antigens in the absence of an allergic Th2 response to OVA.
Figure 2.
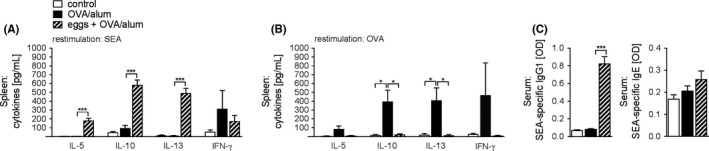
Protection occurs despite the induction of an egg‐specific type 2 induction. (A, B), Cytokine concentration in spleen cell supernatants after 4 d restimulation with SEA (10 μg/mL; (A) or OVA (10 μg/mL; B, Representative of 2 experiments, n = 3‐4. C, SEA‐specific IgG1 and SEA‐specific IgE antibodies in serum measured by ELISA. Representative of 3 experiments, n = 4‐5. Significant differences were determined by one‐way ANOVA following Dunnett's multiple comparisons test and are indicated with *P < 0.05, ***P < 0.001
3.3. Egg‐induced protection against AAI is independent of Treg cells
Schistosoma mansoni infection16, 19 and antigens25, 26 have been described to induce Treg cells in mice and humans. Therefore, we addressed whether egg treatment enhanced the number or activation state of Treg cells following allergen challenge in our model. The frequency and number of Treg cells were significantly increased in the BAL fluid of allergic mice, but remained at baseline in mice treated with eggs (Figure 3A). While the frequency of Treg cells remained unchanged in the medLNs, total numbers increased in allergic mice irrespective of egg treatment (Figure 3B). Additionally, in allergic animals, extracellular regulatory markers, CTLA‐4 and GITR, showed enhanced expression on Treg cells in the BAL (Figure 3C) and medLNs (Figure 3D), but were not further increased by egg treatment. To further dissect the role of Treg cells in egg‐mediated suppression, we depleted Treg cells by means of monoclonal, anti‐CD25‐depleting antibodies (clone PC61) during egg treatment and allergic sensitization (Figure 3E). Successful depletion of Treg cells was confirmed by flow cytometry (FigureS3A). Mice depleted of CD25‐expressing Treg cells still displayed significantly reduced BAL cellularity and number of BAL eosinophils comparable to control mice treated with antibodies against anti‐β‐galactosidase (anti‐βGAL) (Figure 3F), as well as reduced numbers of neutrophils (Figure 3G). In contrast, in egg‐treated mice, Treg cell depletion did seem to affect the number of DCs, T cells and B cells in the BAL fluid at least to some extent, as their numbers were increased and not significantly different anymore between allergic controls and egg‐treated mice (Figure 3G). We observed a similar trend in the secretion of type 2 cytokines IL‐5 and IL‐13 in BAL fluid following Treg cell depletion, which were restored compared to those in allergic control mice (Figure 3H). These data may suggest a selective effect on the lung T‐cell compartment following anti‐CD25 treatment, resulting in enhanced T‐cell activation. Although there is a general trend towards increased airway inflammation following anti‐CD25 treatment in both allergic control and egg‐treated mice, this effect seems to be more pronounced in the egg‐treated group with respect to the lung T‐cell compartment. Anti‐CD25 treatment did however not restore eosinophilic inflammation in egg‐treated mice. Collectively, these data suggest that depletion of Treg cells does not have a major influence on inhibition of AAI by egg treatment and thus cannot explain egg‐induced protection against AAI.
Figure 3.
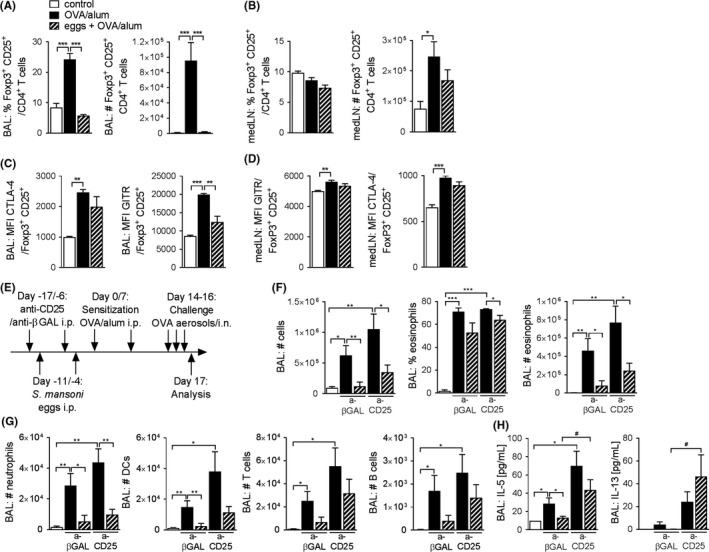
Regulatory T cells are not involved in egg‐induced protection against AAI. (A, B), Percentage and total number of Foxp3+ CD25+ Treg cells in BAL fluid (A) and medLNs (B) assessed by FACS. Representative of multiple experiments, n = 4‐5. (C, D), Geometric mean expression of CTLA‐4 and GITR on Foxp3+ CD25+ Treg cells in BAL fluid (C) and medLNs (D) assessed by FACS. Representative of multiple experiments, n = 4‐5. E, Schematic representation of experimental model. F, Total number of cells, percentage of eosinophils and total number of eosinophils in BAL fluid as assessed by FACS. Representative of 2 experiments, n = 4‐6. G, Total number of neutrophils, dendritic cells (DCs), T cells and B cells in BAL fluid as assessed by FACS. Representative of 2 experiments, n = 4‐6. H, Cytokine concentration in BAL fluid measured by CBA. Representative of 2 experiments, n = 4‐6. Significant differences were determined by one‐way ANOVA following Dunnett's multiple comparisons test as indicated with *P < 0.05, **P < 0.01, ***P < 0.001 or by unpaired t test as indicated by # P < 0.05
3.4. Mature B cells are not crucial for egg‐induced protection against AAI
In addition to Treg cells, we also sought to investigate the role of B cells in egg‐induced protection against AAI. B cells possess various functions ranging from antibody production, formation of memory and antigen presentation to the production of pro‐ and anti‐inflammatory cytokines. The production of regulatory cytokines such as IL‐10 and the production of inhibitory immunoglobulins are the widely recognized regulatory functions exerted by B cells.27 Here, we used μMT mice, which lack mature (B2) B cells,28 to test whether B cells are required for the protective effect observed on OVA/alum‐induced AAI after egg treatment. Both WT and μMT mice responded equally to induction of AAI as shown by total BAL cellularity and the presence of eosinophils in BAL fluid (Figure 4A). Egg treatment significantly inhibited eosinophilia both in WT and μMT mice (Figure 4A), and a similar pattern could be observed for BAL neutrophils, DCs and T cells (Figure 4B). Additionally, while B cells sharply increased in WT mice and decreased with egg treatment, the number of B cells was expectedly low in μMT mice (Figure 4B). In BAL fluid, μMT mice showed a tendency towards reduced IL‐5 and IL‐13 concentrations upon egg treatment similarly to WT animals, albeit not significant (Figure S4). The expression of Th2 cytokines in supernatants of in vitro OVA‐restimulated medLN cell cultures from WT mice was highly elevated in AAI mice and significantly reduced after egg treatment (Figure 5C). Allergic μMT mice produced significantly less IL‐5, IL‐10 and IL‐13 compared to their WT counterparts (Figure 4C), like recently also shown in a house dust mite model of asthma.29 As expected, OVA‐specific IgG1 and IgE antibodies in μMT mice remained at baseline values observed in naïve WT animals (Figure 4D), excluding a major role of inhibitory antibodies in protection. These data show that B2 B cells, while contributing to Th2 cytokine production and the production of antigen‐specific antibody responses, are not required for egg‐mediated protection from AAI.
Figure 4.
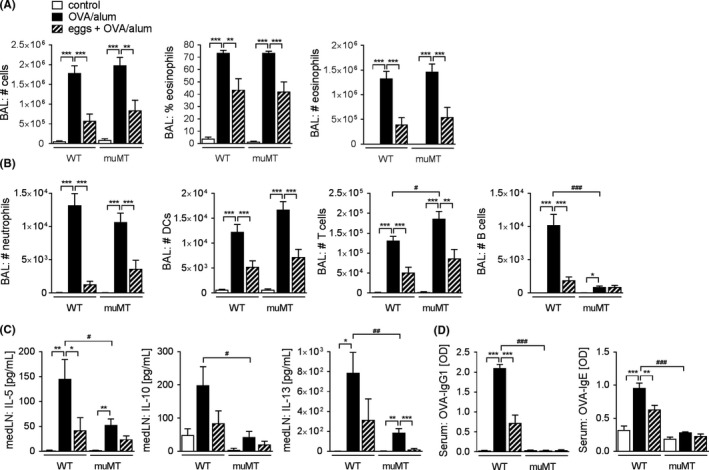
Mature B cells are not involved in egg‐induced protection against AAI. A, Total number of cells, percentage of eosinophils and total number of eosinophils in BAL fluid as assessed by FACS. B, Total number of neutrophils, dendritic cells (DCs), T cells and B cells in BAL fluid as assessed by FACS. C, Cytokine concentration in medLN cell culture supernatants after 4d restimulation with OVA (10 μg/mL) measured by ELISA. D, OVA‐specific IgG1 and OVA‐specific IgE antibodies in serum measured by ELISA. All data are a summary of 2 experiments, n = 5‐12. Significant differences were determined by one‐way ANOVA following Dunnett's multiple comparisons test as indicated with *P < 0.05, **P < 0.01, ***P < 0.001 or by unpaired t test as indicated by # P < 0.05, ## P < 0.01
Figure 5.
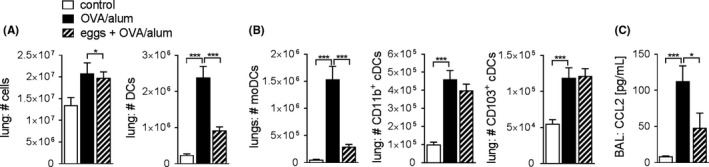
Egg treatment impairs the lung moDC, but not cDC, compartment. A, Total number of lung cells, and total number of lung DCs (CD11c+ MHCII+), assessed by FACS. Representative of 2 experiments, n = 4‐6. B, Total number of moDCs (CD11c+ MHCII+ CD11b+ CD103− CD64+), CD103+ cDC1 (CD11c+ MHCII+ CD11b− CD103+ CD64−) and CD11b+ cDC2s (CD11c+ MHCII+ CD11b+ CD103− CD64−) in the lung, assessed by FACS (see Figure S5 for the gating strategy). Representative of 2 experiments, n = 4‐6. C, Concentration of CCL2 in BAL fluid measured by CBA. Summary of 2 experiments, n = 9‐10. Significant differences were determined by one‐way ANOVA following Dunnett’s multiple comparisons test and as indicated with *P < 0.05, ***P < 0.001
3.5. Egg treatment is associated with decreased recruitment of moDCs in the lung compartment
Different studies have shown that helminths not only affect T and B cells, but also can mediate important effects by acting on DCs.30, 31 Pulmonary DCs play a central role in the immune response to allergens.32 Under steady state conditions, CD103+ and CD11b+ conventional DC (cDC1 and cDC2, respectively) populations can be distinguished, whereas allergic inflammation triggers a strong influx of inflammatory, monocyte‐derived DCs (moDCs).33 Both CD11b+ cDC2 and moDCs can drive allergic Th2 responses in a model of HDM allergy, whereby moDCs were only sufficient in a high‐dose HDM model of AAI.34 moDCs produce various chemokines and present allergen locally in the lung especially in a model of high‐dose allergen exposure.34 Next, we investigated whether prophylactic egg treatment alters the presence and function of different DC subsets in the lung of OVA/alum‐allergic mice. The number of CD11c+ MHCII+ DCs strongly increased in allergic compared to control mice (Figure 5A). Interestingly, egg treatment significantly impaired the number of lung DCs following challenge, whereas the total number of cells in the lung remained unaffected (Figure 5A). The reduction in number of all CD11c+ MHCII+ DCs seems to be solely attributable to an abrogated expansion of the moDC compartment, as both the numbers of CD11b+ cDC2 and CD103+ cDC1s proved to be unaffected by egg treatment (Figure 5B). Monocytes, which can differentiate into moDCs under inflammatory conditions, migrate in a CCR2/CCL2‐dependent manner, whereas CD11b+ cDC2 and CD103+ cDC1s do not.34 We found the concentration of CCL2 in BAL fluid to be strikingly increased in allergic mice and significantly reduced upon egg treatment (Figure 5C), providing an indication that the reduced number of moDCs in lung tissue is the result of reduced CCL2‐mediated influx.
4. DISCUSSION
In this study, we sought to further characterize the potential of S. mansoni to protect from allergic asthma, despite profound egg‐specific Th2 responses, by using isolated S. mansoni eggs in a setting without adult worms. We show that S. mansoni eggs are capable of protecting against experimental AAI, which is in line with previous reports16, 35 and in contrast to earlier work postulating that protection can only be achieved in the absence of female egg‐laying worms. We found that protection from AAI can also be achieved by eggs isolated from infected mice instead of hamsters (data not shown), excluding confounding factors from contaminations of egg preparations with traces of hamster tissue. The data on administration of isolated eggs and the cellular mechanisms eggs can induce in the context of an allergic inflammation is still very limited. This study aims to advance the current knowledge as it provides new insight into the putative mechanism of protection in the presence of egg‐specific Th2 responses.
We show that egg treatment induces a fully developed Th2 response to egg antigens while the OVA‐specific Th2 response, normally induced by alum, is completely absent. This is in line with earlier observations in chronic S. mansoni infections14 and after treatment with excretory‐secretory products of T. suis.36 Mangan et al describe a “helminth‐modified pulmonary Th2 response” in S. mansoni infection, characterized by elevated pulmonary IL‐10 and IL‐13, but reduced IL‐5.13, 37 We found the production of OVA‐specific Th2 cytokines to be reduced upon egg treatment, which argues against a putative “modified Th2 response” in our egg treatment model and is similar to what has been described in humans. People in schistosome‐endemic areas, for which a negative association between chronic infection and allergic sensitization has been shown, often have elevated Th2 responses to the eggs alongside reduced allergic symptoms.7, 8 However, a recent study on a fishing community in Uganda, with a low prevalence of allergy‐related diseases, found a positive correlation between S. mansoni‐specific Th2 cytokines and atopy, and S. mansoni‐specific IgE and atopy, respectively. A significant inverse associations were observed in relation to wheeze, keeping with the original hypothesis.38
Previous reports describe Treg cells, Breg cells and IL‐10 to be important for protection by natural infections.14, 16, 17, 18, 19 We observed that the number of pulmonary Treg cells was increased in the BAL fluid of allergic mice during the challenge phase, which has similarly been reported by others,36, 39 but returns to baseline rather than continues to rise in treated animals. Following allergen challenge, egg‐treated mice did not induce Treg cell numbers or enhance the expression of regulatory activity markers in the lung compartment compared to untreated, allergen‐challenged mice. In addition, IL‐10 in BAL fluid of egg‐treated animals was unchanged following egg treatment. The fact that Treg cells did not exceed the baseline levels from naïve control mice combined with the lack of any activity markers suggests that there is no active suppression by Treg cells during the allergen challenge phase. Mice depleted of CD25‐expressing Treg cells during egg treatment and allergic sensitization display a similar degree of AAI suppression, despite the inflammation being generally increased upon Treg depletion. Probably, as a result of a dysregulated Treg to effector T‐cell balance, this suggests that egg‐induced Treg cells do not play a decisive role in egg‐induced protection from AAI in our hands. These findings seem, at least in part, contrary to a previous report on the putative role of Treg cells in a similar model of egg administration.16, 19 Discrepancies in the results may be related to factors such as the length of exposure to parasitic products (infection versus isolated injections) or the use of different mouse strains.
To study the role of B cells in protection, we treated both WT and μMT mice with S. mansoni eggs. μMT mice lack mature, conventional B2 B cells.28 Most studies report that μMT mice mount an allergic response similar to their WT counterparts.40, 41, 42 In line with these data, we also found the allergic response to be unaffected in μMT mice, apart from a significant reduction in the allergen‐specific Th2 cytokine production by medLN cells. This could be, at least in part, due to the amount of allergen used, as we have previously reported an important role for B cells in low‐dose HDM‐induced AAI.29 Despite the difference in OVA‐specific Th2 responses, egg treatment equally protects from AAI both in WT and μMT mice, indicating that mature B cells are not crucial for protection. While this does not formally exclude a role for all Breg cell subsets, which can be present in both the B1 and B2 B‐cell compartment,43, 44, 45 we believe they are unlikely to play a major role as we have previously studied the induction of Breg cells by S. mansoni infection, SEA and the single egg molecule IPSE/alpha‐1 and predominantly identified Breg cells within the splenic marginal zone (MZ) B‐cell compartment as well as the pulmonary B‐cell compartment.46, 47 Both MZ B cells, which belong to the B2 B‐cell lineage, and pulmonary B cells are absent in μMT mice and thus unlikely crucial for protection. Additionally, μMT mice are also unable to mount an allergen‐specific antibody response,45 excluding a role of inhibitory antibodies in protection.
Dendritic cells play a central role in the induction of adaptive immune responses in the context of AAI. Both CD11b+ cDCs and moDCs can drive allergic Th2 responses in a model of HDM allergy, with moDCs being sufficient in models of high‐dose allergen exposure.34 Moreover, Schistosoma infection has been shown to functionally impair myeloid DCs in humans.30 Here, we show that during the challenge phase of OVA/alum‐induced AAI, control mice depict a sharp influx of moDCs, as well as increased numbers of CD103+ cDC1 and CD11b+ cDC2s. Interestingly, egg treatment selectively affects the lung moDC compartment, whereas both cDC populations remained unchanged. The reduced concentration of CCL2 in BAL fluid of egg‐treated mice suggests that the recruitment of moDCs into the lung is impaired. During allergic sensitization with alum‐supplemented allergen by i.p. injection, inflammatory monocytes are recruited to the peritoneal cavity within hours and ingest allergens in a uric acid‐dependent manner. They migrate to the lung‐draining medLNs and develop there into moDCs that contribute to the development of a Th2 response.48 It is unclear whether this inflammatory monocyte response to allergic sensitization in the peritoneal cavity was targeted by egg treatment or whether the reduced pulmonary moDC levels are the consequence of reduced inflammation and reduced CCL2 levels. Preliminary data suggest that moDCs isolated from allergic and egg‐treated mice behave similarly, and both have a poor T‐cell stimulatory capacity (data not shown). This may suggest that the observed reduced number of moDCs is the consequence rather than the cause of reduced AAI in egg‐treated mice.
To date, a few single, immunomodulatory molecules from helminths have been identified, including ES‐62 and Av17 from the filarial nematode Acanthocheilonema viteae, 49, 50 AIP‐2 from hookworms51 as well as HpARI52 and Hp‐TGM53 from H. polygyrus. For Schistosoma eggs, no immunomodulatory molecule has been characterized in a similar manner to date. The S. mansoni egg secretome has been described to contain 188 proteins.54 Some of these proteins have been shown to fulfil distinct functions,47, 55 whereas the role of others remains poorly characterized.56, 57 Cardoso et al26 describe three single molecules, Sm22.6, PIII and Sm29, to all suppress allergic airway inflammation, all of which are not present in the egg stage of the life cycle. The only description of a Schistosoma ssp. egg‐derived molecule inhibiting AAI is the S. japonicum molecule SjP40, which induces IFN‐γ production.58 As we find low levels of IFN‐γ produced in response to the eggs, we do not expect that the S. mansoni homologue SmP40 mediates protection in our study. It will be the subject of future studies to identify single molecules secreted from S. mansoni eggs capable of modulating allergic asthma and further defining the immunological mechanism of suppression. To date, most studies have focussed on identifying single, egg‐derived antigens as vaccine candidates rather than immunomodulatory agents.59 This study shows that, although eggs are inducers of a strong Th2 response, it is worthwhile to study the immunomodulatory capacities of egg‐derived antigens.
In summary, here the presented data show that the suppressive effect of S. mansoni infection on allergic asthma can be replicated by isolated eggs. This effect occurs despite a strong Th2 response to the eggs itself and is likely independent of Treg and B cells during allergen challenge. Egg treatment strongly and selectively affects the lung moDC compartment. Understanding the complex interactions early during allergic sensitization—and how helminths interfere there—is critical for the development of preventative strategies for allergies and allergy‐related diseases.
Supporting information
ACKNOWLEDGEMENTS
We thank A. van Schadewijk for technical assistance. The work was supported by a ZonMW‐VIDI grant (91714352) from Nederlandse Organisatie voor Wetenschappelijk Onderzoek appointed to HHS, a consortium grant of the Dutch Lung Foundation (5115015) appointed to HHS and an EMBO long‐term postdoctoral fellowship appointed to MJS.
Obieglo K, Schuijs MJ, Ozir‐Fazalalikhan A, et al. Isolated Schistosoma mansoni eggs prevent allergic airway inflammation. Parasite Immunol. 2018;40:e12579 10.1111/pim.12579
REFERENCES
- 1. Pawankar R, Canonica GW, Holgate ST, Lockey RF, Blaiss M. The WAO White Book on Allergy. World Allergy Organization, 2011. http://www.worldallergy.org/UserFiles/file/WAO-White-Book-on-Allergy_web.pdf. Accessed January 24, 2018.
- 2. Burbank AJ, Sood AK, Kesic MJ, Peden DB, Hernandez ML. Environmental determinants of allergy and asthma in early life. J Allergy Clin Immunol. 2017;140(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bloomfield SF, Rook GA, Scott EA, Shanahan F, Stanwell‐Smith R, Turner P. Time to abandon the hygiene hypothesis: new perspectives on allergic disease, the human microbiome, infectious disease prevention and the role of targeted hygiene. Perspect Public Health. 2016;136(4):213‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smits HH, Hiemstra PS, Prazeres da Costa C, et al. Microbes and asthma: Opportunities for intervention. J Allergy Clin Immunol. 2016;137(3):690‐697. [DOI] [PubMed] [Google Scholar]
- 5. Scudellari M. News Feature: cleaning up the hygiene hypothesis. Proc Natl Acad Sci U S A. 2017;114(7):1433‐1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lambrecht BN, Hammad H. The immunology of the allergy epidemic and the hygiene hypothesis. Nat Immunol. 2017;18(10):1076‐1083. [DOI] [PubMed] [Google Scholar]
- 7. Cooper PJ. Interactions between helminth parasites and allergy. Curr Opin Allergy Clin Immunol. 2009;9(1):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wammes LJ, Mpairwe H, Elliott AM, Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. 2014;14(11):1150‐1162. [DOI] [PubMed] [Google Scholar]
- 9. Feary J, Britton J, Leonardi‐Bee J. Atopy and current intestinal parasite infection: a systematic review and meta‐analysis. Allergy. 2011;66(4):569‐578. [DOI] [PubMed] [Google Scholar]
- 10. Leonardi‐Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta‐analysis. Am J Respir Crit Care Med. 2006;174(5):514‐523. [DOI] [PubMed] [Google Scholar]
- 11. Cruz AA, Cooper PJ, Figueiredo CA, Alcantara‐Neves NM, Rodrigues LC, Barreto ML. Global issues in allergy and immunology: parasitic infections and allergy. J Allergy Clin Immunol. 2017;140(5):1217‐1228. [DOI] [PubMed] [Google Scholar]
- 12. Caldas IR, Campi‐Azevedo AC, Oliveira LF, Silveira AM, Oliveira RC, Gazzinelli G. Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection. Acta Trop. 2008;108(2–3):109‐117. [DOI] [PubMed] [Google Scholar]
- 13. Mangan NE, van Rooijen N, McKenzie AN, Fallon PG. Helminth‐modified pulmonary immune response protects mice from allergen‐induced airway hyperresponsiveness. J Immunol. 2006;176(1):138‐147. [DOI] [PubMed] [Google Scholar]
- 14. Smits HH, Hammad H, van Nimwegen M, et al. Protective effect of Schistosoma mansoni infection on allergic airway inflammation depends on the intensity and chronicity of infection. J Allergy Clin Immunol. 2007;120(4):932‐940. [DOI] [PubMed] [Google Scholar]
- 15. Mo HM, Lei JH, Jiang ZW, et al. Schistosoma japonicum infection modulates the development of allergen‐induced airway inflammation in mice. Parasitol Res. 2008;103(5):1183‐1189. [DOI] [PubMed] [Google Scholar]
- 16. Pacifico LG, Marinho FA, Fonseca CT, et al. Schistosoma mansoni antigens modulate experimental allergic asthma in a murine model: a major role for CD4 + CD25 + Foxp3 + T cells independent of interleukin‐10. Infect Immun. 2009;77(1):98‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3‐positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125(5):1114‐1124. e8. [DOI] [PubMed] [Google Scholar]
- 18. van der Vlugt LE, Labuda LA, Ozir‐Fazalalikhan A, et al. Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells: inhibition of allergic inflammation by IL‐10 and regulatory T cells. PLoS ONE. 2012;7(2):e30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Layland LE, Straubinger K, Ritter M, et al. Schistosoma mansoni‐mediated suppression of allergic airway inflammation requires patency and Foxp3 + Treg cells. PLoS Negl Trop Dis. 2013;7(8):e2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth‐induced regulatory T cells. J Exp Med. 2005;202(9):1199‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson MS, Taylor MD, O'Gorman MT, et al. Helminth‐induced CD19 + CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40(6):1682‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wohlleben G, Trujillo C, Muller J, et al. Helminth infection modulates the development of allergen‐induced airway inflammation. Int Immunol. 2004;16(4):585‐596. [DOI] [PubMed] [Google Scholar]
- 23. Kitagaki K, Businga TR, Racila D, Elliott DE, Weinstock JV, Kline JN. Intestinal helminths protect in a murine model of asthma. J Immunol. 2006;177(3):1628‐1635. [DOI] [PubMed] [Google Scholar]
- 24. Setiady YY, Coccia JA, Park PU. In vivo depletion of CD4 + FOXP3 + Treg cells by the PC61 anti‐CD25 monoclonal antibody is mediated by FcgammaRIII+ phagocytes. Eur J Immunol. 2010;40(3):780‐786. [DOI] [PubMed] [Google Scholar]
- 25. Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39(4):1098‐1107. [DOI] [PubMed] [Google Scholar]
- 26. Cardoso LS, Oliveira SC, Goes AM, et al. Schistosoma mansoni antigens modulate the allergic response in a murine model of ovalbumin‐induced airway inflammation. Clin Exp Immunol. 2010;160(2):266‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221‐241. [DOI] [PubMed] [Google Scholar]
- 28. Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell‐deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350(6317):423‐426. [DOI] [PubMed] [Google Scholar]
- 29. Dullaers M, Schuijs MJ, Willart M, et al. House dust mite‐driven asthma and allergen‐specific T cells depend on B cells when the amount of inhaled allergen is limiting. J Allergy Clin Immunol. 2017;140(1):76‐88. e7. [DOI] [PubMed] [Google Scholar]
- 30. Everts B, Adegnika AA, Kruize YC, Smits HH, Kremsner PG, Yazdanbakhsh M. Functional impairment of human myeloid dendritic cells during Schistosoma haematobium infection. PLoS Negl Trop Dis. 2010;4(4):e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Everts B, Smits HH, Hokke CH, Yazdanbakhsh M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur J Immunol. 2010;40(6):1525‐1537. [DOI] [PubMed] [Google Scholar]
- 32. Kopf M, Schneider C, Nobs SP. The development and function of lung‐resident macrophages and dendritic cells. Nat Immunol. 2015;16(1):36‐44. [DOI] [PubMed] [Google Scholar]
- 33. GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 2008;1(6):442‐450. [DOI] [PubMed] [Google Scholar]
- 34. Plantinga M, Guilliams M, Vanheerswynghels M, et al. Conventional and monocyte‐derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell‐mediated immunity to house dust mite allergen. Immunity. 2013;38(2):322‐335. [DOI] [PubMed] [Google Scholar]
- 35. Yang J, Zhao J, Yang Y, et al. Schistosoma japonicum egg antigens stimulate CD4 CD25 T cells and modulate airway inflammation in a murine model of asthma. Immunology. 2007;120(1):8‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebner F, Hepworth MR, Rausch S, et al. Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy. 2014;69(11):1489‐1497. [DOI] [PubMed] [Google Scholar]
- 37. Fallon PG, Mangan NE. Suppression of TH2‐type allergic reactions by helminth infection. Nat Rev Immunol. 2007;7(3):220‐230. [DOI] [PubMed] [Google Scholar]
- 38. Nkurunungi G, Kabagenyi J, Nampijja M, et al. Schistosoma mansoni‐specific immune responses and allergy in Uganda. Parasite Immunol. 2018;40(1):e12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McSorley HJ, O'Gorman MT, Blair N, Sutherland TE, Filbey KJ, Maizels RM. Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. 2012;42(10):2667‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Korsgren M, Erjefalt JS, Korsgren O, Sundler F, Persson CG. Allergic eosinophil‐rich inflammation develops in lungs and airways of B cell‐deficient mice. J Exp Med. 1997;185(5):885‐892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacLean JA, Sauty A, Luster AD, Drazen JM, De Sanctis GT. Antigen‐induced airway hyperresponsiveness, pulmonary eosinophilia, and chemokine expression in B cell‐deficient mice. Am J Respir Cell Mol Biol. 1999;20(3):379‐387. [DOI] [PubMed] [Google Scholar]
- 42. Hamelmann E, Vella AT, Oshiba A, Kappler JW, Marrack P, Gelfand EW. Allergic airway sensitization induces T cell activation but not airway hyperresponsiveness in B cell‐deficient mice. Proc Natl Acad Sci U S A. 1997;94(4):1350‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X. Regulatory functions of innate‐like B cells. Cell Mol Immunol. 2013;10(2):113‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194(4):1395‐1401. [DOI] [PubMed] [Google Scholar]
- 45. Ghosh S, Hoselton SA, Schuh JM. Mu‐chain‐deficient mice possess B‐1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. J Immunol. 2012;189(3):1322‐1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van der Vlugt L, Obieglo K, Ozir‐Fazalalikhan A, Sparwasser T, Haeberlein S, Smits HH. Schistosome‐induced pulmonary B cells inhibit allergic airway inflammation and display a reduced Th2‐driving function. Int J Parasitol. 2017;47(9):545‐554. [DOI] [PubMed] [Google Scholar]
- 47. Haeberlein S, Obieglo K, Ozir‐Fazalalikhan A, et al. Schistosome egg antigens, including the glycoprotein IPSE/alpha‐1, trigger the development of regulatory B cells. PLoS Pathog. 2017;13(7):e1006539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kool M, Soullie T, van Nimwegen M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Melendez AJ, Harnett MM, Pushparaj PN, et al. Inhibition of Fc epsilon RI‐mediated mast cell responses by ES‐62, a product of parasitic filarial nematodes. Nat Med. 2007;13(11):1375‐1381. [DOI] [PubMed] [Google Scholar]
- 50. Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down‐regulates T cell proliferation and enhances interleukin‐10 production. Eur J Immunol. 1997;27(9):2253‐2260. [DOI] [PubMed] [Google Scholar]
- 51. Navarro S, Pickering DA, Ferreira IB, et al. Hookworm recombinant protein promotes regulatory T cell responses that suppress experimental asthma. Sci Transl Med. 2016;8(362):362ra143. [DOI] [PubMed] [Google Scholar]
- 52. Osbourn M, Soares DC, Vacca F, et al. HpARI Protein Secreted by a Helminth Parasite Suppresses Interleukin‐33. Immunity. 2017;47(4):739‐751. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnston CJC, Smyth DJ, Kodali RB, et al. A structurally distinct TGF‐beta mimic from an intestinal helminth parasite potently induces regulatory T cells. Nat Commun. 2017;8(1):1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cass CL, Johnson JR, Califf LL, et al. Proteomic analysis of Schistosoma mansoni egg secretions. Mol Biochem Parasitol. 2007;155(2):84‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Everts B, Hussaarts L, Driessen NN, et al. Schistosome‐derived omega‐1 drives Th2 polarization by suppressing protein synthesis following internalization by the mannose receptor. J Exp Med. 2012;209(10):1753‐1767. S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meevissen MH, Balog CI, Koeleman CA, et al. Targeted glycoproteomic analysis reveals that kappa‐5 is a major, uniquely glycosylated component of Schistosoma mansoni egg antigens. Mol Cell Proteomics. 2011;10(5):M110 005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schramm G, Hamilton JV, Balog CI, et al. Molecular characterisation of kappa‐5, a major antigenic glycoprotein from Schistosoma mansoni eggs. Mol Biochem Parasitol. 2009;166(1):4‐14. [DOI] [PubMed] [Google Scholar]
- 58. Ren J, Hu L, Yang J, et al. Novel T‐cell epitopes on Schistosoma japonicum SjP40 protein and their preventive effect on allergic asthma in mice. Eur J Immunol. 2016;46(5):1203‐1213. [DOI] [PubMed] [Google Scholar]
- 59. Hewitson JP, Maizels RM. Vaccination against helminth parasite infections. Expert Rev Vaccines. 2014;13(4):473‐487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


