Abstract
Retinal prosthesis or artificial retina is a promising modality of treatment for outer retinal degeneration, caused by primary and secondary loss of photoreceptor cells, in hereditary retinal dystrophy and age‐related macular degeneration, respectively. Okayama University‐type retinal prosthesis (OUReP) is a photoelectric dye‐coupled polyethylene film which generates electric potential in response to light and stimulates nearby neurons. The dye‐coupled films were implanted by vitreous surgery in the subretinal space of monkey eyes with macular degeneration which had been induced by cobalt chloride injection from the scleral side. A pilot 1‐month observation study involved 6 monkeys and a pivotal 6‐month observation study involved 8 monkeys. Of 8 monkeys in 6‐month group, 3 monkeys underwent dye‐coupled film removal at 5 months and were observed further for 1 month. The amplitude of visual evoked potential which had been reduced by macular degeneration did recover at 1 month after film implantation and maintained the level at 6 months. Optical coherence tomography showed no retinal detachment, and full‐field electroretinograms maintained a‐wave and b‐wave amplitudes, indicative of no retinal toxicity. Pathological examinations after 6‐month implantation showed structural integrity of the inner retinal layer in close apposition to dye‐coupled films. The implanted films which were removed by vitrectomy 5 months later showed light‐evoked surface electric potentials by scanning Kelvin probe measurement. The photoelectric dye‐coupled film (OUReP), which serves as a light‐receiver and a displacement current generator in the subretinal space of the eye, has a potential for recovering vision in diseases with photoreceptor cell loss, such as retinitis pigmentosa and age‐related macular degeneration.
Keywords: Dye‐coupled thin film retinal prosthesis, —Photoelectric dye, —Monkey, —Vitreous surgery, —Macular degeneration, —Visual evoked potential
Blind patients with hereditary retinal diseases, such as retinitis pigmentosa 1, 2, have dead photoreceptor cells, but the other retinal neurons which send axons to the brain remain alive 3. The basic concept of retinal prostheses is to stimulate surviving retinal neurons such as ganglion cells and bipolar cells with artificial devices and to exploit the function of these living neurons, and finally to send messages to the brain, following artificial stimulation in response to light 4.
At the clinical level, the main stream of retinal prosthesis which has been approved by US Food and Drug Administration (FDA) utilizes a multielectrode array 4, 5, 6. Camera‐captured images are collapsed to 60 pixels, and the electric current, corresponding to grayscale tone in each pixel, is outputted from 60 electrodes to stimulate retinal living neurons in the Argus II Retinal Prosthesis System (Second Sight Medical Products, Sylmar, CA, USA). In addition to the Argus II, Retina Implant Alpha AMS (preceding Alpha IMS, Retina Implant AG, Aachen, Germany) and Epi‐retinal IRIS II Bionic Vision System (Pixium Vision, Paris, France) have obtained CE marks in the European market 7, 8, 9. Surgical implantation of these multielectrode arrays requires sophisticated techniques. At the level of animal studies, several different types of retinal prostheses are designed to be cableless 10, 11, and, for example, utilize a wireless subretinal neurostimulator 12 or photovoltaic systems 13, 14.
Okayama University‐type retinal prosthesis (OUReP) is a new type of retinal prosthesis, so‐called photoelectric dye‐coupled thin film retinal prosthesis 15, 16, 17, 18, 19, 20. Stable photoelectric dye molecules with absorption spectrum of visible light 21, 22 are chemically coupled to polyethylene film surface. The dye‐coupled film generates electric potential in response to light and stimulates nearby neuronal cells to induce action potential 23. The dye‐coupled film, implanted in subretinal space of the eye, serves as a light receiver and an electric potential generator, and thus, replaces the function of dead photoreceptor cells in retinal dystrophy to send the signal to the brain through living retinal bipolar cells, ganglion cells and their axons as optic nerve fibers. From a surgical viewpoint, the dye‐coupled film is a soft and thin sheet which can be rolled up and inserted in the subretinal space of the eyes by standard vitreous surgery (vitrectomy) 24, 25.
In our previous studies, we showed vision recovery in retinal dystrophic rats (RCS rats) with dye‐coupled film implantation 26, 27, 28. In this study, the dye‐coupled films were implanted by vitreous surgery in the subretinal space of monkey eyes with cobalt chloride‐induced macular degeneration. The monkeys underwent ophthalmic examinations such as optical coherence tomography, electroretinography, and visual evoked potential for 6 months to test the safety and efficacy of dye‐coupled films. In addition, spectrophotometric absorbance and light‐evoked surface electric potential were measured on the removed dye‐coupled films which had been implanted for 5 months.
Materials and Methods
Preparation of dye‐coupled polyethylene film
Thin films were made from purified polyethylene powder and exposed to fuming nitric acid to introduce carboxyl moieties on the film surface. Photoelectric dye molecules, 2‐[2‐[4‐(dibutylamino)phenyl]ethenyl]‐3‐carboxymethylbenzothiazolium bromide (NK‐5962, Hayashibara, Inc., Okayama, Japan), were coupled to carboxyl moieties of the polyethylene film surface via ethylenediamine, as described previously 16, 18, 26. The dye‐coupled films were manufactured under a quality management system at a clean‐room facility in the Okayama University Incubator.
Animals
Cynomolgus monkeys (purpose‐bred, anti‐B virus antibody‐negative) were used in this study (Table 1): six monkeys for 1‐month observation in a pilot study and eight monkeys for 6‐month observation in a pivotal study. Dye‐coupled films were implanted for 1 month in six monkeys and for 6 months in eight monkeys. Of these eight monkeys in the 6‐month observation group, three monkeys underwent film removal at the time point of 5 months after the implantation to analyze spectrophotometric absorbance and light‐evoked surface electric potential of the implanted films. Schedules for surgeries and examinations (optical coherence tomography, full‐field and focal electroretinography, and visual evoked potential) are summarized in Table 1. All surgeries were done only in the right eye and the left eye served as control.
Table 1.
Schedules for surgery and examinations in monkeys in pilot 1‐month study and pivotal 6‐month study
| Six monkeys for 1‐month observation | |||||||
|---|---|---|---|---|---|---|---|
| Day −12 | Day −5 | Day 0 | Day 15 or 16 | Day 28 or 29 | Day 39 or 40 | ||
| No. 1–1# | CoCl2 injection | VEP, fERG, OCT | Implantation | AfterCataract, VEP only | Capsulectomy | VEP, fERG, OCT | #Film not at degeneration |
| No. 1–2# | CoCl2 injection | VEP, fERG, OCT | Implantation | AfterCataract, VEP only | Capsulectomy | VEP, fERG, OCT | #Film in choroid |
| No. 1–3 | CoCl2 injection | VEP, fERG, OCT | Implantation | AfterCataract, VEP only | Capsulectomy | VEP, fERG, OCT | |
| No. 1–4 | CoCl2 injection | VEP, fERG, OCT | Implantation | AfterCataract, VEP only | Capsulectomy | VEP, fERG, OCT | Space between film and RPE |
| No. 1–5 | CoCl2 injection | VEP, fERG, OCT | Implantation | AfterCataract, VEP only | Capsulectomy | VEP, fERG, OCT | Space between film and RPE |
| No. 1–6 | CoCl2 injection | VEP, fERG, OCT | Implantation | AfterCataract, VEP only | Capsulectomy | VEP, fERG, OCT | |
| Eight monkeys for 6‐month observation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day −16 | Day −13 | Day −5 | Day 0 | Day 19 | Day 25,26 | Day 81,82 | Day 130,131 | Day 133 | Day 148,149 | Day 165,166 | |
| Tests | Tests | Surgery | Surgery | Tests | Tests* | Tests* | Surgery | Tests* | Tests* | ||
| No. 2–1# | CoCl2 injection | Implantation | |||||||||
| #VEP amplitude not reduced in macular degeneration | #Film relocated spontaneously | ||||||||||
| No. 2–2 | CoCl2 injection | Implantation | Space | ||||||||
| No. 2–3# | CoCl2 injection | Implantation | Space | No testing | Space | ||||||
| #Film not at degeneration | |||||||||||
| No. 2–4 | CoCl2 injection | Implantation | Space | Space | |||||||
| No. 2–5 | CoCl2 injection | Implantation | Capsulectomy | ||||||||
| No. 2–6 | CoCl2 injection | Implantation | Space | Film removal | |||||||
| No. 2–7 | CoCl2 injection | Implantation | Space | Space | Space | Film removal | |||||
| No. 2–8 | CoCl2 injection | Implantation | Capsulectomy | Space | Space | Space | Film removal | ||||
Monkeys (No. with #) were excluded from VEP analysis based on the reason also marked with #.
Tests: visual evoked potential (VEP), focal electroretinography (fERG), and optical coherence tomography (OCT).
Tests*: VEP, fERG, OCT, and full‐field ERG.
Space indicates the space between film and retinal pigment epithelium (RPE) detected by OCT.
No testing on Day 148, 149 in No. 2–3# monkey is due to convulsion in anesthesia.
Before dye‐coupled film implantation, macular degeneration extending to the temporal mid‐peripheral retina was induced in the right eye of each monkey by subretinal injection of cobalt chloride solution (cobalt chloride hexahydrate solution 30 μL, 0.4 mg/mL) from scleral incision 29, and was confirmed by extinguished recordings of focal electroretinography. From the viewpoint of surgical intervention, monkey eyes are smaller in size, compared with human eyes. Lateral canthotomy was, therefore, performed to expose the entire circumference of the bulbar conjunctiva around the cornea with a lid speculum, and to allow 25G trocars for vitrectomy to be placed as usual as in human eyes (Fig. 1c).
Figure 1.
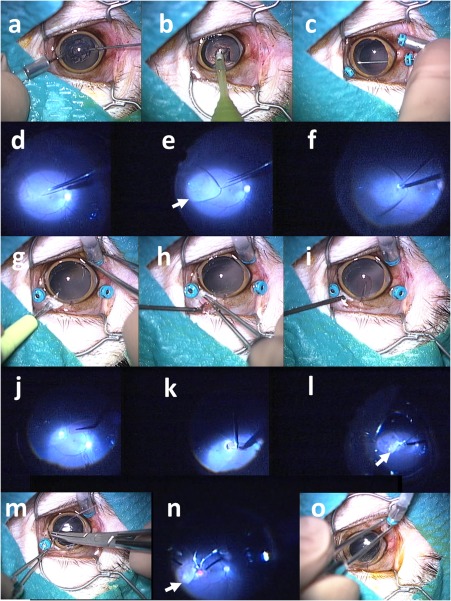
Surgical procedures (scenes) to implant retinal prosthesis OUReP in a monkey (No. 2–2). Total surgical time was 35 min. (a) Lens anterior capsule is cut with 25G vitreous cutter under irrigation with 25G infusion cannula in the anterior chamber. (b) Lens nucleus and cortex is aspirated with phacoemulsification tip from corneal incision. (c) Three 25G trocars are inserted over the conjunctiva through the sclera into the vitreous at 2 mm from the corneal limbus: an inferotemporal trocar is connected with infusion cannula, and the other two trocars are used for the vitreous cutter and light guide. Posterior capsule is cut with vitreous cutter. (d) After vitreous gel has been cut, subretinal fluid infusion is started with 38G tip. (e) Bleb retinal detachment (arrow) is made by irrigating solution infusion with 38G tip. (f) A retinal tear (retinotomy) is made by retinal coagulation with 25G bipolar diathermy. (g) Scleral incision is made with a knife after conjunctival incision. (h and i) A sheet (2.5 × 5 mm) of dye‐coupled film is inserted through scleral incision with 20G subretinal forceps. (j) Film is inserted into the vitreous with 20G subretinal forceps. (k) Film is inserted into subretinal space through a retinal tear with 20G subretinal forceps. (l) Fluid‐air exchange in the vitreous cavity is done with 25G vitreous cutter in aspiration mode to reattach the retina. (m) Scleral and conjunctival incisions are sutured. (n) Laser photocoagulation is applied around the retinal tear. (o) After gas tamponade with 30% sulfur hexafluoride, trocars are removed. Note subretinal dye‐coupled film (arrows in l and n).
At cataract surgery (Fig. 1a,b), anterior capsulectomy was accomplished by cutting with a 25G vitreous cutter inserted from a corneal limbal side‐port under continuous irrigation with a 25G infusion cannula also inserted from another limbal side‐port, as done in congenital cataract surgery in human eyes 30. At vitrectomy 25, retinal detachment (retinal bleb) was successfully induced in the area with retinal degeneration by subretinal injection of intraocular irrigating solution with a 38G polyimide tip (Fig. 1d,e). A sheet of dye‐coupled film in 2.5 × 5 mm square size was grasped with a 20G subretinal forceps and inserted through 3 mm‐wide scleral wound into the vitreous and finally to the subretinal space through a coagulation‐induced retinal tear (Fig. 1k). The detached retina was reattached with fluid‐air exchange, retinal laser photocoagulation, and gas tamponade with 30% sulfur hexafluoride, as done in humans (Fig. 1l,n). Removal of the implanted dye‐coupled films was accomplished by vitrectomy, using a disposable 25G forceps, in a similar procedure. The detailed procedures for surgeries and ophthalmic examinations are given in the Appendix.
All monkeys were sacrificed by bleeding with overdose of intravenous thiopental (sodium pentobarbital 64.8 mg/mL, 0.4 mL/kg of body weight, Tokyo Kasei Kogyo, Tokyo, Japan), and both eyes were enucleated. After the cornea and iris were removed by circumferential incision of the eye ball, the posterior segment was cut meridionally to view the entire retina. The posterior segment was then fixed with phosphate buffered 2.5% formaldehyde and 3% glutaraldehyde, stored in 10% neutral pH formalin, and embedded in paraffin. Paraffin sections were cut and stained with hematoxylin and eosin for pathological examinations.
This study was approved by the Animal Care and Use Committee at Okayama University and also by the Committee at Shin Nippon Biomedical Laboratories, Inc., based on the Animal Welfare and Management Act in Japan. The 6‐month implantation study was based on the Good Laboratory Practice in Japan. All experimental procedures were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Visual evoked potential and statistical analysis
Recordings with 128‐time summation from the right visual cortex (Channel 1) in response to repetitive photic stimuli to the right eye were used for the right eye amplitude while recordings from the left visual cortex (Channel 2) in response to left eye stimuli were used for the left eye amplitude. This is based on the fact that the uncrossed retinal nerve fibers, temporal to the macula, go to the right visual cortex while the crossed retinal nerve fibers, nasal to the macula, go to the left visual cortex.
In statistical analyses, two monkeys, one each in 1‐month study (No. 1–1) and in 6‐month study (No. 2–3) were excluded because the films were implanted at the normal retinal site, but not at the site of retinal degeneration. One monkey in 1‐month study (No. 1–2) was excluded because the film was found by pathology to be implanted in the choroid (Fig. 5). One monkey in 6‐month group (No. 2–1) was also excluded because the amplitude of visual evoked potential was not reduced after retinal degeneration had been induced by cobalt chloride injection. Thus, the analysis set for 1‐month observation included four monkeys and the analysis set for 6‐month observation included three monkeys with continuous film implantation and three monkeys with film removal on Day 133.
Figure 5.
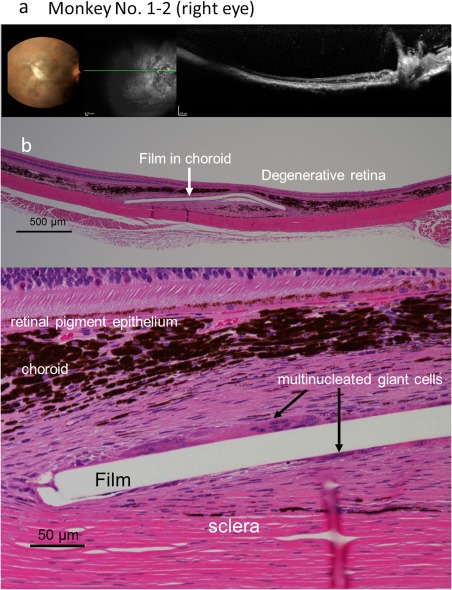
Fundus photographs and optical coherence tomography (a), and pathology (b) of the right eye of Monkey No. 1–2 with 1‐month implantation of a dye‐coupled film. Horizontal section (green line) of optical coherence tomography is shown in the middle black‐and‐white fundus photograph. The film is not detected by optical coherence tomography and is later located in the choroid at pathology. Hematoxylin‐eosin stain. Scale bar = 500 µm (upper panel of b) and 50 µm (lower panel of b).
The amplitude of the first positive peak (P1) and the amplitude of the first negative peak (N1) were summed to obtain the P1‐N1 amplitude, and the amplitude of N1 and the amplitude of the second positive peak (P2) were summed to obtain the N1‐P2 amplitude (Fig. 7a). These P1‐N1 and N1‐P2 amplitudes were compared between time points before and after dye‐coupled film implantation by repeat‐measure analysis of variance (ANOVA) and paired t‐test. Statistical analyses were also done for the percent ratio of right‐eye‐stimulated and right‐lobe‐recorded amplitudes over left‐eye‐stimulated and left‐lobe‐recorded amplitudes. The right eyes underwent surgeries while the left eyes had no intervention to serve as controls.
Figure 7.
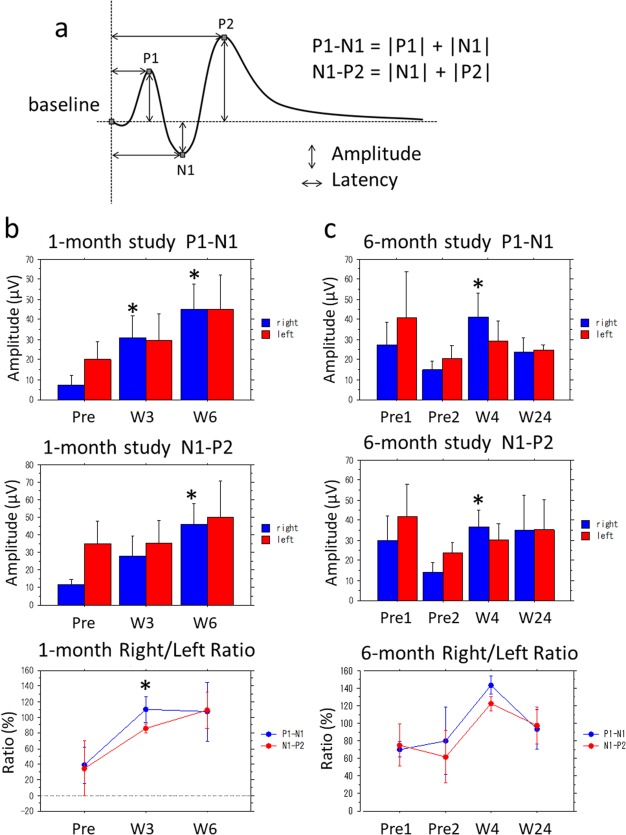
Statistical analyses of visual evoked potential amplitudes. (a) The definition of amplitude P1‐N1 and N1‐P2, indicating the sum of first positive peak (P1) amplitude and first negative peak (N1) amplitude, and the sum of N1 amplitude and second positive peak (P2) amplitude, respectively. (b) P1‐N1, N1‐P2 amplitudes, and percent ratio of right eye/left eye amplitude in 4 monkeys with 1‐month dye‐coupled film implantation. (c) P1‐N1, N1‐P2 amplitudes, and percent ratio of right eye/left eye amplitude in 3 monkeys with 6‐month dye‐coupled film implantation. Reduced amplitudes (Pre in 1‐month study and Pre 2 in 6‐month study) in the right eyes by macular degeneration become larger after film implantation (*P < 0.05, paired t‐test). The left eyes had no intervention and served as controls. Note that amplitudes (Pre 2) are reduced by the development of macular degeneration, compared with amplitudes (Pre 1) before the induction of macular degeneration in 6‐month study. The right eye/left eye ratios for P1‐N1 and N1‐P2 amplitudes in 1‐month study and 6‐month study (bottom graphs) change significantly in the time course of 1 month and 6 months, respectively (P = 0.0002 and P < 0.0001, repeat‐measure analysis of variance, ANOVA).
Spectrophotometry and light‐evoked surface potential measurement
Absorbance spectra of dye‐coupled films were measured by an ultraviolet and visible light spectrophotometer with an integrating sphere unit (V‐750 and PIV‐756, JASCO Corporation, Tokyo, Japan). Plain polyethylene films were used to obtain baseline absorbance. Absorbance was measured in the wavelength ranging from 300 nm to 800 nm at 1 nm spectral bandwidth. Absorbance values at 508 nm were used for comparison between the implanted film and the nonimplanted same lot.
Light‐evoked surface electric potential on dye‐coupled films in the dry condition was measured by the scanning Kelvin Probe system (SKP5050, KP Technology, Ltd., Highlands and Islands, UK) in the surface photovoltage mode 25. The entire measuring system was placed in a humidity‐controlling large box. The dye‐coupled film was fixated on a sample device. The surface potential was measured at changing light intensity with a light source (Surface Photovoltage Spectroscopy SPS040, KP Technology). The slope of surface potential changes in response to increase of light intensity as well as the surface potential in light intensity of dial setting at 2500 (300 lux equivalent) and at 5000 were used as the outcome.
Results
Surgical feasibility and outcome
All monkeys in the 1‐month study and 6‐month study were healthy in the observation period. Rhegmatogenous retinal detachment, as a surgical complication, was found only in one monkey (No. 1–1) in the 1‐month study. All six monkeys for 1‐month observation developed small pupillary areas by anterior capsular contraction with iris adhesion 2 weeks after lensectomy and vitrectomy, and underwent anterior and posterior capsulectomy with iris synechialysis to enlarge the pupillary areas. Based on this experience in the 1‐month study, anterior and posterior capsules were resected to the periphery as far as possible (Fig. 1c) at the initial surgery in eight monkeys for 6‐month observation. Under the circumstances, of these eight monkeys, only two monkeys (No. 2–5 and 2–8) had to undergo anterior and posterior capsulectomy, together with vitrectomy to release vitreoretinal adhesion, 2 weeks after the initial surgery.
The implanted films were at stable position in subretinal space (Fig. 2) of all monkeys, except for one monkey (No. 2–1) which showed apparent relocation of the film in the subretinal space between Day 25 and Day 81 after film implantation (Fig. 3). On Day 133, dye‐coupled films were removed by vitrectomy in three monkeys (No. 2–6, 2–7, 2–8) to prove the feasibility and safety of film‐removing surgical techniques (Fig. 4). The three removed dye‐coupled films which had been implanted for 5 months were submitted for spectrophotometric and Kelvin probe surface potential measurements, as described below.
Figure 2.
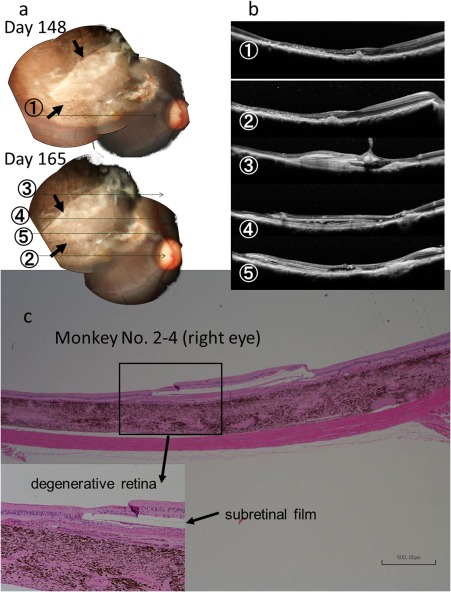
Fundus photographs (a), optical coherence tomography (b), and pathology (c) of the right eye of Monkey No. 2–4 with 6‐month implantation of a dye‐coupled film. Horizontal sections of optical coherence tomography are shown as lines in fundus photographs. Arrows in fundus photographs indicate the edges of the film. The film is located under the degenerative retina at pathology. Hematoxylin‐eosin stain. Scale bar = 500 µm in c.
Figure 3.
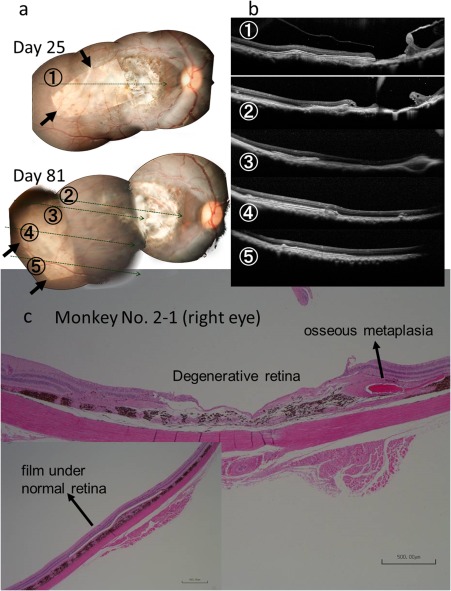
Fundus photographs (a), optical coherence tomography (b), and pathology (c) of the right eye of Monkey No. 2–1 with 6‐month implantation of a dye‐coupled film. Horizontal sections of optical coherence tomography are shown as lines in fundus photographs. Arrows in fundus photographs indicate the edges of the film. The film in this monkey has been relocated spontaneously between time points of Day 25 and Day 81. The film is located under the normal retina at pathology (inset). Hematoxylin‐eosin stain. Scale bar = 500 µm in c (bar = 500 µm at inset).
Figure 4.
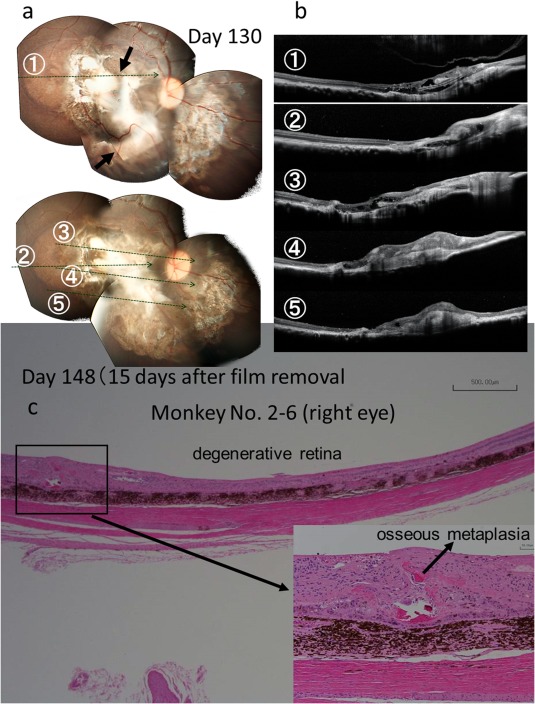
Fundus photographs (a), optical coherence tomography (b), and pathology (c) of the right eye of Monkey No. 2–6 with a dye‐coupled film which has been removed after 5‐month implantation. Horizontal sections of optical coherence tomography are shown as lines in fundus photographs. Arrows in fundus photographs indicate the edges of the film. A small area of osseous metaplasia is found in the retina (inset). Hematoxylin‐eosin stain. Scale bar = 500 µm in c (bar = 50 µm at inset).
Optical coherence tomography
All monkeys for 1‐month and 6‐month observation showed that the neuroretina was attached closely to the dye‐coupled films throughout the course of observation (Figs. 2, 3, 4). The film was not visualized by optical coherence tomography in one monkey (No. 1–2), and was later confirmed by pathological examinations to be located in the choroid (Fig. 5). The films were located not in the macular degenerative areas, but in the superior part of the normal retina of two monkeys, one each in the 1‐month study (No. 1–1) and in the 6‐month study (No. 2–3). Space with fluid was detected on the choroidal side of dye‐coupled films in two of six monkeys for the 1‐month observation and in four of eight monkeys for the 6‐month observation. In 6‐month study, one monkey (No. 2–2) developed the temporary fluid on Day 148 and another monkey (No. 2–3) developed the fluid on Day 130 which continued on Day 165. The other two monkeys (No. 2–7, 2–8) had the persistent fluid on Day 25, Day 81, and Day 130, and the fluid disappeared on Day 148 and Day 165 after the film removal.
Electroretinography
Full‐field electroretinography was performed only from Day 81 in eight monkeys for 6‐month observation to assess retinal toxicity caused by film implantation. Amplitudes of a‐wave and b‐wave of full‐field electroretinography were maintained throughout the course (Fig. 6), indicative of no retinal toxicity. Focal electroretinography at the degeneration site only was planned initially in 1‐month study and 6‐month study. In the 1‐month study, apparent waves were not recorded by focal electroretinography in macular degenerative areas with film implantation (Fig. 6). The aim of focal electroretinography was changed from the assessment of efficacy to the assessment of safety for film implantation in the 6‐month study. Focal electroretinography was recorded in the degenerative macular area with film implantation and also in the normal retinal area on the nasal side of the fundus in the 6‐month study. All monkeys showed positive response of focal electroretinography in the normal retinal area on the nasal side of the fundus, indicative of no retinal toxicity.
Figure 6.
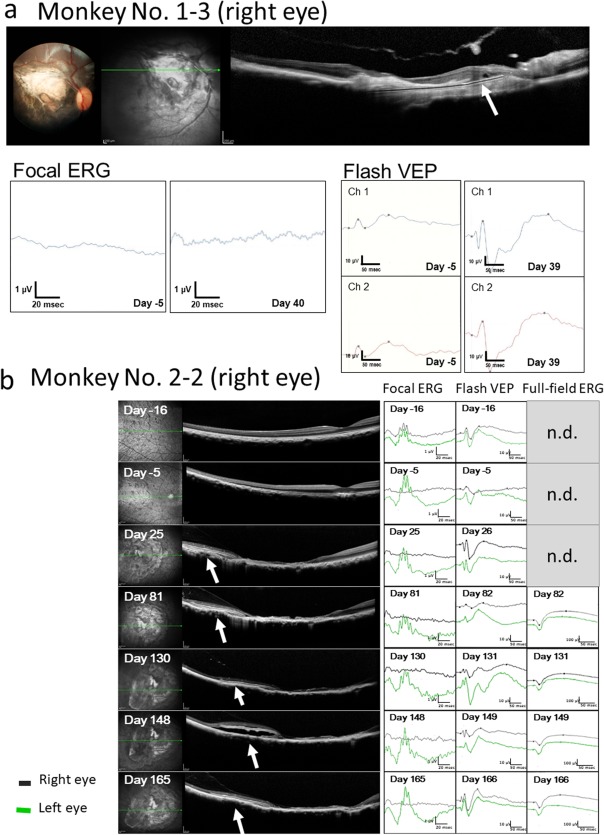
(a) Focal electroretinograms (ERG) and visual evoked potential (flash VEP) in the right eye of Monkey No. 1–3 with 1‐month dye‐coupled film implantation. Focal ERG is extinguished after the induction of macular degeneration (Day −5) and also not detected at 1 month of film (white arrow in optical coherence tomography in a) implantation (Day 40). In contrast, reduced VEP amplitude at Day −5 becomes larger at 1 month of film implantation (Day 39). Channel 1 (Ch 1) and Channel 2 (Ch 2) correspond to the right and left visual cortex recordings, respectively, in photic stimuli to the right eye. (b) Focal ERG, flash VEP, and full‐field ERG in the right eye of Monkey No. 2–2 with 6‐month dye‐coupled film (white arrows in optical coherence tomography) implantation. Focal ERG is noted before the induction of macular degeneration (Day −16), extinguished after the development of macular degeneration (Day −5) and also not detected throughout the 6‐month course until Day 165. In contrast, reduced VEP amplitude on Day −5 becomes larger at 1 month and 6 months of film implantation (Day 26 and Day 166). Full‐field ERG which was scheduled to measure only from Day 82 shows normal waves throughout the course (n.d., not determined). Right eye recordings (black lines) of VEP are from the right visual cortex (Channel 1) in response to right eye photic stimuli while left eye recordings (green lines) are from the left visual cortex (Channel 2) in response to left eye stimuli. Vertical line (amplitude) and horizontal line (time) are 1 μV and 20 msec in focal ERG, 10 μV and 50 msec in flash VEP, and 100 μV and 50 msec in full‐field ERG, respectively. Note that space between film and retinal pigment epithelium appears temporarily on Day 148 in optical coherence tomography.
Visual evoked potential
The P1‐N1 and N1‐P2 amplitudes were significantly larger at 1 month after film implantation both in the 1‐month study and in the 6‐month study, compared with the amplitudes before film implantation (P < 0.05, paired t‐test, Fig. 7b,c). In 6‐month study (Fig. 7c), the P1‐N1 and N1‐P2 amplitudes were larger, although not significantly, 6 months after film implantation, compared with the amplitudes before film implantation. The right eye/left eye ratios for the P1‐N1 and N1‐P2 amplitudes changed significantly in the time course of 1 month and 6 months in 1‐month study and in 6‐month study, respectively (P = 0.0002 and P < 0.0001, repeat‐measure ANOVA). In other words, the right eye/left eye ratios for the P1‐N1 and N1‐P2 amplitudes which dropped at the development of macular degeneration were returned to and maintained at the value of 100% in the follow‐up of 6 months.
Pathology
In six monkeys for 1‐month observation, all films were located in the subretinal space between the neural retina and choroid, except for one monkey (No. 1–2) which had the film implanted in the choroid (Fig. 5b). This monkey corresponded to one with no detection of the film by optical coherence tomography (Fig. 5a). A few multinucleated giant cells, derived probably from retinal pigment epithelial cells, were found around the film (Fig. 5b). The inner layer of the neural retina showed structural integrity at the site of film implantation. No inflammatory cells were found in the choroid and retina.
In five monkeys for 6‐month continuous film implantation in 6‐month study, all films were located in the subretinal space between the neural retina and choroid (Figs. 2c, 3c, and 4c). A few multinucleated giant cells, derived probably from retinal pigment epithelial cells, were found around the film. The inner layer of the neural retina showed structural integrity at the site of film implantation even after 6 months. Three monkeys with film removal at 5 months, followed by further 1‐month observation in 6‐month study, showed subretinal osseous metaplasia (Fig. 4c) in the areas where the films had been implanted.
Absorbance and light‐evoked surface potential
Three pieces of dye‐coupled films which had been implanted for 133 days in three monkey eyes and removed by vitrectomy were assessed by spectrophotometric absorbance and Kelvin probe measurements of light‐evoked surface electric potentials. The absorbance at 508 nm of the films removed from monkeys (No. 2–6, No. 2–7, and No. 2–8) was 0.03205, 0.02156, and 0.02926, respectively (Fig. 8). The percentage against the nonimplanted same lot with the absorbance of 0.07905 was 40.5%, 27.3%, and 37.0%, respectively. In Kelvin probe measurements, all three implanted films showed proportional increase of light‐evoked surface electric potential in response to increasing light intensity (Fig. 8). Potential values at light intensity of 2500 arbitrary units (which roughly equals to 300 lux) were 25.3 mV (66% against 38.4 mV of the nonimplanted same lot), 28.1 mV (73%), and 23.9 mV (62%) for the implanted films from monkeys No. 2–6, No. 2–7, and No. 2–8, respectively. Potential values at light intensity of 5000 arbitrary units were 232.7 mV (67% against 345.2 mV of the nonimplanted same lot), 184.2 mV (53%), and 205.7 mV (60%), respectively.
Figure 8.
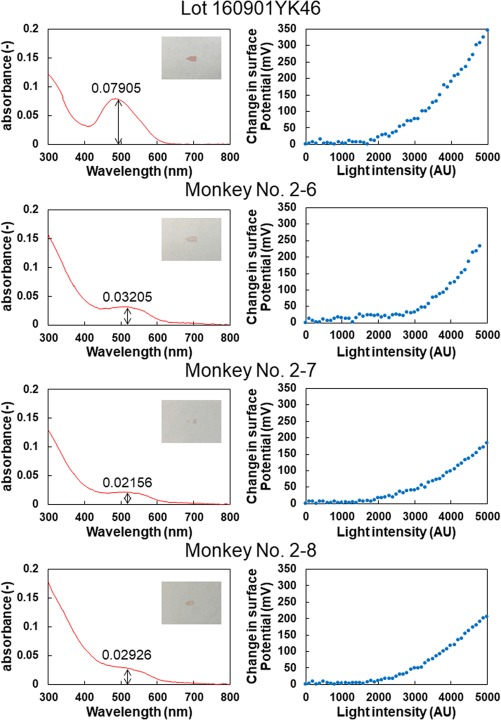
Spectrophotometric absorbance (left column) and surface electric potential changes of the dye‐coupled films in response to increase in light intensity, measured with scanning Kelvin Probe (right column). Top row to bottom rows, measurements of the same nonimplanted lot, the 5‐month implanted films removed from the eyes of 3 monkeys (No. 2–6, 2–7, and 2–8).
Discussion
Cobalt chloride‐induced macular degeneration in monkeys has been developed as a primate model for age‐related macular degeneration 29. Age‐related macular degeneration is classified as outer retinal degeneration in the macular area, secondary to retinal pigment epithelial dysfunction, often coupled with abnormal vessel formation (neovascularization) from choroidal vasculature. The destruction of outer retinal layer with photoreceptor cell loss in the macular area leads to reduced amplitudes of visual evoked potentials as the macular area sends a largest number of nerve fibers to the brain. Indeed, visual evoked potentials in monkeys were reduced in amplitudes after the development of macular degeneration which extended to the mid‐peripheral retina on the temporal side of the fundus. As the temporal retina, including the macular area, sends uncrossed nerve fibers to the right visual cortex in monkeys, visual evoked potential recordings on the right side of the brain were used as the response to photic stimulation in the right eye.
In this study, we showed surgical feasibility to implant or remove the dye‐coupled films by vitrectomy. Optical coherence tomography, as a noninvasive clinical examination, confirmed the attachment of dye‐coupled films to the neural retina throughout the course. Pathological examinations after the sacrifice of animals revealed the absence of inflammation and structural integrity of the inner retinal layer which was in close contact with the dye‐coupled films for 6 months. Full‐field electroretinographic response in the entire retina and focal electroretinographic response in the normal retinal area on the nasal side of the fundus for 6 months provide additional data for no toxicity to the retinal tissue on a physiological basis.
As for the efficacy, the amplitudes of visual evoked potentials increased significantly at 1 month after the dye‐coupled film implantation both in pilot 1‐month observation study and in pivotal 6‐month observation study. The amplitudes also showed increase, although not significant, at 6 months after the film implantation. It should be noted that the right eye/left eye ratios for the P1‐N1 and N1‐P2 amplitudes were kept at the value of 100% throughout the follow‐up after the ratios dropped in the induction of macular degeneration. These facts indicate that photic stimuli to the degenerative right eye resulted in the same level of amplitudes as photic stimuli to the normal left eye.
In humans and monkeys, the amplitude and latency of visual evoked potentials vary largely from individual to individual and also are influenced greatly by anesthetic levels. In this study, visual evoked potentials were, of course, recorded in monkeys under general anesthesia, and thus, the recordings would have intrinsic limitations in their interpretation. In monkeys, head skin moves well in relation with the skull, and thus, plate electrodes on the skin surface for visual evoked potential recording would not be positioned exactly at the same place at several time points of recording. These limitations would explain variable amplitudes of visual evoked potential in monkeys. Under the circumstances, the right eye/left eye ratios for visual evoked potential amplitudes were used to assess the response in this study.
In the present study, monkeys were used because they have macular structure of the retina. We did not adopt cranial‐fixed electrodes for visual evoked potential recording from the viewpoint of animal welfare. We induced macular degeneration and implanted the dye‐coupled films only in the right eye, mainly due to consideration for animal welfare. The macular degeneration in the right eye induced by cobalt chloride injection usually extended to the mid‐peripheral retina on the temporal side in one quadrant of the fundus. Under the circumstances, the monkeys maintained the visual field corresponding to the remaining three quadrants of the fundus. Thus, we did not plan a behavioral test in the present study.
Recently, government authorities, such as Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, tend to require monkey studies for safety tests of novel pharmaceuticals and medical devices. The present study was indeed designed to answer questions, raised by PMDA, for long‐term surgical safety of the dye‐coupled films. We basically advise against using nonhuman primates only for surgical implantation studies. Porcine or feline models would give just as much information in terms of histopathology if behavioral results are not required. We, therefore, designed the present study also to test visual evoked potential as part of efficacy tests, after we had finished implantation studies in dogs 25 and rabbits 31.
It should be noted that three sheets of dye‐coupled films which had been implanted for 5 months showed decreases in absorbance to 35% as a mean, compared with the nonimplanted same lot. In contrast, light‐evoked surface electric potential on the 5‐month implanted films maintained a 67% level as a mean, compared with the nonimplanted same lot. This discrepancy between the absorbance and light‐evoked surface electric potential would be explained by the presence of noncovalently‐bound dye molecules on the film surface. These noncovalently‐bound dye molecules, which are only attached to the film surface by ionic binding, would leave the film surface during the period of implantation. The noncovalently‐bound dye molecules do contribute to the absorbance of the film but do not contribute to light‐evoked surface electric potential 31.
In general, retinal prostheses are largely classified into epiretinal stimulation and subretinal stimulation, based on epiretinal or subretinal positioning of the devices. In recent trends, subretinal implants with different kinds of modalities are emerging as subretinal stimulation can be directed not only to retinal ganglion cells but also to remaining bipolar cells 10, 11. The photoelectric dye‐coupled polyethylene film is unique in that the structure is simple and thus would escape mechanical failure. In the present study, levels of light‐evoked surface electric potential of the 5‐month implanted dye‐coupled films were reduced to about two thirds of the nonimplanted same lot. The functional durability of the dye‐coupled films remains to be determined in years after the subretinal implantation.
Conclusions
The present study confirmed the safety and efficacy of the photoelectric dye‐coupled polyethylene film as a retinal prosthesis in a monkey model of age‐related macular degeneration. The present results could be also applicable in the treatment of retinitis pigmentosa, as retinitis pigmentosa shares the loss of photoreceptor cells with age‐related macular degeneration as a common pathological background. The dye‐coupled films would be durable at least for 6 months. All biological safety tests for medical devices have been completed to prove no toxicity for the dye‐coupled film (data not shown). The photoelectric dye in itself was also proven to have no biological toxicity (data not shown). The filing of a first‐in‐human clinical trial for OUReP in patients who have lost vision by retinitis pigmentosa is now negotiated at Pharmaceuticals and Medical Devices Agency (PMDA) in Japan, counterpart of US Food and Drug Administration (FDA).
Author Contributions
TM, JS, TA, YY, and KK were involved in conception or design of the work, TM performed all surgeries and CM acted as a surgical assistant. TM, TU, KY, CM, TA, and YY performed data collection, data analysis and interpretation. TM drafted the article. All authors were involved in critical revision of the article and final approval of the version.
Conflict of Interest
The authors declare that they have no competing financial interests in this study.
Data Availability
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the staff at Shin Nippon Biomedical Laboratories, Ltd., Kagoshima City, Japan, for helping us conduct experiments in monkeys. We also thank Dr. Toshinori Furukawa at Kurashiki University of Science and the Arts, Dr. Shigiko Takei and Dr. Daisuke Ido at Ina Research, Inc., for their advice on surgeries. This study was supported by a grant (Seeds C 2016) for the Translational Research Network Program from the Japan Agency for Medical Research and Development (AMED).
for Detailed Methods in Surgeries and Examinations
Cobalt Chloride Injection
Monkeys were anesthetized with intramuscular injection of medetomidine (0.04 mg/kg of body weight, Dormitor, Orion Pharma Animal Health, Espoo, Finland), midazolam (0.3 mg/kg, Dormicum, Astellas Pharma, Tokyo), and butorphanol (0.4 mg/kg, Betorphal, Meiji Seika Pharma, Tokyo). Instillation of 0.5% levofloxacin (0.5% Cravit, Santen Pharmaceutical, Osaka, Japan) and 1% atropine (Nitten Pharmaceutical, Nagoya, Japan) was done once daily for 3 days before intervention, and mydriasis was further obtained by 0.5% tropicamide and 0.5% phenylephrine eye drops (Mydrin‐P, Santen Pharmaceutical) on the day of intervention. After instillation of 0.4% oxybuprocaine (Benoxil, Santen Pharmaceutical), the bulbar conjunctiva was opened on the temporal side, and the sclera was incised with a 20‐gauge knife (V‐Lance Knife, Alcon Laboratories, Inc., Fort Worth, TX, USA). Cobalt chloride hexahydrate solution 30 μL (0.4 mg/mL, Nacalai tesque, Kyoto) was injected to the subretinal space under a surgical microscope with a fundus‐viewing contact lens placed on the cornea. The scleral incision and the conjunctiva were sutured with 8‐0 Vicryl (polyglactin 910) suture (Ethicon, Johnson & Johnson, New Brunswick, NJ, USA). Intramuscular atipamezole (5 mg/mL, 0.02 mL/kg, Antisedan, Orion Pharma Animal Health) were given to reverse anesthesia. Monkeys received once daily instillation of 0.5% levofloxacine and 1% atropine, and intramuscular injection of buprenorphine hydrochloride (0.2 mg/mL, 0.05 mL/kg, Lepetan, Otsuka Pharmaceutical, Osaka) and dihydrostreptomycin sulfate procaine benzylpenicillin (0.05 mL/kg, MycillinSol, Kyoritsu Pharmaceutical, Tokyo) for 3 days.
Surgical Procedures
Before anesthesia, monkeys received intramuscular injection of atropine sulfate (0.5 mg/mL, 0.02 mL/kg of body weight, Mitsubishi Tanabe Pharma, Osaka, Japan) to suppress air way mucus secretion, buprenorphine hydrochloride (Lepetan, 0.2 mg/mL, 0.05 mL/kg, Otsuka Pharmaceutical) for analgesia, and dihydrostreptomycin sulfate procaine benzylpenicillin mixture (MycillinSol, 0.05 mL/kg, Kyoritsu Pharmaceutical) as antibiotics. Monkeys were then sedated with intramuscular ketamine hydrochloride (50 mg/kg, 0.2 mL/kg, Supriya Lifescience, Mumbai, India). After intubation, anesthesia was maintained with nitric oxide gas and oxygen (2:1 mixture) with isoflurane (0.5–2.0%, DS Pharma Animal Health, Osaka). Blood pressure, heart rate, electrocardiogram, body temperature, and percutaneous oxygen saturation were monitored with Animal Monitor Life Scope (BSM‐2391 or BSM‐3592, Nihon Kohden, Tokyo). Mydriasis in the right eye was induced by 0.5% tropicamide and 0.5% phenylephrine eye drops (Mydrin‐P) on the day of surgery.
After disinfection with 10% povidone iodine (Negmin Solution, Pfizer Japan, Tokyo) on the skin around the eye and then with 40‐time saline‐diluted povidone iodine on the ocular surface, the monkey's head was positioned with the cornea aligned in the horizontal plane, and covered with a surgical drape. Topical anesthesia was further obtained with 4% lidocaine (Xylocaine Ophthalmic Solution, AstraZeneka, London, UK). The surgery was done under a surgical microscope (OPMI VISU150, Carl Zeiss Meditec, Tokyo, Japan) with an ophthalmic surgical machine (Constellation Vision System, Alcon Laboratories). Anterior capsulectomy (Fig. 1a) was done with a 25‐gauge vitreous cutter under irrigation with a 25‐gauge infusion cannula through two side ports which were made at the corneal limbus with a 20‐gauge knife (V‐Lance Knife, Alcon). Phacoemulsification and aspiration of the lens in the capsular bag (Fig. 1b) was done through a 2.4 mm‐wide corneal incision made on the superior side with a disposable knife (Safety Knife, Kai Medical, Seki, Japan). The corneal incision was sutured with 8‐0 Vicryl suture. Three 25‐gauge trocars (Fig. 1c) were inserted into the vitreous through the conjunctiva and sclera 2 mm from the corneal limbus in the superotemporal and superonasal quadrant for instrument insertion and in the inferotemporal quadrant to place the infusion cannula.
The wide‐field fundus was viewed with a +128‐diopter front lens by Resight 500 fundus viewing system (Carl Zeiss Meditec). Posterior capsulectomy (Fig. 1c) and core vitrectomy was done. Retinal detachment was then induced by infusing irrigation solution (BSS‐Plus Intraocular Irrigating Solution, Alcon) into subretinal space with a 38‐gauge polyimide tip (PolyTip Cannula 25G/38G, MedOne Surgical, Inc., Sarasota, FL, USA) attached to a 10‐mL syringe for the viscous fluid control (VFC) system at the setting of low intraocular pressure (Fig. 1d,e). A retinotomy was made by 25‐gauge diathermy (Grieshaber Diathermy Probe DSP 25Ga, Alcon) at the edge of retinal detachment (Fig. 1f). The conjunctiva was opened, 3 mm‐wide scleral incision was placed with a microsurgery knife (Straight/Stab 22.5°, Kai Medical), 2 mm posteriorly in parallel with the corneal limbus (Fig. 1g), and wound hemostasis was accomplished with a wet‐field hemostatic eraser bipolar instrument (Beaver‐Visitec International, Inc., Waltham, MA, USA). A sheet of the dye‐coupled film in 2.5 × 5 mm square size (Fig. 1h,i) was grasped with a 20‐gauge subretinal forceps (Synergetics 39.21S, Bausch + Lomb Retina, St. Louis, MO, USA), and inserted into the vitreous (Fig. 1j) and then brought under the detached retina through a retinotomy (Fig. 1k). The scleral incision for film insertion was sutured with 8‐0 Vicryl suture (Fig. 1m). The subretinal fluid was aspirated with a vitreous cutter, and the fluid in vitreous cavity was exchanged with air to reattach the retina (Fig. 1l). Laser photocoagulation (Fig. 1n) was applied around the retinal tear caused by retinotomy, and 30% sulfur hexafluoride gas (ISPAN, Alcon) with air was injected into vitreous cavity from the infusion cannula. Trocars were removed (Fig. 1o), and the conjunctiva was sutured with 8‐0 Vicryl suture (Fig. 1m).
At surgery to remove dye‐coupled films implanted in the subretinal space, three 25‐gauge trocars were placed and retinal detachment was induced by infusing irrigation fluid into the subretinal space by a 38‐gauge tip. A retinotomy was made by diathermy and a subretinal dye‐coupled film was grasped with a 25‐gauge forceps (Grieshaber Revolution DSP 25Ga ILM Forceps, Alcon) and brought to vitreous cavity. The film was brought out of the eye ball with a 25‐gauge forceps through a newly made 3‐mm‐wide scleral incision 2 mm posterior to the corneal limbus. Retinal reattachment was obtained by fluid‐air exchange, laser photocoagulation, and gas injection. The films were immersed in distilled water for material analyses. Microscopic surgical view was recorded with a digital processor miniature 3CCD color camera (THD‐311, Ikegami Tsushinki Co., Tokyo, Japan).
After the surgery, monkeys received intramuscular injection of dihydrostreptomycin sulfate procaine benzylpenicillin (0.05 mL/kg, MycillinSol) for 3 days and intramuscular injection of ketoprofen (2 mg/kg, Capisten, Kissei Pharmaceutical, Matsumoto, Japan) for 2 days. Postoperative instillation of 0.5% levofloxacin (Cravit), 0.1% betamethasone (Rinderon, Shionogi & Co., Osaka, Japan), and 1% atropine (Nitten) three times daily were continued for postoperative one month.
Electroretinography and Visual Evoked Potential
Monkeys were sedated with intramuscular injection of a 7:1 mixture (0.2 mL/kg of body weight) of ketamine hydrochloride (50 mg/mL) and xylasine (20 mg/mL, Celactal 2%, Bayer Animal Health, Tokyo, Japan). Mydriasis was induced with 0.5% tropicamide and 0.5% phenylephrine eye drops (Mydrin‐P), and dark adaptation was obtained for at least 40 min. After ocular surface anesthesia with 0.4% bupivacaine eye drops, a 13‐mm‐diameter contact lens (base curve 6.0) with ring electrodes for recording and reference (Kyoto Contact Lens, Kyoto) was placed on the corneal surface with hydroxyethylcellulose gel (Scopisol, Senju Pharmaceutical, Osaka). A needle electrode for ground was placed on the parietal skin. Maximal response of full‐field electroretinography to standard flash (3.0 cd × sec/m2) was recorded with PuREC (PC‐100A, Mayo Corporation, Inazawa City, Japan) and SG‐2002 Stand‐Alone Ganzfeld System (LKC Technologies, Gaithersburg, MD, USA).
Focal electroretinography at photopic condition in the background of 1.5 cd/m2 was recorded with the same contact lens electrode (Kyoto Contact Lens), in response to 15‐degree circular light stimulus (30 cd/m2, 5 Hz, 100 msec) in macular degenerative area where the dye‐coupled film was placed, and also in the normal retinal area on the nasal side of the fundus (Kowa ER‐80, Kowa, Nagoya, and PuREC PC‐100A, Mayo Corporation).
Visual evoked potential was measured by PuREC (PC‐100A Mayo) with plate electrodes placed on the right upper and left upper side of the occipital nodule, in combination with subcutaneous needle electrodes placed on the frontal center for reference, and on the parietal center for ground. After dark adaptation and mydriasis for at least 15 min, flash light stimuli in the intensity of 3.082 cd × sec/m2 at the frequency of 60 Hz were given by SG‐2002 Stand‐Alone Ganzfeld System (LKC Technologies) to the right eye and then to the left eye. The amplitude and latency of P1 N1, and P2 in the 128 summation of recordings with high pass filter at 1 Hz and low pass filter at 100 Hz were automatically detected and used for analyses.
Ultrasonography and Optical Coherence Tomography
Under anesthesia with intramuscular ketamine and xylasine, the presence or the absence of retinal detachment was screened by ultrasonography (SSD‐ALPHA7, Hitachi‐Aloka Medical, Tokyo). The anterior segments were observed by portable slit‐lamp biomicroscopy (SL‐15, Kowa), and the fundus examination was done with binocular funduscopy (IO‐alpha Small Pupil, Neitz Instruments, Tokyo). The images of horizontal retinal sections in macular degenerative area where the dye‐coupled film was implanted were obtained by optical coherence tomography (Heidelberg Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany).
References
- 1. Matsuo T, Morimoto N. Visual acuity and perimacular retinal layers detected by optical coherence tomography in patients with retinitis pigmentosa. Br J Ophthalmol 2007;91:888–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamaki M, Matsuo T. Optical coherence tomographic parameters as objective signs for visual acuity in patients with retinitis pigmentosa, future candidates for retinal prostheses. J Artif Organs 2011;14:140–50. Erratum 2011;14:385. [DOI] [PubMed] [Google Scholar]
- 3. Loewenstein JI, Montezuma SR, Rizzo JF III. Outer retinal degeneration: an electronic retinal prosthesis as a treatment strategy. Arch Ophthalmol 2004;122:587–96. [DOI] [PubMed] [Google Scholar]
- 4. Humayun MS, de Juan E Jr, Dagnelie G. The bionic eye: a quarter century of retinal prosthesis research and development. Ophthalmology 2016;123:S89–97. [DOI] [PubMed] [Google Scholar]
- 5. Humayun MS, Dorn JD, da Cruz L, et al. Interim results from the international trial of Second Sight's visual prosthesis. Ophthalmology 2012;119:779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Da Cruz L, Dorn JD, Humayun MS, et al. Five‐year safety and performance results from the Argus II retinal prosthesis system clinical trial. Ophthalmology 2016;123:2248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stingl K, Bartz‐Schmidt KU, Besch D, et al. Subretinal visual implant Alpha IMS – clinical trial interim report. Vision Res 2015;111:149–60. [DOI] [PubMed] [Google Scholar]
- 8. Stingl K, Schippert R, Bartz‐Schmidt KU, et al. Interim results of a multicenter trial with the new electronic subretinal implant Alpha AMS in 15 patients blind from inherited retinal degenerations. Front Neurosci 2017;11:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roux S, Matonti F, Dupont F, et al. Probing the functional impact of sub‐retinal prosthesis. Elife 2016;5:e12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chuang AT, Margo CE, Greenberg PB. Retinal implants: a systematic review. Br J Ophthalmol 2014;98:852–6. [DOI] [PubMed] [Google Scholar]
- 11. Ghezzi D. Retinal prostheses: progress toward the next generation implants. Front Neurosci 2015;9:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly SK, Shire DB, Chen J, et al. A hermetic wireless subretinal neurostimulator for vision prostheses. IEEE Trans Biomed Eng 2011;58:3197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorach H, Goetz G, Smith R, et al. Photovoltaic restoration of sight with high visual acuity. Nat Med 2015;21:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maya‐Vetencourt JF, Ghezzi D, Antognazza MR, et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat Mater 2017;16:681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuo T. A simple method for screening photoelectric dyes towards their use for retinal prostheses. Acta Med Okayama 2003;57:257–60. [DOI] [PubMed] [Google Scholar]
- 16. Uchida T, Ishimaru S, Shimamura K, Uji A, Matsuo T, Ohtsuki H. Immobilization of photoelectric dye on the polyethylene film surface. Mem Fac Eng Okayama Univ 2005;39:16–20. [Google Scholar]
- 17. Matsuo T, Dan‐oh Y, Suga S, (Inventors). US Patent. US 7,101,533 B2. Date of Patent: Sep. 5, 2006.
- 18. Uji A, Matsuo T, Ishimaru S, et al. Photoelectric dye‐coupled polyethylene film as a prototype of retinal prostheses. Aritif Organs 2005;29:53–7. [DOI] [PubMed] [Google Scholar]
- 19. Uji A, Matsuo T, Uchida T, Shimamura K, Ohtsuki H. Intracellular calcium response and adhesiveness of chick embryonic retinal neurons to photoelectric dye‐coupled polyethylene films as prototypes of retinal prostheses. Artif Organs 2006;30:695–703. [DOI] [PubMed] [Google Scholar]
- 20. Tamaki T, Matsuo T, Hosoya O, et al. Glial reaction to photoelectric dye‐based retinal prostheses implanted in the subretinal space of rats. J Artif Organs 2008;11:38–44. [DOI] [PubMed] [Google Scholar]
- 21. Okamoto K, Matsuo T, Tamaki T, Uji A, Ohtsuki H. Short‐term biological safety of a photoelectric dye used as a component of retinal prostheses. J Artif Organs 2008;11:45–51. [DOI] [PubMed] [Google Scholar]
- 22. Liu S, Matsuo T, Hosoya O, Uchida T. Photoelectric dye used for Okayama University‐type retinal prosthesis reduces the apoptosis of photoreceptor cells. J Ocul Pharmacol Ther 2017;33:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang F, Bladon J, Lagoy RC, et al. A photosensitive surface capable of inducing electrophysiological changes in NG108‐15 neurons. Acta Biomater 2015;12:42–50. [DOI] [PubMed] [Google Scholar]
- 24. Matsuo T, Uchida T, Takarabe K. Safety, efficacy, and quality control of a photoelectric dye‐based retinal prosthesis (Okayama University‐type retinal prosthesis) as a medical device. J Artif Organs 2009;12:213–25. [DOI] [PubMed] [Google Scholar]
- 25. Matsuo T, Uchida T, Nitta M, et al. Subretinal implantation of Okayama University‐type retinal prosthesis (OURePTM) in canine eyes by vitrectomy. J Vet Med Sci 2017;79:1939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alamusi Matsuo T, Hosoya O, Tsutsui KM, Uchida T. Behavior tests and immunohistochemical retinal response analyses in RCS rats with subretinal implantation of Okayama‐University‐type retinal prosthesis. J Artif Organs 2013;16:343–51. [DOI] [PubMed] [Google Scholar]
- 27. Alamusi Matsuo T, Hosoya O, Tsutsui KM, Uchida T. Vision maintenance and retinal apoptosis reduction in RCS rats with Okayama University‐type retinal prosthesis (OURePTM) implantation. J Artif Organs 2015;18:264–71. [DOI] [PubMed] [Google Scholar]
- 28. Alamusi Matsuo T, Hosoya O, Uchida T. Visual evoked potential in RCS rats with Okayama University‐type retinal prosthesis (OURePTM) implantation. J Artif Organs 2017;20:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shirai H, Mandai M, Matsushita K, et al. Transplantation of human embryonic stem cell‐derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci USA 2016;113:E81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsuo T. Intraocular lens implantation in unilateral congenital cataract with minimal levels of persistent fetal vasculature in the first 18 months of life. Springerplus 2014;3:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuo T, Uchida T, Yamashita K, et al. Visual evoked potential in rabbits' eyes with subretinal implantation by vitrectomy of Okayama University‐type retinal prosthesis (OURePTM). J Vet Med Sci 2018;80:247–259. doi:10.1292/jvms.17‐0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.


