Abstract
Background
The heart rate variability (HRV)‐derived Analgesia Nociception Index (ANI™) is a continuous noninvasive tool to assess the nociception/antinociception balance in unconscious patients. It has been shown to be superior to haemodynamic variables in detecting insufficient antinociception in children, while little is known about its predictive value.
Methods
The primary objective of this prospective observational pilot study in paediatric surgical patients under sevoflurane anaesthesia was to compare the predictive value of the ANI and heart rate to help decide to give additional opioids. The paediatric anaesthesiologist in charge was blinded to ANI values.
Results
In patients with an ANI value <50 (indicating insufficient antinociception) at the moment of decision, ANI values dropped from ±55 (indicating sufficient antinociception) to ±35, starting 60 s before decision. Within 120 s after administration of fentanyl (1 μg/kg), ANI values returned to ±60. This phenomenon was only observed in the ANI values derived from HRV data averaged over 2 min. Heart rate remained unchanged. In patients with ANI values ≥50 at the time of decision, opioid administration had no effect on ANI or heart rate. The same accounts for morphine for postoperative analgesia and fentanyl in case of intraoperative movement.
Conclusions
This study provides evidence of a better predictive value of the ANI in detecting insufficient antinociception in paediatric surgical patients than heart rate. The same accounts for depicting re‐establishment of sufficient antinociception after opioid drug administration.
Significance
In paediatric surgical patients anaesthetized with sevoflurane, the heart rate variability‐derived Analgesia Nociception Index (ANI) appears to be a better predictor of insufficient antinociception than heart rate. The ANI also appears to depict re‐establishment of sufficient antinociception better than heart rate.
1. Introduction
It has been one of the biggest desires of (paediatric) anaesthetists for many years to have a valid noninvasive tool available, which provides them with continuous information regarding the nociception/antinociception balance of their unconscious patients. In the absence of this long‐awaited tool, assessment of the nociception/antinociception balance in anaesthetized patients is performed using surrogate parameters, such as heart rate and blood pressure, together with other clinical signs such as sweating or movement. These parameters are known to have low sensitivity and specificity in detecting inadequate antinociception, possibly resulting in under‐ or overdosing of opioid drugs (Daccache et al., 2017). Probably, the most frequently applied heuristic approach to help decide whether or not an anaesthetized patient needs additional opioids is the clinical impression of the anaesthetist. This assumption appears to be so self‐evidently true that there are no published data available in the scientific literature supporting it.
During the last two decades, a variety of analgesia monitoring systems have become commercially available. To detect analgesia or nociception/antinociception, these devices use surrogate parameters, such as skin conductance, plethysmography, pupillometry and heart rate variability analysis (Gruenewald and Ilies, 2013).
Recent studies performed in anaesthetized children suggest that the heart rate variability‐derived Analgesia Nociception Index (ANI™; Mdoloris Medical Systems, Loos, France) may provide a more sensitive assessment of the nociception/antinociception balance than haemodynamic parameters (Migeon et al., 2013; Sabourdin et al., 2013). These paediatric studies are in accordance with the results of studies investigating the performance of the ANI in anaesthetized adult patients (Gruenewald et al., 2013, 2015; Boselli et al., 2015).
While there is a growing body of evidence showing the ability of the ANI to provide valid information regarding the nociception/antinociception balance in anaesthetized patients, little is known about its value in predicting inadequate antinociception.
The primary objective of this prospective observational pilot study in paediatric surgical patients under sevoflurane anaesthesia was to compare the predictive value of ANI and heart rate to help the anaesthetist decide to give additional opioid analgesics. Major secondary objectives were the impact of per‐protocol opioid administration for postoperative and caudal analgesia on the course of the ANI. Changes in ANI values were compared to concomitant changes in heart rate for all analyses.
2. Methods
2.1. Participants and data collection
Paediatric patients, aged between 2 and 12 years, scheduled for elective surgery under sevoflurane anaesthesia at Erasmus MC – Sophia Children's Hospital, Rotterdam, the Netherlands, were eligible for inclusion in this prospective observational study.
Technical requirements of the ANI algorithm resulted in the definition of the following primary exclusion criteria: cardiac rhythm other than sinus rhythm, or an internal cardiac pacemaker, high frequency or jet ventilation during surgery.
2.1.1. The Analgesia Nociception Index (ANI)
The Analgesia Nociception Index (ANI) is calculated by the PhysioDoloris™ monitor (Mdoloris Medical Systems, Loos, France) using the ECG signal recorded by and extracted from the standard anaesthesia patient monitoring system (Dräger Infinity®, Drägerwerk AG & Co. KGaA, Lübeck, Germany) without the need for additional ECG electrodes.
The basic principle of the ANI is the real‐time analysis of the parasympathetic (pS) activity of the autonomous nervous system by means of heart rate variability (HRV) analysis. A fast wavelet transform rejects any other HRV signal than the high‐frequency band (0.15–0.4 Hz), expressing the physiological respiratory sinus arrhythmia. Based on these filtered data, the ANI, a dimensionless numerical value, is calculated. The ANI ranges from 0 to 100 and is related to the relative pS activity, with high index values indicating a high level of pS activity and vice versa (Daccache et al., 2017). The ANI serves as a surrogate parameter of the nociception/antinociception balance in unconscious patients. An ANI value of 50 has been suggested as a threshold to distinguish between adequate and inadequate antinociception in anaesthetized patients (Daccache et al., 2017). An in‐depth description of the ANI algorithm has been published by Jeanne et al. (2009).
The PhysioDoloris monitor displays two ANI values, which differ with regard to the cycle of their moving average windows: The fast ANIi (i = instant) is derived from a moving average time of 120 s, whereas the ANIa (a = average) is derived from a 240 s window (Jeanne et al., 2014).
2.1.2. Intraoperative collection of study data
Analgesia Nociception Index and heart rate data, recorded during the anaesthetic, were downloaded from the PhysioDoloris monitor as .txt‐files. Data derived from the Dräger Infinity patient monitoring system were exported as .xls files. Only data recorded during the surgery were used for subsequent analysis.
During the surgical procedure, the paediatric anaesthetist in charge was blinded to the screen of the PhysioDoloris monitor and the conduct of the anaesthetic was totally unrestricted. Each decision to give an opioid analgesic, together with the motivation to do so, was announced to a researcher who recorded this decision and the subsequent drug administration.
2.2. Ethical consideration
This prospective observational pilot study was approved by the Medical Ethics Review Board of Erasmus University Medical Center, Rotterdam, the Netherlands (MEC‐2016‐501, 9 August 2016). The study was conducted in accordance with the Declaration of the World Medical Association. Written informed consent was obtained from the parents or legal guardians of all participating patients.
2.3. Statistical analysis
Analyses were performed using data related to predefined events. An event was defined as the intraoperative administration of opioid drugs, either due to clinical judgement of insufficient antinociception or per‐protocol administration for postoperative analgesia.
Repeated measures one‐way ANOVA with subsequent Holm–Sidak's multiple comparison test was performed to investigate the course of ANIi, ANIa and HR values for the primary objective of the study during a 120‐s period both before the clinical judgement of insufficient antinociception and after subsequent opioid drug application. ANOVA was performed separately for cases when the ANI was either <50 or ≥50 at the time of decision to administer additional opioid analgesia. For the secondary objectives, ANOVA, using the same set of parameters, was performed to investigate the impact of opioid drug administration due to patient movement during surgery, per‐protocol application of morphine for postoperative analgesia and following caudal epidural analgesia.
Unfortunately, we had no scientifically sound data available on which we could have based a valid sample size calculation. We assumed that data from at least 100 patients should provide us with sufficient information to further develop our research efforts regarding monitoring of the nociception/antinociception balance in anaesthetized children. The duration of the project was set to 3 months, which, based on our average annual number of anaesthetics performed in children between 2 and 13 years, should be sufficient to include ≥100 participants.
2.4. Software
Statistical analysis was performed using Prism 7 for Mac OS X (Version 7.0D, GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Participant demographics and general patient information
A total of 131 patients (female/male: 63/68) could be recruited for participation during a three‐month inclusion period (03‐06/2017). Patient age and weight were 84.7 ± 33.7 months and 25.9 ± 11.4 kg, respectively. Information regarding the surgical procedures and anaesthesia techniques is given in Table 1. According to departmental standards, patients received intravenous paracetamol 20 mg/kg and diclofenac 1 mg/kg during the procedure for postoperative analgesia.
Table 1.
Distribution of surgical procedures performed, together with anaesthesia technique
| Type of surgery | Anaesthesia technique | |
|---|---|---|
| General anaesthesia | General anaesthesia with caudal block | |
| Ear nose & throat surgery | 33 | |
| Eye surgery | 13 | |
| General paediatric surgery | 26 | 5 |
| Neurosurgery | 3 | |
| Oral & maxillofacial surgery | 14 | |
| Orthopaedic surgery | 7 | 1 |
| Plastic surgery | 20 | |
| Radiology intervention | 1 | |
| Urological surgery | 1 | 4 |
Due to permanent data logging errors, three patients had to be excluded from data analysis. Data sets from 35 events (intraoperative opioid drug administration) had to be excluded from analysis due to incidental data logging errors. Data sets from 52 events (intraoperative opioid drug administration) and 10 patients who had a supplemental caudal block were available for analysis.
3.2. Course of ANI values around opioid administration
Data from 14 events recorded in patients with ANI values <50 at the time of decision, indicating insufficient antinociception, were available for analysis. ANIi values at the time of decision to give opioid drugs due to a perceived rise in heart rate were lower than 60, 90 and 120 s before. Opioid drug administration (fentanyl, on average 0.8 μg/kg) resulted in a significant rise in ANIi, starting at 60 s and reaching predecision values after 120 s. ANIa and heart rate remained unchanged during the entire period (see Fig. 1).
Figure 1.
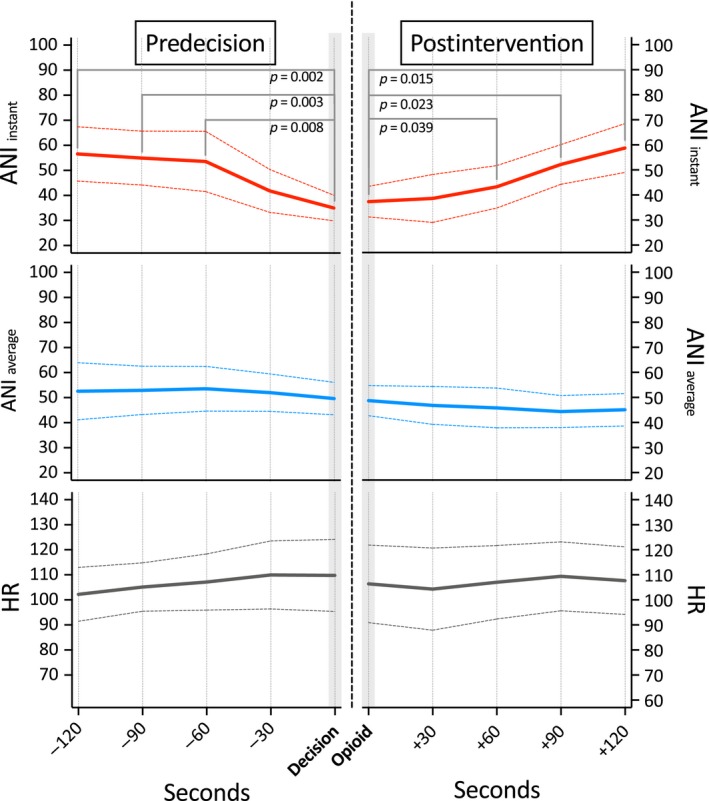
Course of the Analgesia Nociception Index (ANI) and heart rate (HR) values before and after i.v. opioid drug administration in patients with ANI i values <50 (indicating insufficient antinociception) at the moment of decision. (ANI i: values averaged over 120 s; ANI a: values averaged over 240 s).
Data from 13 events recorded in patients with ANI values ≥50 at the time of decision, indicating sufficient antinociception, showed stable values ANIi and ANIa and heart rate values during the entire period before and after opioid drug administration (fentanyl, on average 1.0 μg/kg). For details, see Fig. 2.
Figure 2.
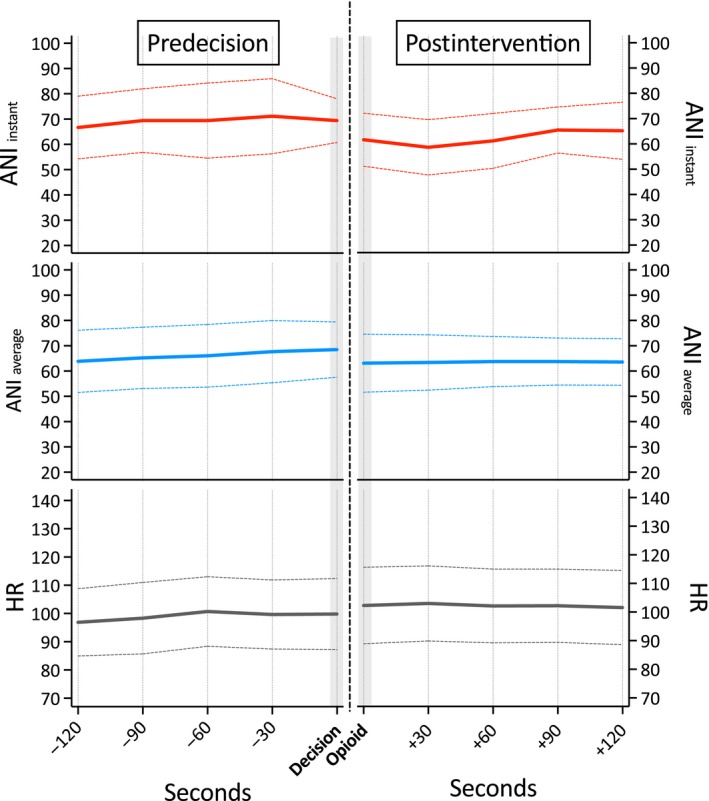
Course of the Analgesia Nociception Index (ANI) and heart rate (HR) values before and after i.v. opioid drug administration in patients with ANI i values ≥50 (indicating sufficient antinociception) at the moment of decision. (ANI i: values averaged over 120 s; ANI a: values averaged over 240 s).
Opioid administration due to patient movement during surgery (nine events) had no effect on ANIi, ANIa and heart rate. For details, see Fig. 3.
Figure 3.
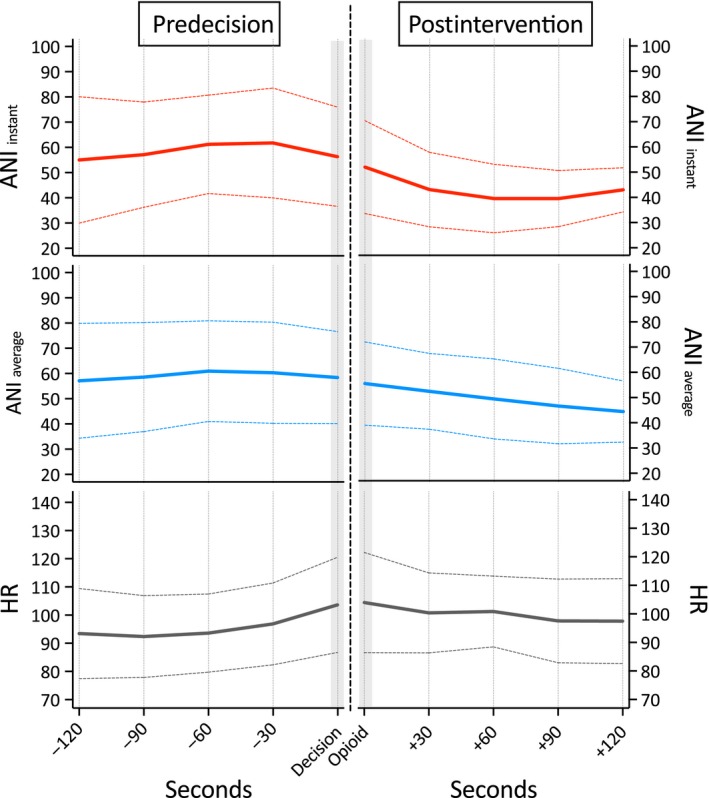
Course of the Analgesia Nociception Index (ANI) and heart rate (HR) values before and after i.v. opioid drug administration due to movement during surgery. (ANI i: values averaged over 120 s; ANI a: values averaged over 240 s).
Per‐protocol administration of morphine 0.1 mg/kg for postoperative analgesia had no effect on ANIi, ANIa and heart rate, as revealed by analysis of 16 events. For details, see Fig. 4.
Figure 4.
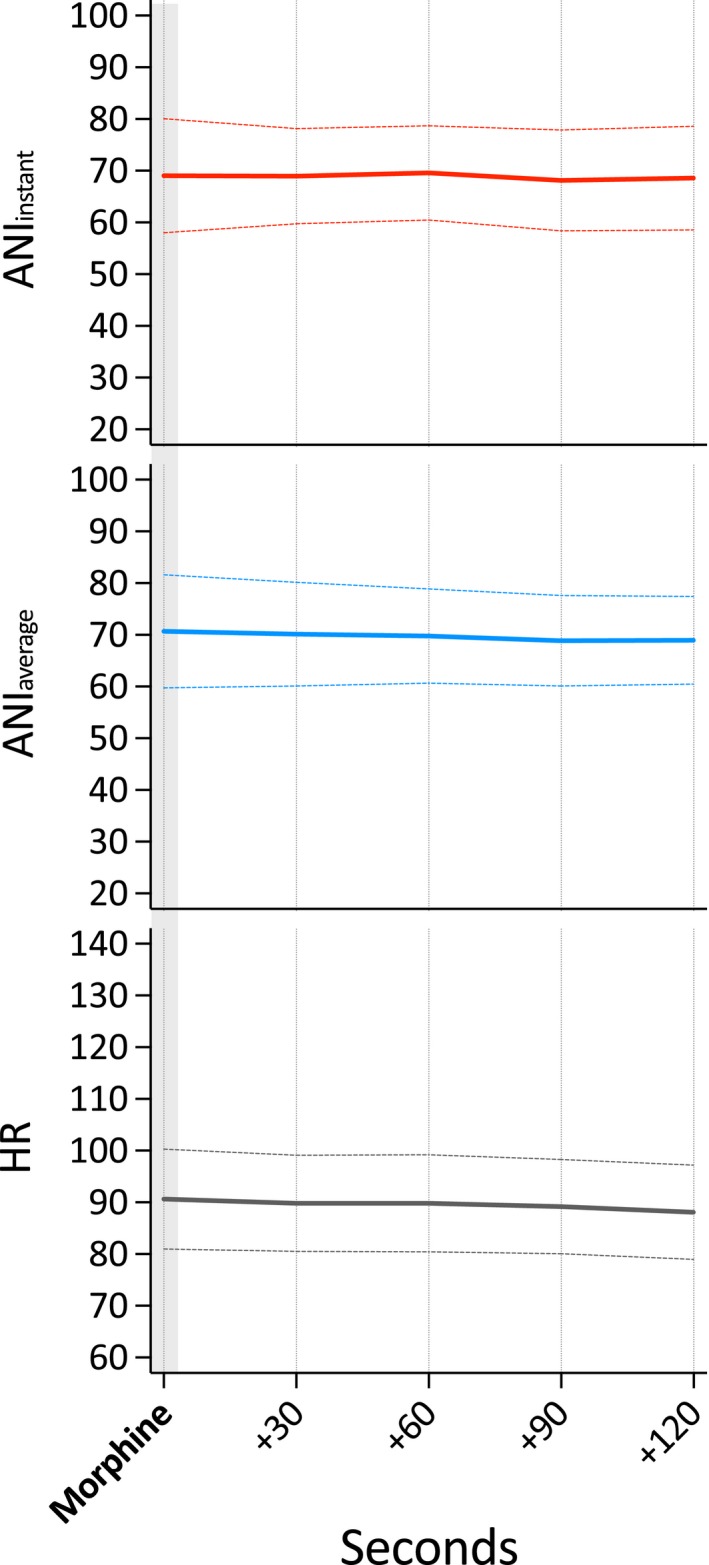
Course of the Analgesia Nociception Index (ANI) and heart rate (HR) values following per‐protocol i.v. morphine administration for postoperative analgesia. (ANI i: values averaged over 120 s; ANI a: values averaged over 240 s).
3.3. Course of ANI values in patients with supplemental caudal block
Within 2 min after surgical incision, which was allowed to be performed no earlier than 10 min after caudal–epidural injection of ropivacaine 0.2%, 1–1.2 mL/kg, we observed a decline in average ANIa values from 82 ± 19 to 68 ± .21 (p = 0.019), still indicative of sufficient antinociception. These change could not be observed in average ANIi or heart rate values (Fig. 5).
Figure 5.
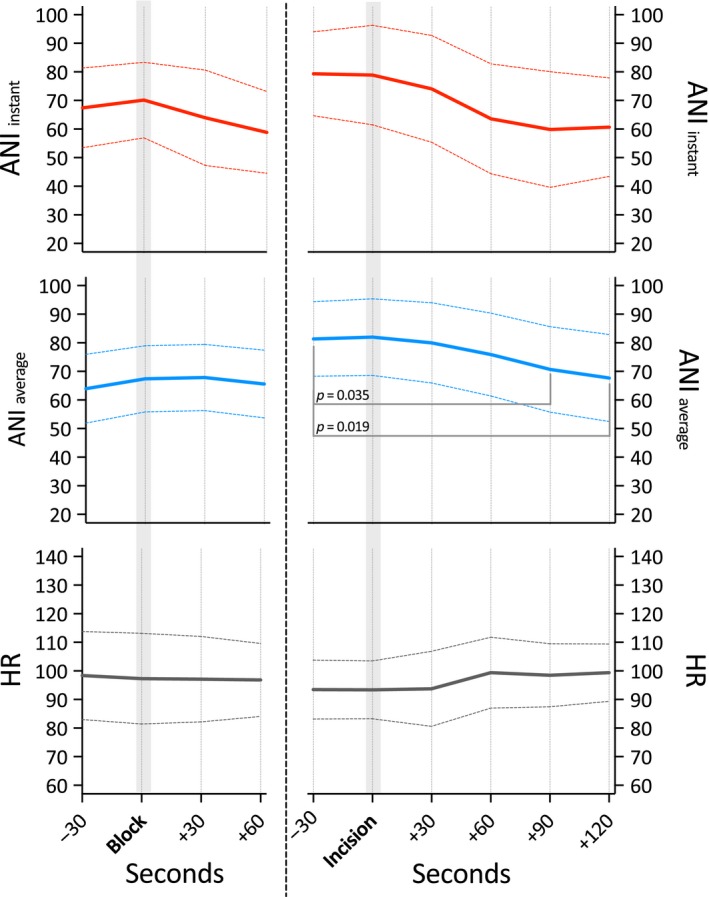
Course of the Analgesia Nociception Index (ANI) and heart rate (HR) values before and after caudal block (left) and surgical incision (right). (ANI i: values averaged over 120 s; ANI a: values averaged over 240 s).
4. Discussion
The results of this observational pilot study provide evidence of the predictive value of the ANIi in detecting insufficient antinociception in paediatric surgical patients under sevoflurane anaesthesia. There is also evidence that the ANIi is depictive of re‐establishment of sufficient analgesia after opioid drug administration.
Although these are certainly a promising findings, we have to acknowledge the following significant methodological issue associated with the attempts to investigate the validity of nociception/antinociception monitoring systems: The most frequently chosen approach to investigate the validity of nociception/antinociception monitors is to compare their values to heart rate and/or blood pressure, regarded as the golden standard, although it is nowadays commonly accepted that heart rate and blood pressure are poor predictors of insufficient antinociception (Daccache et al., 2017). Our study was designed under the prerequisite of acknowledging the assumption that the ANI is capable of detecting noxious stimuli with a higher sensitivity than the clinical standard parameters, heart rate and blood pressure. A growing body of evidence suggests that this assumption may be true:
Sabourdin et al. (2013) investigated the reaction of the ANI to a standardized tetanic stimulation in paediatric patients anaesthetized with desflurane–remifentanil and found the ANI to be a more sensitive indicator of nociception than heart rate and blood pressure.
In another paediatric study, performed in children anaesthetized with sevoflurane, Migeon et al. (2013) found the ANI to be a useful tool to assess efficacy of regional anaesthesia, both as a stand‐alone tool and in combination with heart rate changes.
Bobkowski et al. (2017) reported a significant impact of patient age on HRV in children aged between 3 and 12 years, except from HRV contributors derived from high‐frequency oscillations. The ANI algorithm is entirely based on high‐frequency oscillations (Daccache et al., 2017), making it very unlikely that patient age significantly contributes to outcome in our study.
Gruenewald et al. (2013) reported that the ANI was capable of reflecting noxious stimulation in adult patients anaesthetized with propofol–remifentanil and sevoflurane–remifentanil (Gruenewald et al., 2015). The authors concluded the ANI may be an indicator of the nociception/antinociception balance, without being of predictive value (Gruenewald et al., 2013). Their study design was to apply standardized painful tetanic stimulation under increasing remifentanil effect site concentrations. As opposed to Gruenewald et al., we found evidence of a predictive value of the ANIi to detect insufficient antinociception. This difference in outcome is most likely due to the differences in study design. Whereas Gruenewald et al. applied a controlled 30‐s stimulus prior to the start of the surgical procedure, our study aimed to investigate the performance of the ANI during the surgical procedure.
Jeanne et al. (2012) performed a study in adult patients under propofol–remifentanil anaesthesia and concluded that it was likely that the ANI is more sensitive to moderate noxious stimulation than heart rate and blood pressure.
Our study focused on the variation of the ANI around an event defined by the attending anaesthetist (need for opioid analgesia). The design of our study allowed the anaesthetist, who was blinded to ANI values, to be totally unrestricted regarding the parameters he applied to assess the appropriateness or inappropriateness of the level of antinociception during the surgical procedure. Perceived rise in heart rate was the most commonly reported reason to give additional opioid drug. Interestingly, these perceived rises in heart rate were very small, most frequently several beats/min only. In patients presenting with ANIi values <50 at the time of decision to give additional opioids, this ANIi value was significantly lower than 60, 90 and 120 s before. Furthermore, within 120 s after opioid drug administration, it returned to the same level of around 60 units like 120 s before the diagnosis of insufficient antinociception. As opposed to the ANIi, the ANIa remained unchanged. In patients presenting with ANIi values ≥50 at the time of decision to give additional opioids, none of the investigated parameters (ANIi, ANIa and heart rate) showed any changes before and after opioid drug administration. The same accounts for the course of ANIi, ANIa and heart rate after per‐protocol administration of morphine for postoperative analgesia, where the mean baseline ANIi was about 70 units, indicating sufficient antinociception.
Patient movement during surgery can be regarded as a sign of insufficient antinociception. In our series of nine events when opioid drugs were given due to patient movement, there were no significant changes in either ANIi, ANIa or heart rate. Movement could as well have been a result of too light anaesthesia. Unfortunately, depth of hypnosis monitoring was not applied in any patient, so we can only speculate on this.
The results of our study allow us to suggest the following theory:
In paediatric surgical patients under sevoflurane anaesthesia, ANIi values of ±35, especially after a decline from ±55 within 2 min, most likely reflect insufficient antinociception, even without an evident raise in heart rate. A single dose of ±1 μg/kg fentanyl resulted in a return to ANIi values of ±58, suggesting sufficient antinociception, within 2 min. The ANIa, averaged over 4 min did not show this phenomenon. It seems as if a 4 min averaging period is too long to reflect the typically instantaneous changes in the nociception/antinociception balance during a surgical procedure.
4.1. Limitations
When designing our study, we originally also wanted to investigate the impact of per‐protocol administration of opioid drugs immediately before the surgical incision on ANI and heart rate. Unfortunately, many disruptive nonpainful interventions (patient positioning, application of neutral electrodes for electrocoagulation, etc.) routinely take place during this particular period, resulting in poor ECG signal quality. We therefore decided to restrict our data analysis period to the duration of the surgical procedure.
We decided to restrict our analysis to the course of heart rate, leaving blood pressure unnoticed, which could be regarded as a shortcoming of our study design. This decision was due to the fact that, in our daily practice of paediatric anaesthesia, noninvasive blood pressure is measured at 5‐min intervals. Compared to the ANI and heart rate, which were available at 1‐s intervals, this would have been a far too long interval to result in any meaningful conclusion.
4.2. Future directions
Future studies in paediatric surgical patients should focus on defining optimum doses of opioid drugs to re‐establish ANI values indicating sufficient intraoperative antinociception after a decline in ANIi values below the threshold of 50. Furthermore, the ANI may help to prevent excessive opioid drug administration. Too much opioid drug can result in acute μ‐receptor desensitization, contributing to opioid drug failure to provide postoperative analgesia. Of special interest for paediatric care is the impact of opioid drugs on postanaesthesia respiratory patterns. ANI‐derived opioid intervention guidelines would enable us to conduct outcome studies in paediatric surgical patients, focusing on postoperative pain and opioid drug side effects.
An integrated approach using ANI monitoring and processed EEG would cover both nociception/antinociception and depth of hypnosis. The ANI may bring us closer to monitoring depth of anaesthesia as a whole. Well‐designed future clinical studies will have to show if it is possible to develop such an integrated approach to depth of anaesthesia monitoring (in children).
5. Conclusions
In paediatric surgical patients anaesthetized with sevoflurane, a decline of the ANIi from values around 60 to values around 35 is indicative of insufficient antinociception. After opioid drug administration, the ANIi returns to values around 60, suggesting sufficient antinociception. This phenomenon does not necessarily need to be accompanied by haemodynamic changes, suggesting the superiority of the ANIi over heart rate as a predictive tool to detect insufficient antinociception and as an indicator of re‐establishment of sufficient antinociception after opioid drug administration.
Acknowledgements
The authors thank Mr. Theus van Dam, Thoraxcenter, Erasmus MC, for his technical support in setting up the data logging environment.
Author contributions
F. Weber, N.J.E. Geerts and H.G. Roeleveld were responsible for design and conduct of study, data analysis and manuscript preparation. A.T. Warmenhoven was responsible for design of study, data analysis and manuscript preparation. C.A. Liebrand was responsible for data analysis and manuscript preparation.
Funding sources
The ANI monitor was provided on loan by the manufacturer, Mdoloris Medical Systems, Loos, France. Mdoloris was neither involved in the design and conduct of the study, nor in data analysis or preparation of the manuscript.
Conflicts of interest
F. Weber, N.J.E. Geerts, H.G. Roeleveld, A.T. Warmenhoven and C.A. Liebrand have no conflict of interests to declare.
References
- Bobkowski, W. , Stefaniak, M.E. , Krauze, T. , Gendera, K. , Wykretowicz, A. , Piskorski, J. , Guzik, P. (2017). Measures of heart rate variability in 24‐h ECGs depend on age but not gender of healthy children. Front Physiol 8, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boselli, E. , Bouvet, L. , Begou, G. , Torkmani, S. , Allaouchiche, B. (2015). Prediction of hemodynamic reactivity during total intravenous anesthesia for suspension laryngoscopy using Analgesia/Nociception Index (ANI): A prospective observational study. Minerva Anestesiol 81, 288–297. [PubMed] [Google Scholar]
- Daccache, G. , Jeanne, M. , Fletcher, D. (2017). The analgesia nociception index: Tailoring opioid administration. Anesth Analg 125, 15–17. [DOI] [PubMed] [Google Scholar]
- Gruenewald, M. , Ilies, C. (2013). Monitoring the nociception‐anti‐nociception balance. Best Pract Res Clin Anaesthesiol 27, 235–247. [DOI] [PubMed] [Google Scholar]
- Gruenewald, M. , Ilies, C. , Herz, J. , Schoenherr, T. , Fudickar, A. , Hocker, J. , Bein, B. (2013). Influence of nociceptive stimulation on analgesia nociception index (ANI) during propofol‐remifentanil anaesthesia. Br J Anaesth 110, 1024–1030. [DOI] [PubMed] [Google Scholar]
- Gruenewald, M. , Herz, J. , Schoenherr, T. , Thee, C. , Steinfath, M. , Bein, B. (2015). Measurement of the nociceptive balance by Analgesia Nociception Index and Surgical Pleth Index during sevoflurane‐remifentanil anesthesia. Minerva Anestesiol 81, 480–489. [PubMed] [Google Scholar]
- Jeanne, M. , Logier, R. , De Jonckheere, J. , Tavernier, B. (2009). Validation of a graphic measurement of heart rate variability to assess analgesia/nociception balance during general anesthesia. Conf Proc IEEE Eng Med Biol Soc 2009, 1840–1843. [DOI] [PubMed] [Google Scholar]
- Jeanne, M. , Clement, C. , De Jonckheere, J. , Logier, R. , Tavernier, B. (2012). Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput 26, 289–294. [DOI] [PubMed] [Google Scholar]
- Jeanne, M. , Delecroix, M. , De Jonckheere, J. , Keribedj, A. , Logier, R. , Tavernier, B. (2014). Variations of the analgesia nociception index during propofol anesthesia for total knee replacement. Clin J Pain 30, 1084–1088. [DOI] [PubMed] [Google Scholar]
- Migeon, A. , Desgranges, F.P. , Chassard, D. , Blaise, B.J. , De Queiroz, M. et al. (2013). Pupillary reflex dilatation and analgesia nociception index monitoring to assess the effectiveness of regional anesthesia in children anesthetised with sevoflurane. Paediatr Anaesth 23, 1160–1165. [DOI] [PubMed] [Google Scholar]
- Sabourdin, N. , Arnaout, M. , Louvet, N. , Guye, M.L. , Piana, F. , Constant, I. (2013). Pain monitoring in anesthetized children: First assessment of skin conductance and analgesia‐nociception index at different infusion rates of remifentanil. Paediatr Anaesth 23, 149–155. [DOI] [PubMed] [Google Scholar]


