Abstract
Sphingolipids are found in abundance at synapses and have been implicated in regulation of synapse structure, function, and degeneration. Their precise role in these processes, however, remains obscure. Serine Palmitoyl‐transferase (SPT) is the first enzymatic step for synthesis of sphingolipids. Analysis of the Drosophila larval neuromuscular junction (NMJ) revealed mutations in the SPT enzyme subunit, lace/SPTLC2 resulted in deficits in synaptic structure and function. Although NMJ length is normal in lace mutants, the number of boutons per NMJ is reduced to ∼50% of the wild type number. Synaptic boutons in lace mutants are much larger but show little perturbation to the general ultrastructure. Electrophysiological analysis of lace mutant synapses revealed strong synaptic transmission coupled with predominance of depression over facilitation. The structural and functional phenotypes of lace mirrored aspects of Basigin (Bsg), a small Ig‐domain adhesion molecule also known to regulate synaptic structure and function. Mutant combinations of lace and Bsg generated large synaptic boutons, while lace mutants showed abnormal accumulation of Bsg at synapses, suggesting that Bsg requires sphingolipid to regulate structure of the synapse. In support of this, we found Bsg to be enriched in lipid rafts. Our data points to a role for sphingolipids in the regulation and fine‐tuning of synaptic structure and function while sphingolipid regulation of synaptic structure may be mediated via the activity of Bsg.
Keywords: lipid rafts, neuromuscular junction, synaptic adhesion, synaptic bouton, RRID:AB_2338959, RRID:AB_2713991, RRID:AB_2314867, RRID:AB_528203, RRID:AB_528484, RRID:AB_2166869, RRID:AB_330924, RRID:AB_10694715
Abbreviations
- NMJ
Neuromuscular Junction
- HRP
Horse Radish Peroxidase
- TEM
Transmission Electron Microscopy
- SPT1
Serine Palmitoyl Transferase
- SPTLC1/2
Serine Palmitoyl Transferase Light Chain 1/2
- EMS
ethyl methyl sulphonate
- HSAN1
Hereditary Sensory and Autonomic Neuropathy Type 1
- LSD
Lysosomal Storage Disease
- TOF
Train of Five
1. INTRODUCTION
In 1967, Derry and Wolfe (1967) identified a prominent enrichment of glycosphingolipids within synaptic structures in the mammalian brain. To date our understanding of the role of these enigmatic lipids in synapse structure and function has yet to be fully elucidated. Sphingolipids are major lipid components of the plasma and endomembrane system and have been implicated in many forms of neuropathy and neurodegeneration (for review see Sabourdy et al., 2015). Sphingolipids are proposed to generate structure in membranes due to their rigidity and association with cholesterol (see Munro, 2003). They are also known to be potent signaling molecules regulating processes such as apoptosis, proliferation, migration, and responses to oxidative stress (reviewed in LLahiri & Futerman, 2007).
Numerous neurological and neurodegenerative conditions are directly attributable to the inability to synthesize or catabolize sphingolipids. The failure to synthesize all or particular sphingolipids gives rise to a number of neurological conditions such as infant‐onset symptomatic epilepsy (loss of GM3 ganglioside synthesis (Simpson et al., 2004), bovine spinal muscular atrophy (loss of 3‐ketohydrosphingosine reductase (Krebs et al., 2007) and hereditary sensory and autonomic neuropathy type 1, HSAN1, recessive and dominant mutations in serine palmitoyl transferase subunit 1 ((SPTLC1), Bejaoui et al., 2001; Dawkins, Hulme, Brahmbhatt, Auer‐Grumbach, & Nicholson, 2001). Conversely failure to catabolize sphingolipids in the lysosome generates a subset of lysosomal storage diseases/disorders (LSD's) known as sphingolipidoses, of which there are approximately 14 identified separate genetic conditions (reviewed in Kacher & Futerman, 2006). Sphingolipids are now suggested to have a prominent role in the onset and progression of Alzheimer's disease (Grimm et al., 2005) while the production after bacterial infection of autoimmune antibodies to gangliosides present at the neuromuscular synapse is likely to cause the dramatic and often lethal paralysis seen in Guillain‐Barré and Miller‐Fisher syndromes (Roberts, Willison, Vincent, & Newsom‐Davis, 1994; Willison et al., 1997). The presence of sphingolipids at the synapse is further attested by the ability of tetanus and botulinal toxins to effect their entry to synapses via co‐attachment to synaptic glycosphingolipids (Nishiki et al., 1996; Deinhardt, Berninghausen, Willison, Hopkins, & Schiavo, 2006).
While the presence of sphingolipids (in particular, glycosphingolipids) at the synapse is well established, little is known about their functional or structural role in the operational life of the synapse. Some in vitro studies have addressed the role of sphingolipids at synapses in the context of sphingolipid/cholesterol microdomains and indicate roles in the function and localization of neurotransmitter receptors, (Brusés, Chauvet, & Rutishauser, 2001; Hering, Lin, & Sheng, 2003) and synaptic exocytosis (Salaün, Gould, & Chamberlain, 2005; Darios et al., 2009; Chan & Sieburth, 2012; Chan, Hu, & Sieburth, 2012). The prominence of sphingolipids in neurological disease suggests that absence or accumulation of sphingolipids can exert an influence in synaptic function and indicates an inappropriately large gap in our knowledge regarding the actions of these lipids at the synapse. In the above outlined context, roles for sphingolipids in synapse structure and function remain to be determined. To this end, we have undertaken an analysis of sphingolipid function at a model synapse, the third instar neuromuscular junction of Drosophila. We have examined mutations in SPT2/SPTLC2 (Serine Palmitoyltransferase, Long Chain Base Subunit 2), which encodes an essential subunit of the Serine Palmitoyltransferase (SPT) heterodimer necessary for the initial step in sphingolipid synthesis, for defects in neuromuscular synapse structure. We present evidence to suggest that sphingolipids are essential for synaptic structure and function, and structural regulation may be mediated partially through function of the Ig domain adhesion protein Basigin/CD147 (Bsg).
2. MATERIALS AND METHODS
2.1. Fly stocks and maintenance
Drosophila stocks were raised on standard yeast, sugar, and agar medium at 25°C (4% yeast, 8% sucrose, 1.2% agar, 3.6 mM calcium chloride, 0.65g/l ferrous sulphate, 6.5 g/l potassium sodium tartrate, 0.4 g/l sodium chloride and 0.4 g/l manganese chloride, 0.065% Nipagin, 0.0005% Bavistin). Spt2/lace alleles were a kind gift from John Roote (The University of Cambridge, UK), hiw stocks were gifted by Aaron DiAntonio (Washington University, USA) and UAS‐lace‐HA, lacek05305 and lace2 were a gift from Takashi Adachi‐Yamada (Gakushuin University, Japan), Bsg mutants and rescue transgenes were a gift from Anne Ephrussi (EMBL, Heidelberg). All other stocks were obtained from the Bloomington Drosophila Stock Center.
2.2. Immunohistochemistry
Third instar wandering larvae were dissected in PBS and fixed in 3.7% formaldehyde/PBS for 7 min. Following washes larvae were stained using the appropriate antibody in 0.1% PBT. Primary antibodies were used at the following concentrations Cy3 conjugated α‐HRP (1:200, goat, Jackson ImmunoResearch, Stratech Scientific), α‐syt (1:2,000, rabbit, West, Lu, Marie, Gao, & Sweeney, 2015), α‐nc82 (1:50, mouse, DSHB), α‐dlg (1:50, mouse, DSHB), α‐GluRIIb (1:2,500, rabbit, a kind gift from Aaron DiAntonio, Washington University, St Louis, MO (Marrus, Portman, Allen, Moffat, & DiAntonio, 2004; DiAntonio et al., 1999), α‐Bsg (1:200, Rat, a kind gift from Anne Ephrussi, EMBL, Heidelberg; Besse et al., 2007). Cy3, Cy5 and FITC conjugated secondary antibodies were used at 1:200 (goat, Jackson ImmunoResearch, Stratech Scientific). A comprehensive list of antibodies can be found in Supporting Information Table S1.
2.3. Electrophysiology
Intracellular microelectrode recordings were made from Muscles 6 and 7 in abdominal segments 3 and 4 of filleted third instar larval preparations bathed in HL3 saline, using standard techniques (Powers, Grizzaffi, Ribchester, & Lnenicka, 2016). The concentrations of Ca2+ and Mg2+ in the saline were reduced (to 0.4 and 10 mM, respectively) in order to depress mean quantal content of excitatory junction potential (EJP; evoked transmitter release), thereby increasing sensitivity to differences in synaptic strength between the Drosophila lines we tested (Dodge & Rahamimoff, 1967; Guan et al., 2017). The reduced EJP amplitude under these conditions also obviated correction of EJP amplitudes for non‐linear summation (McLachlan & Martin, 1981). Preparations were mounted in a recording chamber (bath volume approximately 1 ml) on the stage of an Olympus BX50WI upright, fixed stage microscope and visualized using 10× or 20× water‐dipping objectives. Glass capillary microelectrodes with resistances 15–40 MΩ were pulled using a Brown‐Flaming P87 puller (Sutter Instruments, Novato), filled with 3M KCl and mounted on an MP‐85 Huxley‐type micromanipulator (Sutter Instruments). The reference electrode was an Ag/AgCl pellet connected to the system ground. Membrane potentials were recorded using pClamp 10 (Clampex) software via an HS2A headstage (0.1× gain) connected to a Geneclamp 500 amplifier and Digidata 1550B interface (all Molecular Devices, Sunnyvale). Segmental nerves were aspirated into a micropipette with a heat‐polished tip, aperture 10–15 µm, and stimulated with trains of four or five (TOF) supramaximal pulses (nominally 10V, 0.1–0.2 ms duration; interval 50 ms, that is, 20 Hz; programmed in Clampex) triggering a DS2 stimulator (Digitimer, Welywn Garden City, UK). Three pulse trains were delivered at 5 s intervals and each train was preceded by either a positive or negative rectangular 100 ms, 1 nA current pulse delivered through the recording microelectrode. The voltage deflection (after subtraction of electrode resistance) was used to calculate input resistance and qualitatively check membrane time constant as indicators of membrane integrity. Recordings from muscles with input resistances less than 1.5 MΩ or time constant less than 5 ms were rejected (Powers et al., 2016). Spontaneous EJPs (miniEJPs) were recorded in the absence of nerve stimulation over a period of up to 60 s. EJP recordings were analyzed using pClamp 10.6 and miniEJPs were measured using Minianalysis (Synaptosoft, Atlanta). Mean frequency of miniEJPs was estimated from the inverse of their mean intervals. EJP and miniEJP amplitudes were corrected to an arbitrary standard membrane potential of −65 mV before calculating quantal content by the direct method (Ribchester, 2011). An index of synaptic facilitation (f: positive values indicating facilitation, negative values indicating depression) was calculated from the change in quantal content of the first (m 1) and either the fifth (m 5) or occasionally the fourth EJP, according to the formula f = m 5/m 1−1.
2.4. Imaging and quantification
Imaging and quantification of synaptic structure was performed as described in (West et al., 2015). Briefly, synaptic bouton numbers at muscles 6/7 hemisegment A3, were determined by counting each distinct, spherical, anti‐synaptotagmin‐positive varicosity contacting the muscle. As synaptic bouton number has been shown to increase proportionally with muscle surface area synaptic bouton numbers were normalized against muscle surface area by dividing the bouton number by the muscle surface area and multiplying by mean wild‐type muscle surface area as described by (Milton et al., 2011). Muscles and synapses were imaged at room temperature using a camera (AxioCam HRC) on an inverted fluorescence microscope (Axiovert 200; Carl Zeiss) using Plan Neofluar 10×/0.3 NA and 40×/0.75 NA lenses, with Axio‐ Vision Rel. 4.8 software (Carl Zeiss). Measurements were made from images using ImageJ (National Institutes of Health). Confocal images were obtained using a confocal microscope (LSM 710 Axio Observer Z1; Carl Zeiss). Z‐stacked images of single NMJ's were obtained using a Plan Apochromat 63×/1.4 NA oil objective. Z‐stack projections of muscle 4 NMJ's were analyzed using ImageJ to quantify bouton diameter, NMJ length, and satellite bouton number. Bouton diameter was measured as the width across a bouton at the widest point (Milton et al., 2011). NMJ length was measured using the NeuronJ ImageJ plugin.
Transmission electron microscopy was performed as described previously in (West et al., 2015). Third instar wandering larvae were dissected and fixed in 0.1 M NaPO4, pH 7.4, 1% glutaraldehyde, and 4% formaldehyde, pH 7.3, overnight. Fixed larval preparations were washed 3× in 0.1 M NaPO4 before incubation in OsO4 (1% in 0.1 M NaPO4; 2 hr). Preparations were washed 3× in distilled water before incubation in 1% uranyl acetate. Preparations were washed (3× distilled water) and dehydrated through a graded ethanol series; 20% increments starting at 30% followed by two 100% changes and then 2× 100% propylene oxide. Preparations were incubated in a graded series of epon araldite resin (in propylene oxide); 25% increments culminating in 3× 100% changes. Individual muscles were then dissected out. These were then transferred into embedding molds, and the resin was polymerized at 60°C for 48 hr. Resin mounted preparations were sectioned (60–70 nm) using glass knives upon a microtome (Ultracut UCT; Leica) and placed onto grids. Preparations were subsequently incubated in uranyl acetate (50% in ethanol), washed in distilled water, and incubated in lead citrate. Sections were imaged using a transmission electron microscope (TECNAI 12 G2; FEI) with a camera (Soft Imaging Solutions MegaView; Olympus) and Tecnai user interface v2.1.8 and analySIS v3.2 (Soft Imaging Systems). Quantification of active zone length, number of synaptic vesicles localized within 250 nm of the T‐bar active zone, synaptic vesicle diameter and mitochondrial size was performed using ImageJ. Representative images were taken from at least three animals per genotype.
2.5. Lipid raft extraction and Western blotting
Methodology for purification of lipid rafts was adapted from (Fernandez‐Funez et al., 2009) and (Zhai, Chaturvedi, & Cumberledge, 2004). Briefly 50 third instar larvae (w1118) were sonicated in 250 μl cold TNET buffer (100 mM Tris, 0.2 mM EGTA, 150 mM NaCl, 0.3 M Sucrose, pH 7.5, 1% Triton‐X, 1x protease inhibitor) and incubated on ice for 30 min. Debris was removed by centrifugation at 3000g for 10 min. and 200 μl of crude supernatant extract mixed with 400 μl of 60% OptiprepTM in 5 ml. 5% OptiprepTM was underlaid with 1.8 ml 30% OptiprepTM which was underlaid by the OptiprepTM and extract mixture in 5.1 ml ultracentrifuge tubes. Gradients were spun at 43,865 RPM for 1 hr at 4°C in a Beckman Coulter OptimaTM L‐100 XP Ultracentrifuge using a VTi90 Rotor. Following centrifugation 10 500 μl fractions were collected from the bottom and analyzed via western blotting. Antibodies against the α Subunit of the Na+/K+ ATPase (1 : 100,000, mouse, DSHB) and Syntaxin (1:50, mouse, DSHB) were used as negative and positive controls for lipid rafts, respectively. Anti‐Bsg (1:1,500, rat) was a kind gift from Dr. Anne Ephrussi (EMBL Heidelberg, Germany). HRP‐conjugated secondary antibodies were from Cell signaling technology. A comprehensive list of antibodies can be found in Supporting Information Table S1.
3. RESULTS
3.1. SPTLC2/lace function is essential in Drosophila
SPT acts in the first enzymatic step in the de novo synthesis of sphingolipids, catalyzing the condensation of l‐serine with palmitoyl‐coA to generate 3‐ketosphinganine and further subsequent sphingolipid derivatives. SPT is composed of two subunits, SPTLC1 and SPTLC2. Previously we have demonstrated that expression of Drosophila SPTLC1 (dSPT1) bearing a neomorphic mutation associated with HSAN1, and aberrant sphingolipid production, induced morphological aberrations in synapse growth at the Drosophila third instar larval NMJ (Oswald, West, Lloyd‐Evans, & Sweeney, 2015). In order to determine the role of sphingolipids in the regulation of synaptic morphology we looked to characterize further the role of SPTLC2 in regulating the structure and growth of the Drosophila larval neuromuscular synapse. Lethal and hypomorphic mutations in the Drosophila SPTLC2 gene, lace, have previously been identified (Ashburner, 1982; Adachi‐Yamada et al., 1999). Here, using iPCR, we mapped the insertion sites of the two P‐element insertions within the lace locus (Figure 1). l(2)lacWk05305 was mapped to 97 nucleotides upstream of the start ATG codon while l(2)lacWK00706 mapped 52 nucleotides downstream of the first exon (Figure 1b). The lace2 allele has previously been identified as an EMS induced point mutation leading to the amino acid change S429N (Sasamura, Matsuno, & Fortini, 2013). The lace8 allele is an additional EMS induced mutation while lace1 is a spontaneous mutation (Ashburner, 1982). Using this series of mutants, we screened for allelic combinations that generated an early/late pupal lethality (Figure 1a), giving an optimal penetration of the phenotype and a reduction in sphingolipid content for an analysis of the 3rd instar NMJ. Lacek05305/lace2 transheterozygotes and lacek05305 homozygotes were identified as giving an optimal lethal phase for studying synaptic growth and structure. In addition it has previously been demonstrated that lacek05305/lace2 transheterozygotes and lace k05305 homozygotes present with just 5.5% and 2.5% sphingolipid content (Herr et al., 2003), respectively, compared to wildtype. A schematic of the lace alleles utilized in this study is given in Figure 1b. All mutant combinations were found to be lethal.
Figure 1.
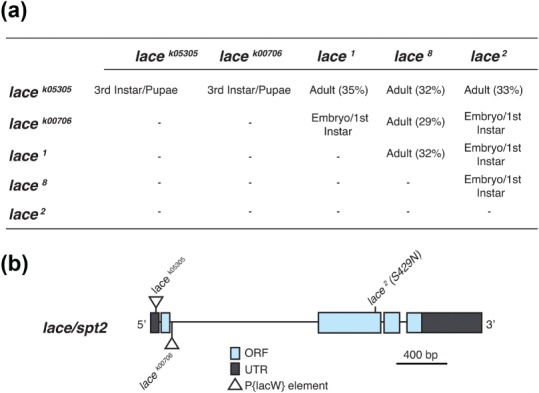
lace is essential for survival in Drosophila. (a) Complementation analysis between lace mutants was used to identify allelic combinations that gave an optimal penetration, generating an early/late pupal lethality, for analysis of the 3rd instar neuromuscular junction. Percentages shown represent the number of unbalanced transheteroygous flies that eclosed for those crosses where transheterozygotes survived to adulthood, baseline = 50%. (b) Gene schematic showing the location of the two P‐element insertions mapped to the lace locus in this study (l(2)lacWK05305 and l(2)lacWK00706) as well as the previously mapped EMS induced lace2 mutation
3.2. Lace mutants display aberrant NMJ structure
Using the Drosophila third instar larval NMJ as a model synapse we identified both transheterozygous and homozygous lace mutants displaying significant perturbations to gross morphological NMJ structure. This was characterized by a significant decrease in synaptic bouton number coupled with an increase in bouton size (Figure 2a–e). It was also observed that mutants showed the presence of spur‐like structures emanating from terminal boutons (Figures 2d), suggesting either partially formed synaptic extensions, or collapse of a bouton. Lace mutants showed no significant variance in the length of the NMJ arbour or muscle surface area when compared to wildtype (Figure 3a, b).
Figure 2.
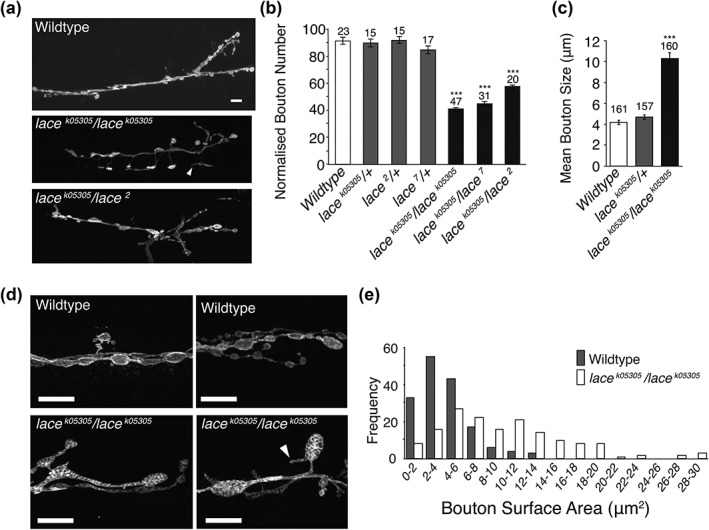
Loss of sphingolipid synthesis leads to enlarged bouton structure at the NMJ. (a, b) Drosophila third instar larvae presenting with homozygous or transheterozgous mutations in lace display a significant reduction in synaptic bouton number (ANOVA p < .001, with post‐hoc Dunnett's comparison to wildtype controls: *** p < .001). (c–e) Reduced synaptic bouton number is coupled with a significant increase in mean synaptic bouton size, associated with an increased frequency of synaptic boutons displaying a surface area > 8 μm2 in lace mutants. lace mutants also displayed spur like protrusions from terminal boutons (arrow heads, a and d). Scale bars = 10 μm
Figure 3.
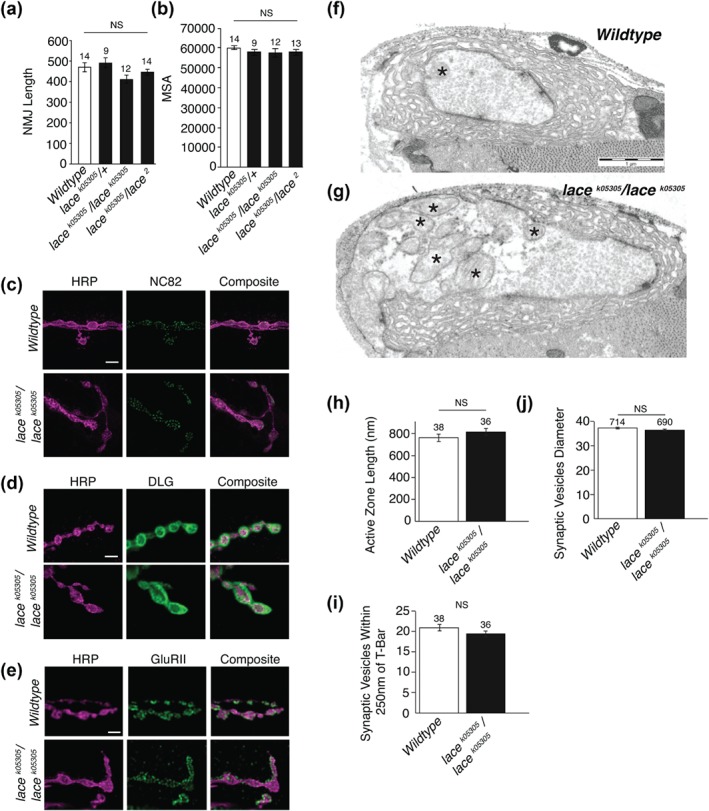
Synaptic components appear unchanged in lace mutants. (a, b) lace mutants show no change in total NMJ length or muscle surface area. a; ANOVA; p < .064, b; ANOVA; p < .616. (c–e) Pre‐ (nc82) and post‐ (discs large and GluRII) synaptic markers appear unchanged at an immunohistochemical level in lace mutants. Scale bars = 5 μm. (f–j) Ultrastructural examination reveals no significant perturbation to active zone length (h), synaptic vesicle number (i) or synaptic vesicle diameter (j). Enlarged mitochondria (asterisk) are observed in lace mutants (g). Scale bars = 1 μm
Despite significant perturbations at the gross morphological level, at a sub‐cellular level pre‐ and post‐synaptic structures appear unperturbed, with no observable alteration to either the pre‐synaptic active zone marker nc82/bruchpilot or post‐synaptic markers GluRIIA and Discs‐large (DLG) (Figure 3c–e). There was also no difference in Futsch or FasII (Supporting Information Figure S1). Mutants did, however, show an apparent disruption to plasma membrane antigens recognized by the anti‐horse radish peroxidase (HRP) antibody, leading to an uneven distribution of HRP labelling (Figures 2d, 3c–e, & Supporting Information Figure S2a). Perturbed HRP staining was not observed in wildtype animals.
At ultrastructural level individual synaptic components also appear normal (Figure 3f–j), showing no significant aberration to active zone size, synaptic vesicle number or synaptic vesicle size (Figure 3h–j). One notable observation, however, is the number of enlarged mitochondria observed throughout the nervous system of lace mutants (Figure 3g, Supporting Information Figure S2). No significant difference in the total number of mitochondria was observed between genotypes (data not shown). Taken together these initial findings suggest that sphingolipid is essential for maintaining synapse structure at a gross morphological level.
3.3. NMJ length is maintained with fewer boutons in the absence of sphingolipids
Boutons are added to the neuromuscular junction during progression through the larval instars (Zito, Parnas, Fetter, Isacoff, & Goodman, 1999). The counting of boutons commonly stands proxy for NMJ size in many studies of the larval neuromuscular junction (Schuster, Davis, Fetter, & Goodman, 1996). On initial observation, the lace mutant NMJ appeared to be of normal length despite having a reduced bouton count. Having identified a defect in bouton structure, we then examined NMJ length in relation to bouton structure. By counting boutons per NMJ arbour while simultaneously measuring the length of the NMJ we found that in sphingolipid depleted NMJ's the overall length of the NMJ was indistinguishable from wildtype while boutons per NMJ was found to be significantly reduced by ∼50%, compared to wild‐type (Figures 2 and 4a–c). lace mutants showed no reduction in muscle surface area (Figure 3b), indicating that reduced bouton number was not as a result of reduced muscle size. Branching patterns of NMJ's were also indistinguishable between sphingolipid depleted NMJ's and wild‐type (Figure 4d).
Figure 4.
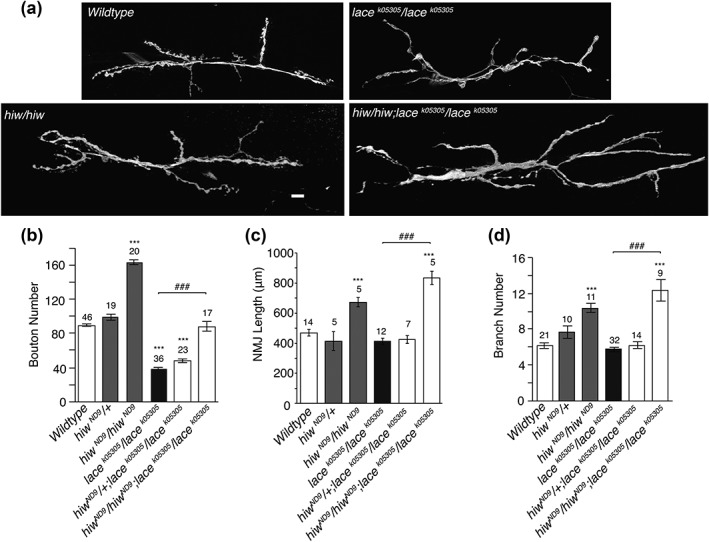
lace mutants are capable of further synaptic growth. (a, b) Combining lace mutants with the synaptic overgrowth mutant highwire revealed double mutants show a ∼50% reduction in bouton number, compared to Hiw alone. This is comparable to the ∼50% reduction observed in lace mutants, compared to wild type. Hiw;lace double mutants, however, remain capable of further synaptic growth showing a significant increase in synaptic length (c) and branching (d), comparable to that seen in Hiw single mutants. ANOVA p < .001, with post‐hoc Dunnett's comparison to wildtype controls: *** p < .001 and Tukey between group's comparison: ### p < .001
To ascertain whether sphingolipid deficient lace mutant NMJ's were capable of further synaptic growth, we combined the lacek05305/lacek05305 mutant with the synaptic overgrowth mutant highwire (hiw) (Wan et al., 2000; Collins, Wairkar, Johnson, & DiAntonio, 2006). NMJ's in the hiwND9;lacek05305/lacek05305 mutant combination were found to be capable of growth well above wildtype length (Figure 4). In the hiw/lace mutant combination, boutons per unit‐length were generated at around 50% of the hiw figure alone. This is similar to the comparison between wild‐type and lace where lace produces ∼50% less boutons per unit length compared to wild‐type. Collectively this data indicates a defect in NMJ synaptic structure, but not overall NMJ length regulation, in the absence of sufficient sphingolipid.
3.4. Expression of lace rescues synaptic structure
Having ascertained that sphingolipid is essential to the generation or maintenance of mature synaptic structure, we examined the relative sphingolipid contribution of the pre‐ and post‐synaptic compartments. To facilitate this, we employed the lacek05305/lacek05305 mutant and rescued lace function in either the pre‐synaptic compartment alone, using the pan‐neuronal elav‐GAL4 driver, or the post‐synaptic compartment alone, using the muscle expressing MHC‐GAL4 driver. We also performed a global rescue using Tubulin‐GAL4 driven expression of UAS‐lace. Here we found that presynaptic expression of lace (elav‐GAL4) failed to recover bouton structure or number in the lacek05305/lacek05305 background (Figure 5). In contrast rescue of lace function in the post‐synaptic muscle compartment induced a nearly complete rescue of both reduced synaptic bouton number and bouton enlargement (Figure 5). Global expression of UAS‐lace was sufficient to completely rescue all aspects of NMJ perturbation in lace mutants (Figure 5). We also examined a role for glia in the sphingolipid regulation of NMJ structure. Glial expression of lace, using the repo‐gal4 driver, was sufficient to rescue both reduced synaptic bouton number and enlarged bouton size in lacek05305/lacek05305 mutants (Supporting Information Figure S3). Interestingly the post‐synaptic rescue of lace function induced the formation of excessive “satellite” boutons (Figure 5d). Satellite boutons are small boutons sprouting from the main synaptic arbour (Beumer, Rohrbough, Prokop, & Broadie, 1999; Koh, Verstreken, & Bellen, 2004; Marie et al., 2004). These data suggest a partial non‐cell‐autonomous role for sphingolipids in the regulation of synaptic growth and structure. Feeding Drosophila larvae sphingosine, the product of serine palmitoyl transferase activity can rescue some phenotypes caused by loss of SPT (Adachi‐Yamada et al., 1999). Our data points to an ability to rescue the sphingolipid deficiency NMJ phenotype with global, muscular or glial expression, but not neuronal expression.
Figure 5.
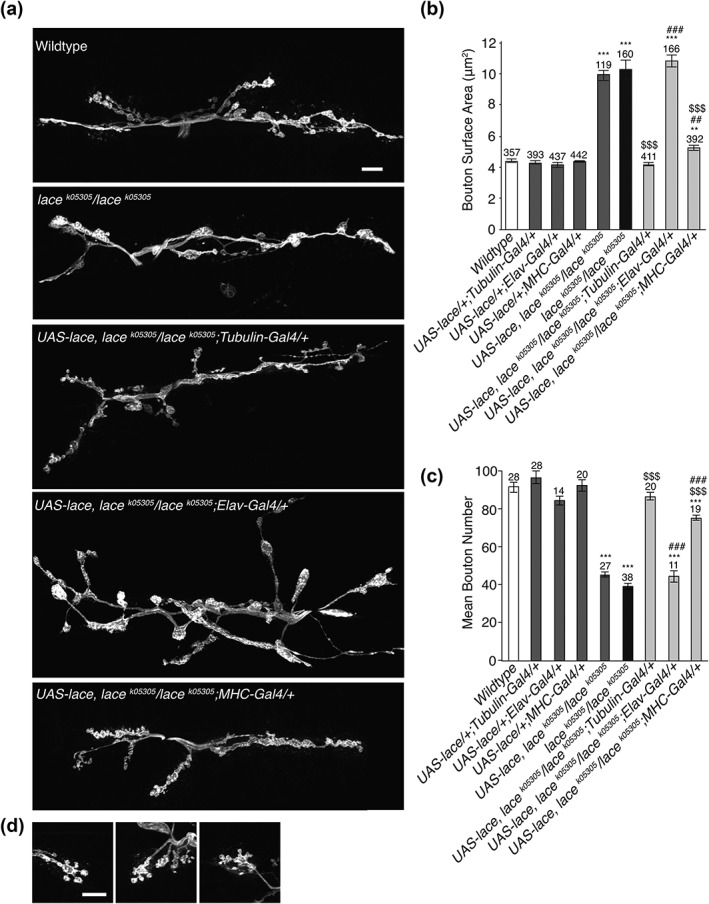
Aberrant synaptic architecture can be partially alleviated by post synaptic expression of lace. (a–c) Global (Tubulin‐Gal4) expression of UAS‐lace was sufficient to completely alleviate both the increase in synaptic bouton size (b) and reduction in synaptic bouton number (c) observed in lace mutants. Post‐synaptic (MHC‐Gal4) expression of UAS‐lace elicits an almost complete rescue of both enlarged synaptic bouton size and reduced bouton number. Pre‐synaptic (Elav‐Gal4) expression of lace is insufficient to rescue aberrant synaptic architecture. (a) ANOVA; p < .001 with post hoc Dunnett's comparison to wildtype: *** p <.001, ** p < .01 and Tukey comparison within groups vs Gal4 control ### p <.001, ## p < .01 or vs lace mutant $$$ p <.001. (b) ANOVA; p < .000 with post hoc Dunnett's comparison to wildtype: *** p <.001, and Tukey comparison within groups vs Gal4 control ### p <.001 or vs lace mutant $$$ p <.001
3.5. Lace mutants show increased synaptic strength
Sphingolipids have previously been implicated in the synaptic vesicle cycle (Salaün et al., 2005; Darios et al., 2009; Chan & Sieburth, 2012; Chan et al., 2012) and in the localization and function of neurotransmitter receptors (Brusés et al., 2001; Hering et al., 2003). Having observed significant perturbations to NMJ morphology, but not ultrastructure, we carried out an electrophysiological analysis in lace mutants to determine the role that sphingolipids might play in the regulation of synaptic activity.
Mutant lace larvae showed a significant increase (∼50%) in both evoked EJP amplitude (Figure 6a, b, Supporting Information Tables S2 and S3) and quantal content (Figure 6c) at both muscles 6 and 7, compared to wild‐type controls. No significant difference in input resistance or resting membrane potential was observed between genotypes (Figure 6d, e). Expression of lace under the control of the global driver tubulin‐Gal4 was sufficient to alleviate both elevated evoked EJP amplitude and quantal content in lace mutant larvae (Figure 6b, c). At the Ca2+/Mg2+ concentrations used in the present experiments, consistent synaptic facilitation during Train‐of‐Five (TOF) stimulation was observed in wildtype larvae but not in lace mutants (Figure 6f). Specifically, lace mutant larvae show a consistent and constant EJP size during the TOF stimulus at NMJs in both muscles 6 and 7, compared to wild‐type larvae (Figure 6f). This difference in EJP consistency during the TOF stimulus was partially rescued by global (tubulin‐Gal4) expression of lace in the lace mutant background (Figure 6f). Mutant lace synapses also showed a significant increase in mini frequency (Muscle 7, Figure 6g) and Quantal size (Muscle 6, Figure 6h), compared to wildtype. These phenotypes, however, were not rescued by expression of wildtype lace (tubulin‐Gal4, Figure 6g, h).
Figure 6.
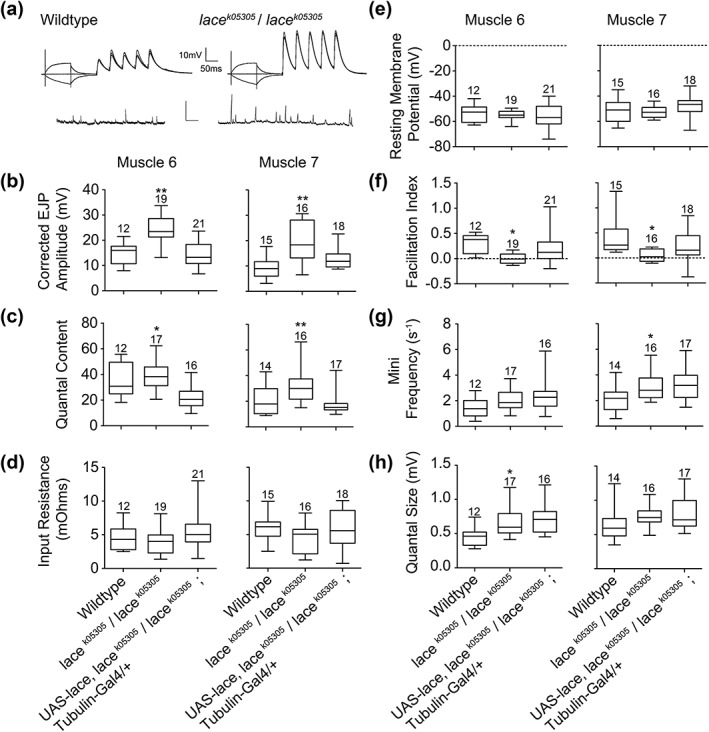
lace mutants display increased synaptic strength. (a) representative intracellular recording traces, showing evoked EJP responses to TOF stimulation and spontaneous (mini) EJPs. (b) lace mutant (lacek05305/lacek05305) larvae show significantly (ANOVA with post‐hoc Tukey comparison between groups, ** p<.01) elevated evoked EJP amplitudes at muscles 6 and 7 compared to wildtype larvae and larvae expressing lace globally (tubulin‐gal4) in a lace mutant background (rescues). Box and whisker plots demonstrate the median, interquartile range and the range of recorded values. (c) lace mutant (lacek05305/lacek05305) larvae show a significant increase (ANOVA with post‐hoc Tukey comparison between groups, * p<.05, ** p<.01) in quantal content at muscles 6 and 7 compared to wildtype larvae and larvae expressing lace globally (tubulin‐gal4) in a lace mutant background (rescues). Box and whisker plots demonstrate the median, interquartile range and the range of recorded values. (d, e) No significant variance in input resistance or resting membrane potential was observed between genotypes. (f) Synaptic facilitation index (f: positive values indicating facilitation, negative values indicating depression) calculated from the change in quantal content of the first (m 1) and either the fifth (m 5) or occasionally the fourth EJP, according to the formula f=m 5/m 1−1. Box and whisker plots demonstrate the median, interquartile range and the range of recorded values (ANOVA with post‐hoc Tukey comparison between groups, * p<.05). (g) lace mutant (lacek05305/lacek05305) larvae show a significant (ANOVA with post‐hoc Tukey comparison between groups, * p<.05) increase in spontaneous (mini) release frequency at muscle 7 compared to wildtype larvae. Box and whisker plots demonstrate the median, interquartile range and the range of recorded values. (h). lace mutant (lacek05305/lacek05305) larvae show a significant (ANOVA with post‐hoc Tukey comparison between groups, * p<.05) increase in quantal size at muscle 6, compared to wildtype larvae. Box and whisker plots demonstrate the median, interquartile range and the range of recorded values
3.6. Lace mutants reveal a relationship between lace and Basigin in the regulation of synapse structure
Previous studies have identified that the Ig family protein Basigin/CD147 (Bsg) is required pre‐ and post‐synaptically to restrict synaptic bouton size and regulate bouton number. Bsg mutants were shown to display significantly enlarged boutons and a reduction in synaptic bouton number, while the overall NMJ size remained close to wild‐type (Besse et al., 2007), a phenotype similar to lace. Bsg mutants also show an elevated evoked EJP amplitude, mini amplitude and mini frequency similar to lace mutants (Besse et al., 2007) with an additional release asynchrony. As with sphingolipids, Bsg has also been implicated in the regulation of actin cytoskeleton dynamics, with mutants showing accumulation of mitochondria (Curtin, Meinertzhagen, & Wyman, 2005), perturbed synaptic structure and an early lethal phase (Besse et al., 2007). As such we asked whether a functional interaction existed between Bsg and the loss of sphingolipid function generated in lace mutants and whether Bsg localization was also altered in lace mutants.
Here we show that heterozygous lace/Bsg mutant combinations phenocopy both lace and Bsg mutants, displaying an ∼50% reduction in synaptic bouton number, coupled with significantly enlarged synaptic boutons (Figure 7a–c). As has previously been shown (Besse et al., 2007) heterozygous mutations in either Bsg or lace alone show no variance from wildtype.
Figure 7.
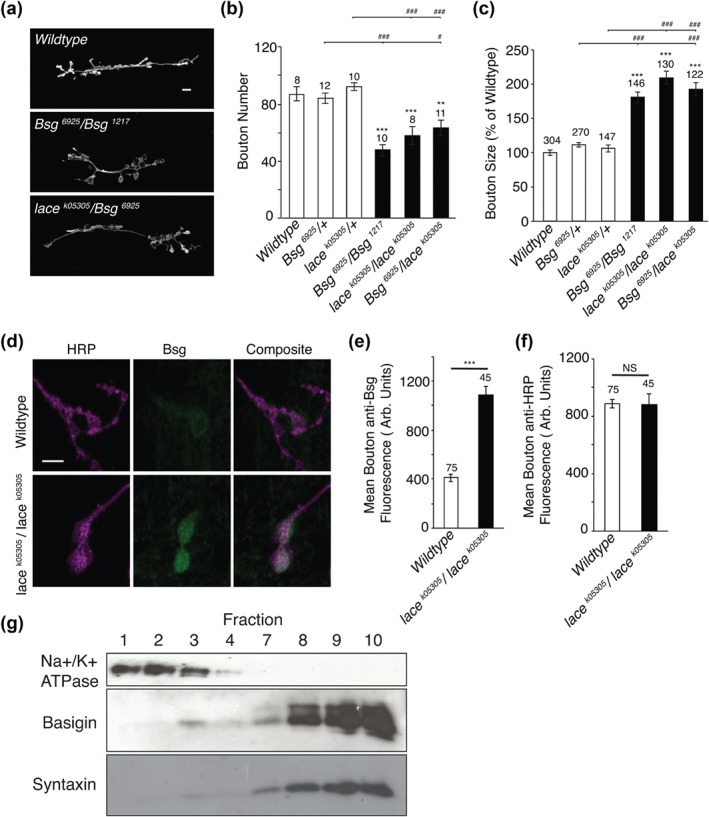
lace mutants reveal a functional interaction between lace and Basigin. (a–c) Heterozygous lace/Bsg double mutants phenocopy both lace and Bsg transheterozygotes, displaying a significant reduction in synaptic bouton number and enlargement of synaptic boutons. ANOVA; p < .001 with post hoc Dunnett's comparison to wildtype: *** p < .001, ** p < .01 and Tukey comparison within groups ### p< .001, # p < .05. (d–f) Bsg was shown to accumulate at NMJs in lace mutants, showing a significant increase in mean anti‐Bsg fluorescence compared to wildtype. Mean relative HRP fluorescence was also quantified, as a control, and showed no variance from wildtype. Student's t‐test; p < .000 (d) and p < .745 (e). (g) Gradient fractionation revealed to Bsg to be present within floating fractions 9 and 10, along with the lipid raft marker Syntaxin, demonstrating Bsg to be present in lipid raft microdomains in Drosophila larvae
Having identified an apparent genetic relationship between Bsg and lace we next looked to determine the abundance and localization of Bsg in lace mutants. Here we show that, as previously identified, Bsg is present at the NMJ. However, we also demonstrate there to be a significant increase in the amount of Bsg accumulating at lace mutant NMJ's when compared to wildtype (Figure 7d–f). Relative HRP was also quantified as a control (Figure 7f) with no significant variance observed between lace mutants and wildtype.
Sphingolipids are major constituents of lipid rafts, specialized membrane microdomains that act to regulate membrane dynamics, endocytic process and cell signaling events, amongst other processes. Previous studies have implicated Bsg in the regulation of signaling complexes within lipid‐raft like domains in cancer (Grass, Tolliver, Bratoeva, & Toole, 2013). We then proposed that the accumulation of Bsg observed at the NMJ of lace mutants may relate to its presence within lipid raft microdomains. To determine whether Bsg was present in microdomains in Drosophila, lipid rafts were isolated via optiprep gradient fractionation. The presence of Bsg within lipid raft fractions was confirmed by identification of its presence within floating fractions 9 and 10, which were also positive for the known lipid raft marker Syntaxin (Figure 7g; Chamberlain, Burgoyne, & Gould, 2001; Lang et al., 2001). The transmembrane ion pump Na+/K+ ATPase, which is excluded from lipid rafts and enriched in non‐lipid raft membranes (Fernandez‐Funez et al., 2009), was not. Taken together these findings suggest that Bsg is localized within lipid raft micro‐domains and that a functional interaction exists between sphingolipids and Bsg in the regulation of synaptic structure.
4. DISCUSSION
4.1. The role of sphingolipids at synapses
The enrichment of sphingolipids at synapses has been long known (Derry & Wolfe, 1967). Assigning functions for these enigmatic lipids at the synapse has remained problematic. Ablation of gangliosides in mouse has identified subtle defects in neurotransmission (Zitman et al., 2010, 2008, 2011) while loss of G3‐ganglioside synthesis results in an infantile onset epilepsy (Simpson et al., 2004), the mechanism for which remains obscure. A specific role for sphingosine has been identified in promoting SNARE protein fusion and synaptic exocytosis (Darios et al., 2009).
Many sphingolipid species present in the outer leaflet of the plasma membrane are found in association with cholesterol as “lipid rafts.” Neurons receive supplementary cholesterol from glia which is essential for supporting synapse maturation and additional synaptogenesis (Mauch et al., 2001) suggesting cholesterol, and potentially lipid rafts, are rate limiting for these processes. Depletion of both cholesterol and sphingolipids together has been shown to reduce and enlarge dendritic spines with eventual loss of synapses in hippocampal neurons in culture possibly due to reduced association with lipid rafts of synapse structure promoting proteins such as Post‐Synaptic Density protein 95 (PSD95) (Hering et al., 2003). In this present study, we have reduced synthesis of sphingolipids with a mutation in SPTLC2 and examined the development of neuromuscular synapses in the Drosophila larval preparation. This approach has allowed us to study the genetic depletion of sphingolipids at an identified synapse in vivo and investigate a role for sphingolipids in the regulation of synaptic structure and activity. As part of this study, we have also identified a potential role for the Ig domain cell adhesion protein Bsg in sphingolipid dependent regulation of synaptic structure.
4.2. Sphingolipids are required for normal synapse structure
On examination of sphingolipid deficient synapses, we observed a disruption to the normal synaptic structure. We found that synaptic boutons were enlarged and the overall numbers of boutons reduced by ∼50% while the length of the neuromuscular synapse remained indistinguishable from wildtype. This phenotype is highly reminiscent of the reduction of synapse number, but increase in synapse size observed in hippocampal neurons in culture depleted for lipid rafts (Hering et al., 2003). Nevertheless, we were surprised that beyond the structural deficit of the synapse, the ultrastructure of the synapse was remarkably intact, suggesting a role in fine‐tuning of synaptic properties.
Synapses depleted for sphingolipids were capable of greater growth when combined with the synaptic overgrowth mutation highwire (hiw) (Wan et al., 2000). Our data suggests the mutations in lace and sphingolipid depletion decouples bouton structure from normal synaptic length. Large boutons are observed in mutants of mothers against dpp (mad), thick veins (tkv), saxophone (sax) medea (med), and glass‐bottom‐boat (gbb), components of the TGF‐ß pathway that is known to regulate synaptic growth (Aberle et al., 2002; Sweeney & Davis, 2002; Rawson, Lee, Kennedy, & Selleck, 2003; McCabe et al., 2004). However these mutations reduce synaptic length by ∼50% and ultrastructural synaptic defects such as nonplasma membrane attached active zones (T‐bars), large endosomal vesicles and ripples in pre‐synaptic peri‐active membranes are observed (McCabe et al., 2004). One obvious ultrastructural defect that is present in sphingolipid depleted synapses is enlarged mitochondria (Figure 3g, Supporting Information Figure S2). Enlarged mitochondria are observed in a number of sensory neuropathies (see Vital & Vital, 2012; for review) and it is of interest that dominant mutations in SPTLC1 (Bejaoui et al., 2001; Dawkins et al., 2001) and SPTLC2 (Penno et al., 2010; Oswald et al., 2015) that generate aberrant sphingolipids give rise to Hereditary and Sensory Neuropathy Type 1 (HSAN1) where enlarged mitochondria are often observed. This may be attributable to a recognized role for sphingolipids in mitochondrial fission (Ciarlo et al., 2010).
To dissect the spatial requirement for sphingolipid regulation of synapse structure we rescued the lace mutant with a rescue transgene, expressed globally, pre‐ or post‐synaptically. We found that we could rescue synaptic bouton size and number with a global expression of the rescue transgene (Figure 5), but no aspects of the phenotype could be rescued with a pre‐synaptic expression. Perturbed NMJ morphology could also be rescued by glial or post‐synaptic expression of lace, however post‐synaptic muscle expression generated a partial rescue, with an excess of “satellite” boutons, a phenotype normally associated with integrin dysfunction (Beumer et al., 1999) or endocytic defects (Koh et al., 2004; Marie et al., 2004). Previous data feeding lace mutant larvae with sphingosine, the product of the SPT enzyme, partially rescued lace mutant associated phenotypes (Adachi‐Yamada et al., 1999). Taken together with our analysis, there is a strong suggestion that sphingolipid precursors such as sphingosine may be able to act non‐cell autonomously, and traffic between cells to support synapse structure and function, but not when supplied from the nervous system.
4.3. Sphingolipids are required for regulation of synaptic output
Analysis of EJP and miniEJP characteristics at the 3rd instar larval NMJ reveals mutations in lace produce, at the Ca2+/Mg2+ concentrations we used, a small but significant increase in synaptic strength, accompanied by a change in short‐term plasticity, with synaptic depression predominating over synaptic facilitation. NMJs with high‐quantal content EJPs normally show synaptic depression during paired or short‐train repetitive stimulation, while those with a low basal quantal content show synaptic facilitation (Lnenicka & Keshishian, 2000; Lnenicka, Theriault, & Monroe, 2006). Further analysis is required, for instance using a range of Ca2+ concentrations, to establish whether this apparent change in synaptic plasticity is commensurate with a greater basal synaptic strength in the lace mutant larvae, or whether it represents a specific effect of the mutation, disrupting the normal link between mechanisms that couple basal quantal content to short‐term synaptic plasticity.
In vitro and in vivo analysis has suggested a role for sphingolipids in synaptic vesicle endocytosis (Salaün et al., 2005; Shen et al., 2014) and exocytosis (Darios et al., 2009; Chan & Sieburth, 2012; Chan et al., 2012) in addition to a role in neurotransmitter distribution (Brusés et al., 2001; Hering et al., 2003). We observe no evident defects in neurotransmitter receptor distribution. Interestingly, ablation of major subsets of gangliosides and subsequent analysis of synaptic function at the NMJ in a mouse model reveals a more pronounced run‐down of neurotransmitter release upon sustained stimulation, consistent with the data we have presented here (Zitman et al., 2008, Zitman et al., 2011). We cannot however, directly attribute the apparent deficit in synaptic facilitation we observed here in lace mutants to exo‐ or endocytosis, at this point.
4.4. Sphingolipids interact with Basigin to regulate synaptic structure at the synapse
We noted a strong phenotypic similarity at the larval neuromuscular synapse between lace mutants and mutations in the small Ig domain adhesion protein Basigin/CD147 (Besse et al., 2007). Bsg is a glycoprotein localized in the plasma membrane that is known to genetically interact with integrins (Curtin et al., 2005) during development of the Drosophila eye. In Bsg mutants, synaptic boutons at the larval neuromuscular junction are enlarged in size and reduced in number with a modest reduction in synaptic span (Besse et al., 2007). Bsg has previously been localized to sphingolipid enriched lipid rafts in invading epithelial breast cells (Grass et al., 2013) and we observed that Bsg is abundant in the lipid raft associated membrane fraction, co‐sedimenting with syntaxin, a known component of lipid rafts (Fernandez‐Funez et al., 2009). We cannot say at this juncture if Bsg function is directly regulated by sphingolipids. Indeed, recruitment of Bsg to lipid rafts can be critical for the recruitment of other protein factors such as claudin‐5 in retinal vascular epithelial cells (Arima et al., 2016). However, given the genetic interaction between Bsg and lace, with bsg;lace transheterozygous double mutants phenocopying both lace and bsg mutants, our data suggests Bsg and sphingolipids genetically interact to regulate synaptic structure. We interpret this interaction as indirect; the loss of sphingolipid generated in the lace mutant affecting Bsg function to regulate synapse structure and function
Synaptic sphingolipids have previously been implicated in synaptic vesicle release (Darios et al., 2009; Chan & Sieburth, 2012; Chan et al., 2012), endocytosis (Salaün et al., 2005), neurotransmitter receptor localization (Hering et al., 2003; Brusés et al., 2001) and maintenance of synaptic activity (Zitman et al., 2008, Zitman et al., 2011). However other roles at the synapse for these enigmatic lipids remain elusive. Two potential functions for sphingolipid at the synapse are suggested by our study. Mitochondrial uptake of Ca2+ shapes Ca2+ dependent responses (Mammucari et al., 2018). The enlarged mitochondria we observe in lace mutants may impinge on Ca2+ uptake to affect synaptic facilitation. A further deficit in Ca2+ handling at the synapse is suggested by the recent finding that Bsg is an obligatory subunit of plasma membrane Ca2+‐ATPases (PMCAs). PMCAs extrude Ca2+ to the extracellular space, and knock‐out of Bsg considerably affects Ca2+ handling by PMCAs (Schmidt et al., 2017). Sphingolipid deficient synapses in the lace mutant have deficits in Bsg function which may in turn have an effect on Ca2+ dynamics via PMCA function.
Ablation of sphingolipid synthesis at a Drosophila model synapse supports a role for sphingolipids in maintenance of synaptic activity and regulation of synaptic structure. Our analysis also points to sphingolipid dependent regulation of synaptic structure via function of the small Ig‐domain protein Bsg. The precise regulation of synapse structure and function is a potent mechanism underlying synaptic plasticity and we suggest that the presence of sphingolipids at synapse may partially reflect this function.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Data were collected by R.J.H.W., R.R.R., M.P.F, and L.B. All Authors contributed toward the design, implementation and analysis of the experiments. Statistical analysis and assembly of figures was performed by R.J.H.W and R.R.R. The manuscript was written by R.J.H.W., R.R.R and S.T.S.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Figure S1
Supporting Information Figure S2
Supporting Information Figure S3
Supporting Information Tables
ACKNOWLEDGMENTS
We thank the Bloomington Drosophila stock center, Developmental Studies Hybridoma Bank Iowa, the University of Cambridge, Department of Genetics Stock Centre, Thomas Hummel, Takashi Adachi‐Yamada, John Roote, Aaron DiAntonio and Anne Ephrussi for antibodies and Drosophila stocks. Thanks also go to Meg Stark and the Technology Facility, University of York, (York, England, UK) for assistance with transmission EM and confocal microscopy and Dr Paul Pryor (University of York, UK) for his expertise and assistance with lipid raft isolation.
West RJH, Briggs L, Perona Fjeldstad M, Ribchester RR, Sweeney ST. Sphingolipids regulate neuromuscular synapse structure and function in Drosophila . J Comp Neurol. 2018;526:1995–2009. 10.1002/cne.24466
Funding information Medical Research Council UK, Grant/Award Number: G0400580 and MR/M013596/1
REFERENCES
- Aberle, H. , Haghighi, A. P. , Fetter, R. D. , McCabe, B. D. , Magalhães, T. R. , & Goodman, C. S. (2002). Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron, 33, 545–558. [DOI] [PubMed] [Google Scholar]
- Adachi‐Yamada, T. , Gotoh, T. , Sugimura, I. , Tateno, M. , Nishida, Y. , Onuki, T. , & Date, H. (1999). De novo synthesis of sphingolipids is required for cell survival by down‐regulating c‐Jun N‐terminal kinase in Drosophila imaginal discs. Molecular and Cellular Biology, 19, 7276–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arima, M. , Cui, D. , Kimura, T. , Sonoda, K.‐H. , Ishibashi, T. , Matsuda, S. , & Ikeda, E. (2016). Basigin can be a therapeutic target to restore the retinal vascular barrier function in the mouse model of diabetic retinopathy. Scientific Reports, 6, 38445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, M. (1982). The genetics of a small autosomal region of Drosophila melanogaster containing the structural gene for alcohol dehydrogenase. III. Hypomorphic and hypermorphic mutations affecting the expression of hairless. Genetics, 101, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejaoui, K. , Wu, C. , Scheffler, M. D. , Haan, G. , Ashby, P. , Wu, L. , … Brown, R. H. (2001). SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nature Genetics, 27, 261–262. [DOI] [PubMed] [Google Scholar]
- Besse, F. , Mertel, S. , Kittel, R. J. , Wichmann, C. , Rasse, T. M. , Sigrist, S. J. , & Ephrussi, A. (2007). The Ig cell adhesion molecule Basigin controls compartmentalization and vesicle release at Drosophila melanogaster synapses. The Journal of Cell Biology, 177, 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beumer, K. J. , Rohrbough, J. , Prokop, A. , & Broadie, K. (1999). A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila. Development (Cambridge, England), 126, 5833–5846. [DOI] [PubMed] [Google Scholar]
- Brusés, J. L. , Chauvet, N. , & Rutishauser, U. (2001). Membrane lipid rafts are necessary for the maintenance of the (alpha)7 nicotinic acetylcholine receptor in somatic spines of ciliary neurons. The Journal of Neuroscience, 21, 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, L. H. , Burgoyne, R. D. , & Gould, G. W. (2001). SNARE proteins are highly enriched in lipid rafts in PC12 cells: Implications for the spatial control of exocytosis. Proceedings of the National Academy of Sciences of the United States of America, 98, 5619–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. P. , Hu, Z. , & Sieburth, D. (2012). Recruitment of sphingosine kinase to presynaptic terminals by a conserved muscarinic signaling pathway promotes neurotransmitter release. Genes & Development, 26, 1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, J. P. , & Sieburth, D. (2012). Localized sphingolipid signaling at presynaptic terminals is regulated by calcium influx and promotes recruitment of priming factors. The Journal of Neuroscience, 32, 17909–17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarlo, L. , Manganelli, V. , Garofalo, T. , Matarrese, P. , Tinari, A. , Misasi, R. , … Sorice, M. (2010). Association of fission proteins with mitochondrial raft‐like domains. Cell Death and Differentiation, 17, 1047–1058. [DOI] [PubMed] [Google Scholar]
- Collins, C. A. , Wairkar, Y. P. , Johnson, S. L. , & DiAntonio, A. (2006). Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron, 51, 57–69. [DOI] [PubMed] [Google Scholar]
- Curtin, K. D. , Meinertzhagen, I. A. , & Wyman, R. J. (2005). Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. Journal of Cell Science, 118, 2649–2660. [DOI] [PubMed] [Google Scholar]
- Darios, F. , Wasser, C. , Shakirzyanova, A. , Giniatullin, A. , Goodman, K. , Munoz‐Bravo, J. L. , … Davletov, B. (2009). Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron, 62, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins, J. L. , Hulme, D. J. , Brahmbhatt, S. B. , Auer‐Grumbach, M. , & Nicholson, G. A. (2001). Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit‐1, cause hereditary sensory neuropathy type I. Nature Genetics, 27, 309–312. [DOI] [PubMed] [Google Scholar]
- Deinhardt, K. , Berninghausen, O. , Willison, H. J. , Hopkins, C. R. , & Schiavo, G. (2006). Tetanus toxin is internalized by a sequential clathrin‐dependent mechanism initiated within lipid microdomains and independent of epsin1. The Journal of Cell Biology, 174, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry, D. M. , & Wolfe, L. S. (1967). Gangliosides in isolated neurons and glial cells. Science (New York, N.Y.), 158, 1450–1452. [DOI] [PubMed] [Google Scholar]
- Dodge, F. A. , & Rahamimoff, R. (1967). Co‐operative action a calcium ions in transmitter release at the neuromuscular junction. The Journal of Physiology, 193, 419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio, A. , Petersen, S. A. , Heckmann, M. & Goodman, C. S. (1999). Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. Journal of Neuroscience, 19, 3023–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Funez, P. , Casas‐Tinto, S. , Zhang, Y. , Gómez‐Velazquez, M. , Morales‐Garza, M. A. , Cepeda‐Nieto, A. C. , … Rincon‐Limas, D. E. (2009). In vivo generation of neurotoxic prion protein: Role for hsp70 in accumulation of misfolded isoforms. PLoS Genetics, 5, e1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass, G. D. , Tolliver, L. B. , Bratoeva, M. , & Toole, B. P. (2013). CD147, CD44, and the epidermal growth factor receptor (EGFR) signaling pathway cooperate to regulate breast epithelial cell invasiveness. The Journal of Biological Chemistry, 288, 26089–26104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, M. O. W. , Grimm, H. S. , Pätzold, A. J. , Zinser, E. G. , Halonen, R. , Duering, M. , … Hartmann, T. (2005). Regulation of cholesterol and sphingomyelin metabolism by amyloid‐beta and presenilin. Nature Cell Biology, 7, 1118–1123. [DOI] [PubMed] [Google Scholar]
- Guan, Z. , Bykhovskaia, M. , Jorquera, R. A. , Sutton, R. B. , Akbergenova, Y. , & Littleton, J. T. (2017). A synaptotagmin suppressor screen indicates SNARE binding controls the timing and Ca(2+) cooperativity of vesicle fusion. Elife, 6, 2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering, H. , Lin, C.‐C. , & Sheng, M. (2003). Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. The Journal of Neuroscience, 23, 3262–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr, D. R. , Fyrst, H. , Phan, V. , Heinecke, K. , Georges, R. , Harris, G. L. , & Saba, J. D. (2003). Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development (Cambridge, England), 130, 2443–2453. [DOI] [PubMed] [Google Scholar]
- Kacher, Y. , & Futerman, A. H. (2006). Genetic diseases of sphingolipid metabolism: Pathological mechanisms and therapeutic options. FEBS Letters, 580, 5510–5517. [DOI] [PubMed] [Google Scholar]
- Koh, T.‐W. , Verstreken, P. , & Bellen, H. J. (2004). Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron, 43, 193–205. [DOI] [PubMed] [Google Scholar]
- Krebs, S. , Medugorac, I. , Röther, S. , Strässer, K. , & Förster, M. (2007). A missense mutation in the 3‐ketodihydrosphingosine reductase FVT1 as candidate causal mutation for bovine spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America, 104, 6746–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri, S. , & Futerman, A. H. (2007). The metabolism and function of sphingolipids and glycosphingolipids. Cellular and Molecular Life Sciences, 64, 2270–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, T. , Bruns, D. , Wenzel, D. , Riedel, D. , Holroyd, P. , Thiele, C. , & Jahn, R. (2001). SNAREs are concentrated in cholesterol‐dependent clusters that define docking and fusion sites for exocytosis. The Embo Journal, 20, 2202–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lnenicka, G. A. , & Keshishian, H. (2000). Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. Journal of Neurobiology, 43, 186–197. [PubMed] [Google Scholar]
- Lnenicka, G. A. , Theriault, K. , & Monroe, R. (2006). Sexual differentiation of identified motor terminals in Drosophila larvae. Journal of Neurobiology, 66, 488–498. [DOI] [PubMed] [Google Scholar]
- Mammucari, C. , Raffaello, A. , Vecellio Reane, D. , Gherardi, G. , De Mario, A. , & Rizzuto, R. (2018). Mitochondrial calcium uptake in organ physiology: From molecular mechanism to animal models. Pflugers Archiv. 10.1007/s00424-018-2123-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie, B. , Sweeney, S. T. , Poskanzer, K. E. , Roos, J. , Kelly, R. B. , & Davis, G. W. (2004). Dap160/intersectin scaffolds the periactive zone to achieve high‐fidelity endocytosis and normal synaptic growth. Neuron, 43, 207–219. [DOI] [PubMed] [Google Scholar]
- Marrus, S. B. , Portman, S. L. , Allen, M. J. , Moffat, K. G. , & DiAntonio, A. (2004). Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. The Journal of Neuroscience, 24, 1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch, D. H. , Nägler, K. , Schumacher, S. , Göritz, C. , Müller, E. C. , Otto, A. , & Pfrieger, F. W. (2001). CNS synaptogenesis promoted by glia‐derived cholesterol. Science (New York, N.Y.), 294, 1354–1357. [DOI] [PubMed] [Google Scholar]
- McCabe, B. D. , Hom, S. , Aberle, H. , Fetter, R. D. , Marqués, G. , Haerry, T. E. , … Haghighi, A. P. (2004). Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron, 41, 891–905. [DOI] [PubMed] [Google Scholar]
- McLachlan, E. M. , & Martin, A. R. (1981). Non‐linear summation of end‐plate potentials in the frog and mouse. The Journal of Physiology, 311, 307–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton, V. J. , Jarrett, H. E. , Gowers, K. , Chalak, S. , Briggs, L. , Robinson, I. M. , & Sweeney, S. T. (2011). Oxidative stress induces overgrowth of the Drosophila neuromuscular junction. Proceedings of the National Academy of Sciences of the United States of America, 108, 17521–17526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S. (2003). Lipid rafts: elusive or illusive?. Cell, 115, 377–388. [DOI] [PubMed] [Google Scholar]
- Nishiki, T. , Tokuyama, Y. , Kamata, Y. , Nemoto, Y. , Yoshida, A. , Sato, K. , … Kozaki, S. (1996). The high‐affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Letters, 378, 253–257. [DOI] [PubMed] [Google Scholar]
- Oswald, M. C. W. , West, R. J. H. , Lloyd‐Evans, E. , & Sweeney, S. T. (2015). Identification of dietary alanine toxicity and trafficking dysfunction in a Drosophila model of hereditary sensory and autonomic neuropathy type 1. Human Molecular Genetics, 24, 6899–6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penno, A. , Reilly, M. M. , Houlden, H. , Laurá, M. , Rentsch, K. , Niederkofler, V. , … Hornemann, T. (2010). Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. Journal of Biological Chemistry, 285, 11178–11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, A. S. , Grizzaffi, J. , Ribchester, R. , & Lnenicka, G. A. (2016). Regulation of quantal currents determines synaptic strength at neuromuscular synapses in larval Drosophila. Pflugers Archive, 468, 2031–2040. [DOI] [PubMed] [Google Scholar]
- Rawson, J. M. , Lee, M. , Kennedy, E. L. , & Selleck, S. B. (2003). Drosophila neuromuscular synapse assembly and function require the TGF‐β type I receptor saxophone and the transcription factor Mad. Journal of Neurobiology, 55, 134–150. [DOI] [PubMed] [Google Scholar]
- Ribchester, R. R. (2011). Quantal analysis of endplate potentials in mouse flexor digitorum brevis muscle. Current Protocols in Mouse Biology, 1, 429–444. [DOI] [PubMed] [Google Scholar]
- Roberts, M. , Willison, H. , Vincent, A. , & Newsom‐Davis, J. (1994). Serum factor in Miller‐Fisher variant of Guillain‐Barré syndrome and neurotransmitter release. Lancet (London, England), 343, 454–455. [DOI] [PubMed] [Google Scholar]
- Sabourdy, F. , Astudillo, L. , Colacios, C. , Dubot, P. , Mrad, M. , Ségui, B. , … Levade, T. (2015). Monogenic neurological disorders of sphingolipid metabolism. Biochimica et Biophysica Acta, 1851, 1040–1051. [DOI] [PubMed] [Google Scholar]
- Salaün, C. , Gould, G. W. , & Chamberlain, L. H. (2005). Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. The Journal of Biological Chemistry, 280, 19449–19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamura, T. , Matsuno, K. , & Fortini, M. E. (2013). Disruption of Drosophila melanogaster lipid metabolism genes causes tissue overgrowth associated with altered developmental signaling. PLoS Genetics, 9, e1003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt, N. , Kollewe, A. , Constantin, C. E. , Henrich, S. , Ritzau‐Jost, A. , Bildl, W. , … Shulte, U. (2017). Neuroplastin and Basigin are essential auxiliary subunits of plasma membrane Ca2+‐ATPases and key regulators of Ca2+ clearance. Neuron, 96, 827–838. [DOI] [PubMed] [Google Scholar]
- Schuster, C. M. , Davis, G. W. , Fetter, R. D. , & Goodman, C. S. (1996). Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron, 17, 641–654. [DOI] [PubMed] [Google Scholar]
- Shen, H. , Giordano, F. , Wu, Y. , Chan, J. , Zhu, C. , Milosevic, I. , … De Camilli, P. (2014). Coupling between endocytosis and sphingosine kinase 1 recruitment. Nature Cell Biology, 16, 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, M. A. , Cross, H. , Proukakis, C. , Priestman, D. A. , Neville, D. C. A. , Reinkensmeier, G. , … Crosby, A. H. (2004). Infantile‐onset symptomatic epilepsy syndrome caused by a homozygous loss‐of‐function mutation of GM3 synthase. Nature Genetics, 36, 1225–1229. [DOI] [PubMed] [Google Scholar]
- Sweeney, S. T. , & Davis, G. W. (2002). Unrestricted synaptic growth in spinster—A late endosomal protein implicated in TGF‐β‐mediated synaptic growth regulation. Neuron, 36, 403–416. [DOI] [PubMed] [Google Scholar]
- Vital, A. , & Vital, C. (2012). Mitochondria and peripheral neuropathies. Journal of Neuropathology and Experimental Neurology, 71, 1036–1046. [DOI] [PubMed] [Google Scholar]
- Wan, H. I. , DiAntonio, A. , Fetter, R. D. , Bergstrom, K. , Strauss, R. , & Goodman, C. S. (2000). Highwire regulates synaptic growth in drosophila. Neuron, 26, 313–329. [DOI] [PubMed] [Google Scholar]
- West, R. J. H. , Lu, Y. , Marie, B. , Gao, F.‐B. , & Sweeney, S. T. (2015). Rab8, POSH, and TAK1 regulate synaptic growth in a Drosophila model of frontotemporal dementia. The Journal of Cell Biology, 208, 931–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison, H. J. , O'Hanlon, G. , Paterson, G. , O'Leary, C. P. , Veitch, J. , Wilson, G. , … Vincent, A. (1997). Mechanisms of action of anti‐GM1 and anti‐GQ1b ganglioside antibodies in Guillain‐Barré syndrome. The Journal of Infectious Diseases, 176(Suppl 2), S144–S149. [DOI] [PubMed] [Google Scholar]
- Zhai, L. , Chaturvedi, D. , & Cumberledge, S. (2004). Drosophila wnt‐1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. The Journal of Biological Chemistry, 279, 33220–33227. [DOI] [PubMed] [Google Scholar]
- Zitman, F. M. P. , Todorov, B. , Furukawa, K. , Furukawa, K. , Willison, H. J. , & Plomp, J. J. (2010). Total ganglioside ablation at mouse motor nerve terminals alters neurotransmitter release level. Synapse, 64, 335–338. [DOI] [PubMed] [Google Scholar]
- Zitman, F. M. P. , Todorov, B. , Jacobs, B. C. , Verschuuren, J. J. , Furukawa, K. , Willison, H. J. , & Plomp, J. J. (2008). Neuromuscular synaptic function in mice lacking major subsets of gangliosides. Neuroscience, 156, 885–897. [DOI] [PubMed] [Google Scholar]
- Zitman, F. M. P. , Todorov, B. , Verschuuren, J. J. , Jacobs, B. C. , Furukawa, K. , Furukawa, K. , … Plomp, J. J. (2011). Neuromuscular synaptic transmission in aged ganglioside‐deficient mice. Neurobiology of Aging, 32, 157–167. [DOI] [PubMed] [Google Scholar]
- Zito, K. , Parnas, D. , Fetter, R. D. , Isacoff, E. Y. , & Goodman, C. S. (1999). Watching a synapse grow: Noninvasive confocal imaging of synaptic growth in Drosophila. Neuron, 22, 719–729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Figure S1
Supporting Information Figure S2
Supporting Information Figure S3
Supporting Information Tables


