Abstract
Hepatitis B virus (HBV) RNA in serum is a novel biomarker for intrahepatic HBV replication and treatment response. For its proper use, it is essential to identify factors influencing serum HBV RNA level. Using a rapid amplification of complimentary DNA (cDNA) ends (RACE) PCR technique (lower limit of detection [LLD], 800 copies/mL [c/mL]), serum HBV RNA levels were measured in samples of 488 untreated individuals with chronic HBV infection who were eligible to treatment according to currently used recommendations. We explored the association of serum levels of HBV RNA with patient‐ and virus‐associated factors. HBV genotype distribution was 21/10/20/46/3% for A/B/C/D/other. Mean HBV RNA serum level was 5.9 (1.6) log10 c/mL (hepatitis B e antigen [HBeAg]‐positive chronic hepatitis B [CHB], 6.5 [1.2] log c/mL; HBeAg‐negative CHB, 4.1 [1.2] log c/mL; P < 0.001). By multivariable linear regression, factors associated with lower HBV RNA level were HBeAg negativity (β = –0.69; P < 0.001), HBV genotypes A (β = –0.13; P = 0.002), B (β = –0.07; P = 0.049), and C (β = –0.61; P < 0.001) in comparison to D, and presence of HBV basal core promoter mutation either alone (β = –0.14; P = 0.001) or in combination with precore mutation (β = –0.22; P < 0.001). Higher serum alanine aminotransferase (ALT) was associated with higher HBV RNA (β = 0.23; P < 0.001). HBV RNA correlated strongly with HBV DNA (HBeAg‐pos, r = 0.72; P < 0.001; HBeAg‐neg, r = 0.78; P < 0.001) and moderately with quantitative hepatitis B surface antigen (qHBsAg; HBeAg‐pos, r = 0.54; P < 0.001; HBeAg‐neg, r = 0.19; P = 0.04) and quantitative hepatitis B surface antigen (qHBeAg; r = 0.41; P < 0.001). Conclusion: In this multiethnic cohort of 488 untreated individuals with CHB, factors associated with serum HBV RNA level were HBeAg status, serum ALT, HBV genotype, and presence of basal core promotor mutations. For the future use of serum HBV RNA as a clinical marker, it seems mandatory to take these factors into consideration. (Hepatology 2018).
Abbreviations
- ALT
alanine aminotransferase
- BCP
basal core promoter
- cDNA
complimentary DNA
- cccDNA
covalently closed circular DNA
- CHB
chronic hepatitis B infection
- CI
confidence interval
- c/mL
copies/mL
- HBV
hepatitis B virus
- HBeAg
hepatitis B e antigen
- HBsAg
hepatitis B surface antigen
- LLD
lower limit of detection
- NA(s)
nucleos(t)ide analogue(s)
- PC
precore
- PCR
polymerase chain reaction
- PEG‐IFN
peginterferon
- qHBeAg
quantitative hepatitis B surface antigen
- qHBsAg
quantitative hepatitis B surface antigen
- ULN
upper limit of normal
- WT
wild type
Currently available treatment strategies for chronic hepatitis B virus (HBV) infection can inhibit viral replication to a great extent as reflected by suppression of HBV DNA in serum. However, loss of hepatitis B surface antigen (HBsAg), which is regarded as a functional cure, is achieved in only a minority of patients, and treatment does not eliminate the intrahepatic covalently closed circular DNA (cccDNA), which is the main transcriptional template of HBV. Also, the HBV genome that is integrated in the host genome remains largely unaffected. Therapeutic agents currently under investigation may have a stronger effect on cccDNA and are potentially more effective, and therefore serum markers that reflect cccDNA activity are needed. These markers may allow a reliable prediction of response, ideally before treatment initiation or early during treatment, and can also help to individualize the use of already available treatments.1
HBV RNA in serum could fulfill criteria of such a biomarker, as the 4 major types of HBV RNAs are direct transcriptional products of the cccDNA. HBV RNA can be detected in serum and can be quantified by using PCR techniques.2 The nature of HBV RNA in serum has not fully been researched, but it most likely includes the 3.5‐kilobase pregenomic RNA, which is the template for reverse transcription to HBV DNA and for translation of core protein and polymerase. It is probably released from infected hepatocytes in virion‐like capsids.3, 4, 5
The potential of serum HBV RNA as a baseline marker for treatment response was recently demonstrated by studies revealing its strong correlation with cccDNA activity on the one hand and its response to currently available treatments on the other hand. Thus, before and during treatment with nucleos(t)ide analogue (NA) or pegylated interferon alfa (PEG‐IFN), a correlation between serum HBV RNA and the transcriptional activity of cccDNA was demonstrated.6 In addition, serum HBV RNA levels were found to be associated with the probability of response to treatment with either NAs or PEG‐IFN.2, 4, 7, 8, 9 However, before the further development of serum HBV RNA as a response‐predicting marker, it is mandatory to evaluate which factors which may influence HBV RNA levels in individuals who are eligible for treatment. To study this, we measured serum HBV RNA level in a large, multiethnic population of well‐characterized individuals with chronic HBV infection, who are in need for treatment according to recent guidelines.10 We assessed the association with between HBV RNA level and host and viral factors, including levels of other biomarkers.
Patients and Methods
STUDY POPULATION
Full‐length polyadenylated HBV RNA levels were measured in available baseline serum samples of 441 hepatitis B e antigen (HBeAg)‐positive and 133 HBeAg‐negative chronic hepatitis B (CHB) patients who participated in three previously conducted global randomized, controlled trials (99‐01 study, ARES study, and PARC study) and had not received antiviral treatment in the preceding 6 months.11, 12, 13 Specific inclusion and exclusion criteria have been described earlier. Serum samples were stored in –20 or –80°Celsius since the original studies. Serum samples were available for 366 HBeAg‐positive patients and 122 HBeAg‐negative patients. We specifically chose to combine these three cohorts in order to create a large population and to ensure a better generalizability of HBV RNA level in serum as a biomarker for patients with different characteristics.
ENDPOINTS
We explored factors associated with serum level of HBV RNA and the correlation of HBV RNA to other serum markers.
SERUM HBV RNA QUANTIFICATION
HBV RNA was quantified from serum samples using a rapid amplification of complimentary DNA (cDNA) ends (RACE)‐based real‐time PCR technique that has been described. Quantification of polyadenylated HBV RNA was performed using specific primers (including HBV RNA RT primer 5′‐ACC ACG CTA TCG CTA CTC AC (dT17)GWA GCT C) designed according to van Bömmel et al.7 For the current study the assay's lower limit of detection (LLD) for HBV RNA was 800 (2.9 log10) copies/mL (c/mL), with a corresponding linear range of 800 to 106 c/mL. HBV RNA levels measured below the LLD were set to 450 c/mL before statistical analysis.
OTHER LABORATORY MEASUREMENTS
Routine biochemical and hematological tests were performed locally. Serum alanine aminotransferase (ALT) levels were standardized by calculating the value times for the upper limit of normal (ULN) per center and sex. Virological tests were performed at one central laboratory (Erasmus Medical Center, Rotterdam, The Netherlands). HBV DNA was measured using TaqMan‐based PCR assays (Roche Diagnostics, Basel, Switzerland; LLD of 400 c/mL, 35 c/mL, or 20 IU/mL depending on the study cohort). HBV‐DNA results in c/mL were converted into IU/mL using a conversion factor of 5.8 copies per IU. The comparabiltiy of the assays was assessed (Bland‐Altman test, r = 0.12; P = 0.49).14 Serum quantitative hepatitis B surface antigen (qHBsAg) levels were measured using the Architect HBsAg assay (Abbott Laboratories; range, 0.05‐250 IU/mL) or the Elecsys Assay (Roche Diagnostics; LLD, 0.05 IU/mL). Serum HBeAg levels were quantified using the Cobas Elecsys HBeAg assay (Roche Diagnostics; measurement range, 0.2‐100.0 IU/mL). HBV genotype analysis was performed using the INNO‐LiPA HBV genotype assay (Fujirebio Europe, Ghent, Belgium). The presence of precore (PC) and basal core promoter (BCP) mutants was assessed using the INNO‐LiPA HBV PreCore assay (Fujirebio Belgium, Ghent, Belgium), which detects PC mutations at nucleotide position 1896 and BCP mutations at nucleotide positions 1762 and 1764. Results were classified into four groups: wild type (WT; only WT virus detectable); PC (only PC or both PC and WT detectable); BCP (either or both BCP mutations detected, with or without WT); or as PC + BCP when both types of mutants were found.
STATISTICAL ANALYSIS
SPSS software (version 21.0; SPSS, Inc., Chicago, IL) was used to perform statistical analyses. Skewed laboratory values were log‐transformed before analyses and were expressed as mean (SD). After log transformation, HBV RNA level showed a near to normal distribution in both the HBeAg‐positive population (skewness, –0.59 [SE, 0.13]; kurtosis, 0.11 [SE, 0.25]), and the HBeAg‐negative population (skewness, 0.29 [SE, 0.24]; kurtosis, 0.03 [SE, 0.47]). Associations between variables were tested using the Student t test, chi‐square, Pearson correlation, or their nonparametric equivalents, when appropriate. Subgroup analysis of mean HBV RNA levels was performed using analysis of variance with Bonferroni correction for intergroup comparison. We performed linear regression analysis to determine factors associated with HBV RNA level. R2 was calculated as a measure of goodness of fit for the linear model. For linear correlations, Pearson correlation coefficients (r) were provided, and for skewed distributions, the Spearman rank coefficients (ρ) were provided. Logistic regression analysis was performed to determine factors associated with HBV RNA level below LLD. Genotypes other than A, B, C, and D were not analyzed in linear regression. All analyses were performed two‐sided at the 0.05 level of significance.
Results
STUDY COHORT
Study population characteristics are shown in Table 1. Mean age was 33 (SD, 11) years for HBeAg‐positive patients and 42 (SD, 11) for HBeAg‐negative patients (P < 0.001), and the majority of patients were male (n = 362; 74%). Patients were mostly of white (n = 313; 64%) or Asian (n = 153; 31%) origin. All main HBV genotypes were represented. Mean serum HBV‐DNA levels were 8.1 (1.1) log IU/mL for HBeAg‐positive patients and 6.0 (1.2) for HBeAg‐negative patients (P < 0.001), and mean serum qHBsAg levels were 4.3 (0.7) log IU/mL versus 3.8 (0.6) IU/mL (P < 0.001), respectively.
Table 1.
Patient Characteristics
| Characteristics |
All Patients (n = 488) |
HBeAg‐pos (n = 366) |
HBeAg‐neg (n = 122) |
P Value |
|---|---|---|---|---|
| Demography | ||||
| Age, years | 35 (12) | 33 (11) | 42 (11) | <0.001 |
| Male, n (%) | 362 (74) | 274 (74) | 88 (72) | 0.55 |
| Race, n (%) | <0.001 | |||
| White | 313 (64) | 197 (54) | 122 (95) | |
| Asian | 153 (31) | 149 (41) | 4 (3) | |
| Other | 22 (5) | 20 (5) | 2 (2) | |
| HBV genotype, n (%) | <0.001 | |||
| A | 91 (19) | 75 (21) | 16 (13) | |
| B | 50 (10) | 49 (13) | 1 (1) | |
| C | 113 (23) | 110 (30) | 3 (2) | |
| D | 222 (46) | 125 (34) | 97 (80) | |
| Other | 12 (2) | 11 (2) | 5 (4) | |
| INNO‐LiPA result, n (%) | <0.001 | |||
| WT virus | 81 (17) | 75 (21) | 6 (5) | |
| PC mutation | 52 (11) | 38 (11) | 14 (11) | |
| BCP mutation | 102 (21) | 78 (21) | 24 (20) | |
| PC and BCP mutation | 203 (42) | 125 (34) | 78 (64) | |
| Histology | ||||
| Cirrhosis, n (%) | 31 (6) | 27 (7) | 4 (3) | 0.13 |
| Treatment history | ||||
| Previous NA | 58 (12) | 36 (10) | 22 (18) | 0.02 |
| Previous (PEG‐)IFN | 86 (18) | 64 (18) | 22 (18) | 0.89 |
| Laboratory results | ||||
| HBV RNA‡ | 5.9 (1.6) | 6.5 (1.2) | 4.1 (1.2) | <0.001 |
| HBV RNA undetectable, n (%) | 30 (6) | 2 (0.5) | 28 (23) | <0.001 |
| HBV DNA† | 7.5 (1.4) | 8.1 (1.1) | 6.0 (1.2) | <0.001 |
| qHBsAg† | 4.2 (0.7) | 4.3 (0.7) | 3.8 (0.6) | <0.001 |
| qHBeAg† | n.a. | 2.4 (0.9) | n.a. | n.a. |
| ALT (× ULN)* | 3.7 (3.2) | 3.8 (3.4) | 3.2 (2.5) | 0.07 |
Continuous variables are expressed as mean (SD), categorical variables as n (%).
*Multiples of upper limit of the normal range.
†Logarithmic scale, IU/mL.
‡Logarithmic scale, c/mL.
Abbreviation: n.a., not applicable.
SERUM HBV RNA LEVEL IN RELATION TO VIRAL AND CLINICAL CHARACTERISTICS
Mean HBV RNA level was 5.9 (1.6) c/mL and differed by HBeAg status (HBeAg‐positive CHB, 6.5 [1.2] log c/mL; HBeAg‐negative CHB, 4.1 [1.2] log c/mL; P < 0.001; Fig. 1A). HBV RNA levels were below LLD in 2 (0.5%) HBeAg‐positive patients and in 28 (23%) HBeAg‐negative patients (P < 0.001). In these 30 patients, mean HBV‐DNA level was 4.7 (SD, 0.7) log IU/mL. HBV RNA levels were associated with degree of ALT elevation (r = 0.29; P < 0.001; Fig. 1B), and HBV RNA level varied by HBV genotype (P < 0.001), with mean unadjusted HBV RNA levels of 6.0/6.5/6.0/5.6 log c/mL for genotypes A/B/C/D (P = 0.003; Fig. 2A). In patients infected with WT HBV, highest mean unadjusted HBV RNA levels were found (6.7 log c/mL), and presence of BCP mutation either alone or in combination with PC mutation led to lower HBV RNA levels (Fig. 2B; 5.5 log and 5.3 log c/mL, both P < 0.001 in comparison to WT HBV). HBV RNA level did not differ by sex (P = 0.26), presence of cirrhosis (P = 0.55), or treatment history (previous IFN, P = 0.96; previous NA, 0.38), but did negatively correlate with age (r = –0.23; P < 0.001).
Figure 1.
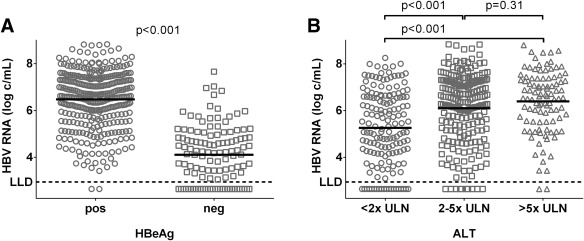
HBV RNA level according to HBeAg status and ALT level. Dots represent individual HBV RNA measurements, with lines representing the unadjusted mean level of HBV RNA (log c/mL) according to HBeAg status (A) and serum ALT level (B) expressed in ×ULN. The LLD for the HBV RNA PCR is 800 c/mL (2.90 log c/mL).
Figure 2.
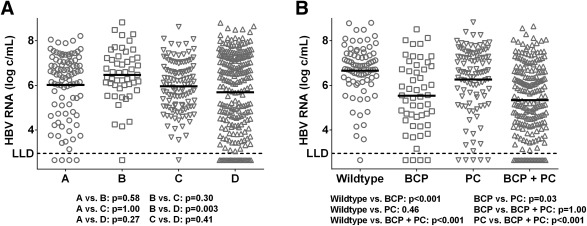
HBV RNA level according to HBV genotype and presence of precore and basal core promoter mutations. Dots represent individual HBV RNA measurements, with lines representing the unadjusted mean level of HBV RNA (log c/mL) according to HBV genotype (A) and presence of PC and BCP mutations (B). The LLD for the HBV RNA PCR is 800 c/mL (2.90 log c/mL).
REGRESSION ANALYSIS OF FACTORS ASSOCIATED WITH HBV RNA LEVEL
By univariable linear regression, factors associated with HBV RNA level were age, HBeAg status, HBV genotype, and presence of any PC or BCP mutation (Table 2). By multivariable linear regression, factors associated with lower HBV RNA level were HBeAg‐negative CHB (β = –0.69; P < 0.001), HBV genotypes A, B, and C in comparison to genotype D (genotype A: β = –0.13; P = 0.002; genotype B, β = –0.07; P = 0.049; genotype C, β = –0.61; P < 0.001), presence of any BCP mutation either alone (β = –0.14; P = 0.001), or in combination with PC mutation (β = –0.22; P < 0.001). Higher serum ALT was associated with higher HBV RNA level (β = 0.23; P < 0.001). This particular linear model explains 56.2% of the variance of the HBV RNA level (r2 = 0.562), which diminishes to 25.3% if HBeAg is excluded from the model. When studying the factors associated with HBV RNA level below LLD using logistic regression analysis, the only factors independently associated with HBV RNA level below LLD were HBeAg‐negative CHB (odds ratio [OR], 86; 95% confidence interval [CI; 9.1‐811]; P < 0.001) and ALT (OR, 0.07; 95% CI [0.01‐0.40]; P < 0.001) (Supporting Table S1). Because HBeAg was negative in 28 of 30 patients with HBV RNA below LLD, analysis was repeated in HBeAg‐negative patients, which did not result in any additional factors independently associated with HBV RNA below LLD.
Table 2.
Univariable and Multivariable Linear Regression Analysis of Factors Associated With Serum HBV RNA Level
| Univariable | Multivariable (Full Model) | ||||||
|---|---|---|---|---|---|---|---|
| B | 95% CI | P Value | B | 95% CI | β | P Value | |
| Age, years | –0.03 | –0.04; –0.02 | <0.001 | 0.003 | –0.007; 0.01 | 0.02 | 0.59 |
| Sex | 0.26 | ||||||
| Male | reference | ||||||
| Female | –0.19 | –0.51; 0.14 | 0.26 | ||||
| HBeAg | <0.001 | <0.001 | |||||
| Pos | reference | reference | |||||
| Neg | –2.37 | –2.62; –2.13 | <0.001 | –2.46 | –2.74; –2.18 | –0.69 | <0.001 |
| HBV genotype | 0.003 | <0.001 | |||||
| A | 0.39 | 0.01; 0.76 | 0.04 | –0.53 | –0.86; –0.19 | –0.13 | 0.002 |
| B | 0.84 | 0.36; 1.31 | 0.001 | –0.36 | –0.72; –0.001 | –0.07 | 0.049 |
| C | 0.32 | –0.03; 0.67 | 0.07 | –0.61 | –0.91; –0.32 | –0.16 | <0.001 |
| D | reference | reference | |||||
| PC/BCP variants | <0.001 | <0.001 | |||||
| WT HBV | reference | reference | |||||
| PC mutation | –0.38 | –1.68; –0.90 | <0.001 | –0.19 | –0.56; 0.18 | –0.05 | 0.44 |
| BCP mutation | –1.12 | –1.66; –0.59 | <0.001 | –0.70 | –1.09; ‐0.30 | –0.14 | 0.001 |
| PC and BCP mutation | –1.29 | –0.82; 0.07 | 0.10 | –0.68 | –1.02; 0.35 | –0.22 | <0.001 |
| ALT (log ×ULN) | 1.46 | 1.02; 1.90 | <0.001 | 1.18 | 0.85; 1.51 | 0.23 | <0.001 |
Patients with genotype other than one of the main genotypes genotypes A, B, C, and D were excluded for this analysis. When a significance level of P < 0.20 was reached in univariable analysis, the factor was assessed in multivariable analysis. No interactions were found between HBeAg status and genotype (P = 0.14), HBeAg status and INNO‐LiPA result (P = 0.81), genotype and INNO‐LiPA result (P = 0.12), HBeAg status and ALT (P = 0.19), genotype and ALT (P = 0.64), or INNO‐LiPA result and ALT (P = 0.42).
Abbreviations: B, unstandardized regression coefficient; β, standardized regression coefficient.
SERUM HBV RNA LEVEL IN RELATION TO SERUM HBV DNA, qHBsAg, AND QUANTITATIVE HBeAg
Overall, HBV RNA correlated highly with HBV DNA (r = 0.85/ρ = 0.84; P < 0.001) and moderately with qHBsAg (r = 0.52/ρ = 0.60; P < 0.001) and quantitative HBeAg (qHBeAg; r = 0.41/ρ = 0.34; P < 0.001). Because HBeAg status is the strongest factor independently associated with HBV RNA level in linear regression, we also studied correlations of HBV RNA to other markers in HBeAg‐positive and HBeAg‐negative patients separately.
In HBeAg‐positive patients, levels of HBV RNA correlated strongly correlated with HBV DNA (r = 0.72; P < 0.001) and moderately correlated with qHBsAg (r = 0.54; P < 0.001) and qHBeAg (r = 0.41; P < 0.001). Correlations of HBV RNA to other HBV markers, however, were HBV‐genotype dependent, with strongest correlation with HBV DNA observed for genotype A, strongest correlation with qHBsAg for genotypes B and C, and a weakest correlation with qHBsAg for genotype D (Fig. 3).
Figure 3.
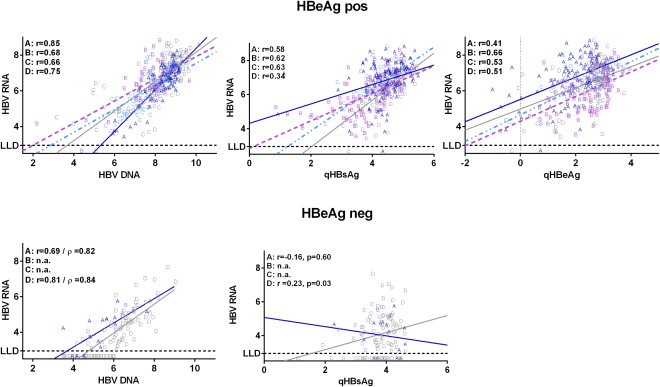
Correlations of HBV RNA with serum levels of HBV DNA, qHBsAg, and qHBeAg. Correlations of serum levels of HBV RNA (log c/mL) with serum levels of HBV DNA, qHBsAg, and qHBeAg (log IU/mL). Letters represent individual serum‐marker measurements according to HBV genotype. For linear correlations, Pearson correlation coefficients (r) were provided, and for skewed distributions, the Spearman rank coefficients (ρ) were provided. The LLD for the HBV RNA PCR is 800 c/mL (2.90 log c/mL). Abbreviation: n.a., not applicable.
In HBeAg‐negative patients, overall correlation of HBV RNA to HBV DNA was comparable to HBeAg‐positive CHB (r = 0.78/ρ = 0.80; P < 0.001). Unlike in HBeAg‐positive CHB, the overall correlation of HBV RNA to qHBsAg was weak (r = 0.19, P = 0.04; ρ = 0.17, P = 0.07). Genotype‐specific correlations of HBV RNA levels could only be determined for HBV genotypes A and D. The correlation of HBV RNA with HBV DNA was comparable for these genotypes (genotype A, r = 0.69/ρ = 0.82; P = 0.003; genotype D, r = 0.81/ρ = 0.84; P < 0.001), but the correlation of HBV DNA with qHBsAg was genotype dependent, indicated by a weak correlation within genotype D–infected patients (r = 0.23/ρ = 0.22; P = 0.03), but no significant correlation within genotype A–infected patients (r = –0.16/ρ = –0.14; P = 0.60; Fig. 3).
Discussion
HBV RNA in serum is a novel and promising marker for cccDNA transcriptional activity and could thus be used to monitor responses both to currently available treatment, and particularly to future HBV treatments targeting the HBV life cycle. However, its interactions with host factors and viral factors, such as markers of HBV transcription, have not yet been systematically studied. In this study, we investigate the association of HBV RNA serum levels with currently used HBV markers and patient characteristics in a large, multiethnic cohort including 441 HBeAg‐positive and 133 HBeAg‐negative individuals who were candidates for antiviral treatment according to current guidelines.10 We found that HBV RNA levels are independently associated with HBeAg status, ALT levels, BCP variants, and HBV genotype. Our results indicate that these factors have to be taken into consideration for a correct interpretation of HBV RNA serum levels.
From recent studies, there is evidence that the level of HBV RNA in serum reflects cccDNA transcriptional activity.6, 15 We identified HBeAg status as the strongest factor associated with serum HBV RNA levels, which is likely an expression of the higher transcriptionally activity of cccDNA in HBeAg‐positive patients as compared to HBeAg‐negative patients. Mean HBV RNA level was 2.4 log10 lower in HBeAg‐negative as compared to HBeAg‐positive patients, and the proportion of patients with undetectable HBV RNA serum level 23% in HBeAg‐negative as compared to only 0.5% in HBeAg‐positive patients (P < 0.001). This is in line with previous findings in smaller patient cohorts.4, 7, 16 The correlation of HBV RNA with HBV‐DNA levels was similar in HBeAg‐positive and HBeAg‐negative individuals. Interestingly, however, the correlation between HBV RNA and HBsAg was moderate in HBeAg‐positive (r = 0.54), but only weak in HBeAg‐negative patients (r = 0.19; Fig. 3). A possible explanation for this difference is that in HBeAg‐negative patients, HBsAg may be derived not only from cccDNA, but also in a profound number from an integrated HBV genome. This hypothesis is supported by data of a recent study assessing the effect of small interfering RNA–based inhibition of messenger HBV RNAs, where a significant decrease in HBsAg levels was found in HBeAg‐positive, but not in HBeAg‐negative, patients.17 Applying this hypothesis to our observation could imply that in HBeAg‐negative patients, HBV RNA and HBsAg are partially derived from different matrices and that HBV RNA (and possibly also HBV DNA) might be a better marker of for remaining cccDNA in HBeAg‐negative disease.
Another factor we found to be associated with lower HBV RNA serum levels was the presence of BCP variants. In presence of these variants, either alone or in combination with PC variants, HBV RNA levels were significantly lower than in WT virus (P < 0.002). We also found lower HBV‐DNA and HBsAg levels in patients with BCP and/or PC variants (data not shown). Other studies, however, have reported inconclusive results regarding the effect of BCP mutations on HBV replication.18, 19, 20, 21 These heterogenic results may be explained by testing patients with different disease phases, genotype distributions, and frequency of BCP mutants.22 It is yet unclear which mechanisms would explain lower HBV‐DNA and HBV RNA level in presence of BCP mutations, but the key may be in HBV RNA packaging. Recently, virus‐like particles have been proposed as a medium for transport of HBV RNA in serum.3, 23 Therefore, lower HBV RNA levels in patients bearing these variants may be a result of decreased availability of those carriers of HBV RNA instead of a direct effect of BCP mutation on HBV replication.21, 24 Further studies are required to elucidate how BCP and PC variants truly affect serum HBV RNA levels.
HBV genotype was associated with both absolute levels of HBV RNA and correlations of HBV RNA with other serum markers. In multivariable regression analysis, genotype D was associated with the highest HBV RNA level, followed by genotypes B, A, and C. The HBV genotype mainly influenced the correlation between HBV RNA and qHBsAg in our cohort. This might be of relevance for the use of serum HBV RNA as a clinical marker. HBV RNA levels were significantly higher in patients with ALT level >2× ULN compared to patients with ALT level <2× ULN, which was similar for HBV DNA and qHBsAg. Age and sex of individuals with chronic HBV infection did not influence HBV RNA serum levels.
In conclusion, our study shows that HBeAg status, serum ALT, HBV genotype, and presence of BCP variants are independently associated with serum HBV RNA level in a multiethnic cohort of patients currently considered eligible for treatment. These factors should be taken into consideration for the interpretation and comparison of HBV RNA serum levels across different infected individuals and in the development of HBV RNA as a potential serum marker for cccDNA transcriptional activity. This is, in particular, relevant for future studies with novel agents aiming targeting the HBV life cycle for functional cure of CHB.
Supporting information
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29872/suppinfo.
Supplementary table 1. Univariable and multivariable logistic regression analysis of factors associated with undetectable level of serum HBV RNA (n=34)
Acknowledgments
The authors thank Anthonie Groothuismink and Buddy Roovers from the Department of Gastroenterology and Hepatology, Erasmus MC University Medical Center (Rotterdam) for retrieval of serum samples.
Potential conflict of interest: Dr. van Bommel consults for, is on the speakers' bureau for, and received grants from Gilead, Bristol‐Myers Squibb, and Roche. Prof. Janssen consults for and received grants from AbbVie, Bristol‐Myers Squibb, Gilead, Janssen, Medimmune, Merck, and Roche. He consults for Benitec and Arbutus.
This study was initiated and sponsored by the Foundation for Liver Research, Rotterdam, The Netherlands. Financial support was provided by F. Hoffmann‐La Roche Ltd., Basel, Switzerland. For the original studies, financial support, study medication, and drug supply were provided by F. Hoffmann‐La Roche Ltd. (Basel, Switzerland), Bristol‐Myers Squibb (BMS; New York, NY), with additional financial support provided by the Virgo consortium, funded by the Dutch government project number FES0908, and by the Netherlands Genomics Initiative (NGI) project number 050‐060‐452. The funding sources did not have any influence on study design, data collection, analysis and interpretation of the data, writing of the report, nor the decision to submit for publication.
REFERENCES
- 1. Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015;64:1972‐1984. [DOI] [PubMed] [Google Scholar]
- 2. van Bömmel F, van Bömmel A, Krauel A, He H, Wat C, Pavlovic V, et al. Serum HBV RNA is an early predictor of HBeAg seroconversion in patients with chronic Hepatitis B (CHB) treated with pegylated interferon alfa‐2a (40KD). Hepatology 2015;62:336A. [Google Scholar]
- 3. Wang J, Shen T, Huang X, Kumar GR, Chen X, Zeng Z, et al. Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. J Hepatol 2016;65:700‐710. [DOI] [PubMed] [Google Scholar]
- 4. Jansen L, Kootstra NA, van Dort KA, Takkenberg RB, Reesink HW, Zaaijer HL. Hepatitis B virus pregenomic RNA is present in virions in plasma and is associated with a response to pegylated interferon alfa‐2a and nucleos(t)ide analogues. J Infect Dis 2016;213:224‐232. [DOI] [PubMed] [Google Scholar]
- 5. Wooddell CI, Yuen MF, Chan HL, Gish RG, Locarnini SA, Chavez D, et al. RNAi‐based treatment of chronically infected patients and chimpanzees reveals that integrated hepatitis B virus DNA is a source of HBsAg. Sci Transl Med 2017;9:eaan0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang J, Du M, Huang H, Chen R, Niu J, Jiang J, et al. Reply to: “Serum HBV pgRNA as a clinical marker for cccDNA activity”: consistent loss of serum HBV RNA might predict the “para‐functional cure” of chronic hepatitis B. J Hepatol 2017;66:462‐463. [DOI] [PubMed] [Google Scholar]
- 7. van Bommel F, Bartens A, Mysickova A, Hofmann J, Kruger DH, Berg T, Edelmann A. Serum hepatitis B virus RNA levels as an early predictor of hepatitis B envelope antigen seroconversion during treatment with polymerase inhibitors. Hepatology 2015;61:66‐76. [DOI] [PubMed] [Google Scholar]
- 8. Huang YW, Takahashi S, Tsuge M, Chen CL, Wang TC, Abe H, et al. On‐treatment low serum HBV RNA level predicts initial virological response in chronic hepatitis B patients receiving nucleoside analogue therapy. Antivir Ther 2015;20:369‐375. [DOI] [PubMed] [Google Scholar]
- 9. Tsuge M, Murakami E, Imamura M, Abe H, Miki D, Hiraga N, et al. Serum HBV RNA and HBeAg are useful markers for the safe discontinuation of nucleotide analogue treatments in chronic hepatitis B patients. J Gastroenterol 2013;48:1188‐1204. [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370‐398. [DOI] [PubMed] [Google Scholar]
- 11. Brouwer WP, Xie Q, Sonneveld MJ, Zhang N, Zhang Q, Tabak F, et al. Adding pegylated interferon to entecavir for hepatitis B e antigen‐positive chronic hepatitis B: a multicenter randomized trial (ARES study). Hepatology 2015;61:1512‐1522. [DOI] [PubMed] [Google Scholar]
- 12. Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, et al. Pegylated interferon alfa‐2b alone or in combination with lamivudine for HBeAg‐positive chronic hepatitis B: a randomised trial. Lancet 2005;365:123‐129. [DOI] [PubMed] [Google Scholar]
- 13. Rijckborst V, ter Borg MJ, Cakaloglu Y, Ferenci P, Tabak F, Akdogan M, et al. A randomized trial of peginterferon alpha‐2a with or without ribavirin for HBeAg‐negative chronic hepatitis B. Am J Gastroenterol 2010;105:1762‐1769. [DOI] [PubMed] [Google Scholar]
- 14. Buster EH, Flink HJ, Cakaloglu Y, Simon K, Trojan J, Tabak F, et al. Sustained HBeAg and HBsAg loss after long‐term follow‐up of HBeAg‐positive patients treated with peginterferon alpha‐2b. Gastroenterology 2008;135:459‐467. [DOI] [PubMed] [Google Scholar]
- 15. Giersch K, Allweiss L, Volz T, Dandri M, Lutgehetmann M. Serum HBV pgRNA as a clinical marker for cccDNA activity. J Hepatol 2017;66:460‐462. [DOI] [PubMed] [Google Scholar]
- 16. Krauel A, Deichsel D, Böhm S, Grossmann M, Berg T, Van Bömmel F. The level of HBV RNA in serum as a novel marker for the phase of chronic hepatitis B virus (HBV) infections. Hepatology 2016;63:895A. [Google Scholar]
- 17. Wooddell CI, Chavez D, Goetzmann JE, Guerra B, Peterson RM, Lee H, et al. Reductions in cccDNA under NUC and ARC‐520 therapy in chimpanzees with chronic hepatitis B virus infection implicate integrated DNA in maintaining circulating HBSAG. Hepatology 2015;62:222A‐223A. [Google Scholar]
- 18. Homs M, Caballero A, Gregori J, Tabernero D, Quer J, Nieto L, et al. Clinical application of estimating hepatitis B virus quasispecies complexity by massive sequencing: correlation between natural evolution and on‐treatment evolution. PLoS One 2014;9:e112306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoo BC, Park JW, Kim HJ, Lee DH, Cha YJ, Park SM. Precore and core promoter mutations of hepatitis B virus and hepatitis B e antigen‐negative chronic hepatitis B in Korea. J Hepatol 2003;38:98‐103. [DOI] [PubMed] [Google Scholar]
- 20. Laras A, Koskinas J, Hadziyannis SJ. In vivo suppression of precore mRNA synthesis is associated with mutations in the hepatitis B virus core promoter. Virology 2002;295:86‐96. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Buckwold VE, Hon MW, Ou JH. Mechanism of suppression of hepatitis B virus precore RNA transcription by a frequent double mutation. J Virol 1999;73:1239‐1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yan L, Zhang H, Ma H, Liu D, Li W, Kang Y, et al. Deep sequencing of hepatitis B virus basal core promoter and precore mutants in HBeAg‐positive chronic hepatitis B patients. Sci Rep 2015;5:17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Revill PA, Locarnini SA. New perspectives on the hepatitis B virus life cycle in the human liver. J Clin Invest 2016;126:833‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chun YK, Kim JY, Woo HJ, Oh SM, Kang I, Ha J, Kim SS. No significant correlation exists between core promoter mutations, viral replication, and liver damage in chronic hepatitis B infection. Hepatology 2000;32:1154‐1162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at http://onlinelibrary.wiley.com/doi/10.1002/hep.29872/suppinfo.
Supplementary table 1. Univariable and multivariable logistic regression analysis of factors associated with undetectable level of serum HBV RNA (n=34)


