Summary
Background
Azathioprine (AZA) and mercaptopurine (MP) are the cornerstone of steroid‐sparing strategies in autoimmune hepatitis (AIH). Up to 20% of patients do not tolerate or respond to these regimens.
Aim
To evaluate retrospectively the tolerability and efficacy of tioguanine (thioguanine) (TG) therapy in selected patients with AIH and AIH variant syndromes.
Methods
Records of 52 patients who received TG therapy were retrieved from nine hospitals in the Netherlands. Indications for TG treatment were intolerable side effects on AZA or MP (n = 38), insufficient response (n = 11) or first‐line treatment (n = 3). Treatment efficacy was defined as normalisation of serum aminotransferases and serum immunoglobulin G.
Results
No serious adverse events occurred in patients treated with TG during a median follow‐up of 18 months (range 1‐194). Treatment was well tolerated in 41 patients (79%), whereas four had tolerable (8%) and seven (13%) intolerable side effects. Thirty‐eight patients were treated with TG after intolerable side effects on AZA or MP; 29 patients continued TG therapy of whom 24 (83%) achieved complete biochemical remission, four (14%) had incomplete and one (3%) had no response; nine discontinued treatment. Seven of 11 patients with insufficient response to AZA or MP were responsive to TG, three with complete and four with incomplete biochemical remission; four discontinued due to intolerance (n = 2) and non‐response (n = 2). TG was effective in all AIH patients as first‐line maintenance treatment.
Conclusion
In our retrospective review of TG therapy in selected patients with AIH or AIH variants who previously failed on AZA or MP, TG appeared tolerable with biochemical efficacy.
1. INTRODUCTION
Autoimmune hepatitis (AIH) is an immune‐mediated inflammation of unknown aetiology primarily targeting hepatocytes, usually requiring lifelong immunosuppressive therapy. Treatment is aimed to prevent disease relapse, relief symptoms and achieve complete biochemical and histological normalisation in order to prevent progression to fibrosis, cirrhosis and end‐stage liver failure requiring liver transplantation.1 Remission is induced using prednisone and often maintained by a corticosteroid‐saving regime using thiopurines: azathioprine (AZA) or mercaptopurine (MP).2 Unfortunately, up to 20% of patients do not respond to or tolerate these conventional thiopurines.3 Currently, rescue medications are limited to mycophenolate mofetil, tacrolimus and ciclosporin. The European AIH guideline commemorate states that patients intolerant to AZA can be offered tioguanine (TG) therapy as alternative to mycophenolate mofetil, although there is only anecdotic evidence available to support its use.1
Tioguanine, a rediscovered thiopurine for inflammatory bowel disease can be interchanged with AZA and MP.4, 5 Based on a single study addressing potential side effects related to TG use, there has been initial concern about the safety of this drug,6 yet well‐designed additional studies in different cohorts of inflammatory bowel disease patients have not found evidence to support this assumption.7
Azathioprine and MP are metabolized by a shared pathway via thiopurine S‐methyltransferase, into hepatotoxic breakdown metabolites 6‐methyl MP and by other enzymatic steps into the pharmacologically active compound 6‐tioguaninenucleotide (6‐TGN). TG is metabolized directly to 6‐TGN and to 6‐methyltioguanine, the latter also by thiopurine S‐methyltransferase. Deficient forms of thiopurine S‐methyltransferase leads to toxic levels of 6‐TGN when a patient is on TG therapy, causing bone marrow suppression. However, the absolute burden of methylated side products is much lower during use of TG when compared to the larger thiopurines AZA and MP. Hence, TG therapy might be as effective as other thiopurines, but be better tolerated in the treatment of AIH.
In 2005, our group published the first data on TG therapy in three AIH patients,8 and more recently another group performed a single ‐centre study in a small and heterogeneous group of patients with AIH and AIH variant syndrome.9 To extend the experience with TG therapy in AIH patients, we have been collecting data on a national level over a 17‐year period, in the Netherlands. Here, we report the tolerability, clinical efficacy, safety and steroid‐reduction of TG therapy in 52 patients with AIH or AIH variant syndromes.
2. METHODS
2.1. Study population
Patients were identified by sending an inquiry to hepatogastroenterologists in 37 hospitals in the Netherlands in collaboration with the Dutch Autoimmune Hepatitis Group. A total of 26 (70%) hospitals responded to the questionnaire (19 referral, 7 tertiary). The study protocol (number 2008.84) was approved by the ethics committee of the VU University Medical Center. Patient data was anonymously provided by the treating physicians.
Patients with a clinical diagnosis of AIH who were actively, or had been, treated with TG were identified in six referral hospitals and three tertiary centres. The diagnostic post‐treatment revised International AIH Group scores were calculated.10 All were initiated on TG therapy between 2001 and 2017 and followed until February 2018 or until TG therapy was discontinued. Three thiopurine naïve AIH patients were treated with TG therapy as first‐line maintenance treatment. Thirty‐nine AIH and 10 AIH variant syndrome patients were switched after previous failure on AZA or MP therapy. The reasons for conventional thiopurine failure and switching to TG therapy were determined by the attending physician and include intolerance (including toxic 6‐methyl MP levels) or insufficient response (failure to achieve or maintain remission).
The AIH variant with features of primary biliary cholangitis (AIH‐PBC) was defined according to the “Paris criteria as AIH with an anti‐mitochondrial antibodies (AMA) titer of >1:80 in combination with compatible histology”.11 AMA negative AIH patients with clinical and histopathological features of PBC were defined as “AMA negative AIH‐PBC”, formerly known as autoimmune cholangitis. AIH with concurrent primary sclerosing cholangitis (AIH‐PSC) was defined as AIH with typical findings of PSC on imaging (magnetic resonance cholangiopancreatography/endoscopic retrograde cholangiopancreatography) and/or a compatible histology.12, 13
2.2. Adverse events
Patient records were analysed for (serious) adverse events (i.e. hospital admissions, acute pancreatitis, side effects). Myelotoxicity was defined as thrombocytopenia (platelet count of <150 × 109/L), leucopenia (white blood cell count of <4.0 × 109/L) or anaemia (female: haemoglobin <7.5 mmol/L, male: haemoglobin <8.5 mmol/L). One hepatopathologist assessed the majority of follow‐up biopsies; nodular regenerative hyperplasia was defined according to the consensus criteria on the existing histopathologic diagnosis of nodular regenerative hyperplasia by Jharap et al.14 Abdominal imaging reports and gastroscopy reports were assessed for signs of portal hypertension.
2.3. Efficacy
Complete biochemical remission was defined as serum alanine aminotransferase (ALT), serum aspartate aminotransferase and, when available, serum immunoglobulin G (IgG) within the normal range. Incomplete response was defined as an ALT of >1 and <2 times the upper limit of normal and nonresponse as an ALT of >2 times the upper limit of normal.1 Biochemical relapse was defined as an increase of ALT >3 times the upper limit of normal or an increase of IgG to >20 g/L.1, 15
Sparing of glucocorticosteroid was considered successful if the dose could be decreased with at least 25% per day with sustained remission or biochemical improvement. Cirrhosis and inflammatory activation were histologically assessed according to the Scheuer classification for grading and staging of chronic hepatitis.16 Drug survival was defined as continuation of TG therapy, patients who were offered a controlled trial of withdrawal were censored at the date TG was stopped. Treatment failure was defined as discontinuation of TG therapy for any other reason.
2.4. Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics for Windows, version 22, IBM Corp., Armonk, NY, USA. Graphs were computed with GraphPad Prism for Windows, version 7.02, GraphPad Software, La Jolla California, USA. The binominal McNemar test was used to compare the number of patients reaching complete biochemical remission versus patients who did not reach complete biochemical remission. Patients with ongoing treatment were censored at the date of last follow‐up. Categorical variables were compared between two groups using the two‐sided Fisher's exact test. Ordinal and continuous variables were compared between two groups with the Mann‐Whitney test (nonparametric). In case three groups were compared, ANOVA was used for continuous variables with a normal distribution. For nonnormally distributed variables the Kruskal‐Wallis test was used. Post‐hoc analyses for correction of multiple testing were performed with the Bonferroni in case of a parametric test and Dunn‐Bonferroni following nonparametric tests. The significance level (α‐level) was set at ≤0.05. Normally distributed variables were described as mean with SD, not‐normally distributed variables were described as median with range or interquartile range (IQR) if stated.
3. RESULTS
A total of 52 patients including AIH (n = 39) and AIH variant syndrome (n = 10) patients who were treated with TG as rescue treatment after failing on AZA or MP as well as three thiopurine‐naïve AIH patients who were treated with TG as first‐line therapy were included in this study. Characteristics and treatment details are summarised in Table 1.
Table 1.
Characteristics at diagnosis and study baseline
| AIH | AIH‐PSC | AIH‐PBC, AMA neg. AIH‐PBC | |
|---|---|---|---|
| N | 42 | 6 | 4 |
| Characteristics at diagnosis | |||
| Female (%) | 79 | 33 | 100 |
| Age (y) | 47 (11‐71) | 32.5 (16‐59) | 61 (24‐67) |
| ALT (U/L) | 558 (57‐2214) | 107 (38‐182) | 152 (78‐1418) |
| IgG (g/L) | 20 (8‐60) | 19 (13‐45) | 15 (9‐20) |
| ANA and or SMA positive, n (%) | 27 (69) | 6 (100) | 4 (100) |
| LKM‐1 positive, n (%) | 1 (3) | 0 | 0 |
| SLA/LP positive, n (%) | 2 (5) | 0 | 0 |
| IAIHG score,a median (range) | 16 (8‐21) | 17 (10‐19) | 13 (7‐15) |
| Study baseline | |||
| Cirrhosis %, n biopsied | 19%, 40 | 0%, 6 | 0%, 3 |
| Months diagnosed | 18 (0‐280) | 57 (10‐190) | 31 (2‐46) |
| Tioguanine therapy | |||
| Initial dose | |||
| mg kg−1 d−1 | 0.23 (0.10‐0.33) | 0.27 (0.18‐0.32) | 0.29 (0.27‐0.38) |
| mg/d | 20 (10‐24) | 21 (10‐24) | 20 (18‐24) |
| Dose at last use (mg/d) | 18 (5‐30) | 19 (10‐21) | 16 (10‐18) |
| Months on tioguanine therapy | 12 (2‐194) | 9 (3‐97) | 83 (18‐93) |
ALT: alanine aminotransferase; AMA: anti‐mitochondrial antibodies; ANA: anti‐nuclear antibody; IgG: immunoglobulin G; LKM‐1: liver kidney microsomal antibody; SLA/LP: soluble liver antigen/liver and pancreas antibody; SMA: smooth muscle antibody.
Post‐treatment revised International AIH Group score.10
3.1. Adverse events
Tioguanine therapy (20 mg/day, range: 10‐24 mg/day) was well‐tolerated in 41 (79%) patients, whereas four experienced tolerable (8%) and seven (13%) experienced intolerable side effects. A description of the adverse events reported on previous AZA and MP (44 of 49 patients; 90%) and those reported on subsequent TG therapy is provided in Table 2. Complaints of arthralgia recurred in one patient after switching to TG therapy, whereas all other patient‐reported side effects on TG therapy differed from those reported on the original thiopurine. Six patients had severe myelosuppression on AZA or MP; switching to TG therapy resulted in normalisation of white blood cell count, haemoglobin and thrombocytes in five patients and in one patient leukopenia (with levels between 3.0 and 4.0 × 109/L) persisted. No serious adverse events (i.e. pancreatitis), requiring hospitalisation or critical care were observed in this study. Four patients with established cirrhosis prior to TG therapy all showed signs of portal hypertension including; oesophageal varices (n = 3) and splenomegaly (n = 3) and collateral veins (n = 1). In addition, splenomegaly was present in one noncirrhotic AIH‐PSC patient who was treated with AZA 125 mg/day, 1 year prior to initiation of TG therapy. None of the patients had a history of variceal bleeding or ascites. No development of portal hypertension or associated events were recorded in patients while they were treated with TG.
Table 2.
Tolerable and intolerable adverse events on AZA, MP and TG therapy
| AZA or MP (N = 49) | TG (N = 52) | |||
|---|---|---|---|---|
| Tolerable | Intolerable | Tolerable | Intolerable | |
| Patients with an AE | 6 | 38 | 4 | 7 |
| Nausea and vomiting | 6 | 20 | 1 | |
| Headache | 2 | 1 | 2 | |
| Fatigue | 2 | 7 | 1 | |
| Myalgia/arthralgia | 1 | 6 | 3 | |
| Itch | 1 | 1 | ||
| Rash | 1 | 1 | ||
| Abdominal pain | 1 | 3 | 1 | |
| Fever | 1 | 5 | 1 | |
| Malaise | 4 | 1 | ||
| Alopecia | 2 | |||
| Hot flushes | 1 | |||
| Diarrhoea | 1 | |||
| Myelotoxicity | 2 | 4 | 1 | |
AE: adverse event; AZA: azathioprine; MP: mercaptopurine; TG: tioguanine.
Patients could report multiple side effects.
3.2. TG Efficacy in AIH patients
Thirty‐three AIH patients were treated with TG after intolerable side effects on AZA or MP (Table 2). At the time of last follow‐up, TG therapy was continued in 26 patients for a median of 22 months (range 3‐194). Biochemical measurements showed complete biochemical remission, incomplete response and nonresponse in 22 (85%), three (11%) and one (4%) patient respectively. Seven (21%) of the 33 patients discontinued treatment due to intolerable side‐effects (after months 2, 5, 9 and 24), noncompliance (after 2 months), nonresponse (after 7 months); one patient was offered a trial of withdrawal after sustained remission according to recent guidelines.1
Insufficient response on AZA or MP was the reason for switch in six AIH patients; four were responsive to TG therapy, two had complete and two had incomplete biochemical remission; two discontinued due to intolerance and nonresponse after 5 and 18 months respectively.
Early biochemical response to TG therapy was measured at 1 and 3 months. At baseline 9 of 39 (23%) patients had complete biochemical remission; this increased to 19 of 38 (50%, P = 0.002), and 19 of 34 (56%, P < 0.001) after 1 and 3 months, respectively (Table 3). Serum ALT levels are shown in Figure 1. The median IgG levels were similar at 1, 3 and 12 months compared with baseline (Figure 2).
Table 3.
Early response on rescue treatment with tioguanine in AIH patients
| Months of treatment | Follow‐up n | Tioguanine therapy | Biochemical response | P‐valuea | ||
|---|---|---|---|---|---|---|
| Stopped n | Ongoing n | Incomplete n (%) | Complete n (%) | |||
| Baseline | 39 | 0 | 39 | — | 9 (23) | |
| 1 | 38 | 0 | 38 | 19 (50) | 19 (50) | 0.002 |
| 3 | 36 | 2 | 34 | 15 (44) | 19 (56) | <0.001 |
Compared with complete biochemical response at baseline.
Figure 1.
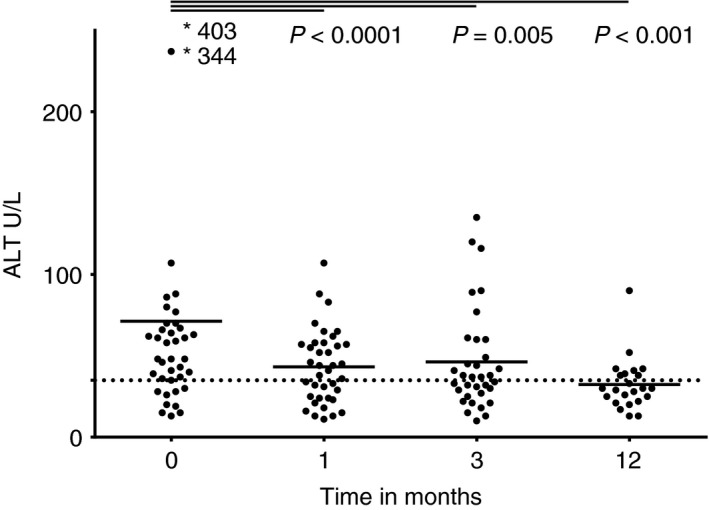
Serum alanine aminotransferase (ALT) in patients with autoimmune hepatitis before and after 1, 3 and 12 mo of tioguanine therapy. The dotted line represents the upper value of normal for female patients
Figure 2.
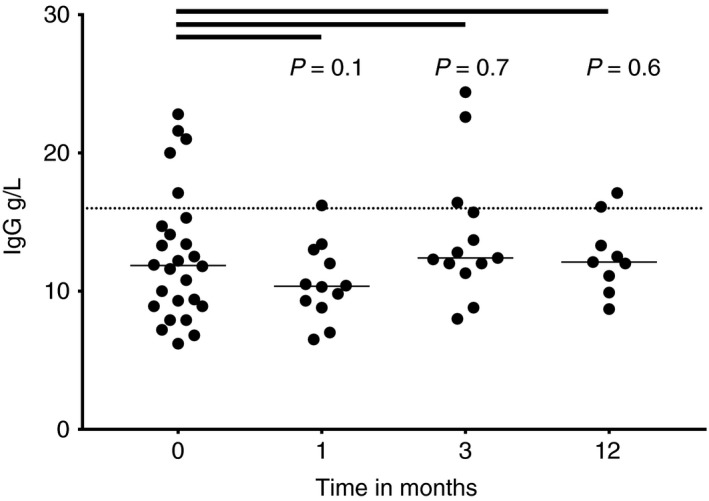
Serum immunoglobulin G (IgG) in patients with autoimmune hepatitis before and after 1, 3 and 12 mo of tioguanine therapy. The dotted line represents the upper value of normal
Prednisolone or budesonide was used by 32 (87%) patients at the time of initiation of TG therapy. A decrease in glucocorticoid dose of >25% was achieved in 18 (56%) patients at the last follow‐up. Complete withdrawal of steroids was achieved in six of these patients. Two (6%) patients required a steroid dose escalation. Steroids were initiated in another two (6%) patients.
Two AIH patients who were in remission for 1 year, temporarily discontinued TG, one patient in a drug withdrawal attempt as agreed with the treating physician and another patient refused to switch between two brands of TG. Both relapsed after 3 and 18 months respectively. After remission was induced with prednisone, complete biochemical remission was again maintained with TG monotherapy (20 mg/day) in both patients with a follow‐up of 6 months.
3.3. AIH variant syndromes
Treatment details of 10 patients with AIH variant syndromes who failed on conventional treatment are depicted in Table 4 and include six AIH‐PSC, two AIH‐PBC and two AMA negative AIH‐PBC patients. TG therapy was initiated in two patients after insufficient response on prednisone combined with mycophenolate mofetil and tacrolimus (patient two) or ciclosporin (patient seven). Patient nine and 10 had toxic 6‐methyl MP levels of >5.700 pmol/8 × 108 red blood cells on conventional thiopurines respectively. Six out of 10 patients achieved complete biochemical remission on TG therapy (Figure 3). At the last follow‐up five AIH variant patients had discontinued TG therapy for various reasons (Table 4).
Table 4.
Characteristics and treatment details of patients with AIH variants
| Patient | Diagnosis | Age, gender | Reason for prior AZA/MP failure | Tioguanine therapy | Biochemical response | Drug survival | Prednisone | Comedication | ||
|---|---|---|---|---|---|---|---|---|---|---|
| mg/d | Mo | Baseline (mg/d) | Last FU (mg/d) | |||||||
| 1 | AIH‐PSC | 17, m | Nonresponse, AZA/MP | 21 | 3 | Nonresponse | No | 20 | 20 | UDCA (14 mg/kg) |
| 2 | AIH‐PSC | 40, f | Nonresponse, AZA | 21 | 31 | Incomplete | Yes | 10 | 7.5 | UDCA (12 mg/kg, tacrolimus |
| 3 | AIH‐PSC | 40, f | Nonresponse, AZA/MP | 24 | 97 | Incomplete | Noa | 10 | 2.5 | UDCA (14 mg/kg) |
| 4 | AIH‐PSC | 51, m | Nonresponse and intolerance, AZA | 20 | 11 | Complete | Intolerant | 30 | 5 | UDCA (24 mg/kg) |
| 5 | AIH‐PSC | 67, m | Intolerance, AZA | 10 | 3 | Incomplete | Yes | 10 | — | — |
| 6 | AIH‐PSC | 29, m | Intolerance, AZA | 20 | 5 | Complete | Yes | — | 10 | UDCA (13 mg/kg) |
| 7 | AIH‐PBC | 67, f | Nonresponse and intolerance, MP | 20 | 86 | Complete | Yes | 30 | 10 | UDCA (13 mg/kg), ciclosporin |
| 8 | AIH‐PBC | 67, f | Intolerance, AZA | 20 | 93 | Complete | Yes | — | — | UDCA (18 mg/kg) |
| 9 | AMA neg. AIH‐PBC | 62, f | Toxic levels and intolerance, AZA/MP | 18 | 18 | Complete | Intolerant | 10 | 10 | UDCA (9 mg/kg), budesonideb |
| 10 | AMA neg. AIH‐PBC | 27, f | Toxic levels, AZA | 24 | 80 | Complete | Noc | 30 | — | UDCA (19 mg/kg) |
AZA: azathioprine; f: female; m: male; MP: mercaptopurine; UDCA: ursodeoxycholic acid.
Initially, nodular regenerative hyperplasia was suspected in histology, however, review of the histology by two hepatopathologists did not confirm this.
Budesonide (6 mg/d) was fully tapered.
A trial of withdrawal was offered after more than 2 years of complete biochemical remission on tioguanine monotherapy, according to recent guidelines.1
Figure 3.
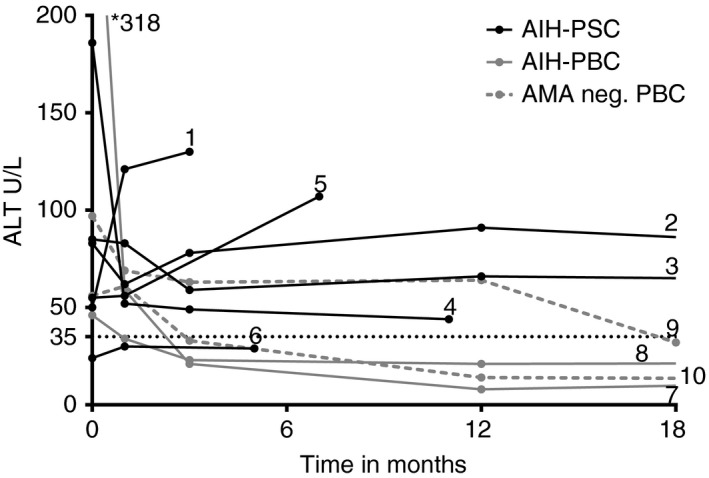
The effect of tioguanine therapy on alanine aminotransferase (ALT) levels in patients with AIH‐PSC, AIH‐PBC and anti‐mitochondrial antibody negative AIH‐PBC. The dotted line represents the upper value of normal for female patients
The median alkaline phosphatase at diagnosis of AIH variant patients was 258 U/L (range 74‐757). Alkaline phosphatase levels during TG therapy are shown in Figure S1.
3.4. First‐line maintenance therapy
In three patients with established AIH10 (post‐treatment International AIH Group scores of 13‐16), TG was initiated as the first steroid sparing agent, based on the judgement of the treating physician. TG therapy was initiated after diagnosis in two and after a flare while on monotherapy budesonide in another.
Two patients achieved complete biochemical remission after 6 months of TG therapy, which led to complete withdrawal of budesonide in one patient and tapering of prednisolone from 30 to 10 mg/day in the other. The third patient was followed for 1 month, in which she tolerated TG treatment.
3.5. Cirrhosis in AIH and AIH variant patients
Cirrhosis was present in eight (16%) and fibrosis in 22 (45%) of 49 patients who were biopsied prior to TG therapy. Two patients were not biopsied at diagnosis, due to coagulopathy and the histology report of another patient could not be retrieved. Twenty‐eight biopsies were performed in 10 patients on TG therapy, after a median treatment of 56 months (range 10‐111). Progression from fibrosis to cirrhosis occurred in one of the 10 biopsied patients. Of relevance, there were no cases of nodular regenerative hyperplasia identified in histology.
3.6. Drug adherence in AIH and AIH variant patients
In 30 patients, a total of 51 serum 6‐TGN levels were measured at least 4 weeks after first TG administration. Twenty‐nine patients were adherent to TG therapy with median 6‐TGN levels of 746 (range 168‐3070) pmol/8 × 108 red blood cells. One patient who was noncompliant after experiencing intolerable side effects and had undetectable 6‐TGN levels.
4. DISCUSSION
The conventional thiopurines AZA and MP have been successfully used for decades in the treatment of various immune mediated diseases, including inflammatory bowel disease and AIH. Nevertheless, about 20% of patients do not respond to conventional thiopurines, which can be attributed in part to an unfavourable metabolism resulting in high levels of breakdown products associated with several side effects and low levels of the active metabolites. Similarly, many patients do not tolerate these drugs. TG has the advantage that it does not require metabolic steps in order to become active and in inflammatory bowel disease, this drug has proven to be an attractive alternative in these patients.17, 18 So far, evidence that supports this strategy in AIH is lacking. In this largest retrospective study to date, we report the efficacy and tolerability of TG therapy in 52 patients with AIH and AIH variant syndromes treated in nine referral and tertiary hospitals in the Netherlands. Results demonstrate that TG therapy is well tolerated and clinically effective in patients who previously failed on AZA or MP. In addition, TG therapy was effective and tolerated in three thiopurine naïve patients.
Our data show that the vast majority of patients with AIH and AIH variants with prior intolerable side effects on conventional thiopurines will tolerate TG therapy. Similarly, nearly all patient‐reported tolerable side effects disappeared after switching to TG therapy. The side effects reported on TG therapy differed from those reported on the original thiopurine and switching resulted in normalisation of full blood count outcomes in all but one patient with myelosuppression in our cohort, which is supported by recent data in the field of inflammatory bowel disease.4, 19 The favourable tolerability might be explained by the one‐step metabolism of TG into the pharmacologically effective 6‐TGNs, without the formation of potentially toxic 6‐methyl MP metabolites seen in the complex metabolism of AZA and MP. Although this metabolism can effectively be skewed towards 6‐TGN formation by adding low‐dose allopurinol or mesalazine,20 direct TG therapy would prevent addition of another drug.
In our cohort, TG was effective in the majority (85%) of AIH patients with prior intolerance on conventional thiopurines. This is in line with a recent small single centre study, where 17 AIH patients who had failed on AZA were switched to TG.9 Although switching patients from one thiopurine to another after incomplete response was ineffective in prior studies,9, 21 two AIH and two AIH‐PSC patients in our cohort achieved complete biochemical remission with TG therapy after insufficient response on other thiopurines.
Patients with AIH who fail AZA have limited rescue options with the most frequently used second line treatment being mycophenolate mofetil. The success rate of TG therapy in our cohort was slightly higher compared to mycophenolate mofetil for those patients who switched due to intolerable side effects (85% vs 43%‐75%) or due to insufficient response on conventional thiopurines (50% vs 20%‐34%).22, 23, 24 A multicentre study with a considerable experience in AIH patients (n = 105) treated with mycophenolate mofetil reported 25% tolerable and 9% intolerable side effects, which is comparable to our data in patients using TG.24 Serious adverse events occurred in three patients using mycophenolate mofetil, which we did not observe in our cohort.
Metabolite measurements show that all but one of the patients tested were adherent to TG therapy, confirming that this is a useful tool for adherence monitoring, but due to infrequent 6‐TGN measurements, insufficient data was available for dose‐response and toxicity profiles analyses.
Concerns regarding nodular regenerative hyperplasia were not substantiated in this cohort, in which despite available histological follow‐up no cases were identified. This can be explained by the relatively low dose compared with the original oncological and inflammatory bowel disease studies in which nodular regenerative hyperplasia was identified. Moreover, a review in patients with inflammatory bowel disease showed that considerably less nodular regenerative hyperplasia occurred when dosages of TG did not exceed 25 mg/day.7 In addition, no association was found between nodular regenerative hyperplasia and clinically significant liver disease, in a recent inflammatory bowel disease study.25 There were no cases of pancreatitis in our cohort.
This study encompasses experience on a national‐level leading to generalisable results of AIH patients who received TG therapy. Other strengths of this study are the large number of patients in a real‐life cohort, the strict complete biochemical remission and the separate analysis of variant syndromes. It should be noted that this study was not powered for safety analysis and despite measures to control for ascertainment bias, adverse events may have been missed. Due to the retrospective nature of this study, it was not possible to control for co‐medication which might have biased the response rate. However, corticosteroid dose could be decreased in most patients, while maintaining remission.
In conclusion, our data show that TG therapy with a median dose of 20 mg/day is tolerated and leads to complete biochemical remission in selected patients with AIH or AIH variants who were previously intolerant and in some patients with prior insufficient response on conventional thiopurines.
ACKNOWLEDGEMENT
Declaration of personal and funding interests: None.
AUTHORSHIP
Guarantor of the article: Gerd Bouma.
Author contributions: GB had the original idea and supervised the study. CN, BV, CB, JV, JD, SP, AT, MT, AI, FtB, MB, FB and SH collected the data. FB analysed the data. FB and YB wrote the manuscript. All authors critically reviewed the manuscript and approved the final version of the manuscript.
Supporting information
van den Brand FF, van Nieuwkerk CMJ, Verwer BJ, et al. Biochemical efficacy of tioguanine in autoimmune hepatitis: a retrospective review of practice in the Netherlands. Aliment Pharmacol Ther. 2018;48:761–767. 10.1111/apt.14939
The Handling Editor for this article was Professor Stephen Harrison, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. European Association for the Study of the L . EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971‐1004. [DOI] [PubMed] [Google Scholar]
- 2. van Gerven NM, de Boer YS, Mulder CJ, van Nieuwkerk CM, Bouma G. Auto immune hepatitis. World J Gastroenterol. 2016;22(19):4651‐4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193‐2213. [DOI] [PubMed] [Google Scholar]
- 4. van Asseldonk DP, Jharap B, Kuik DJ, et al. Prolonged thioguanine therapy is well tolerated and safe in the treatment of ulcerative colitis. Dig Liver Dis. 2011;43(2):110‐115. [DOI] [PubMed] [Google Scholar]
- 5. Meijer B, Mulder CJ, Peters GJ, van Bodegraven AA, de Boer NK. Efficacy of thioguanine treatment in inflammatory bowel disease: a systematic review. World J Gastroenterol. 2016;22(40):9012‐9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dubinsky MC, Vasiliauskas EA, Singh H, et al. 6‐thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125(2):298‐303. [DOI] [PubMed] [Google Scholar]
- 7. Seinen ML, van Asseldonk DP, Mulder CJ, de Boer NK. Dosing 6‐thioguanine in inflammatory bowel disease: expert‐based guidelines for daily practice. J Gastrointestin Liver Dis. 2010;19(3):291‐294. [PubMed] [Google Scholar]
- 8. de Boer NK, van Nieuwkerk CM, Aparicio Pages MN, de Boer SY, Derijks LJ, Mulder CJ. Promising treatment of autoimmune hepatitis with 6‐thioguanine after adverse events on azathioprine. Eur J Gastroenterol Hepatol. 2005;17(4):457‐461. [DOI] [PubMed] [Google Scholar]
- 9. Legue C, Legros L, Kammerer‐Jacquet S, et al. Safety and efficacy of 6‐thioguanine as a second‐line treatment for autoimmune hepatitis. Clin Gastroenterol Hepatol. 2018;16(2):290‐291. [DOI] [PubMed] [Google Scholar]
- 10. Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929‐938. [DOI] [PubMed] [Google Scholar]
- 11. Chazouilleres O, Wendum D, Serfaty L, Montembault S, Rosmorduc O, Poupon R. Primary biliary cirrhosis‐autoimmune hepatitis overlap syndrome: clinical features and response to therapy. Hepatology. 1998;28(2):296‐301. [DOI] [PubMed] [Google Scholar]
- 12. van Gerven NM, Verwer BJ, Witte BI, et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand J Gastroenterol. 2014;49(10):1245‐1254. [DOI] [PubMed] [Google Scholar]
- 13. Boberg KM, Chapman RW, Hirschfield GM, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 2011;54(2):374‐385. [DOI] [PubMed] [Google Scholar]
- 14. Jharap B, van Asseldonk DP, de Boer NK, et al. Diagnosing nodular regenerative hyperplasia of the liver is thwarted by low interobserver agreement. PLoS ONE. 2015;10(6):e0120299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van Gerven NM, Verwer BJ, Witte BI, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58(1):141‐147. [DOI] [PubMed] [Google Scholar]
- 16. Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13(3):372‐374. [DOI] [PubMed] [Google Scholar]
- 17. Taylor KM, Ward MG, Blaker PA, Sparrow MP. Is there a role for thioguanine therapy in IBD in 2017 and beyond? Expert Rev Gastroenterol Hepatol. 2017;11(5):473‐486. [DOI] [PubMed] [Google Scholar]
- 18. Simsek M, Meijer B, van Bodegraven AA, de Boer NKH, Mulder CJJ. Finding hidden treasures in old drugs: the challenges and importance of licensing generics. Drug Discov Today. 2018;23(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 19. Meijer B, Wilhelm AJ, Mulder CJJ, Bouma G, van Bodegraven AA, de Boer NKH. Pharmacology of thiopurine therapy in inflammatory bowel disease and complete blood cell count outcomes: a 5‐year database study. Ther Drug Monit. 2017;39(4):399‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. de Boer YS, van Gerven NM, de Boer NK, Mulder CJ, Bouma G, van Nieuwkerk CM. Allopurinol safely and effectively optimises thiopurine metabolites in patients with autoimmune hepatitis. Aliment Pharmacol Ther. 2013;37(6):640‐646. [DOI] [PubMed] [Google Scholar]
- 21. Hubener S, Oo YH, Than NN, et al. Efficacy of 6‐mercaptopurine as second‐line treatment for patients with autoimmune hepatitis and azathioprine intolerance. Clin Gastroenterol Hepatol. 2016;14(3):445‐453. [DOI] [PubMed] [Google Scholar]
- 22. Baven‐Pronk AM, Coenraad MJ, van Buuren HR, et al. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther. 2011;34(3):335‐343. [DOI] [PubMed] [Google Scholar]
- 23. Hennes EM, Oo YH, Schramm C, et al. Mycophenolate mofetil as second line therapy in autoimmune hepatitis? Am J Gastroenterol. 2008;103(12):3063‐3070. [DOI] [PubMed] [Google Scholar]
- 24. Roberts SK, Lim R, Strasser S, et al. Efficacy and safety of mycophenolate mofetil in patients with autoimmune hepatitis and suboptimal outcomes after standard therapy. Clin Gastroenterol Hepatol. 2018;16(2):268‐277. [DOI] [PubMed] [Google Scholar]
- 25. van Asseldonk DP, Jharap B, Verheij J, et al. The prevalence of nodular regenerative hyperplasia in inflammatory bowel disease patients treated with thioguanine is not associated with clinically significant liver disease. Inflamm Bowel Dis. 2016;22(9):2112‐2120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


