Abstract
Introduction
Diabetes mellitus is a progressive disease with cardiovascular complications. This study evaluated the effects of liraglutide, a glucagon‐like peptide‐1 analogue and the dipeptidyl peptidase 4 inhibitors sitagliptin and linagliptin on cardiac function in type 2 diabetes patients with renal impairment.
Materials and Methods
A total of 139 patients who were referred because of suboptimal glycaemic control were randomly assigned to liraglutide 0.9 mg/d (n = 45), sitagliptin 50 mg/d, (n = 49) or linagliptin 5 mg/d (n = 45) at enrolment and were evaluated. Blood glucose, glycosylated haemoglobin and serum creatinine were assayed every 3 months for 48 months. Echocardiography was performed every 12 months for 48 months.
Results
Compared with baseline, fasting glucose, postprandial glucose, and systolic and diastolic pressure, but not estimated glomerular filtration rate, significantly decreased in all three groups. Albuminuria decreased from 24 to 48 months with liraglutide, but only from 24 to 30 months with sitagliptin and linagliptin. Diastolic function, assessed by E/e′ or left atrial dimension improved only with liraglutide.
Conclusions
Liraglutide was effective for glucose and blood pressure control, reduced albuminuria and improved diastolic function. Diastolic function was not improved by sitagliptin and linagliptin.
1. INTRODUCTION
Diabetes mellitus (DM) is a progressive disease with systemic cardiovascular complications. Cardiovascular comorbidities are important prognostic factors in DM patients, and their frequency increases with the progression of renal impairment. Control of blood pressure (BP) and glucose levels prevent progressive systemic vascular complications. Glycaemic control in patients with DM and renal impairment is difficult because reduced glomerular filtration rate (GFR) leads to accumulation of drugs and their metabolites.1 Consequently, reduced GFR limits the choice of antidiabetic agents. Novel incretin enhancing agents have been evaluated in DM patients with renal impairment.2, 3, 4 The available agents enhance incretin activity by acting as glucagon‐like peptide‐1 (GLP‐1) receptor agonists or as dipeptidyl peptidase‐4 (DPP‐4) inhibitors. This study investigated the effects of a GLP‐1 analogue (liraglutide) and two DPP‐4 inhibitors (sitagliptin and linagliptin) on cardiac function in a series of DM patients with impaired renal function.
2. MATERIALS AND METHODS
2.1. Patients and laboratory testing
This longitudinal observational study prospectively enrolled 139 patients with a mean age of 67.8 ± 9.6 years (range 48‐82 years) with type 2 DM, nonoptimal glycaemic control, and renal impairment, with an estimated GFR (eGFR) between 30 and 60 mL/min/1.73 m2, who were referred to Konan Kosei Hospital between October 2010 and June 2013. Patients with a history of type 1 DM, diabetic ketoacidosis, severely impaired insulin secretion (serum C‐peptide < 2.0 ng/mL), high‐dose insulin (>20 U/d) requirement, or hepatic or cardiac failure and atrial fibrillation were excluded. The patients were randomly allocated to lira group (liraglutide 0.9 mg/d, n = 45), sita group (sitagliptin 50 mg/d, n = 49) or lina group (linagliptin 5 mg/d, n = 45). Allocation was performed using sequentially numbered envelopes. Liraglutide 0.9 mg/d is the approved upper dose in Japan. Patients who died or began dialysis therapy for end‐stage renal disease were withdrawn from the study protocol.
The primary endpoints were the rate of new onset renal replacement therapy, death from cardiovascular events, including acute myocardial infarction (AMI), stroke and diagnosis of heart failure. Secondary endpoints were a ≥ 30% decline in eGFR, ≥30% increase in albuminuria, ≥30% change in the ratio of early diastolic transmitral flow velocity to peak early diastolic mitral annular velocity (E/e′). All patients provided written informed consent before participation. The study protocol was approved by the ethics committees at Konan Kosei Hospital. The study was conducted following the ethical principles of the Declaration of Helsinki and the Japanese Ministry of Health, Labour and Welfare. Reporting of the study conforms to STROBE statement along with references to STROBE statement and the broader EQUATOR guidelines.5
2.2. Blood chemistry
Laboratory tests were performed every 3 months for 48 months after initiation of incretin therapy. Patient characteristics included age, sex, body mass index (BMI) and BP. Blood samples were collected for assay of plasma glucose, haemoglobin A1c (HbA1c), high‐density lipoprotein cholesterol(HDL‐C), low‐density lipoprotein cholesterol(LDL‐C), blood urea nitrogen, creatinine, uric acid(UA)brain natriuretic peptide (BNP), total protein, albumin and C‐reactive protein (CRP). eGFR was estimated as previously described by the Japanese Society of Nephrology6 for men eGFR (mL/min/1.73 m2) = 194 × SCr−1.094 × age−0.287 and eGFR (mL/min/1.73 m2) (Female) = 194 ×SCr−1.094 × age−0.287 × 0.739 for women, where SCr is serum creatinine. Albuminuria was measured as a ratio of urinary albuminuria (mg)/urinary creatinine (g).
2.3. Echocardiography
Echocardiography was performed at the start of incretin therapy and every 12 months thereafter for 48 months. The recordings and measurements were performed following the American Society of Echocardiography guidelines using a standard imaging transducer (Vivid 7; GE, Inc., Stamford, CT, USA) with a linear probe frequency of 5 MHz. The echocardiographic data were independently evaluated by at least four echocardiologists in our hospital. The ratio of the peak early diastolic (E) and the peak atrial systolic (A) transmitral flow velocities (E/A) was calculated. E/e′ was calculated as a marker of left ventricular filling pressure. Routine echocardiographic evaluations included the left ventricular mass index (LVMI), left ventricular ejection fraction (EF), left ventricular fractional shortening (FS), left atrial dimension (LAD), E/e′ and relative wall thickness (RWT). FS was calculated as [(LVEDD − LVEDS)/LVEDD] × 100, where LVEDD is the left ventricle end‐diastolic dimension and LVEDS is the left ventricle end‐systolic dimension. RWT was calculated as two times the posterior wall thickness divided by LVEDD.
LVMI was calculated as LVM = 1.04 [(LVEDD + IVS + PWT)3 − LVEDD3] − 13.6 and LVMI = LVM/body surface area, as described by Devereux and Reicheck,7 IVS is the interventricular septum thickness, and PWT is the posterior wall thickness.
2.4. Statistical analysis
Results are reported as means ± standard deviation except for ACR. The values of ACR were indicated as means ± standard errors. Differences in baseline values were compared using the unpaired t test. Change in values during the study period was analysed by comparison with baseline using the Wilcoxon signed‐rank test. The frequencies of cardiovascular events, ≥30% eGFR decline and ≥30% E/e′ increase were analysed using the χ2 test. P‐values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS version 23 for Windows (SPSS, Inc., Chicago, IL, USA).
3. RESULTS
3.1. Clinical characteristics
Forty‐one patients failed to complete the study. Four transferred to other clinics, one each in the lira and lina groups and two in the sita group. Six were withdrawn for AMI and cerebrovascular accident, one in the lira, three in the sita and two in the lina group (P = 0.894, 0.973 vs lira group). Eleven were withdrawn because they started renal replacement therapy, three in the lira, five in the sita and three in the lina group (P = 0.645, 0.977 vs lira group). Four were withdrawn because they began cancer treatment, two in the lira, one in the sita and one in the lina group (P = 0.810, 0.973 vs lira group). Seven deaths from other causes occurred during the study, one from an accident, and two in each group from pneumonia (P = 0.592, 0.641 vs lira group). Nine were hospitalized for heart failure, three in each group, (P = 0.725, 0.973 vs lira group). The remaining 98 patients, 32 in the liraglutide, 34 in the sitagliptin, and 32 in the linagliptin group, completed the design and were evaluated. Thirty‐eight patients switched from insulin to incretin therapy, 52 switched from other antidiabetic agents to incretin therapy and 8 received incretin for the first time. Other antidiabetic, antihypertensive and statin regimens were not changed when the study started. Six patients, two in each group, had atrial fibrillation at baseline. Five, one in the lira and two each in the sita and lina groups, were newly diagnosed with atrial fibrillation during the study.
The patient baseline characteristics are shown in Table 1. There were no differences in age, DM duration, eGFR, albuminuria‐to‐urinary creatinine ratio (ACR), echocardiographic findings, New York Heart Association classification, or use of antihypertensive and other antidiabetes agents at baseline. As shown in Figure 1A, systolic BP (SBP) and diastolic BP were significantly lower in lira group at 6 months than at baseline (both P < 0.01) and remained lower. SBP and DPB were lower than baseline between 6 and 36 months in the sita and lina groups (both P < 0.01). BMI did not change during the study period in any of the three groups (Table 2), and there were no differences in the incidence of cardiovascular events (AMI, stroke and heart failure) in the three groups (data not shown).
Table 1.
Baseline patient characteristics
| lira group | sita group | lina group | |
|---|---|---|---|
| (liraglutide; n = 32) | (sitagliptin; n = 34) | (linagliptin; n = 32) | |
| Age (y) | 70.5 ± 5.7 | 69.9 ± 8.5 | 69.0 ± 7.7 |
| DM duration (y) | 9.2 ± 7.0 | 8.8 ± 8.3 | 8.3 ± 0.4 |
| BMI (kg/m2) | 23.5 ± 3.5 | 24.2 ± 3.8 | 23.8 ± 4.8 |
| HbAlc (%) | 6.75 ± 0.62 | 6.72 ± 0.72 | 6.71 ± 0.75 |
| FPG (mg/dL) | 140.9 ± 15.8 | 137.9 ± 18.5 | 137.7 ± 8.1 |
| eGFR (mL/min/1.73 m2) | 40.2 ± 11.6 | 46.1 ± 12.8 | 45.2 ± 14.2 |
| ACR (mg/g Cr) | 380.0 ± 62.4 | 300.1 ± 47.0 | 289.7 ± 44.6 |
| SBP (mm Hg) | 141.1 ± 9.7 | 135.8 ± 14.9 | 133.1 ± 16.7 |
| DBP (mm Hg) | 88.2 ± 8.0 | 82.3 ± 14.2 | 85.2 ± 8.1 |
| EF (%) | 66.3 ± 10.2 | 68.9 ± 7.2 | 66.0 ± 7.2 |
| FS (%) | 40.4 ± 10.3 | 37.8 ± 6.0 | 36.8 ± 4.9 |
| E/e′ | 13.4 ± 2.9 | 12.7 ± 3.8 | 12.9 ± 4.1 |
| E/A | 0.63 ± 0.22 | 0.65 ± 0.17 | 0.64 ± 0.19 |
| LAD (mm) | 38.9 ± 6.3 | 37.8 ± 6.0 | 36.8 ± 4.9 |
| RWT (%) | 44.4 ± 8.3 | 42.9 ± 8.2 | 44.5 ± 9.6 |
| LVMI (g/m2) | 134.8 ± 28.8 | 135.5 ± 33.4 | 133.5 ± 30.7 |
| BNP (pg/mL) | 91.6 ± 69.9 | 83.9 ± 59.2 | 90.6 ± 68.6 |
| New York Heart Association, Classification | |||
| I : n (%) | 15 (46.9) | 16 (47.1) | 16 (50.0) |
| H: n (%) | 12 (37.5) | 13 (38.2) | 11 (34.4) |
| M: n (%) | 5 (15.6) | 5 (14.7) | 5 (15.6) |
| Medication | |||
| Antidiabetic agents | |||
| plus none, n(%) | 23 (71.9) | 24 (70.6) | 21 (65.6) |
| plus insulin, n(%) | 3 (9.4) | 3 (8.8) | 3 (9.4) |
| plus aGI, n(%) | 3 (9.4) | 4 (11.8) | 4 (12.5) |
| plus glinide, n(%) | 3 (9.4) | 3 (8.8) | 2 (6.3) |
| Other drug | |||
| ARB, n (%) | 27 (84.4) | 28 (82.3) | 26 (81.3) |
| CCB, n (%) | 20 (62.5) | 21 (61.7) | 21 (65.6) |
| Statin, n (%) | 24 (75.0) | 25 (73.5) | 24 (73.5) |
| Diuretics, n (%) | 17 (53.1) | 18 (52.9) | 18 (56.3) |
Data are expressed as means ± standards deviations except for ACR. ACR are expressed as means ± standards errors.
αGI, alfa glucosidase inhibitor; ARB, angiotensin II receptor blockade; CCB, calcium channel blockade.
Figure 1.
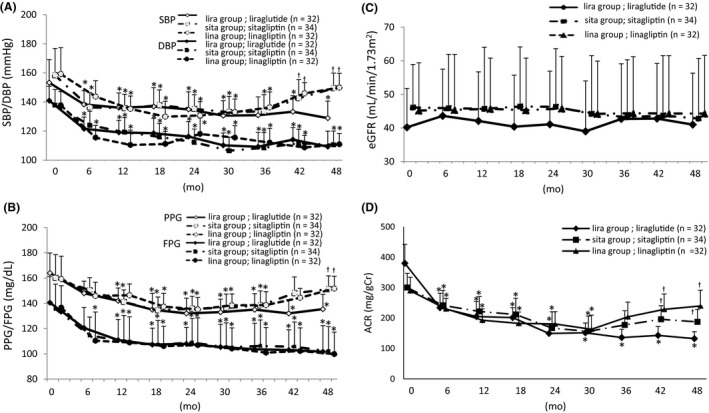
Change in blood pressure, glycaemic control, and renal function. Blood pressure was decreased by liraglutide throughout the study, but only from 12 to 30 mo in sita and lina group (A). Blood pressure (B). Glucose (C). Estimated glomerular filtration rate did not change in any group during the study period (D). Albuminuria decreased in all groups. SBP, systolic blood pressure; DBP, diastolic blood pressure; PPG, postprandial plasma glucose; FPG, fasting plasma glucose; eGFR, estimated glomerular filtration ratio; ACR, ratio of albuminuria to urinary creatinine. *P < 0.01 vs baseline, †P < 0.05 vs lira group
Table 2.
Clinical data at baseline and at 12, 24, 36 and 48 mo after initiation of incretin‐based therapy
| Group | 0 mo | 12 mo | 24 mo | 36 mo | 48 mo |
|---|---|---|---|---|---|
| HbAlc(%) | |||||
| lira group (liraglutide; n = 32) | 6.75 ± 0.62 | 6.40 ± 0.56** | 6.33 ± 0.72 * | 6.35 ± 0.56* | 6.26 ± 0.69* |
| sita group (sitagliptin; n = 34) | 6.72 ± 0.72 | 6.48 ± 0.66** | 6.41 ± 0.74* | 6.49 ± 0.62** | 6.55 ± 0.94** |
| lina group (linagliptin; n = 32) | 6.7 l ± 0.75 | 6.35 ± 0.56* | 6.18 ± 0.58* | 6.25 ± 0.56* | 6.21 ± 0.49* |
| BMI (kg/m2) | |||||
| lira group (liraglutide; n = 32) | 23.5 ± 3.5 | 22.8 ± 3.4 | 22.8 ± 3.2 | 22.7 ± 3.1 | 23.1 ± 3.2 |
| sita group (sitagliptin; n = 34) | 24.2 ± 4.2 | 24.1 ± 4.2 | 24.2 ± 4.2 | 24.3 ± 4.2 | 24.3 ± 4.3 |
| lina group (linagliptin; n = 32) | 23.8 ± 4.8 | 23.0 ± 4.6 | 22.8 ± 4.6 | 22.6 ± 4.8 | 23.2 ± 4.6 |
| BNP (pg/mL) | |||||
| lira group (liraglutide; n | 91.6 ± 69.9 | 61.2 ± 49.4* | 58.8 ± 46.1* | 55.8 ± 46.2* | 65.8 ± 50.9* |
| sita group (sitagliptin; n = 34) | 83.9 ± 59.2 | 66.1 ± 50.7* | 64.4 ± 48.3* | 76.8 ± 53.5 | 73.3 ± 47.2 |
| lina group (linagliptin; n = 32) | 90.6 ± 58.6 | 76.8 ± 56.2 | 70.8 ± 53.0* | 78.3 ± 51.1 | 71.8 ± 46.5* |
| β2MG (mg/dL) | |||||
| lira group (liraglutide; n = 32) | 4.9 ± 3.2 | 4.5 ± 2.4 | 4.6 ± 2.7 | 4.7 ± 2.8 | 4.8 ± 2.7 |
| sita group (sitagliptin; n = 34) | 4.5 ± 4.2 | 4.5 ± 3.4 | 4.4 ± 3.7 | 4.5 ± 3.6 | 4.6 ± 4.4 |
| lina group (linagliptin; n = 32) | 4.6 ± 4.5 | 4.4 ± 3.7 | 4.4 ± 3.8 | 4.6 ± 3.4 | 4.7 ± 4.2 |
| CRP (mg/dL) | |||||
| lira group (liraglutide; n = 32) | 0.26 ± 0.21 | 0.12 ± 0.13 | 0.19 ± 0.10 | 0.08 ± 0.09 | 0.09 ± 0.05 |
| sita group (sitagliptin; n = 34) | 0.22 ± 0.30 | 0.13 ± 0.17 | 0.16 ± 0.20 | 0.15 ± 0.19 | 0.13 ± 0.14 |
| lina group (linagliptin; n = 32) | 0.24 ± 0.18 | 0.17 ± 0.11 | 0.16 ± 0.11*** | 0.17 ± 0.11*** | 0.16 ± 0.11*** |
| UA (mg/dL) | |||||
| lira group (liraglutide; n = 32) | 6.7 ± 2.5 | 6.3 ± 1.9 | 6.3 ± 1.7 | 6.2 ± 1.8 | 6.0 ± 1.6 |
| sita group (sitagliptin; n = 34) | 6.3 ± 1.1 | 6.2 ± 1.0 | 6.2 ± 1.0 | 6.1 ± 1.2 | 6.1 ± 1.1 |
| lina group (linagliptin; n = 32) | 6.7 ± 2.0 | 6.3 ± 2.1 | 6.3 ± 1.7 | 6.0 ± 1.5 | 5.9 ± 1.3 |
| LDL‐C (mg/dL) | |||||
| lira group (liraglutide; n = 32) | 125.5 ± 27.4 | 109.1 ± 36.5 | 105.9 ± 27.5* | 93.5 ± 25.3* | 103.2 ± 20.8** |
| sita group (sitagliptin; n = 34) | 100.9 ± 40.7 | 94.2 ± 28.3 | 97.7 ± 28.1 | 95.8 ± 30.2 | 91.6 ± 26.9 |
| lina group (linagliptin; n = 32) | 115.8 ± 29.5 | 102.2 ± 26.9 | 91.3 ± 29.9 | 91.2 ± 25.8* | 91.5 ± 27.8 |
| HDL‐C (mg/dL) | |||||
| lira group (liraglutide; n = 32) | 51.9 ± 14.6 | 50.3 ± 14.5 | 50.0 ± 14.4 | 51.6 ± 15.7 | 51.6 ± 14.0 |
| sita group (sitagliptin; n = 34) | 51.2 ± 11.4 | 49.8 ± 12.8 | 49.0 ± 11.4 | 50.7 ± 11.4 | 52.4 ± 12.7 |
| lina group (linagliptin; n = 32) | 51.0 ± 18.2 | 49.1 ± 14.6 | 51.8 ± 13.6 | 50.2 ± 17.0 | 53.0 ± 14.9 |
Data are expressed as means ± standards deviations.
BMI, body mass index; BNP, brain natriuretic peptide; CRP, C‐reactive protein; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; UA, uric acid; β2 MG, β2 microgloburin.
*P < 0.01, **P < 0.05 vs baseline, ***P < 0.05 vs lira group.
3.2. Glycaemic control
Fasting plasma glucose, postprandial plasma glucose and HbA1c at baseline in all groups were similar at baseline (Table 1). Fasting plasma glucose was significantly decreased at 12 months in all groups compared with baseline. Postprandial plasma glucose in lira group was significantly decreased after 12 months. In the sita and lina groups, postprandial plasma glucose was decreased from 12 to 36 months (Figure 1B). HbA1c was significantly lower than baseline at 12, 24, 36 and 48 months in all groups. The between‐group differences were not significant (Table 2). The improvements in glycaemic control in all three groups were similar.
In this study, participants with nonoptimal glycaemic control, such as HbA1c > 8.0% or having many times hypoglycaemic events, were enrolled. And HbA1c < 7.0% is assumed appropriate target of glycaemic control, then 21 participants (6, 7, 6 in each groups) with having incretin‐based therapies did not get good control at 2 years. Moreover, 22 (6, 7, 7 in each groups) did not get. But there were no differences in patients who had not get appropriate glycaemic controls among groups at 2 and 4 years.
3.3. Renal function
The mean eGFR remained unchanged in all groups during the study period. There were no differences among the study groups at any time (Figure 1C). Albuminuria, measured by ACR, was significantly lower than baseline in all study groups at 30 months. The ACR increased in the sita and lina groups at 36 months, but continued to decrease in lira group (Figure 1D). The incidence of a ≥ 30% eGFR decline was similar in the study groups (data not shown). Uric acid was not changed throughout the study period in all groups.
3.4. Other lipid profile and C‐reactive protein
The values of HDL‐C were not changed throughout the study period in all groups. But the values of LDL‐C were decreased gradually throughout the study period in all groups. In particular, in liraglutide group, the value of LDL‐C after 24 months and after was significantly lower than that of at baseline (P < 0.005).
The values of CRP were also gradually decreased in all groups without significance. But after 24 months and after, the value in liraglutide group was lower than that in linagliptin groups (P < 0.05).
3.5. Echocardiography and cardiac function
Echocardiography revealed significant decreases in LVMI, E/e′ and LAD in the lira group and stable or increasing values in the sita and lina groups (Figure 2A‐C). The increase in patients with a ≥ 30% elevation of E/e′ in group lira was significantly smaller than that in the sita and lina groups (P < 0.05, lira vs sita group, P < 0.01, lira vs lina group). No between‐group differences were observed in EF, E/A and FS during the study period (Figure 2D‐F). Mean RWT values did not change (data not shown). BNP values significantly decreased in the lira but not in the sita and lina groups (Table 2).
Figure 2.
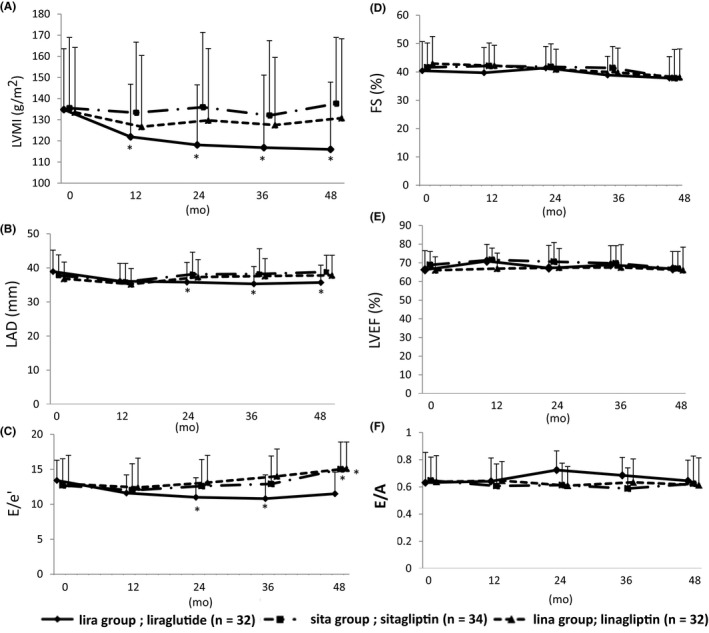
Echocardiography. In lira group, left ventricular mass index (LVMI) (A), left atrial dimension (LAD) (B) and E/e’ (C) decreased throughout the study period. Fraction shortening (FS) (D), left ventricular ejection fraction (LVEF) (E) and E/A ratio (F) did not change in any group. *P < 0.01, **P < 0.05 vs baseline. †P < 0.01 vs lira group
4. DISCUSSION
In this study, liraglutide, but not sitagliptin and linagliptin, significantly improved parameters related to diastolic dysfunction, such as E/e′, LAD and BNP. No between‐group differences in systolic function were associated with the GLP‐1 analogue and DPP‐4 inhibitors. We previously reported that liraglutide caused natriuresis, decreased average blood glucose and BP and improved renal function, as shown by eGFR.8 Those results were consistent with other demonstrations of liraglutide‐induced diuresis, natriuresis9 and increase in eGFR.10 This study investigated the effects of incretin enhancing agents on cardiac diastolic function. Sitagliptin and liraglutide have been shown to improve diastolic function in the short term11, 12, 13, 14 and diabetes mortality increases with E/e′.15 This may be the first report of differential effects of GLP‐1 analogue and DPP‐4 inhibitors on cardiac diastolic function with follow‐up as long as 48 months. The DPP‐4 inhibitors and GLP‐1 analogue did not result in weight gain or increased inflammation. Metabolic abnormalities, poor glycaemic control and ischaemic changes are associated with cardiac diastolic dysfunction.16 Reduction in body weight improves insulin resistance,17 may be independently associated with improved diastolic function.18 Improved glycaemic and BP control induced by incretin‐based therapy also may contribute to improvement of cardiac dysfunction, but in this study, there were no differences in glycaemic control with liraglutide and the DPP‐4 inhibitors until 30 months after starting treatment. Postprandial glucose continued to improve in the lira group. As there was no significant loss of body weight in any study group, weight loss could not have influenced insulin resistance, BP change and cardiac function. Nevertheless, liraglutide had stronger effects on cardiac diastolic function than the DPP‐4 inhibitors did. The strong, sustained BP reduction and glycaemic control by liraglutide may have reduced albuminuria and improved cardiac function, including BNP, LVMI, LAD and E/e′. Liraglutide is also thought to have anti‐inflammatory and vasodilator effects, which attenuate atherosclerosis, and may have contributed to improved BP control and cardiac diastolic function in this study.
In the Functional Impact of GLP‐1 for Heart Failure Treatment (FIGHT) trial, liraglutide did not improve cardiac function in participants with an EF of <25%.19 In the liraglutide on left ventricular function (LIVE) trial, liraglutide did improve diastolic function as indicated by E/e′ in participants with an EF of <45%.20 Liraglutide might not improve cardiac function in an advanced stage but in moderate to mild impairment of cardiac function was improved. In the FIGHT and LIVE trials, participants were given 1.8 mg liraglutide, but 0.9 mg liraglutide, which is the maximum dose approved in Japan, was given in this study, and fewer than 10% of the participants had an EF of <40%. The aim of this study was to compare the effects of 0.9 mg liraglutide and DPP‐4 inhibitors on cardiac and renal function in diabetes patients with renal failure and mild to moderate heart failure.
Nogueira et al21 reported an association of sitagliptin‐mediated improvement in diastolic dysfunction and an increase in plasma GLP‐1. Liraglutide has been found to cause a greater increase in serum GLP‐1 concentration than a DPP‐4 inhibitor.22 The liraglutide‐induced increase in GLP‐1 concentrations might account for stronger effects on cardiac diastolic function than those caused by DPP‐4 inhibitors in this study. The effect of GLP‐1 on diastolic function was larger than that of the DPP‐4 inhibitors; the GLP‐1 concentration was not assayed.
In this study, the duration of DM was related to the E/e′ value as reported by Aaron et al15 and not to the EF value, suggesting that diastolic dysfunction may appear before systolic dysfunction in DM patients. Cardiac diastolic function is thought to be influenced by glycaemic control and control of BP, ischemia and insulin resistance.15 E/e′ is a reliable, noninvasive estimate of cardiac diastolic function and it showed that the GLP‐1 analogue had a more beneficial effect on cardiac diastolic function than DPP‐4 inhibitors. The effects of liraglutide on cardiac diastolic function might have resulted from a reduction of BP, increased nitric oxide (NO) production and anti‐inflammatory effects induced by an increase of serum GLP‐1 concentration. Liraglutide has been reported to reduce oxidative stress23 and improve endothelial function and NO production.24 Those changes would likely be followed by amelioration of albuminuria and renal function. Natriuresis and diuresis were followed by BP reduction and improved glycaemic control; vasodilation and NO production may ameliorate cardiac diastolic dysfunction.
The study results showing that liraglutide injection may induce higher concentrations of serum GLP‐1 than DPP‐4 inhibition treatment are consistent with those of the LEADER,25, 26 TECOS,27 and SAVOR‐TIMI28 trials. Increased serum GLP‐1 concentration could result in greater improvement in cardiac function than that induced by DPP‐4 inhibitors. Diastolic function is thought to worsen with increase in DM duration. In Japan, liraglutide is usually used after other antidiabetic agents, including DPP‐4 inhibitors. If cardiac dysfunction is associated with DM duration, and liraglutide is used earlier in patients with DM, its beneficial effects on cardiac function might be enhanced. Further studies of the prevention of cardiovascular events induced by liraglutide in line with a recent report by Rosenmeier29 are warranted. Some adverse events, including neoplasms, cardiovascular events and initiation of dialysis therapy, occurred in this study, but there were no differences in incidence in the three groups. There were no differences in the incidence of AMI, stroke and heart failure in the three groups (data not shown).
Even though this was a prospective, randomized study, the interpretation of the impact of liraglutide on cardiac function was limited by a small sample size and short duration of follow‐up. Further investigation with larger sample sizes and longer follow‐up are needed to more fully understand the protection of renal function and other benefits of liraglutide in patients with type 2 diabetes and renal impairment.
5. CONCLUSION
In conclusion, in addition to protecting renal function, liraglutide had positive effects on cardiac diastolic function in patients with type 2 DM and moderate‐to‐severe renal impairment for up to 48 months.
CONFLICT OF INTEREST
No authors have a conflict of interest to report.
ACKNOWLEDGEMENTS
We thank patients and staff and acknowledge the input of many other members of the team who assisted in performing the study.
Hiramatsu T, Asano Y, Mabuchi M, Imai K, Iguchi D, Furuta S. Liraglutide relieves cardiac dilated function than DPP‐4 inhibitors. Eur J Clin Invest. 2018;48:e13007 10.1111/eci.13007
REFERENCES
- 1. Abe M, Okada K, Soma M. Antidiabetic agents in patients with chronic kidney disease and end‐stage renal disease on dialysis: metabolism and clinical practice. Curr Drug Metab. 2011;12:57‐69. [DOI] [PubMed] [Google Scholar]
- 2. Jacobsen LV, Hindsberger C, Robson R, Zdravkovic M. Effect of renal impairment on the pharmacokinetics of the GLP‐1 analogue liraglutide. Br J Clin Pharmacol. 2009;68:898‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zavattaro M, Caputo M, Samà M, et al. One‐year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine. 2015;50:620‐626. [DOI] [PubMed] [Google Scholar]
- 4. Terawaki Y, Nomiyama T, Takenoshita H, et al. The efficacy of incretin therapy in patients with type 2 diabetes undergoing hemodialysis. Diabetol Metab Syndr. 2013;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40:35‐53. [DOI] [PubMed] [Google Scholar]
- 6. Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982‐992. [DOI] [PubMed] [Google Scholar]
- 7. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613‐618. [DOI] [PubMed] [Google Scholar]
- 8. Hiramatsu T, Ozeki A, Ishikawa H, Furuta S. Long term effects of liraglutide in Japanese patients with type 2 diabetes among the subgroups with different renal functions: results of 2‐year prospective study. Drug Res (Stuttg). 2017;67:640‐646. [DOI] [PubMed] [Google Scholar]
- 9. Schlatter P, Beglinger C, Drewe J, Gutmann H. Glucagon‐like peptide 1 receptor expression in primary porcine proximal tubular cells. Regul Pept. 2007;141:120‐128. [DOI] [PubMed] [Google Scholar]
- 10. Crajoinas RO, Oricchio FT, Pessoa TD, et al. Mechanism mediating the diuretic and natriuretic actions of the incretin hormone glucagon‐like peptide‐1. Am Physiol Renal Physiol. 2011;301:F355‐F363. [DOI] [PubMed] [Google Scholar]
- 11. Yamada H, Tanaka A, Kusunose K, et al. Effect of sitagliptin on the echocardiographic parameters of left ventricular diastolic function in patients with type 2 diabetes: a subgroup analysis of the PROLOGUE study. Cardiovasc Diabetol. 2017;6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCormic LM, Kydd AC, Read PA, et al. Chronic dipeptidyl peptidase‐4 inhibition with sitaglipyin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circ Cardiovasc Imaging. 2014;7:247‐281. [DOI] [PubMed] [Google Scholar]
- 13. Saponaro F, Sonaglioni A, Rossi A, et al. Improved diastolic function in type 2 diabetes after a six month liraglutide treatment. Diabetes Res Clin Pract. 2016;118:21‐28. [DOI] [PubMed] [Google Scholar]
- 14. Arturi F, Succurro E, Miceli S, et al. Liraglutide improves cardiac function in patients with type 2 diabetes and chronic heart failure. Endocrine. 2017;57:464‐473. [DOI] [PubMed] [Google Scholar]
- 15. From AM, Scott CG, Chen HH. Changes in diastolic dysfunction in diabetes mellitus over time. Am J Cardiol. 2009;103:1463‐1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steferovic PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two‐faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718‐1727. [DOI] [PubMed] [Google Scholar]
- 17. Dinh W, Lankisch M, Nickl W, et al. Insulin resistance and glycemic abnormalities are associated with deterioration of left ventricular diastolic function: a cross‐sectional study. Cardiovasc Diabetol. 2010;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nauck MA. Incretin‐based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med. 2011;124(Suppl 1):S3‐S18. [DOI] [PubMed] [Google Scholar]
- 19. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction. A randomized clinical trial. JAMA. 2016;316:500‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon‐like peptide‐1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)–a multicenter, double‐blind, randomized, placebo‐controlled trial. Eur J Heart Fail. 2016;. 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 21. Nogueira KC, Furtado M, Fukui RT, et al. Left ventricular diastolic function in patients with type 2 diabetes treated with a dipeptidyl peptidase‐4 inhibitor‐ a pilot study. Diabetol Metab Syndr. 2014;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Degn KB, Juhl CB, Sturis J, et al. One week's treatment with the long‐acting glucagon‐like derivative liraglutide (NN2211) markedly improves 27‐h glycemia and alpha‐ and beta‐cell function and reduces endogeneous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187‐1194. [DOI] [PubMed] [Google Scholar]
- 23. Rizzo M, Abate N, Chandalia M, et al. Liraglutide reduces oxidative stress and restores heme oxygenase‐1 and ghrelin levels in patients with type 2 diabetes: a prospective pilot study. J Clin Endocrinol Metab. 2015;100:603‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang B, Zhong J, Lin H, et al. Blood pressure‐lowering effects of GLP‐1 receptor agonists exenatide and liraglutide: a meta‐analysis of clinical trials. Diabetes Obes Metab. 2013;15:737‐749. [DOI] [PubMed] [Google Scholar]
- 25. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 27. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232‐242. [DOI] [PubMed] [Google Scholar]
- 28. Udell JA, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: observations from the SAVOR‐TIMI 53 Trial. Diabetes Care. 2015;38:696‐705. [DOI] [PubMed] [Google Scholar]
- 29. Silvertsen J, Rosenmeier J, Holst JJ, Vilsbøll T. The effect of glucagon‐like peptide 1 on cardiovascular risk. Nat Rev Cardiol. 2012;9:209‐222. [DOI] [PubMed] [Google Scholar]


