Summary
Background
Filaggrin is central to the pathogenesis of atopic dermatitis (AD). The cheeks are a common initiation site of infantile AD. Regional and temporal expression of levels of filaggrin degradation products [natural moisturizing factors (NMFs)], activities of filaggrin‐processing enzymes [bleomycin hydrolase (BH) and calpain‐1 (C‐1)] and plasmin, and corneocyte envelope (CE) maturity in early life are largely unknown.
Objectives
We conducted a cross‐sectional, observational study investigating regional and age‐dependent variations in NMF levels, activity of proteases and CE maturity in stratum corneum (SC) from infants to determine whether these factors could explain the observed predilection sites for AD in early life.
Methods
We measured NMF using a tape‐stripping method at seven sites in the SC of 129 children (aged < 12 months to 72 months) and in three sites in 56 neonates and infants (< 48 h to 3 months). In 37 of these neonates and infants, corneocyte size, maturity, BH, C‐1 and plasmin activities were determined.
Results
NMF levels are low at birth and increase with age. Cheek SC, compared with elbow flexure and nasal tip, has the lowest NMF in the first year of life and is the slowest to reach stable levels. Cheek corneocytes remain immature. Plasmin, BH and C‐1 activities are all elevated by 1 month of age in exposed cheek skin, but not in elbow skin.
Conclusions
Regional and temporal differences in NMF levels, CE maturity and protease activities may explain the predilection for AD to affect the cheeks initially and are supportive of this site as key for allergen priming in early childhood. These observations will help design early intervention and treatment strategies for AD.
Short abstract
What's already known about this topic?
Atopic dermatitis (AD) frequently starts in early infancy, and the first eczematous lesions emerge on the cheeks.
Filaggrin is a major structural protein in the stratum corneum (SC).
Filaggrin deficiency is associated with the development of AD and, in the context of AD, food allergies and asthma.
Filaggrin is metabolized into natural moisturizing factors (NMFs), which can be measured in the SC.
What does this study add?
Regional differences in NMF levels, corneocyte envelope immaturity and protease activities may help explain why infantile AD most often initially affects the cheeks.
Filaggrin processing, corneocyte maturity, and protease activities show regional and temporal differences in infant skin.
These findings may explain disease patterns in early‐life AD.
What is the translational message?
Cheek skin may be highly relevant for allergen priming.
Emollient therapy at the vulnerable cheek site might help to prevent AD and/or food sensitization.
Linked Editorial: https://doi.org/10.1111/bjd.16806.
https://doi.org/10.1111/bjd.16959 available online
Atopic dermatitis (AD) is the most common chronic inflammatory disease of childhood in the developed world.1 Clinical manifestations of AD vary with age. In infancy, the first lesions most commonly emerge on the cheeks and the scalp.2 A Danish study of 411 infants reported that AD lesions were found to begin on the scalp, forehead, ear, neck and cheek in infants, with the cheek being the most commonly involved region.3 While infantile AD typically involves the facial skin, the nasal tip is almost always lesion free (the ‘Yamamoto’ sign).4 These well‐recognized predilection sites for AD suggest distinct regional differences in skin structure and function.
Structural differences between infant and adult skin have been reported.5, 6 Infant corneocytes are smaller than those of adults, correlating with a higher epidermal cell turnover rate compared with adults.7 In contrast to adults, newborn babies do not show variation in corneocyte size between nonacral skin regions. Their corneocytes are uniformly small and are similar in size to those found in the adult forehead.8 This suggests consistently high epidermal turnover across the entire skin surface in neonates. Adaptive changes, both in stratum corneum (SC) water‐handling properties and skin surface pH, occur in early life. In full‐term neonates, transepidermal water loss (TEWL) data suggest a competent epidermal barrier function shortly after birth.5, 9
A defective skin barrier is a key feature of AD.10 Filaggrin is a major structural protein in the SC and its constituent amino acids play a critical role in SC acidification and hydration. Filaggrin deficiency is associated with impaired barrier function and the development of AD.11 Filaggrin deficiency in children seems to define a specific endotype of AD, characterized by a predilection to exposed areas of the body, in particular the cheeks and hands.12 Profilaggrin, the phosphorylated precursor to filaggrin, is expressed in the upper stratum granulosum, where processing is initiated. A complex cascade of dephosphorylation and proteolysis liberates functionally active filaggrin monomers that, within the SC, are further cleaved into short peptides and are finally degraded fully to form a cytoplasmic pool of hygroscopic amino acids and their derivatives, known as natural moisturizing factor (NMF). Following deimination of arginine residues, the cysteine/aspartic proteases caspase‐14, bleomycin hydrolase (BH) and calpain‐1 (C‐1), located mainly in the upper SC, have been shown to be important in enzymatic degradation of filaggrin.13, 14 C‐1 is also involved in the processing of profilaggrin to filaggrin, and also in corneocyte maturation.15, 16
NMF modulates the pH of the SC surface, promotes the retention of water within the corneocytes and may exert antimicrobial activity.1 Important amino acid derivatives include trans‐urocanic acid (t‐UCA) and pyrrolidone carboxylic acid (PCA).1 The enzyme histidase converts histidine (His) to t‐UCA.17 Glutamic acid is converted to PCA, by nonenzymatic degradation (cyclization) in addition to enzymatic processes and overall, constitutes 12% of NMF.18, 19, 20 In addition, non‐filaggrin‐derived compounds such as lactate, urea, sugars and ions contribute to NMF. The amino acids and their derivatives that constitute NMF can be measured and closely correlate with filaggrin content.21 The epidermis expresses several proteases that are involved in a range of key epidermal responses, including proliferation, differentiation, lipid barrier homeostasis and tissue remodelling.22 Plasmin plays an important, but still not completely defined role in the corneocyte maturation process and is a marker for SC stress and inflammation, even in areas lacking clinically observable inflammation.23
The reasons why AD has a predilection for certain body sites and why the affected regions vary with age in childhood remain unknown. Age‐related and regional differences in skin structure and function are likely important. Here, we investigated the regional and temporal expression of filaggrin‐derived NMF together with filaggrin‐processing enzyme activities (BH and C‐1), corneocyte phenotypes and plasmin activities in a large cohort of healthy neonates, infants and children in anatomical locations relevant for AD.
Patients and methods
Study population
In phase I of the study, healthy infants and children were recruited from March to September 2013. These infants and children were scheduled to undergo elective surgical procedures in Our Lady's Children's Hospital, Dublin, Ireland. Children were recruited if they did not have a history suggestive of AD or any other inflammatory skin disease. An experienced paediatric dermatologist (M.A.McA.) examined all infants. Other exclusion criteria from the study included infants and children who had pyrexial illness in the preceding 2 weeks, those who had received immunosuppressive systemic therapy, such as oral corticosteroids, in the preceding 3 months, and patients whose ancestry was not exclusively Irish (four of four grandparents). Non‐Irish children were excluded to allow for accurate FLG mutation stratification. The following seven body sites were tape stripped in phase I: cheek, nasal tip, nape of neck, volar forearm at the elbow flexure, dorsal proximal mid‐upper limb, dorsal hand and the buttock.
Phase II of the study was carried out in The National Maternity Hospital, Holles Street, Dublin, Ireland (from July to September 2014). Infants were recruited, both in the postnatal wards in the 48 h following delivery and in the early weeks of life in outpatient/ambulatory clinics, when attending for elective baby checks. Identical inclusion and exclusion criteria for phase I were applied for phase II. The following three body sites were tape stripped in this cohort: cheek (C), nasal tip (T) and volar forearm at the elbow flexure (E). The number of body sites sampled had to be reduced in view of the very young age of these individuals. The three body sites in this cohort were selected because preliminary data from phase I of the study suggested that these body sites were most likely to provide the greatest amount of information. As this was an exploratory study, a definitive sample size that would allow statistically significant findings was difficult to predict. After phase I yielded significant results, we calculated that the smaller sample size would be informative in phase II. The power analysis was based on the difference in NMF values obtained between skin sites C, T and E. The highest sample size of n = 38 (power 80%; P = 0·05, paired t‐test) was obtained for the difference between C and E (mean 0·21 and SD 0·47). We included more children (n = 59) as we expected that tape stripping might fail in neonates and infants.
The study was conducted in accordance with the Declaration of Helsinki principles and was approved by the Research Ethics Committee of Our Lady's Children's Hospital, Dublin, Ireland. Full written informed consent was obtained from the parents of each individual.
Sampling of the stratum corneum by tape stripping
All sampled sites were emollient free for 24 h before tape stripping. The SC was sampled using the method previously described.21 Circular adhesive tape strips (3·8 cm2, D‐Squame, CuDerm, Dallas, TX, U.S.A.) were attached to the skin and, in all regions apart from the nasal tip, pressed for 10 s with a constant pressure (225 g cm−2) using a D‐Squame pressure instrument D500 (CuDerm). On the nasal tip, the circular adhesive tape strip was cut in half, placed on the skin, and pressure was applied manually for 10 s.24 Eight consecutive tape strips were sampled, all from the same site. Tape strips were stored at −80 °C until analysis.
Filaggrin genotyping
All patients were screened for the nine most common FLG mutations found in the Irish population (R501X, Y2092X, 2282del4, R2447X, S3247X, R3419X, 3702X, S1040X and G1139X) from DNA extracted from a blood sample or buccal swab. The methods used have been previously described.25 All individuals that were heterozygous or homozygous for loss‐of‐function mutations in the FLG gene were excluded from the analysis.
Determination of filaggrin breakdown products in the stratum corneum
NMF component analysis (His, PCA, trans‐UCA and cis‐UCA) was performed and total SC protein determined on the fourth consecutive tape stripping according to the method described in detail elsewhere.21 The four components were combined to give a ‘total NMF’ value. NMF components in the SC from each tape stripping were extracted with 500 μL 25% (w/w) ammonia solution. After evaporation of the ammonia extract, the residue was reconstituted in 250 μL pure water and analysed by high‐performance liquid chromatography ultraviolet analysis. Owing to incomplete extraction recovery of SC proteins by ammonia, the second extraction of proteins from the tape stripping was performed with 0·1 mol L−1 KOH solution over 24 h. The proteins were determined in both extracts by using Pierce Micro BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, U.S.A.). The levels of NMF components in the SC were normalized for proteins and expressed as mmol NMF per g of protein.
Natural moisturizing factor depth profiling
To ensure that NMF measurement in different age groups and sites was not affected by varying SC thickness, NMF profiling was performed on consecutive tape strips in 13 individuals. NMF was assessed in the second, sixth and eighth tape stripping in participants from < 48 h to 3 months of age at the cheek and elbow sites.
Stratum corneum maturation assays
The first tape stripping from 37 individuals at two skin sites, i.e. the cheek and elbow, were investigated for corneocyte envelope (CE) maturity. This was performed using differential Nile red and immunostaining for the late epidermal differentiation marker involucrin, using a modification of the previously described method.26
Measurement of stratum corneum protease activities: calpain‐1 and bleomycin hydrolase and plasmin
The same 37 individuals were assessed for plasmin, C‐1 and BH activities. Protease activities were determined on the fifth tape stripping using previously reported methods.23, 26, 27, 28, 29
Statistical analysis
All calculations were performed using Prism 6 software (GraphPad, San Diego, CA, U.S.A.). The distribution of data was tested using the Shapiro–Wilk normality test. The applied statistical test is indicated in the figure legends.
Results
Demographic data of participants
In total, 188 infants and children were recruited; 112 were male and 76 were female (Table 1). The average age, age range and age groups are outlined in Tables 1 and 2. The demographic characteristics of the 37 participants for whom corneocyte maturity assays were performed and measurements of C‐1, BH and plasmin were taken are outlined in Table S1 (see Supporting Information). FLG mutation status was determined for all participants in phase I. In phase II we were unable to establish the FLG status definitively in 12 participants because of poor DNA quality obtained from this subset of buccal swabs (Table S2; see Supporting Information). These were excluded from analysis.
Table 1.
Age groups and sex of all recruited participants
| N | Male | Female | Mean age | Age range | |
|---|---|---|---|---|---|
| Phase I | 129 | 78 | 51 | 27·9 months | 0·25–70 months |
| Phase II | 59 | 34 | 25 | 20·5 days | 1–84 days |
| Total | 188 | 112 | 76 |
Table 2.
Sex distribution, mean age and age range of all participants in phase I and phase II of study
| Age groups | < 48 h | 48 h to 4 weeks | 1–3 months | 4–11 months | 12–36 months | 36–72 months |
|---|---|---|---|---|---|---|
| Male, n | 13 | 12 | 17 | 14 | 28 | 27 |
| Female, n | 13 | 8 | 9 | 14 | 16 | 16 |
| Total | 26 | 20 | 26 | 28 | 44 | 43 |
Total natural moisturizing factor is low after birth, increases rapidly in postnatal life and is regionally determined, with the cheek skin being slow to mature
In the first 48 h after birth, the total NMF levels were low in all sampled regions. There was an increase in the total NMF levels in the early weeks of life in all regions. The nasal tip and the elbow flexure had a rapid increase in total NMF levels in the first 4 weeks of life, after which NMF stabilizes, with no further increase (Fig. 1). In contrast, in cheek skin there was still a significant increase (P < 0·001, Mann–Whitney two‐sided paired test) in NMF between the time periods of 1–11 months and 12–35 months. Furthermore, the rate of increase in levels of NMF in cheek SC was much slower (Fig. S1; see Supporting Information), and there was a significant (r 2 = 0·28, P < 0·001) linear response of NMF vs. age from 1 month up to 72 months (Fig. S1; see Supporting Information). Of note, there was no variation in NMF levels with SC depth after 48 h of age (Fig. S2; see Supporting Information). NMF levels on the remaining body sites sampled in phase I (nape of neck, dorsal hand, dorsal upper limb and buttock) were stable from 1 month to 72 months of age (Fig. S3; see Supporting Information). In the first year of life, the NMF values for the cheek SC were significantly lower compared with those of the elbow (median of difference 0·15; P < 0·001, Wilcoxon paired t‐test), although this difference was not significant at ages > 12 months (Fig. S4; see Supporting Information).
Figure 1.
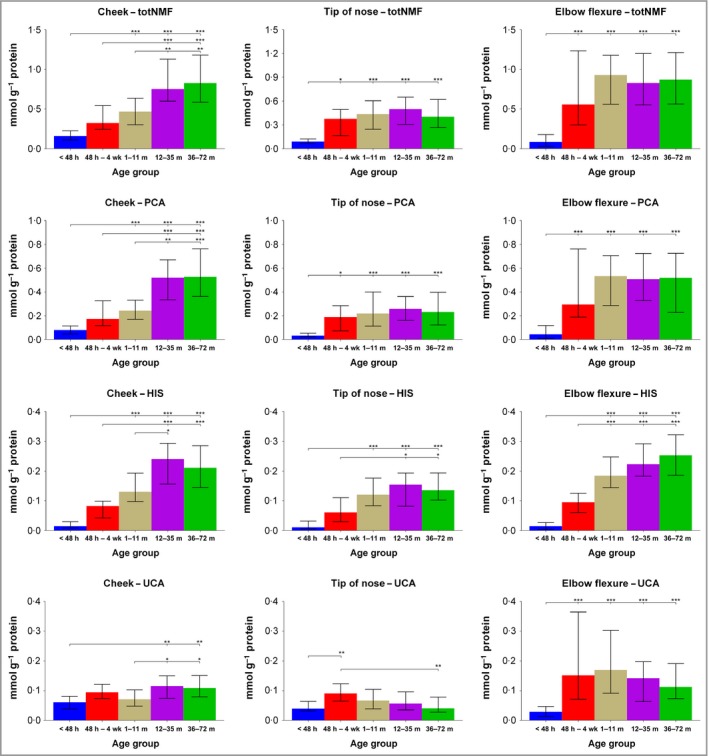
Levels of total natural moisturizing factor (NMF), histidine (His), pyrrolidone carboxylic acid (PCA) and sum of trans‐urocanic acid (UCA) and cis‐UCA in the stratum corneum of children (median with interquartile range) in different age groups [< 48 h (n = 18), 48 h – 4 weeks (n = 18), 1–11 months (n = 41), 12–35 months (n = 25) and 36–72 months (n = 30)] on three body regions. Differences between age groups were determined by Kruskal–Wallis test followed by Dunn's multiple comparisons test. ***P < 0.001, **P < 0.01 and *P < 0.05.
The histidine/urocanic acid ratio and the trans‐urocanic acid/cis‐urocanic acid ratio change rapidly postnatally and transition to a steady state more quickly in exposed skin sites compared with nonexposed sites
The His/UCA ratio rapidly increases in the first 3 months of life in exposed sites, and reaches a steady state from 4 months of age onwards (approximately twofold to threefold). In the nonexposed elbow flexure skin the His/UCA ratio remains low for the first 3 months of life (Fig. 2). The cis‐UCA/total UCA ratio increases rapidly in postnatal life in exposed sites. The ratio of cis‐UCA to the total amount of UCA (cis + trans isomer) in the cheek and nasal tip skin reaches the levels 30–50% around the first year of life while the ratio between cis‐UCA/total UCA in the elbow flexure SC remains low (Fig. 3).
Figure 2.
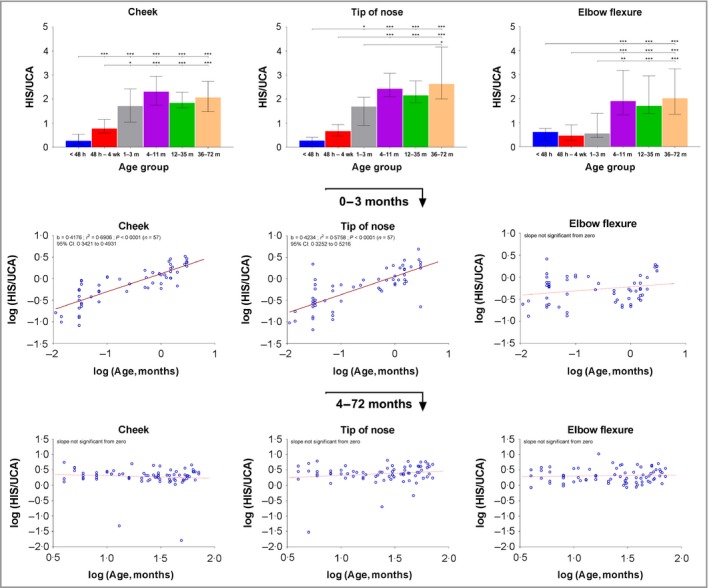
Ratio of histidine (His) to urocanic acid (UCA) (median with interquartile range) (a–c) and linear regression between ratio of His to UCA and age of children [(d–f) up to 3 months of age; (g–i) from 3 to 72 months of age] on three body regions. For regression analysis the values were log‐transformed. Age groups: < 48 h (n = 18), 48 h – 4 weeks (n = 18), 1–3 months (n = 21), 4–11 months (n = 20), 12–35 months (n = 25), 36–72 months (n = 30). ***P < 0.001, **P < 0.01 and *P < 0.05 as determined by Kruskal–Wallis test followed by Dunn's multiple comparisons test. b, slope of the regression line; CI, confidence interval.
Figure 3.
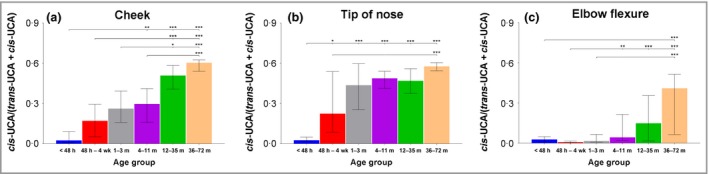
Ratio of cis‐urocanic acid (UCA) to total UCA (cis‐UCA + trans‐UCA) (median with interquartile range). Age groups: < 48 h (n = 18), 48 h – 4 weeks (n = 18), 1–3 months (n = 21), 4–11 months (n = 20), 12–23 months (n = 1), 24–35 months (n = 14), 36–47 months (n = 12) and 48–72 months (n = 18). Differences between age groups were determined by Kruskal–Wallis test followed by Dunn's multiple comparisons test. ***P < 0.001, **P < 0.01 and *P < 0.05.
Bleomycin hydrolase and calpain‐1 activities increase after 1 month of life and are higher in the cheek than the elbow
The late‐stage filaggrin‐processing enzymes, BH and C‐1 activities are similar in the cheek and elbow skin after birth. After 1 month of age the levels increase significantly in the exposed cheek skin, but not at the elbow (Figs 4 and S5; see Supporting Information).
Figure 4.
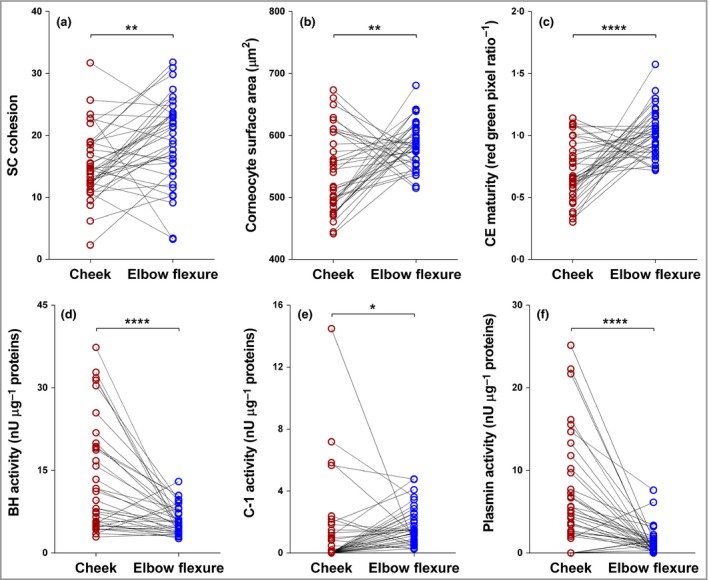
(a–c) Corneocyte envelope (CE) maturity, stratum corneum (SC) cohesion, cornecyte surface area and activity of (d–f) bleomycin hydrolase (BH), calpain‐1 (C‐1) and plasmin in the SC of children up to 11 months of age (n = 37). Difference between cheek region and elbow flexure region were determined by two‐tailed paired t‐test (SC cohesion and CE maturity) or by two‐tailed Wilcoxon matched‐pairs signed‐rank test (corneocyte surface area, BH, C‐1 and plasmin). ****P < 0.0001, **P < 0.01.
Plasmin activity increases at 1 month of age in the cheek and correlates with trans‐urocanic acid/cis‐urocanic acid ratio in cheek skin
Plasmin activities are higher in the cheek compared with the elbow at birth (Fig. 4). At 1 month, the plasmin activity generally increases in the exposed cheek skin. Plasmin activities correlate with trans‐UCA/cis‐UCA ratios at the cheek (Fig. 5).
Figure 5.
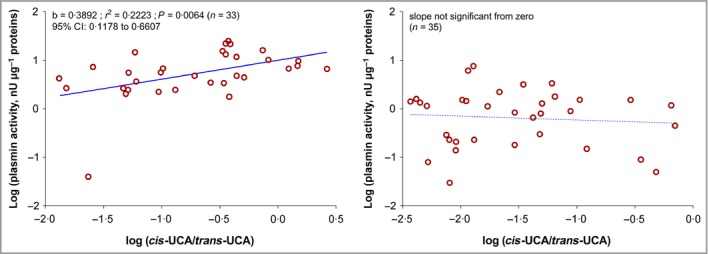
Linear regression plasmin activity vs. ratio of cis‐urocanic acid (UCA) to total UCA (cis‐UCA + trans‐UCA) on two body sites. The values were log‐transformed. b, slope of the regression line; CI, confidence interval.
Cheek corneocytes have a more immature phenotype in contrast with elbow corneocytes
The cheek corneocytes have an immature phenotype from birth, which does not change with age. This contrasts with the elbow corneocytes, which have a mature phenotype from birth (Fig. 4). In keeping with an immature phenotype, the cheek corneocytes had higher cohesion – specifically, less SC protein was removed, which meant that the corneocytes were more tightly cohesive (Fig. 6).
Figure 6.
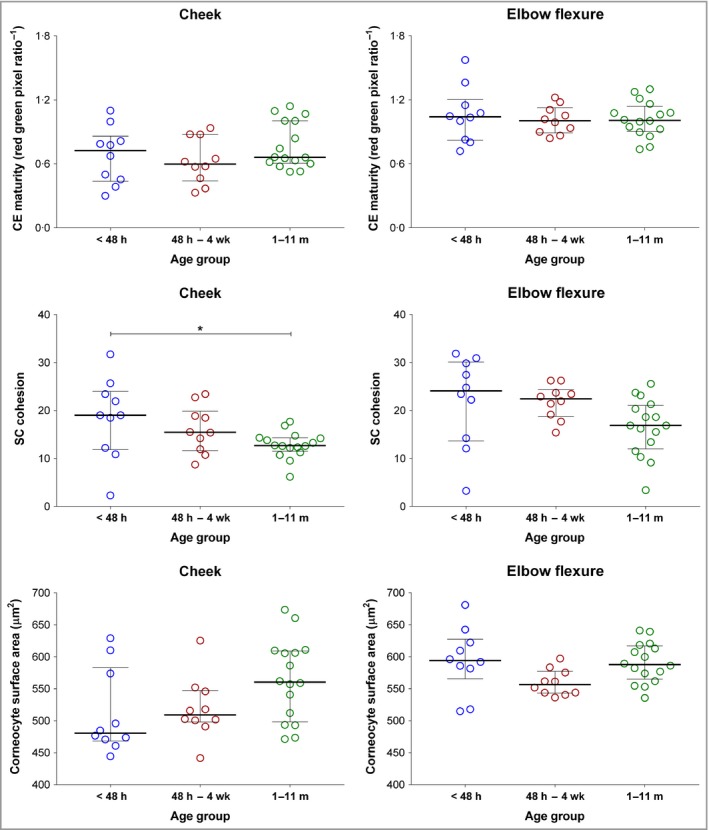
Corneocyte envelope (CE) maturity, stratum corneum (SC) cohesion and cornecyte surface area in the SC of children (median with interquartile range) in three age groups across two body regions. Age groups: < 48 h (n = 10); 48 h – 4 weeks (n = 10) and 1–11 months (n = 16). Differences between age groups were determined by one‐way anova followed by Tukey's multiple comparison test (for CE maturity and cornecyte surface area on cheek region Kruskal–Wallis test followed by Dunn's multiple comparisons test was used). *P < 0.05.
Discussion
Our results show that NMF is low at birth at all investigated skin sites and increases significantly over the first month of life, suggesting that the SC rapidly adapts to the dramatic environmental shift from in utero to ex utero. Our findings are in keeping with a previous study that measured free amino acids in the chest or back skin of 30 neonates and infants.30 Similarly, Chittock et al. reported low NMF in the skin of 115 neonates at birth, with a significant increase in NMF by 4 weeks of age.31 Low NMF in infant skin at birth is in keeping with reports in variations between neonatal and adult rat skin. Scott and Harding reported that during late fetal development filaggrin accumulates throughout the entire thickness of rat SC and immediately after birth filaggrin proteolysis occurs in the outer SC.32
We found that the cheek skin was a unique site with respect to NMF levels and corneocyte maturity in infancy. NMF levels in cheek SC were much slower to increase compared with other sites, including exposed sites such as the nasal tip (Fig. 7). Furthermore, in the first year of life the cheek NMF levels were significantly lower than the corresponding levels in the elbow SC. The cheek is frequently the site of initial inflammation in infantile AD. Absolute low levels of NMF and a slow increase in NMF levels may play a role in disease initiation at this site, as may high plasmin activity. The elbow flexure was notable for reaching a steady state of NMF early in infancy. This site is not typically involved in infantile eczema, but is a classic site of childhood AD. The reason why this site is vulnerable in childhood AD is unclear, but may reflect the local microbiome rather than NMF levels. It has been shown that age strongly affects the microbiome in infants, with bacterial community structure and diversity shifting over time.33 Furthermore, compared with exposed skin sites, elbow flexure skin had a consistently lower ratio of cis‐UCA to total UCA. Cis‐UCA has previously been suggested as an immunosuppressant.34
Figure 7.
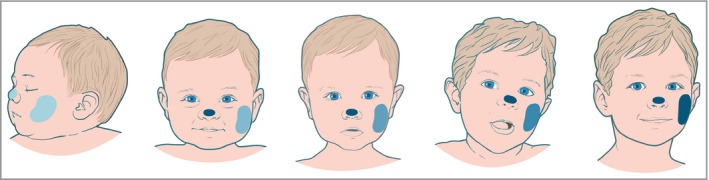
Cheek skin is slow to mature with respect to natural moisturzing factor (NMF) levels and corneocyte maturity. While other exposed sites, such as the nasal tip, rapidly reach steady‐state maturity in the early months of life, the cheek skin starts life with the lowest NMF and only reaches steady‐state NMF levels at approximately 7 years of age. NMF levels are graphically represented by depth of blue colour.
We also report other important insights into postnatal SC biology. In early postnatal life, there is a rapid increase in the ratio between His and its metabolite UCA (Fig. 2), which levels off after approximately 3 months. This might be caused by the differences related to age and skin site in the expression and activity of histidase, which has an optimum pH of about 8·5 and decreases towards a lower pH.35 The pH of the skin surface is highest in the first 2 weeks after birth (pH = 6·0) and gradually decreases with age.36
To provide a more comprehensive definition of the generation of NMF in the SC in early postnatal life, along with SC stress, we measured the activities of SC proteases BH and C‐1 and plasmin at the cheek and elbow sites in a selection of participants. The active forms of BH and C‐1 are critical in the final stages of filaggrin degradation.14, 15, 16, 37, 38, 39 We demonstrated increased activity of BH in the exposed cheek skin after 1 month of life. In contrast, levels at the elbow remained unchanged with increasing age. At birth plasmin activities, which indicate SC stress and impaired barrier function, are high in the cheek compared with the elbow. At 1 month of age plasmin activities increase in the exposed cheek skin, but not at the elbow. Furthermore, plasmin activity correlated with the cis‐UCA/total UCA ratio in the cheek skin, but not at the elbow. Raj et al. hypothesized that these atmospheric conditions result in a feedback mechanism to upregulate filaggrin degradation proteases, thereby producing NMF in an effort to improve barrier function and/or epidermal water retention.29 Our data support this hypothesis, in addition to demonstrating that it is relevant in infant skin during early postnatal life.
CE maturity is critical for skin barrier function. We show that the cheek CE is immature from birth and does not improve rapidly with age. Therefore, cheek CE immaturity is present from birth and persists into adulthood.29, 40, 41 In adults, facial TEWL is much higher than that of the forearm and upper arm.42 It is notable that the facial skin, unlike most other body sites, is continually exposed to environmental stress. The nasal tip is a site of interest in this study; while this site matures rapidly to steady‐state NMF levels, these levels settle at relatively low levels, suggesting that at this site factors other than NMF or filaggrin expression protect against the development of AD. At this site sebum production is much higher, as is skin hydration, which are factors that may offset low NMF.
Furthermore, low NMF and low maturity of the CE in the infant cheek SC may be important for allergen sensitization at this site, and subsequent food allergy. There is clear evidence that epicutaneous exposure to peanut through an impaired skin barrier increases the risk of peanut sensitization and confirmed peanut allergy.43, 44, 45 Early‐life exposure to peanut antigen in household dust is a risk factor for peanut sensitization and allergy in children46 and murine models support the concept of an impaired skin barrier as a route for sensitization.43, 44, 45, 47 The demonstration of low NMF levels, a more inflammatory plasmin profile and decreased corneocyte maturity in the exposed cheek skin of infants supports the hypothesis of this site being key to food sensitization and allergy in early life, either independently or in the setting of AD. Saliva and food protein exposure causing irritation at this site are likely cofactors in this pathology.
The early postnatal weeks are particularly dynamic for SC maturation. The infant cheek SC has delayed NMF normalization, higher cohesion and reduced CE maturation, and shows evidence of increased SC stress from birth. We propose that this combination of factors facilitates the initiation of AD inflammation and allergic sensitization. Our results support the concept of improving SC moisturization and skin barrier function in sites of vulnerability in infants in an effort to prevent the onset of AD.48, 49 The use of moisturizers may compensate for the decrease in NMF levels. Even small increases in filaggrin copy number that drive NMF have been shown to be protective against developing AD.50
In summary, regional SC biochemical and cellular characteristics may explain the initial clinical patterns of AD in infancy. Cheek SC early in life is likely permissive for the development of AD, allergen penetration and food allergy.
Supporting information
Table S1 Demographics of participants included in stratum corneum protease and corneocyte envelope maturity study.
Table S2 Filaggrin status of all recruited participants.
Fig S1. Levels of total natural moisturizing factor (NMF) in the stratum corneum (SC) of children (median with interquartile range) (a–c) and regression analysis of total NMF vs. age of children [up to 4 weeks of age (d–f); from 1 to 72 months of age; panels (g–i)] on three body regions.
Fig S2. Level of total natural moisturizing factor (NMF) at different stratum corneum (SC) depth (mean + SEM) in different age groups [< 48 h (n = 7) and 48 h to 4 weeks (n = 2) and 1–11 months (n = 4)] on two body regions, cheek and elbow flexure (depth: 2 = second SC tape, 6 = sixth SC tape, 8 = eighth SC tape).
Fig S3. Levels of total natural moisturizing factor (NMF), histidine, pyrrolidone carboxylic acid (PCA) and sum of trans‐ and cis‐urocanic acid (UCA) in the stratum corneum (SC) of children (median with interquartile range) across four body regions in the following different age groups: 1–11 months (n = 25); 12–35 months (n = 25) and 36–72 months (n = 27).
Fig S4. Natural moisturizing factor (NMF) values between cheek (C) and elbow flexure (E) during the first year of life and for all ages > 1 year.
Fig S5. Bleomycin hydrolase (BH), calpain‐1 (C‐1) and plasmin activities in the stratum corneum (SC) of children (median with interquartile range) across two body regions in the following three age groups: < 48 h (n = 10); 48 h to 4 weeks (n = 10) and 1–11 months (n = 16).
Powerpoint S1. Journal Club Slide Set.
Video S1 Author Video.
Acknowledgments
We are very grateful to the patients and their families for participating in this study. We appreciate the support of Stephanie McCollum and Linda Campbell in genotyping.
Funding sources The Irvine group is funded by the National Children's Research Centre, Dublin, Ireland. M.A.McA. is supported by the National Children's Research Centre, Dublin. A.D.I. and W.H.I.McL. are supported by the Wellcome Trust Programme Grants (090066/B/09/Z and 092530/Z/10/Z). The Centre for Dermatology and Genetic Medicine, University of Dundee is supported by a Wellcome Trust Strategic Award (098439/Z/12/Z to W.H.I.McL.). The Lane group is funded by DSM Ltd (Switzerland) and a University College London Overseas Research Studentship.
Conflicts of interest None to declare.
https://doi.org/10.1111/bjd.16959 available online
References
- 1. Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011; 365:1315–27. [DOI] [PubMed] [Google Scholar]
- 2. Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387:1109–22. [DOI] [PubMed] [Google Scholar]
- 3. Halkjaer LB, Loland L, Buchvald FF et al Development of atopic dermatitis during the first 3 years of life: the Copenhagen prospective study on asthma in childhood cohort study in high‐risk children. Arch Dermatol 2006; 142:561–6. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto K. Studies on autosensitization dermatitis. Keio J Med 1961; 10:59–78. [DOI] [PubMed] [Google Scholar]
- 5. Ludriksone L, Garcia Bartels N, Kanti V et al Skin barrier function in infancy: a systematic review. Arch Dermatol Res 2014; 306:591–9. [DOI] [PubMed] [Google Scholar]
- 6. Stamatas GN, Nikolovski J, Mack MC, Kollias N. Infant skin physiology and development during the first years of life: a review of recent findings based on in vivo studies. Int J Cosmet Sci 2011; 33:17–24. [DOI] [PubMed] [Google Scholar]
- 7. Stamatas GN, Nikolovski J, Luedtke MA et al Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr Dermatol 2010; 27:125–31. [DOI] [PubMed] [Google Scholar]
- 8. Hoath SB, Pickens WL, Scarborogh TE et al Characterisation of vernix caseosa: relevance to stratum corneum biology In: Stratum Corneum, the Vital Structure (Marks MP, Deveque JL, eds), Cardiff: The Stratum Corneum Group, 2005. [Google Scholar]
- 9. Kelleher MM, O'Carroll M, Gallagher A et al Newborn transepidermal water loss values: a reference dataset. Pediatr Dermatol 2013; 30:712–16. [DOI] [PubMed] [Google Scholar]
- 10. Sandilands A, Sutherland C, Irvine AD, McLean WH. Filaggrin in the frontline: role in skin barrier function and disease. J Cell Sci 2009; 122:1285–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol 2013; 131:280–91. [DOI] [PubMed] [Google Scholar]
- 12. Carson CG, Rasmussen MA, Thyssen JP et al Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLOS ONE 2012; 7:e48678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamata Y, Itoh Y, Kajiya A et al Quantification of neutral cysteine protease bleomycin hydrolase and its localization in rat tissues. J Biochem 2007; 141:69–76. [DOI] [PubMed] [Google Scholar]
- 14. Kamata Y, Taniguchi A, Yamamoto M et al Neutral cysteine protease bleomycin hydrolase is essential for the breakdown of deiminated filaggrin into amino acids. J Biol Chem 2009; 284:12829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamazaki M, Ishidoh K, Suga Y et al Cytoplasmic processing of human profilaggrin by active mu‐calpain. Biochem Biophys Res Commun 1997; 235:652–6. [DOI] [PubMed] [Google Scholar]
- 16. Hsu CY, Henry J, Raymond AA et al Deimination of human filaggrin‐2 promotes its proteolysis by calpain 1. J Biol Chem 2011; 286:23222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takahashi M, Tezuka T. The content of free amino acids in the stratum corneum is increased in senile xerosis. Arch Dermatol Res 2004; 295:448–52. [DOI] [PubMed] [Google Scholar]
- 18. Barrett JG, Scott IR. Pyrrolidone carboxylic acid synthesis in guinea pig epidermis. J Invest Dermatol 1983; 81:122–4. [DOI] [PubMed] [Google Scholar]
- 19. DeLapp NW, Dieckman DK. Gamma glutamyl peptidase: a novel enzyme from hairless mouse epidermis. J Invest Dermatol 1988; 90:490–4. [DOI] [PubMed] [Google Scholar]
- 20. Rawlings AV. Molecular basis for stratum corneum maturation and moisturization. Br J Dermatol 2014; 171(Suppl. 3):19–28. [DOI] [PubMed] [Google Scholar]
- 21. Dapic I, Jakasa I, Yau NLH et al Evaluation of an HPLC method for the determination of natural moisturizing factors in the human stratum corneum. Anal Lett 2013; 46:2133–44. [Google Scholar]
- 22. Clements JA, Willemsen NM, Myers SA, Dong Y. The tissue kallikrein family of serine proteases: functional roles in human disease and potential as clinical biomarkers. Crit Rev Clin Lab Sci 2004; 41:265–312. [DOI] [PubMed] [Google Scholar]
- 23. Voegeli R, Rawlings AV, Doppler S et al Profiling of serine protease activities in human stratum corneum and detection of a stratum corneum tryptase‐like enzyme. Int J Cosmet Sci 2007; 29:191–200. [DOI] [PubMed] [Google Scholar]
- 24. Breternitz M, Flach M, Prässler J et al Acute barrier disruption by adhesive tapes is influenced by pressure, time and anatomical location: integrity and cohesion assessed by sequential tape stripping. A randomized, controlled study. Br J Dermatol 2007; 156:231–40. [DOI] [PubMed] [Google Scholar]
- 25. Sandilands A, Smith FJ, Irvine AD, McLean WH. Filaggrin's fuller figure: a glimpse into the genetic architecture of atopic dermatitis. J Invest Dermatol 2007; 127:1282–4. [DOI] [PubMed] [Google Scholar]
- 26. Mohammed D, Matts PJ, Hadgraft J, Lane ME. Variation of stratum corneum biophysical and molecular properties with anatomic site. AAPS J 2012; 14:806–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Voegeli R, Rawlings AV, Doppler S, Schreier T. Increased basal transepidermal water loss leads to elevation of some but not all stratum corneum serine proteases. Int J Cosmet Sci 2008; 30:435–42. [DOI] [PubMed] [Google Scholar]
- 28. Mohammed D, Matts PJ, Hadgraft J, Lane ME. Depth profiling of stratum corneum biophysical and molecular properties. Br J Dermatol 2011; 164:957–65. [DOI] [PubMed] [Google Scholar]
- 29. Raj N, Voegeli R, Rawlings AV et al Variation in the activities of late stage filaggrin processing enzymes, calpain‐1 and bleomycin hydrolase, together with pyrrolidone carboxylic acid levels, corneocyte phenotypes and plasmin activities in non‐sun exposed and sun‐exposed facial stratum corneum of different ethnicities. Int J Cosmet Sci 2016; 38:567–75. [DOI] [PubMed] [Google Scholar]
- 30. Visscher MO, Utturkar R, Pickens WL et al Neonatal skin maturation–vernix caseosa and free amino acids. Pediatr Dermatol 2011; 28:122–32. [DOI] [PubMed] [Google Scholar]
- 31. Chittock J, Cooke A, Lavender T et al Development of stratum corneum chymotrypsin‐like protease activity and natural moisturizing factors from birth to 4 weeks of age compared with adults. Br J Dermatol 2016; 175:173–20. [DOI] [PubMed] [Google Scholar]
- 32. Scott IR, Harding CR. Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol 1986; 115:84–92. [DOI] [PubMed] [Google Scholar]
- 33. Kennedy EA, Connolly J, Hourihane JO et al Skin microbiome before development of atopic dermatitis: Early colonization with commensal staphylococci at 2 months is associated with a lower risk of atopic dermatitis at 1 year. J Allergy Clin Immunol 2017; 139:166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gibbs NK, Norval M. Urocanic acid in the skin: a mixed blessing? J Invest Dermatol 2011; 131:14–17. [DOI] [PubMed] [Google Scholar]
- 35. Kuroda Y, Ito M, Ogawa T et al A new sensitive method for assay of histidase in human skin and detection of heterozygotes for histidinemia. Clin Chim Acta 1979; 96:139–44. [DOI] [PubMed] [Google Scholar]
- 36. Fluhr JW, Darlenski R, Lachmann N et al Infant epidermal skin physiology: adaptation after birth. Br J Dermatol 2012; 166:483–90. [DOI] [PubMed] [Google Scholar]
- 37. Takeda A, Higuchi D, Yamamoto T et al Purification and characterization of bleomycin hydrolase, which represents a new family of cysteine proteases, from rat skin. J Biochem 1996; 119:29–36. [DOI] [PubMed] [Google Scholar]
- 38. Takeda A, Nonaka M, Ishikawa A, Higuchi D. Immunohistochemical localization of the neutral cysteine protease bleomycin hydrolase in human skin. Arch Dermatol Res 1999; 291:238–40. [DOI] [PubMed] [Google Scholar]
- 39. Schwartz DR, Homanics GE, Hoyt DG et al The neutral cysteine protease bleomycin hydrolase is essential for epidermal integrity and bleomycin resistance. Proc Natl Acad Sci USA 1999; 96:4680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Raj N, Voegeli R, Rawlings AV et al Variation in stratum corneum protein content as a function of anatomical site and ethnic group. Int J Cosmet Sci 2016; 38:224–31. [DOI] [PubMed] [Google Scholar]
- 41. Kashibuchi N, Hirai Y, O'Goshi K, Tagami H. Three‐dimensional analyses of individual corneocytes with atomic force microscope: morphological changes related to age, location and to the pathologic skin conditions. Skin Res Technol 2002; 8:203–11. [DOI] [PubMed] [Google Scholar]
- 42. Tagami H. Location‐related differences in structure and function of the stratum corneum with special emphasis on those of the facial skin. Int J Cosmet Sci 2008; 30:413–34. [DOI] [PubMed] [Google Scholar]
- 43. Strid J, Hourihane J, Kimber I et al Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol 2004; 34:2100–9. [DOI] [PubMed] [Google Scholar]
- 44. Strid J, Hourihane J, Kimber I et al Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy 2005; 35:757–66. [DOI] [PubMed] [Google Scholar]
- 45. Bartnikas LM, Gurish MF, Burton OT et al Epicutaneous sensitization results in IgE‐dependent intestinal mast cell expansion and food‐induced anaphylaxis. J Allergy Clin Immunol 2013; 131:e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brough HA, Simpson A, Makinson K et al Peanut allergy: effect of environmental peanut exposure in children with filaggrin loss‐of‐function mutations. J Allergy Clin Immunol 2014; 134:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fallon PG, Sasaki T, Sandilands A et al A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009; 41:602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horimukai K, Morita K, Narita M et al Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014; 134:e6. [DOI] [PubMed] [Google Scholar]
- 49. Simpson EL, Chalmers JR, Hanifin JM et al Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014; 134:818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brown SJ, Kroboth K, Sandilands A et al Intragenic copy number variation within filaggrin contributes to the risk of atopic dermatitis with a dose‐dependent effect. J Invest Dermatol 2012; 132:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Demographics of participants included in stratum corneum protease and corneocyte envelope maturity study.
Table S2 Filaggrin status of all recruited participants.
Fig S1. Levels of total natural moisturizing factor (NMF) in the stratum corneum (SC) of children (median with interquartile range) (a–c) and regression analysis of total NMF vs. age of children [up to 4 weeks of age (d–f); from 1 to 72 months of age; panels (g–i)] on three body regions.
Fig S2. Level of total natural moisturizing factor (NMF) at different stratum corneum (SC) depth (mean + SEM) in different age groups [< 48 h (n = 7) and 48 h to 4 weeks (n = 2) and 1–11 months (n = 4)] on two body regions, cheek and elbow flexure (depth: 2 = second SC tape, 6 = sixth SC tape, 8 = eighth SC tape).
Fig S3. Levels of total natural moisturizing factor (NMF), histidine, pyrrolidone carboxylic acid (PCA) and sum of trans‐ and cis‐urocanic acid (UCA) in the stratum corneum (SC) of children (median with interquartile range) across four body regions in the following different age groups: 1–11 months (n = 25); 12–35 months (n = 25) and 36–72 months (n = 27).
Fig S4. Natural moisturizing factor (NMF) values between cheek (C) and elbow flexure (E) during the first year of life and for all ages > 1 year.
Fig S5. Bleomycin hydrolase (BH), calpain‐1 (C‐1) and plasmin activities in the stratum corneum (SC) of children (median with interquartile range) across two body regions in the following three age groups: < 48 h (n = 10); 48 h to 4 weeks (n = 10) and 1–11 months (n = 16).
Powerpoint S1. Journal Club Slide Set.
Video S1 Author Video.


