Abstract
The biology and regulation of YAP and TAZ, two closely related transcriptional regulators, are receiving increasing attention owing to their fundamental roles in organ growth, tissue repair and cancer. In particular, the widespread activation of YAP/TAZ in carcinomas, and the crucial role of YAP/TAZ activation for many "hallmarks" of cancer are indicating YAP/TAZ as prime targets for designing anti-cancer drugs. Here, we start from the known modalities to regulate YAP/TAZ to highlight possible routes of therapeutic intervention.
The current surge of interest on YAP/TAZ biology and regulation is fueled by the realization that YAP/TAZ are pervasively activated in human tumors [1,2] and by some striking genetic evidence in mouse models, collectively indicating that inactivation of YAP/TAZ in several adult organs - including breast, liver, pancreas, skin and intestine [3–7] - renders those tissues immune to cancer emergence or progression. Notably, YAP/TAZ inactivation has no overt adverse effects on the basal homeostasis of the surrounding healthy tissue. The cellular and molecular bases of these phenomena remain poorly understood.
A conceptually unifying notion that integrates the several roles of YAP/TAZ in cancer is their unique ability to install cancer-stem cell (CSC) properties in otherwise benign tumor cells [8,9]. Moreover, YAP/TAZ are required to preserve stemness properties in established CSC populations. It has been proposed that tumors progress by increasing their CSC content; YAP/TAZ appear to contribute to this phenomenon by generating new CSCs.
The connection between YAP/TAZ and stemness entails the ability of YAP/TAZ to promote self-renewal and the capacity to proliferate in spite of growth-suppressive environmental conditions. It is worth noting that some of these attributes are not specific to CSCs, as they are also associated to normal somatic SCs; consistently, YAP/TAZ are also essential for physiological tissue repair upon injury [7,10]. This suggests the tempting possibility that carcinomas may have actually hijacked one ancestral property of YAP/TAZ - that is, endowing stemness to build and repair organs in order to induce recurrence and metastasis. Whereas somatic SCs require specific signals from localized tissue niches in order to activate YAP/TAZ, these signals may be abundant and widespread within the tumor microenvironment or bypassed by cancer-specific mutations. For these reasons, major emphasis in YAP/TAZ research has been placed on the mechanisms of their regulation.
Although the field remains riddled with may open questions, the available evidence is nevertheless sufficient to pinpoint YAP/TAZ as prime candidates for the development of effective cancer treatments. Here we will review some of the most promising research avenues and proofs-of-concept toward these goals.
YAP/TAZ regulation by the Hippo cascade
The function of YAP/TAZ has been classically understood in the context of Hippo signaling, whose core components are the evolutionary conserved kinases LAST1/2 and MST1/2 (related to Drosophila Hippo kinase) and their associated co-factors SAV1 and MOB1/2 [2,11]. When the pathway is turned ON, YAP/TAZ are OFF, as LATS1/2 phosphorylate and negatively regulate YAP/TAZ restricting their stability or nuclear localization. The Hippo cascade represents a key limiting factor for YAP/TAZ, as experimental inactivation of Hippo kinase activity during embryonic development results in aberrantly oversized organs or defective differentiation (reviewed in [2,11]).
That said, Hippo pathway mutations are virtually absent in human carcinomas, rendering this cascade an unlikely candidate to explain the wide and robust activation of YAP/TAZ in most, if not all solid tumors [1,2]. Of note, loss-of-function mutations in LATS or in NF2 (an upstream Hippo pathway component acting as MST positive regulator) are actually selected in specific tumors, such as mesothelioma, schwannomas and meningiomas [1]. Any therapeutic intervention aimed at restoring the Hippo pathway in these tumors is challenging, as it would entail the design of compounds aiming at reactivation of LAST kinase activity.
YAP/TAZ and mechanotransduction
In addition to the inhibitory module provided by the Hippo kinases, YAP/TAZ have been more recently discovered as central mediators of cellular mechanotransduction. Cells are in fact constantly targeted by mechanical forces from their attachment to their surrounding extracellular matrix (ECM) or to other cells, and this molds cell shape and cytoskeletal organization [12–14]. Patterns of mechanical forces are intrinsic to the 3D architecture and geometry of our tissues, and represent dominant modalities to regulate cell fate.
Mechanical signals represent an overarching determinant in the control of YAP/TAZ activity [15,16]; differently from the Hippo pathway, cell mechanics represent a positive and permissive modality to activate YAP/TAZ, offering unanticipated routes for therapeutic interventions. YAP and TAZ are activated by ECM stiffness, stretched cell shape, an F-actin organization typified (at least in vitro) by stress-fibers and elevated cytoskeletal tension (reviewed in [12]); in contrast YAP/TAZ are inhibited by soft ECM environments, round cell shape, and by cell physical confinement within a restricted space. YAP/TAZ activation by cell mechanics is influenced by Rho-GTPase, ROCK and integrity of the actomyosin cytoskeleton, in a manner largely independent from LATS [15–17]. Mechanotransduction affects YAP/TAZ activity in all cells, irrespectively of their load of oncogenic mutations; for example, a soft ECM is effective at restricting YAP/TAZ in normal, benign and metastatic cancer cells.
In most adult epithelial organs, YAP/TAZ levels and activity are barely detectable or confined to specific tissue niches. This is consistent with the view that most cells in living tissues rest in a "soft" mechanical condition, experiencing low mechanical forces incompatible with YAP/TAZ activation and cell proliferation, and consistent with the notion that the structural and physical features of our tissues are intrinsically tumor suppressive [18,19]. This scenario changes dramatically during tumors progression, whereby disturbed tissue architecture, accumulation of stromal cells, increased compression forces, ECM stiffening with changes in ECM composition and activation of cancer-associated fibroblasts (CAFs) all represent conditions potentially able to induce stable YAP/TAZ expression in a large fraction of cancer cells.
YAP/TAZ mechanotransduction may be also active in the cells of the tumor microenvironment. For example YAP is activated in CAFs, by mechanisms involving Src and ROCK [20]. YAP activation in CAFs leads to ECM stiffening and thus generates a mechanically activated feedback loop that promote increased extracellular matrix protein deposition, tissue rigidity, and YAP/TAZ-driven aberrant proliferation in tumor cells.
The mechanism(s) linking mechanically-activated cytoskeletal changes to YAP/TAZ are largely unknown; yet, the identification of YAP/TAZ as downstream effectors of cellular mechanotransduction bears several implications for cancer biology and its possible treatment.
Role of YAP/TAZ in installing drug resistance and escaping oncogene addiction
A key property of CSCs, and indeed of tumor cells with activated YAP/TAZ, is resistance to chemotherapeutic drugs. YAP/TAZ activation sustains survival of breast cancer stem cells treated with conventional chemotherapeutics, such as paclitaxel and doxorubicin, and protects cancer cells against DNA-damaging agents, including cisplatin and radiations. [1,8,9].
Furthermore, YAP/TAZ promote resistance to molecularly targeted therapies in tumor cells harboring specific oncogenic lesions. Lin and colleagues reported that YAP promotes resistance to RAF and MEK inhibitors in several tumor cell lines harboring BRAF, KRAS or NRAS activating mutations [21]. As such, YAP downregulation enhanced RAF and MEK inhibitor efficacy in mutant cells that had acquired resistance to monotherapy with RAF or MEK inhibitors. Immunohistochemistry (IHC) analysis in human melanoma specimens with BRAF mutation revealed a tendency for primary tumors with high YAP expression to display an incomplete response or no response to therapy with RAF/MEK inhibitors. Thus, YAP acts as a parallel survival input for tumor cells and its upregulation dampens the efficacy of oncogene-specific, molecularly targeted agents.
YAP mechanotransduction pathway has been recently implicated in the acquisition of drug resistance in melanoma and breast cancer. In melanoma, resistance to the BRAF-inhibitor vemurafenib is paralleled by cell stretching, increased content of actin stress fibers and mechano-dependent YAP activation [22]. Consistently, the responsiveness of HER2-positive breast cancer cells to lapatinib is enhanced on soft matrices, and blunted in stiff matrices in a YAP/TAZ-dependent manner [23]. Of note, in vivo studies have demonstrated that BRAF inhibitors do not only act in melanoma cells but also in the surrounding tumor fibroblasts, in fact activating them to produce a stiff, collagen-rich extracellular matrix (ECM) [24]. An intriguing hypothesis is that these two events - the cell-autonomous increase in responsiveness to ECM rigidity, and the stiffening of surrounding ECM - might occur simultaneously and concur to the activation of YAP in therapy-resistant cancer cells [25]. It is thus plausible that attenuation of YAP/TAZ-dependent mechanotransduction through inhibitors of integrin signaling or cytoskeletal modulators - including drugs targeting FAK, Src family members, Rho-GTPases or ROCK (see below) - may represent an intriguing avenue to blunt cancer chemoresistance.
Just like YAP/TAZ activation can bypass drug resistance, it can also bypass oncogene addiction. For example, pancreas-specific expression of KRASG12D from an inducible transgene leads to the development of pancreatic ductal adenocarcinomas (PDAC) in mice, but tumors regress and disappear when the transgene is experimentally turned off. Yet, some tumors spontaneously relapse in the absence of any KRASG12D overexpression through amplification of the YAP1 locus, as such providing genetic evidence that YAP-overexpressing cellular clones are positively selected upon KRAS-inactivation [26]. In line with this notion, the relapsed tumor cells require YAP/TAZ to sustain tumor growth. In other words, as tumor cells gain independency from their initiating oncogenic lesions by activating YAP/TAZ, they also gain a higher dependency on YAP/TAZ themselves. This opens interesting therapeutic scenarios for synthetic lethal approaches, whereby attenuation of YAP/TAZ activity in combination with drugs targeting cancer-relevant mutations may effectively kill tumor cells, avoid relapse and, at the same time, spare normal cells.
Routes for anti-YAP/TAZ therapeutic interventions
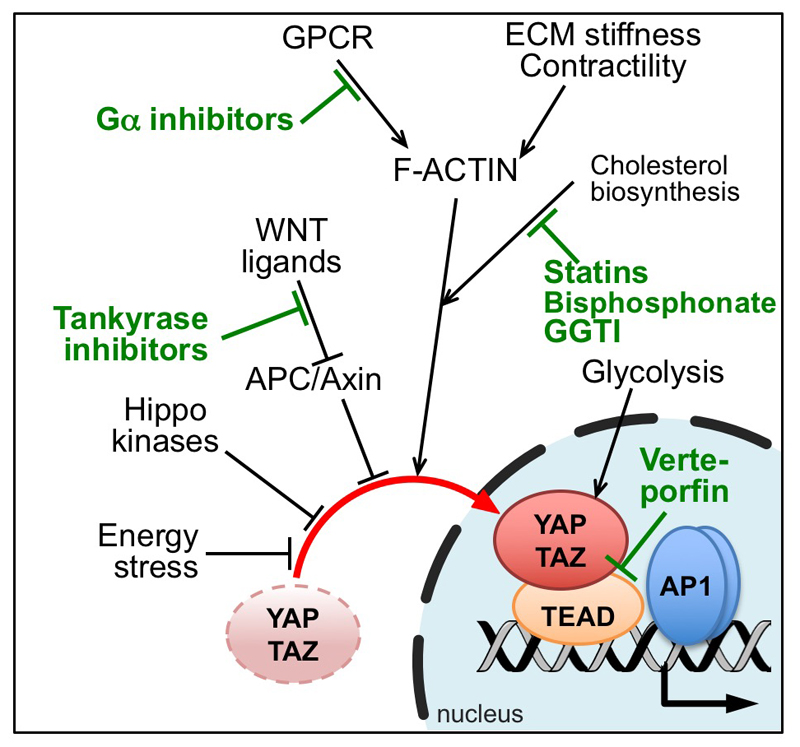
YAP/TAZ are at the nexus of several signaling cascades (Figure 1), in turn suggesting a number of potential therapeutic options.
Figure 1.
Summary of positive and negative regulators of YAP/TAZ. Drugs of potential therapeutic usage are indicated in green. See Figure 2 for a more detailed representation of molecular pathways regulating F-actin and mechanical signals, and their targeting drugs.
Targeting mechanotransduction
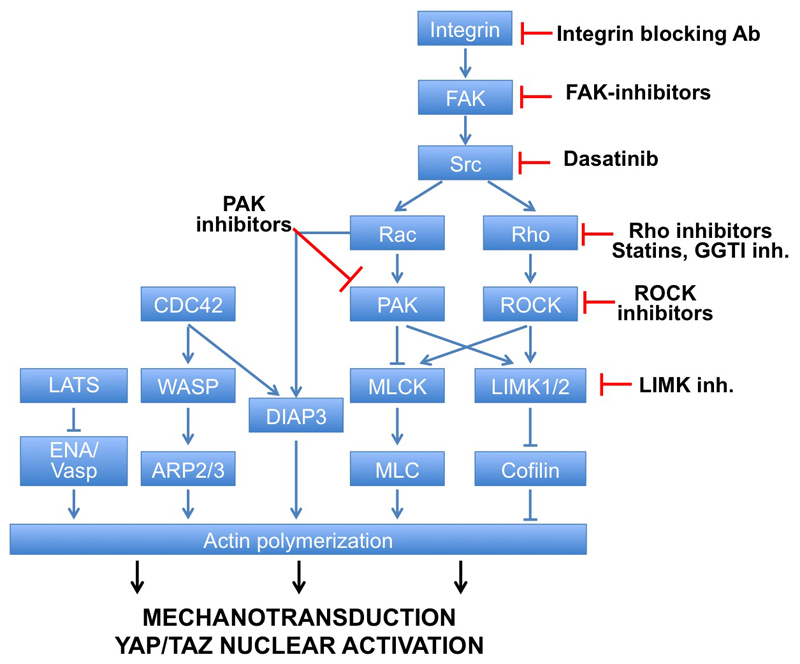
The actin cytoskeleton offers a potentially rich and still largely unexplored set of opportunities to control YAP/TAZ activity [12]. The polymerization, organization and tensile states of cellular F-Actin rely on the activity of Rho, Rac and CDC42 GTPases. ROCK kinases are downstream of Rho, and control F-actin polymerization by regulating LIMKs (LIMK1/2), that are also downstream of Rac/PAK signaling. LIMKs are inhibitors of Cofilin/ADF, proteins involved in F-actin capping and severing that ultimately promote F-actin depolymerization (Figure 2). Capping and severing proteins have been recently shown to serve as potent regulators of YAP/TAZ activity: loss-of-Cofilin/CapZ/Gelsolin can overcome YAP/TAZ inhibition triggered by contact-inhibition, in a manner independent from LATS kinase activity [15,27]. Intriguingly, conditional inactivation of Cofilin and its related protein ADF in mouse models triggers dramatic organ overgrowths [28]. Consistently, ROCK and PAK have been already reported to sustain YAP/TAZ function [16,20]; some cell types appears however insensitive to ROCK inhibition [29], possibly because of redundant function with MLCK. Cofilin/ADF are opposed by anti-capping factors, such as ENA/Vasp and by ARP2/3.
Figure 2.
Summary of positive and negative regulators of F-actin and mechanical signals. Drugs of potential therapeutic usage for YAP/TAZ inhibition are indicated.
The above outline indicates several routes to blunt YAP/TAZ activity by targeting mechanotransduction, at least in vitro (Figure 2). These include direct disruption of F-actin microfilaments, or inhibition of upstream activators of F-actin [16]. However, toxicity of anti-cytoskeletal treatments makes most of them unlikely to be translated in the clinical practice. Therapeutic options are currently limited to inhibitors of Rho and its upstream inducers, such as Src, FAK and integrins. For example, the SRC-family inhibitor dasatinib (already in clinical practice for treatment of leukemias) has been recently shown to oppose YAP/TAZ transcriptional effects in vitro and in vivo [20,30].
YAP/TAZ regulation by the cytoskeleton is a broadly general phenomenon allowing integration with other pathways known to indirectly control F-actin stability and organization. A point in case is the mevalonate pathway. This metabolic cascade generates geranylgeranyl moieties essential for the post-translational modification and membrane localization of Rho-GTPases. The key enzyme of this cascade, HMG-CoA reductase, is inhibited by statins, a class of drugs widely used to treat hypercholesterolemia. Indeed, statins caused YAP/TAZ inhibition and cytoplasmic relocalization in several cancer cell types; moreover, TAZ-dependent preservation of cancer stem cells in vitro and their growth as tumor xenografts in vivo were reduced upon statin treatment [17,31,32].
The anti-YAP/TAZ effect of statin appeared stronger in vitro than in vivo, suggesting that it may be difficult to keep statin concentration above the minimal threshold of effectiveness within tumors. Statins are however significantly more potent at inhibiting YAP/TAZ activity when combined with Src inhibitors [33]. It is suggestive that, in epidemiological studies, statin-taking patients display reduced risk to develop cancer [34]. In addition to statins, bisphosphonates (an FDA approved drug) and GGTI (inhibitors of geranylgeranyl transferase-1) represent additional compounds targeting the mevalonate pathway that are relevant for Rho-GTPase signaling, and that display YAP/TAZ inhibiting abilities (Figure 1) [17,35].
Targeting the energy metabolism
Differently from normal differentiated cells, which rely primarily on mitochondrial function to generate ATP, most cancer cells rely on aerobic glycolysis. This so-called “Warburg effect” impacts on YAP/TAZ regulation likely by distinct mechanisms. For example, inhibition of glycolysis with 2-deoxy-D-glucose blunted YAP/TAZ transcriptional activity and transforming capacity in vitro [36–38]. Alternatively, AMPK, a kinase that senses cellular ATP levels, also partially inhibits YAP/TAZ. AMPK activation by ICAR or metformin (AMPK agonists used in diabetic patients) downregulates expression of YAP/TAZ target genes[37,39] and reduces the outgrowth of transplanted LATS1/2 double knockout MEFs [37]. Envisioning a pro-AMPK approach to hit YAP/TAZ in tumor cells is however unlikely, given that, at odd with its proposed anti-YAP/TAZ effects, AMPK is itself a tumor promoter fostering cancer cell survival [40].
Targeting GPRC signaling
Signaling from G-protein-coupled-receptor (GPCR) and their ligands has been recently shown to regulate YAP/TAZ activity, once again involving a central role for Rho and the actin cytoskeleton. GPCRs represent a large family of receptors; depending on the specific subset of G-proteins involved, this regulation can be inhibitory or activating: Gα12/13-, Gα11-, Gα1/o-coupled receptors induce YAP/TAZ, whereas Gαs-coupled receptors oppose them [41]. Consistently with their role as Rho inducers, GPCRs require a sufficient “mechanical tone” in the target cells in order to sustain YAP/TAZ activity, as GPCR ligands are unable to overcome YAP/TAZ mechanical inhibition in cells plated in soft ECMs [15]. In addition, Gαs-coupled GPCRs raise PKA/cAMP levels, leading to activation of Hippo/LATS signaling [41].
GPCRs are also a prominent family of validated pharmacological targets suggesting that their selective inhibition may represent a way to blunt YAP/TAZ activity, particularly in tumors driven by mutations in the GPCR pathways. A paradigm for this scenario is the frequent mutation of Gαq and Gα11 in uveal melanoma [42,43]. Overexpression of oncogenic Gαq in melanocytes activates YAP and induces melanoma formation. Clearly, GPRCs can activate other pathways whose biological effect may or not be coherent with their YAP/TAZ regulation. In fact, apparently at odd with their anti-YAP/TAZ effects, Gαs-coupled GPRCs are activated by mutations in some tumor types [44], and their inhibition by β-blockers has been proposed as anti-cancer treatment [45]. It is plausible that other, pro-tumorigenic Gαs-GPCR downstream responses may overrule YAP/TAZ activation in these contexts.
Turning off Wnt/YAP-TAZ signaling by sustaining the activity of the destruction complex using Tankyrase inhibitors
Recent studies found that cytoplasmic YAP/TAZ associate to the destruction complex, involved in the degradation and sequestration of β-catenin and other proteins [5]. YAP/TAZ bind directly to Axin, APC and β-catenin [5,46,47], and this contributes to their cytoplasmic sequestration and to TAZ degradation [5,48]. Mutation or experimental depletion of Axin1/2 or APC, or Wnt stimulation, all inhibit the destruction complex, fostering YAP/TAZ nuclear accumulation and target genes transcription [5,48,49]. Consistently, conditional knockout of APC in the intestinal epithelium leads to massive YAP/TAZ nuclear stabilization. Strikingly, crypt overgrowth and intestinal adenoma formation downstream of APC inactivation depends on YAP/TAZ [5,46,50]. YAP/TAZ activation by the Wnt pathway can be prevented by reactivation of the destruction complex by treatment with tankyrase inhibitors [48], that is, compounds that promote Axin stabilization. Interestingly, these compounds can restore the activity of the destruction complex also in colorectal cancer (CRC) cells bearing truncations of APC [51], suggesting that these compounds may have a dual anti-β-catenin and anti-YAP/TAZ activity that might be exploited in CRC patients.
Targeting YAP/TAZ transcriptional mechanisms
The mechanisms by which nuclear YAP/TAZ control gene expression represent a largely unexplored but potentially promising area to design new modalities of therapeutic interventions. Since all upstream regulators ultimately impact on YAP/TAZ nuclear availability and transcriptional responses, designing compounds able to interfere at these levels may represent an “universal” anti-YAP/TAZ approach; this approach may also display the added advantage of reduced toxicity compared to drugging upstream signaling molecules likely endowed with pleiotropic functions.
YAP/TAZ are transcriptional co-activators that do not bind DNA directly [2]. Only very recently, ChIP-seq and transcriptomic experiments provided the first global picture of the nuclear partners of YAP/TAZ in several cancer cell lines [52–54]. This work collectively indicates that TEAD is by far the primary DNA binding platform for YAP/TAZ.
The structure of the YAP-TEAD binding domains has been resolved, indicating extensive interactions through evolutionary conserved interfaces [55,56]. However, it remains undetermined whether this interaction could be exploited for rational design of small ligands. Verteporfin (VP - belonging to the porphyrin family), the only compound able to inhibit the physical association between YAP and TEAD [57], actually emerged from an unbiased functional screen of FDA-approved drugs able to blunt YAP/TAZ activity, and not from rational design. In transgenic mice, VP appears to limit liver overgrowth resulting from either YAP overexpression or activation of endogenous YAP by Nf2 knockout [57]. VP has been successfully used in vivo to restrain the growth of uveal melanoma cells in an orthotopic mouse model [43], and of pre-established xenografts of prostate cancer cells bearing activated YAP [58]. That said, a recent study reports that VP suppresses the proliferation of cancer cells by inducing toxicity, acting irrespectively of YAP [59].
The potential validity of an anti-YAP/TEAD strategy is corroborated by evidence that a short peptide competing with YAP for the binding to TEAD, could inhibit YAP-dependent tumorigenic potential of gastric cancer cells both in vitro and in vivo [60]. This peptide was designed on the sequence of VGLL4, a protein that interacts with TEAD, in a way that is mutually exclusive with YAP [60,61]. In spite of the mild therapeutic efficacy shown by the VGLL4-mimicking peptide, the results obtained with this approach are encouraging, and strengthen the view that the YAP/TAZ-TEAD interface is a promising drug target.
How do YAP/TAZ control the expression of target genes? YAP/TAZ bind to enhancers that are “in touch” with their cognate promoters through chromatin loops [52]; in these structures, YAP/TAZ would promote elongation of nascent mRNA molecules by recruiting the Mediator complex [53], a multi-subunit complex that communicate regulatory signals from DNA-bound TFs directly to the basic transcriptional machinery. Indeed, YAP/TAZ depletion impairs recruitment of the Mediator complex, and diminishes PolII levels on gene bodies [53]. An encouraging result was obtained with flavonoids, compounds able to oppose CDK9, a subunit of the positive transcription elongation factor, P-TEFb, which promotes mRNA elongation. These treatments could at least partially limit the rapid liver overgrowth induced by transgenic YAP overexpression in mice [53]. YAP/TAZ also promote acetylation of histones located in enhancers, raising the prospect that small molecule inhibitors of histone acetyltransferases may limit YAP/TAZ [52,54].
An unexpected discovery that emerged from YAP/TAZ/TEAD ChIP-seq explorations has been the elucidation that these factors do not work in isolation: in order to turn on their oncogenic growth program, YAP/TAZ must cooperate with AP-1 (Activator Protein 1) dimers [52,54,62]. YAP/TAZ, TEAD and AP-1 form a transcription factor complex, and synergistically activate target genes to promote cell transformation. Notably, the AP1-YAP/TAZ/TEAD cooperation is extensive, occurring in the majority of YAP/TAZ-bound chromatin sites. It is thus plausible that AP-1 inhibitors, such a retinoids, may be effective at limiting the pro-oncogenic properties of YAP/TAZ.
YAP has been recently proposed to serve as tumor suppressor in hematological malignancies [63]. Consistently, a pro-apoptotic role of YAP has also been reported, at least in some contexts [64]. The models proposed entail functional cooperation between YAP and p53 family members for activation of pro-apoptotic target genes [64]. Mutation of p53 induces neomorphic or “gain-of-function” properties and, intriguingly, YAP has been shown to contribute to the pro-proliferative effects of mutant-p53 proteins [17,65].
Considering these insights, we speculate that a deeper understanding of the molecular mechanisms of YAP/TAZ nuclear function might offer further opportunities to target their oncogenic functions.
Conclusions
The prominence of YAP/TAZ for many aspects of cancer biology indicates these factors as ideal targets for development of anticancer treatments. From the above discussion, inhibition of YAP/TAZ remains challenging, as many protein-protein interactions specifically involved in the control of YAP/TAZ activity may be difficult to target. Several enzymes operating in pathways that activate YAP/TAZ, such as in mechanotransduction, do warrant further investigation, although their targeting with small molecules may be associated to toxicity.
The nuclear mechanisms underlying YAP/TAZ-mediated transcription may represent so far underestimated opportunities of intervention. For example, YAP/TAZ control cancer cell fates, hinting to their involvement as epigenetic modifiers; although presently unknown, dissecting these regulatory layers may link YAP/TAZ inhibition to an arsenal of potent epigenetic drugs. Also underdeveloped is the possibility to target YAP/TAZ-downstream effectors. We and others have recently identified a host of YAP/TAZ direct target genes involved in the control of cell cycle progression. In addition, YAP/TAZ induce receptors and secreted ligands that are traditionally ideal targets for biologic drugs. More speculatively, how YAP/TAZ mRNA levels are regulated has been almost entirely neglected, including the existence of post-transcriptional regulations by miRNAs or ceRNAs that are also appealing targets for innovative drugs. Finally, the ability of YAP/TAZ to reprogram cancer cells and to operate from complex enhancers indicate that these factors must act combinatorially as segments of a stemness network operating in somatic cells, whose other components remain to be discovered. Clearly, more research in this field is likely to disclose so far unexpected and potentially very effective anti-YAP/TAZ pharmacological strategies for cancer treatment.
Acknowledgments
We thank all members of SP lab for discussion. FZ is supported by a fellowship from Italian Association for Cancer Research (AIRC). This work is supported by AIRC Special Program Molecular Clinical Oncology “5 per mille” and an AIRC PI-Grant to S.P and by Epigenetics Flagship project CNR-Miur grants to S.P. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 670126-DENOVOSTEM). Author Contributions: all authors collected and discussed the material, FZ prepared figures. All authors contributed to write the MS.
References
- 1.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–257. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 2.Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev. 2014;94:1287–1312. doi: 10.1152/physrev.00005.2014. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [*These articles showed that, in mouse models, YAP/TAZ are essential for tumor initiation, but are dispensable for normal tissue homeostasis in mammary gland, pancreas and intestine epithelia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. doi: 10.1126/scisignal.2005049. [*These articles showed that, in mouse models, YAP/TAZ are essential for tumor initiation, but are dispensable for normal tissue homeostasis in mammary gland, pancreas and intestine epithelia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [* These articles showed that, in mouse models, YAP/TAZ are essential for tumor initiation, but are dispensable for normal tissue homeostasis in mammary gland, pancreas and intestine epithelia.] [DOI] [PubMed] [Google Scholar]
- 6.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene. 2015;34:681–690. doi: 10.1038/onc.2014.5. [DOI] [PubMed] [Google Scholar]
- 10.Lee MJ, Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ Regulate Skin Wound Healing. J Invest Dermatol. 2014;134:518–525. doi: 10.1038/jid.2013.339. [DOI] [PubMed] [Google Scholar]
- 11.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30:1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspar P, Tapon N. Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol. 2014;31:74–83. doi: 10.1016/j.ceb.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 14.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 16.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 17.Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 18.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–256. doi: 10.1038/ng.3218. [**These important studies demonstrated that YAP/TAZ activities increase in tumors to confer resistance against oncogene-targeted therapies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MH, Kim J, Hong H, Lee SH, Lee JK, Jung E, Kim J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 2015 doi: 10.15252/embj.201592081. [*These important studies demonstrated that YAP/TAZ activities increase in tumors to confer resistance against oncogene-targeted therapies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CH, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, LaBarge MA. Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors. Mol Biol Cell. 2015;26:3946–3953. doi: 10.1091/mbc.E15-07-0456. [* These important studies demonstrated that YAP/TAZ activities increase in tumors to confer resistance against oncogene-targeted therapies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirata E, Girotti MR, Viros A, Hooper S, Spencer-Dene B, Matsuda M, Larkin J, Marais R, Sahai E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin beta1/FAK signaling. Cancer Cell. 2015;27:574–588. doi: 10.1016/j.ccell.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanconato F, Piccolo S. Eradicating tumor drug resistance at its YAP-biomechanical roots. EMBO J. 2015 doi: 10.15252/embj.201593584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Jo H, Hong H, Kim MH, Kim JM, Lee JK, Heo WD, Kim J. Actin remodelling factors control ciliogenesis by regulating YAP/TAZ activity and vesicle trafficking. Nat Commun. 2015;6 doi: 10.1038/ncomms7781. 6781. [DOI] [PubMed] [Google Scholar]
- 28.Kanellos G, Zhou J, Patel H, Ridgway RA, Huels D, Gurniak CB, Sandilands E, Carragher NO, Sansom OJ, Witke W, et al. ADF and Cofilin1 Control Actin Stress Fibers, Nuclear Integrity, and Cell Survival. Cell Rep. 2015;13:1949–1964. doi: 10.1016/j.celrep.2015.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohgushi M, Minaguchi M, Sasai Y. Rho-Signaling-Directed YAP/TAZ Activity Underlies the Long-Term Survival and Expansion of Human Embryonic Stem Cells. Cell Stem Cell. 2015;17:448–461. doi: 10.1016/j.stem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A. 2014;111:E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Wang Y, Zhu Y, Yuan C, Wang D, Zhang W, Qi B, Qiu J, Song X, Ye J, et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol Oncol. 2015;9:1091–1105. doi: 10.1016/j.molonc.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taccioli C, Sorrentino G, Zannini A, Caroli J, Beneventano D, Anderlucci L, Lolli M, Bicciato S, Del Sal G. MDP, a database linking drug response data to genomic information, identifies dasatinib and statins as a combinatorial strategy to inhibit YAP/TAZ in cancer cells. Oncotarget. 2015;6:38854–38865. doi: 10.18632/oncotarget.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karp I, Behlouli H, Lelorier J, Pilote L. Statins and cancer risk. Am J Med. 2008;121:302–309. doi: 10.1016/j.amjmed.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Mi W, Lin Q, Childress C, Sudol M, Robishaw J, Berlot CH, Shabahang M, Yang W. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. 2015;34:3095–3106. doi: 10.1038/onc.2014.251. [DOI] [PubMed] [Google Scholar]
- 36.Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang J, Mills GB. AMPK: a contextual oncogene or tumor suppressor? Cancer Res. 2013;73:2929–2935. doi: 10.1158/0008-5472.CAN-12-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Hayre M, Vazquez-Prado J, Kufareva I, Stawiski EW, Handel TM, Seshagiri S, Gutkind JS. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer. 2013;13:412–424. doi: 10.1038/nrc3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, Matsuo K, Squires KC, Coleman RL, Lutgendorf SK, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121:3444–3451. doi: 10.1002/cncr.29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/beta-catenin signalling. EMBO J. 2012;31:1109–1122. doi: 10.1038/emboj.2011.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 49.Byun MR, Hwang JH, Kim AR, Kim KM, Hwang ES, Yaffe MB, Hong JH. Canonical Wnt signalling activates TAZ through PP1A during osteogenic differentiation. Cell Death Differ. 2014;21:854–863. doi: 10.1038/cdd.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–718. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 51.Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009;461:614–620. doi: 10.1038/nature08356. [DOI] [PubMed] [Google Scholar]
- 52.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [ *This work identified the genomic regios bound by YAP/TAZ, and the downstream targets in malignant cancer cells. AP-1 was indentified as an essential factor cooperating with YAP/TAZ for oncogenic growth.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, Gurung B, Pepe-Mooney B, Zhang T, Geeven G, Gray NS, de Laat W, et al. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Mol Cell. 2015;60:328–337. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, Agarinis C, Schmelzle T, Bouwmeester T, Schubeler D, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015;11:e1005465. doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, Loh PG, Song H. Structural and functional insights into the TEAD-YAP complex in the Hippo signaling pathway. Protein Cell. 2010;1:1073–1083. doi: 10.1007/s13238-010-0138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pobbati AV, Han X, Hung AW, Weiguang S, Huda N, Chen GY, Kang C, Chia CS, Luo X, Hong W, et al. Targeting the Central Pocket in Human Transcription Factor TEAD as a Potential Cancer Therapeutic Strategy. Structure. 2015;23:2076–2086. doi: 10.1016/j.str.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, Bolouri H, Kutyavin VI, Morrissey C, True LD, et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell. 2015;27:797–808. doi: 10.1016/j.ccell.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H, Ramakrishnan SK, Triner D, Centofanti B, Maitra D, Gyorffy B, Sebolt-Leopold JS, Dame MK, Varani J, Brenner DE, et al. Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci Signal. 2015;8:ra98. doi: 10.1126/scisignal.aac5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Li H, Rajurkar M, Li Q, Cotton JL, Ou J, Zhu LJ, Goel HL, Mercurio AM, Park JS, et al. Tead and AP1 Coordinate Transcription and Motility. Cell Rep. 2016;14:1169–1180. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri A, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G, Pandolfi PP, Givol D, Strano S, Lu X, et al. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803–814. doi: 10.1016/j.molcel.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 65.Di Agostino S, Sorrentino G, Ingallina E, Valenti F, Ferraiuolo M, Bicciato S, Piazza S, Strano S, Del Sal G, Blandino G. YAP enhances the pro-proliferative transcriptional activity of mutant p53 proteins. EMBO Rep. 2015 doi: 10.15252/embr.201540488. [DOI] [PMC free article] [PubMed] [Google Scholar]